Abstract
Many studies on extraction, purification, and modification processes of natural dyes and antimicrobials, and their subsequent application on textiles in recent years, demonstrates the revival of natural dyeing and finishing. Natural dyes have been widely used in textile coloration since ancient times. However, with advent of man-made synthetic dyes in the mid-nineteenth century, the dye market has been captured due to variety of competitive properties of synthetic dyes. Such properties are lower cost, color variety, ability to dye synthetic fibers, and availability in large industrial scale. Subsequently the use of natural dyes fainted. However, most synthetic dyes raise serious problems for human health and cause environmental risks. As a consequence there is now a worldwide interest for the production of dyes from natural sources such as plants and microorganisms.
The use of natural dyes in textile processing is increasing because of higher dyestuff quality, environmental compatibility and lower costs. In addition many natural dyes have inherently antimicrobial properties. Natural dyes are extracted from microorganisms and plant organs such as bark, leaf, root, fruit, seed, and flowers. Natural dyes are not only a rich and varied source of dyestuff, but also could be considered as safe, environmentally friendly and low cost treatments with additional benefit of coloring in a single stage. In this article, we review extraction and application of natural dyes on textiles as effective coloring and antibacterial agents. Extraction and treatment methods are discussed
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
For centuries, natural colorants have been used for numerous purposes varying from tribal or funeral ceremonies, body painting, decorative art and clothing or domestic decoration. At first, colorants were mainly extracted from minerals, insects and plants (Guinot et al. 2006). But since 1856, the manufacture of synthetic colorants became more important as a result of: (I) moderate washing and light fastness of the natural colorants; (II) high cost of the production of natural dye; (III) the advancements in chemical synthesis and characterization techniques and; (IV) the greater production efficiency of synthetic dyes in terms of quality, quantity and the potential to produce low-cost raw materials (Samanta and Agarwal 2009). Consequently, natural colorants were increasingly replaced by synthetic ones, produced in cheaper ways, with moderate to excellent fastness. It is estimated that some 700,000 t of dyes are consumed annually and world production of pigments is also about five million tons, with inorganic pigments accounting for approximately 96 % (McCarthy 1997). Almost all these synthetic colorants being synthesized from petrochemical sources through hazardous chemical processes pose threat towards the environment and human body health (Kasiri et al. 2008; Safapour et al. 2010; Kasiri and Khataee 2011; Samanta and Konar 2011).
Global concern for environmental pollution has put stringent rules forward for high level pollutant industries. Textile industry with huge amount of hazardous wastewater production is one of such industries. Numerous researches in recent years have focused on development of totally new technologies and/or modification of conventional technologies for cleaner and environmentally friendlier production processes.
Hence, in the last few years the use of non toxic, antimicrobial and eco-friendly natural dyes on textiles, preferably natural fiber products, has become a matter of increasing importance due to the increased awareness of synthetic dyes environmental hazards (Hill 1997; Dawson 2009). Some of the advantages of natural dyes are eco-friendliness, i.e., they do not create any environmental problems at the stage of production or use and maintains the ecological balance (Sivakumar et al. 2011).
On the other hands, textiles, by virtue of their characteristics and proximity to human body, provide an excellent medium for the growth and multiplication of microorganisms, where they could find the required nutrition including; water, carbon, nitrogen and some inorganic salts. This could help microorganisms to transfer, propagation of infection and causing microbial diseases (Gupta and Bhaumik 2007).
Most of natural dyes have inherently antimicrobial properties and could consequently possess high medicinal activity. Natural dyes are extracted from different parts of plants including bark (e.g. Purple bark, Sappan wood, Shillicorai, Khair, Red and Sandalwood), leaf (e.g. Indigo, Henna, Eucalyptus, Tea, Cardamon, Coral Jasmine, Lemon Grass), root (e.g. Turmeric, Madder, Onions, Beet-root), fruits or seeds (e.g. Latkan, Pomegranate rind, Beetle nut, Myrobolan) and flower (e.g. Marigold, Dahlia, Tesu, Kusum) that contain coloring materials like tannin, flavonoids, quinonoids, etc (Fig. 6.1).
Natural dyes are extracted from different parts of plants such as (a) bark (Purple bark; http://www.wickedsmileys.com/GothicGreetingCards/index2.html), (b) leaf (Eucalyptus; http://herbs.ygoy.com/what-is-eucalyptus/), (c) flower (Marigold; http://flowerwallpapers.co/marigold/download-yellow-marigolds-flower-images-inpretty-orange-marigolds-wallpaper.html), (d) fruits (Pomegranate rind; http://www.anniesremedy.com/herb_detail518.php?gc=518b) and (e) root (Madder; http://microsites.merton.gov.uk/riverandcloth/image/madder-roots.html)
The natural dyes also come from some types of microorganisms such as fungi, algae and bacteria. These dyes can offer not only a rich and varied source of dyestuff, but also could be considered as safe, environmentally friendly and low cost treatments with additional benefit of coloring in a single stage (Samanta and Agarwal 2009; Samanta and Konar 2011).
Application of natural dyes has, of course, some limitations. It is difficult to reproduce shades by using natural dyes. Natural dyeing requires skilled workmanship and is therefore expensive. They have poor fastness and the colors produced are of poor quality. Moreover, these dyes require time-consuming extractions, are difficult to use and the dyed textile may change color when exposed to the sun, sweat and air (Hill 1997).
For using natural dyes in an industrial scale, the appropriate and standardized extraction methods and dyeing techniques must be developed without sacrificing the required quality and quantity of dyed textiles. Therefore, to obtain stable shades with acceptable color fastness behavior and reproducible color yield, appropriate extraction, identification, dyeing and finishing techniques need to be derived from the current scientific studies.
In this review, an attempt has been made to give the latest scientific overview on extraction and application of natural dyes/antimicrobials on textiles as effective coloring and antibacterial agents. Different methods of extraction of natural dyes/antimicrobials will be discussed and examples of early applications of these dyes on textile processing, properties, advantages and disadvantages will be reviewed.
6.2 Natural Dyes/Antimicrobials
Natural dyes/antimicrobials are dyes, colorants, and agents derived from natural resources. The resurgence of natural dyes in the early twenty-first century is due to some their peculiar benefits. However, there are some drawbacks restricting the application of these beneficial natural materials (Dawson 2008). In this section, some main benefits and restrictions of natural dyes/antimicrobials have been summarized.
6.2.1 Benefits
According to the results of previous works, growing global interest toward dyes/antimicrobials from natural resources is due to some main following benefits:
-
Some natural dyes have intrinsic additional properties such as antibacterial, moth proof, anti-allergy, anti-ultra violet, etc.
-
Natural dyes produce different uncommon, eye-catching, and soothing shades on textiles.
-
Wide range of shades can be produced with an individual natural dye either in mixture with mordants or by change in dyeing condition.
-
Different natural constituents along with natural dye may enhance dyeing/finishing process efficiency as auxiliary, mordant, fixing component.
-
Natural dyes/antimicrobials are obtained from inexpensive renewable resources with huge potential.
-
From two aspects, natural dyes/antimicrobials are environmentally friendlier than synthetic ones: first, in natural materials all synthesis processes are accomplished by nature with no pollution of environment. Second, these materials are readily biodegradable and do not produce hazardous wastewater upon degradation in environment, and so, there is no need for treatment of wastewater before discharging into the environment.
-
Majority of natural dyes/antimicrobials are extracted from wild and self growing plants with no additional cost for their cultivation.
-
Color characteristics of some natural dyes mellow with time. This characteristic imparts unique properties and appearance to the stuff dyed, e.g., old carpet piles dyed with natural dyes.
-
Most of natural antimicrobial components do not have negative effects on beneficial non-target microorganisms.
-
Most of natural antimicrobials do not have side effects on human body.
-
Extraction, purification, and application of natural dyes/antimicrobials have become easier and more possible with different modern characterization techniques developed.
6.2.2 Restrictions
Despite various benefits of natural dyes/antimicrobials, they have some drawbacks limiting their application on textiles. Some important restrictions can be summarized as follows:
-
Generally, natural dyes/antimicrobials have low wash, rub, light, sweat, and gas fastness on textiles probably due to weak bonding, weak interaction, etc. with textiles.
-
To improve fastness of natural dyes/antimicrobials, some additional chemicals are used such as metallic mordants, crosslinking chemicals, etc. Discharge of wastewater of dyeing and finishing processes containing these hazardous materials poses some serious environmental risks.
-
Only little information is available about extraction, purification, chemical structure, and application methods of natural dyes/antimicrobials onto textiles.
-
Color matching and reproduction of color is another disadvantage of natural dyes since the quantity and quality of natural materials steadily change with climate, plant genus, etc.
-
There is no standard method of natural dyeing.
-
The efficiency of the natural dyes/antimicrobials extraction process is generally low, only few grams of natural component per kg of raw materials are normally obtained.
6.3 Extraction of Natural Dyes
Extraction of dyes/antimicrobials from the natural sources could be one of the most important steps for treatment of textiles to achieve the desired dyeing properties and/or antimicrobial activities. Moreover, obtaining a standard extraction process and optimizing the extraction variables for a particular natural source, are economically important and consequently, affect the end-products price.
Natural dyes/antimicrobials could be extracted from different types of microorganisms as well as various parts of the plants such as bark, leaf, root, fruit, seed, flower, etc. There are different methods to extract these materials from above-mentioned natural sources including; aqueous extraction i.e. using water for the extraction with or without addition of salt/acid/alkali/alcohol in the extraction bath, chelating with alum and precipitating with acid, enzyme assisted extraction, alcoholic/organic solvent extraction by using relevant extracting equipment, such as soxhlet apparatus, with use of alcohol, hexane or benzene solvents, adsorption of the pigments on silica gel and finally, ultrasonic extraction method. In the following paragraphs, four main important techniques, i.e. aqueous extraction methods, alcoholic/organic solvent extraction, ultrasonic extraction and enzyme assisted extraction will be reviewed.
6.3.1 Aqueous Extraction Methods
Due to the chemical structure of the most natural dyes/antimicrobials, simple set-up and cost-effective aqueous extraction method is the most widely used extraction technique. Routinely, dried and finely cut materials of natural source are grinded in powdered form and then the dye/antimicrobial is extracted in water employing different standard processes.
For instance, the aqueous solution could be filtered and concentrated under reduced pressure (rotary evaporator) to give a crude extract (Farizadeh et al. 2009).
Extraction time, pH of the solution, and temperature are the most important parameters influencing the yield of extraction. In an interesting work, the natural dye was extracted from petals under different operating conditions such as extraction time (45–120 min), temperature (60–90 °C) and mass of the petals (0.5–2 g) by conventional extraction technique, where response surface methodology (RSM) was used for optimization of the extraction process (Sinha et al. 2012).
6.3.2 Alcoholic/Organic Solvent Extraction Methods
The organic nature of some dyes/antimicrobials from natural sources implies the application of non-aqueous extraction methods. During this method, a mixture of organic solvents, usually alcohols with hexane, is used for extraction of natural agents.
As an example, the roots of the authentic sample of Ratanjot were air-dried and ground to coarse powder. The powdered roots were extracted with n-hexane in soxhlet apparatus (Fig. 6.2) till the color of the decoction became very light. Solvent was removed and the percentage yield of the hexane extraction from roots was estimated (Arora et al. 2009).
A schematic representation of a soxhlet extractor; (1) stirrer bar (2) still pot (3) distillation path (4) thimble (5) solid (6) siphon top (7) siphon exit (8) expansion adapter (9) condenser (10) cooling water in (11) cooling water out (http://en.wikipedia.org/wiki/Soxhlet_extractor)
Similarly, for extraction of carminic acid, a natural colorant that can be obtained from the dried bodies of females of the Dactylopius coccus Costa insect species, Borges et al. (2012) have tried the following method. Three different mixtures of solvents i.e. methanol:water, ethanol:water and pure ethanol were employed to achieve extracts with different compositions. Moreover, extractions were performed at three different extraction temperatures (100 °C, 150 °C, and 200 °C) and 30 min as extraction time. All extractions were performed in 11 ml extraction cells, containing 2.0 g of sample.
Another example has been explained at flavonoid dye extraction from marigold flower (Guinot et al. 2008). Dried ground flowers were soaked by decoction for 10 min in distilled water (1:100 w/v). Extracts of the dried ground flowers were obtained by maceration (1:100 w/v) at 60 °C for 30 min and 60 min under mechanical stirring (180 rpm). The solvents used were ethanol:water mixtures of various compositions, i.e. 2:8, 3:7, 5:5 v/v. The resulting suspensions were filtered through gauze and the filtrates were made up to their initial volume with the appropriate solvent. Finally, 2 ml of filtrate were centrifuged for 10 min (7,176 g) before analysis.
6.3.3 Ultrasonic Extraction Method
State of arts process like ultrasonic, enzymatic and enzyme mediated ultrasound extraction techniques are nowadays widely being studied for their high efficiency over the conventional methods. In this method, the energy of the ultrasonic waves enhances the yield of extraction.
An example of application of this extraction technique has been explained by Mishra et al. (2012). The petals of the flowers were picked and dried at 50 °C in tray drier. Methanol (15 ml/g of petals) was added in dried petals and then pH was adjusted to the desired value. The beakers was then placed in ultrasonic bath and sonicated for 5–30 min at 27–30 MHz and 160 V. After sonication, the contents of beakers were filtered through standard test sieve to remove solid materials. The filtrated dark red liquid was vacuum evaporated in a rotary vacuum evaporator to about half of the original volume and then, the concentrated red liquid was spray-dried.
In this method, extraction bath pH, salt concentration, ultrasonic power, extraction time and temperature are the most influencing parameters. Kamel et al. (2005) have studied the extracting of lac as a natural dye with ultrasonic assisted method varying amounts of the dye materials (2–12 %) at different temperatures (50–80 °C) using different ultrasonic powers (100–500 W) and for different time intervals (20–120 min), yielding different amount of dyeing agent.
Similarly, plant extracts (1:100 w/v) were obtained from dried leaves and stems in ethanol/water (3:7 v/v) with ultrasonication (15 min, 24 kHz, R.E.U.S-GEX 180). After passing through filter paper, the filtrate was centrifuged for 10 min (7,176 g) (Guinot et al. 2009).
As another example of ultrasonic extraction described by Sivakumar et al. (2011), 1 g of the plant samples was taken and added to 50 ml water taken in the beaker. The beaker was covered using aluminum foil to prevent loss of solvent by evaporation. The ultrasonic probe was positioned in the beaker and the ultrasound power set at 80 W, while the temperature was maintained at around 45 °C.
6.3.4 Enzyme Assisted Extraction Method
As a recipe for enzymatic extraction technique, a 2 % solution of pectinase:cellulase (2:1) was sprayed on pomegranate rind (25 g) for better soaking and contact and then, left overnight. The enzyme treated material was washed with little amount of distilled water and pH of the solution was adjusted to 10. The sample was shaken at 150 rpm for 20–80 min at desired temperature. The contents of beakers were filtered through standard test sieve to remove solid materials and the concentrated dye extract was vacuum evaporated in a rotary vacuum evaporator to about half of the original volume and finally, the concentrated liquid was spray-dried (Tiwari et al. 2010).
6.4 Application of Natural Dyes/Antimicrobials in Textiles
6.4.1 Treatment Methods
In application of materials from natural sources onto textiles, fastness properties such as wash, light, perspiration, etc. are often in low and undesired level. This can be due to heterogeneity, diversity and complexity of natural matters as well as their weak or negligible interactions with textiles. Low fastness properties limit the application area of such useful, eco-safe, low cost natural materials in the treatments of textiles in spite of their various beneficial properties. Hence, to surpass this drawback, additional treatments are often needed to enhance the durability and value of textile products. Treatment processes can be performed on textiles (Das et al. 2007; Pruthi et al. 2008; Ammayappan and Moses 2009; Çalıs et al. 2009; Giri Dev et al. 2009; Kiumarsi et al. 2009; Mongkholrattanasit et al. 2011) or on natural matters (Iqbal et al. 2008; Syamili et al. 2012) or on both (Iqbal et al. 2008), where the first one is more common and frequently used. In literature survey to prepare the present review, we have found some interesting treatment methods to enhance the properties and values of final products. Although addressing all available treatment methods is not possible here, some important examples will be briefly reviewed in the following paragraphs.
6.4.1.1 Structure Modification of Textiles
In the dyeing process with indicaxanthin natural dye, Guesmi et al. (2013) used modified acrylic fibers with amino and hydroxyl functional groups. They reported that the affinity of the dye and dyeability of fibers were strongly dependent on the pH value of dyeing bath with the highest amount of dye up-take at pH 3. It was attributed to the action of functional groups available in both fiber and dye structures controlling ionic interaction of carboxyl groups of dye with the protonated amino groups of the modified acrylic fibers.
6.4.1.2 Treatment with Chemicals
In a series of studies, cotton was dyed with Berberine as natural colorant (Kim et al. 2004; Kim and Son 2005; Son et al. 2008). Berberine is a quaternary ammonium salt and strong yellow colored natural dye with both dyeing and antimicrobial properties (Fig. 6.3). Berberine has high affinity for protein fibers such as wool and silk whereas it shows low affinity for cellulosic fibers because of lack of ionic charges on cotton surface compared to protein fibers. Accordingly, cotton was reacted with an anionic bridging agent to produce negative charges on the surface, being responsible for electrostatic interactions with cationic natural dye, in order to enhance the affinity of Berberine to cotton. Dyeing tests demonstrated significantly enhanced properties in both Berberine exhaustion and fastness properties.
In another work, Glyoxal as crosslinking chemical were employed to enhance the application of Azadirachta indica, a natural antibacterial agent, on polyester/cotton blend fabrics (Joshi et al. 2007). Antibacterial properties retained after different wash cycles demonstrated the efficiency of chemicals in crosslinking yield of bioactive matter on the substrate. Similarly, citric acid was used as crosslinking agent in antibacterial finishing of cotton with green tea leaves extracts to increase the durability against wash (Syamili et al. 2012).
Another example of treatment with chemicals is the work of Ammayappan and Moses (2009). They reported that pre-treatment of cotton with hydrogen peroxide and wool fibers with formic acid increase antibacterial activity of fibers treated with aloevera, chitosan, and curcumin as natural antimicrobials. It was attributed to the increase in the accessible areas in the case of cotton, and disulphide linkage modification and change in wettability in the case of wool/hair fibers. Moreover, additional reasons stated were the increase in the quantity of antimicrobials absorbed and decrease of impurities for all fibers after treatment with selective chemicals.
6.4.1.3 Treatment with Chitosan
Chitosan is a linear polysaccharide produced commercially by deacetylation of chitin (Fig. 6.4), the structural element in the crustaceans such as crabs and shrimp and cell walls of fungi. It has been widely used in various industries because of its multifunctional properties. Chitosan is also extensively used in treatment of textiles such as dyeing, finishing, printing, etc.
Chemical structure of chitosan, a linear polysaccharide
For instance, chitosan has been used in pre-treatment of cotton fibers prior to dyeing with lac dye (Rattanaphani et al. 2007). Due to lack of cationic sites in cotton fibers, lac dye has low affinity for cotton. It was shown that treatment with chitosan produced cationic sites on cotton and subsequently the affinity of lac dye toward fiber, dye up-take, and fatness properties have been enhanced. It was discussed that chitosan could be a suitable eco-friend alternative in treatment of cotton compared to use other toxic chemicals.
In another work, cotton has been pre-treated with chitosan and then dyed with turmeric (Kavitha et al. 2007). Treatment with chitosan enhances antibacterial activity and improves some mechanical properties of fibers. Similar attempt has been made to pre-treat wool fibers with this biopolymer prior dyeing with henna dye (Giri Dev et al. 2009). Increasing in henna dye up-take and antimicrobial finish were properties imparted to wool fibers after treatment with chitosan.
6.4.1.4 Treatment with Enzyme
Protease is an enzyme, biological molecules that catalyze chemical reactions, that conducts proteolysis. In the study of antimicrobial characteristics of some natural dyes on wool fibers, effects of protease enzyme treatment on dyeing and antimicrobial yield were studied (Raja and Thilagavathi 2011). It was demonstrated that alkaline protease enzyme process enhances the quantity of natural dye exhausted. It was ascribed to partial or complete damage of cuticle facilitating easier penetration of natural dye molecules into fibers.
6.4.1.5 Treatment with Ultraviolet (UV) Radiation
Iqbal et al. (2008) have studied the effect of UV irradiation on the henna dyed cotton fabrics. Henna and cotton were separately UV irradiated and then after coloration, the results were compared with non-irradiated samples. At first step, untreated fabrics were dyed with both virgin and irradiated henna. It was observed that both color strength and shade of fabrics dyed with irradiated henna are higher compared to non-irradiated henna. It was concluded that lawsone, the coloring matter in henna, upon irradiation is hydrolytically degraded and thereby considerable increase in the sorption of degraded dye occurs. In the next step, effect of radiation treatment of cotton substrate on its dyeability with henna was investigated and similarly higher color strength values were obtained for treated samples. It was inferred that increase in the interaction between dye molecules and cellulose macromolecules, resulted from developed spaces within fibers after irradiation, was responsible for higher dye up-take values. Finally, Iqbal et al. (2008) concluded that to obtain the highest color strength both henna dye and cotton must be UV irradiated.
6.4.1.6 Treatment with Ultrasound
Nowadays, ultrasound energy is exploited in dyeing of textiles so-called sonicator dyeing. Sonicator dyeing has some significant advantages over the conventional dyeing, e.g., decrease in temperature and time of dyeing, and decrease in quantity of dye and electrolyte required. Wool fibers were dyed with lac as a natural dye using both conventional and ultrasonic techniques (Kamel et al. 2005). About 47 % increase in lac dye up-take was reported using ultrasonic dyeing. Also, the amount of dye extracted by ultrasonic technique was 41 % higher than conventional extraction methods. Similar study was conducted on the dyeing of cationized cotton fabric with same dye using both conventional and ultrasonic methods, exhibiting 66.5 % improvement in lac dye up-take using ultrasonic dyeing technique (Kamel et al. 2007).
In another study, cotton was dyed with natural dye Eclipta using both conventional and sonicator dyeing. The efficiency of sonicator dyeing was reported to be 7–9 % higher than conventional method (Vankar et al. 2007).
Guesmi et al. (2013) also compared dyeing properties of modified acrylic fibers with indicaxanthin natural dye using both conventional and sonicator dyeing and reported 49.62 % higher natural dye up-take, better wash and light fastness with less process time and energy consumption for sonicator dyeing technique.
Similarly, improved natural dye exhaustion, fastness properties as well as waste reduction of dyeing were reported in the ultrasonic dyeing study of cotton with natural dye from plant species Symplocos spicata (Vankar et al. 2008).
6.4.1.7 Treatment with Plasma
Plasma treatment is a modern method of textiles treatment. Plasma is the fourth state of the matter in the form of partly ionized gases. When a gas under controlled pressure interacts with electromagnetic field, plasma is produced. Plasma technology is gradually being replaced by chemical methods in treatment of textiles. Low-temperature plasma is widely used in non-destructive surface modification of textiles where a wide range of properties can be obtained. Ghoranneviss et al. (2010) have studied dyeing properties of plasma treated wool fibers with two natural dyes, namely, madder and weld. It was proved that the treatment of wool fibers with plasma enhances natural coloration process and properties. Moreover, plasma treatment was introduced as a suitable replacement for metallic mordant treatment with some additional benefits. Chen and Chang (2007) used low temperature microwave plasma to treat cotton fabric prior to grafting with onion skin and onion pulp extracts in order to impart antimicrobial properties to cotton fabrics. It was discussed that functional groups produced on the fiber surface after low-temperature oxygen plasma treatment significantly increase the hydrophilic nature of cotton and thereby the grafting yield of both onion skin and pulp extracts enhances.
6.4.2 Examples of Early Applications
Numerous reports have been published on natural dyes/antimicrobials application, including variability in the quantity and quality in the natural source, their use in combinations, their dyeing and antimicrobial properties, the effect of mordants and auxiliaries, their fastness, improvements in extraction methods and the development of alternative sources. Natural dyes/antimicrobials are extracted mainly from two different resources: plants and microorganisms. In the following parts, examples of early applications of dyes/antimicrobials from these two resources will be reviewed.
6.4.2.1 Dyes/Antimicrobials from Plants
Plants are the major source of natural dyes/antimicrobials in the nature. These materials are extracted from different parts of the plants such as bark, leaf, root and flower containing commonly coloring materials like tannin, flavonoids, quinonoids, etc. During the following paragraphs, the examples of applications are classified according to the different parts of plants used as the natural source.
6.4.2.1.1 Root
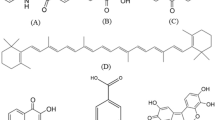
Root is one of the common parts of the plants that has been extensively used for dyes/antimicrobials extraction (Table 6.1). Madder, the ground root of the Rubia species with anthraquinone substituted chemical structure, has traditionally been an important natural source of red and brown color on wool and cotton along with different mordants (Gupta et al. 2001a, 2004a). Chemical structure of some colorants presented in madder root is shown in Fig. 6.5.
Gupta et al. (2001a) have tried the dyeing of nylon with the pigment extracted from indian madder. Nordamncanthal, a bright red pigment with a hydroxyanthraquinone chemical structure, was extracted from madder by chelating with alum and precipitating with acid. This pigment was used for dyeing of nylon and polyester, and the results showed good affinity for the fibers studied.
The authors have also studied the dyeing process of nylon with another extract of madder (Gupta et al. 2001b). Purpurin with 1,2,4-trihydroxyanthraquinone structure was extracted from madder in a similar way and was used for dyeing of nylon fabrics. The authors have reported that this red pigment has also good affinity, like nordamncanthal, for the nylon fibers.
The light fastness of madder extracts applied on nylon has been reported in a related work (Gupta et al. 2004a). The authors have extracted two pigments, purpurin and munjistin, from the indian madder by soaking and extraction with alum and applied it for dyeing of nylon with ferrous sulphate, copper sulphate, alum and stannous chloride as mordants. The authors have reported a good light fastness for the fabrics while the dye fastness of fabrics with munjistin was poor.
Extraction of dyes from madder at different conditions has been studied by Farizadeh and his colleagues (2009), too. The extracted pigments with distilled water were analyzed by thin layer chromatography (TLC) and high performance liquid chromatography (HPLC) techniques and identified as alizarin, purpurin and 1,4-dihydroxyanthraquinone. Extracted pigments were used for dyeing of wool fabrics at different temperatures and dyebath pHs and the results proved that the affinity of the dye on the fabrics dependent mainly on the pH and temperature of the bath, where increasing of temperature led to decrease the values of partition ratio and affinity.
In an attempt, dyeing process of wool with madder and liposomes as an auxiliary in dyeing has also been optimized (Montazer et al. 2007). The pigment were extracted with steeping the madder in water, heated and passed through a filter. The dyeing process was optimized by response surface methodology, where the bath temperature, time, and liposome percentage were chosen as input variables and the fabrics color strength as the response. The dyeing process was optimized, but it was also found that wash, light, wet and dry rub fastness properties of samples dyed with madder including liposomes have not changed significantly.
On the other hand, wool yarns were dyed with madder along with aluminum potassium sulfate as the mordant and then treated with different ammonia solutions by Montazer and Parvinzadeh (2004). It has been reported that a change of color is observed on the dyed samples when wash-fastness tests were carried out, while the light-fastness tests showed more fading of the madder-dyed yarns after ammonia treatment.
They have also studied the effect of ammonia on the coloristic properties of wool dyed with madder and other natural dyes containing anthraquinone, naphthaquinone, flavone and tannin structures (Montazer et al. 2004). Wool was first mordanted with aluminium potassium sulphate and then dyed using madder, cochineal, walnut husks, weld, red and white onion skins, and pomegranate shells. The results showed that the color changes could be due to the extension of dye molecule conjugated system by one lone pair electron in ammonia solution.
Ratanjot, Arnebia nobilis Rech.f is the root of herbal plant that has been widely used as a potent resource for obtaining both herbal drugs and color matter (Arora et al. 2009). It contains a variety of naturally occurring chemicals, mainly a naphthoquinone derivative called alkannin (Fig. 6.6). Alkannin has a deep red color in a greasy or oily environment and a violet color in an alkaline environment (Papageorgiou et al. 1999).
Arora et al. (2009) have tried to extract coloring matters from Ratanjot. Due to variety of plant species, they used different identification methods such as macroscopic, microscopic, chemical and TLC analysis of the roots. The extraction was performed using soxhlet system with n-hexane and the TLC analysis showed the presence of five naphthoquinone derivatives with red and pink colors.
In another work, Arora et al. (2012a) have used ratanjot extract for coloration of variety of textiles, i.e., cotton, wool, silk, nylon, polyester, and acrylic. Interestingly, the strong sensitivity of extracted natural dye solution to pH and temperature changes was observed. With gradual increasing of pH in the range 3–12, continuous changes in color from red to purple and then to dark blue was observed. It was attributed to the conversion of dye structure from quinonoid in acidic solution to benzenoid in alkaline solution. Dyeing results showed that as the dyeing pH increases, the dye up-take decreases which was ascribed to decomposition of dye structure in alkaline solution.
Wool, cotton, silk, nylon, polyester, and acrylic were dyed with ratanjot extract in similar condition and different shades were obtained such as pink on polyester, blue on nylon, and purple on the rest fibers. Different results for affinity and dye up-take were observed for fibers. Very low dye up-take on cotton fabric was attributed to non-polar character of dye and low affinity to cotton fibers. Wool had higher dye up-take compared to silk possibly because of lower crystallinity of wool fibers. Polyester showed lower dye up-take at 130 °C compared to 100 °C, not due to lower affinity of the dye to polyester but owing to more decomposition of dye at enhanced temperatures decreasing effective dye concentration in the solution (Arora et al. 2012a).
Interestingly, by means of oxidation/reduction test, the reason of completely different observed shades was addressed to be the structure transformation of dye from normal state (quinonoid) to the reduced reversible state (benzenoid) on all substrates. Fastness tests data demonstrated poor light fastness whereas wash, rub and perspiration fastnesses were excellent. Finally, the findings of the study showed that Arnebia nobilis Rech.f can be used as a good natural dye resource for dyeing and production of variety of eco-friend textiles where light fastness is not key factor in dye selection (Arora et al. 2012a).
Using Arnebia nobilis Rech.f dye, kinetics and thermodynamics of dyeing on wool has also been studied by Aora et al. (2012b) and the results were compared with those of juglone, lawsone, and Rheum emodi from literature. Lower diffusion coefficient than other three natural dyes with exothermic dyeing process was reported for this dye.
Turmeric, Curcuma longa, is a rhizomatous herbaceous perennial plant of the ginger family native to tropical south Asia. The dried rhizomes are ground into a deep orange-yellow powder commonly used as spice in cuisine and for dyeing. Turmeric’s active ingredient is curcumin which is the principal curcuminoids, natural phenols that are responsible for the yellow color of turmeric. Other two curcuminoids present in turmeric are desmethoxycurcumin and bis-desmethoxycurcumin, known as curcumin I-III (Chan et al. 2009). Chemical structures of curcuminoids are shown in Fig. 6.7.
Turmeric has been used to impart either color or antibacterial property on textiles (Popoola 2000; Han and Yang 2005; Kavitha et al. 2007; Ammayappan and Moses 2009). Han and Yang (2005) used curcumin as antimicrobial finish for wool against two bacteria Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) with no additional treatment through conventional dyeing process. Very low concentration of curcumin was effective in bacterial growth inhibition on wool, e.g., only 0.05 % and 0.2 % curcumin were able to impart inhibition rate of 70 % and 95 % against E. coli, respectively. An equation was also established with good accuracy to predict the inhibition rate of bacteria growth using color strength values (K/S). The advantage of the developed equation was in the measuring of antibacterial property indirectly with no need to perform antimicrobial tests.
Using chitosan and turmeric, an attempt has also been made to develop green textiles for medical application. Kavitha et al. (2007) have studied the effects of chitosan pretreatment and subsequent dyeing with turmeric on the properties of cotton yarns. Improvements in mechanical properties such as tensile strength, flexural rigidity, and shear strength as well as natural dye up-take were achieved through chitosan treatment. Excellent antibacterial activity against two pathogenic bacteria, E. coli and S. Aureus, was another beneficial result of the treatment of cotton yarns with chitosan and turmeric.
In an interesting attempt to develop an eco-friend antibacterial finish using materials from green resources, curcumin along with two other natural bioactive agents, aloevera and chitosan, have been applied lonely or in binary or in ternary combinations on cotton, wool and rabbit hair (Ammayappan and Moses 2009). Then, their bactericidal properties in both pretreated and untreated substrates were examined. It was found that antibacterial activity of substrates finished after pretreatment with selective chemicals is higher than directly finished fabrics. Such behaviors were explained through enhanced wettability, critical surface tension change, and available area in the fiber responsible for better diffusion, adhesion and up-take of antibacterial agent.
When natural antibacterials applied individually on substrates, the antibacterial activity was in descending order: aloevera > curcumin > chitosan (Ammayappan and Moses 2009). The higher antibacterial activity of aloevera was concluded to be related to diverse ingredients, such as acemannon, anthraquinone, salicylic acid, amino acids, zinc, saponins, and many others compared to curcumin with individual benzenoid structure (only phenolic groups) and chitosan with polysaccharide structure (only hydroxyl and amino groups). Antimicrobial performance of binary or ternary combined agents was much better than corresponding individuals. It was explained through some determining factors, e.g., (I) the number and concentration of components present in natural material; (II) the number and diversity of functional groups and their determining role on affinity/binding to fiber; (III) Their mutual interaction with other chemical components and/or fibers and; (IV) synergistic, antagonistic or neutral effects of ingredients on each other in combined applications. Wash fastness tests proved that antibacterial finish durability on substrates are quite high where up to 25 wash cycles completely retained.
Choo and Lee (2002) have also tried to recreate traditional korean dyeing methods using colorants extracts from nine plants, used either singly or in combination, to produce a wide range of colors. In addition to madder, the extracts of indigo, turmeric, sapanwood, phellodendron, chinese yellow berries, gardenia, safflower and gromwell were used as dyeing agents and the fibers dyed were studied by microchemical tests, visible spectroscopy, thin layer chromatography and high performance liquid chromatography.
In a similar study, reconstruction of traditional textile dyeing techniques was the subject of the scientific research done by Zarkogianni et al. (2010). In this work, extractions of cochineal, madder, alkanna, henna, brazilwood, red sandalwood, safflower, indigo and logwood were obtained by aqueous extraction of the powdered mixture of plant/insect. These nine pigments were used for dyeing of wool and cotton fabrics after mordanting of the textile material with a variety of metallic salts. The results demonstrated significant improvement of the dye adsorption on the cotton fabrics and very good washing fastness of the wool samples.
The antimicrobial properties of 11 natural dyes from madder and other plants against three types of gram-negative bacteria were studied experimentally in an interesting work (Gupta et al. 2004b). The pigments were extracted from different parts of acacia, indigo, lac, kamala, pomegranate, gall nuts, cutch, myrobolan, himalayan rhubarb, indian madder and golden dock and were used for dyeing of wool fibers. The authors have reported that certain dyes are able to reduce microbial growth almost completely in the case of Escherichia coli and Proteus vulgaris, and since the selected dyes showed reasonably good wash fastness it could be assumed that the antimicrobial effect will be durable in practice.
The roots of a chinese herb, Rhizoma coptidis, is a source of natural cationic yellow colorant called berberine (Fig. 6.7). In a research work by Ke et al. (2006), the plant root was minced and dipped in distilled water for 30 min, then boiled in distilled water to extract the brownish yellow pigment and used for dyeing of wool fibers with mordanting.
Wool fabrics dyed with extraction of R. coptidis under different conditions showed various yellow colors with deep shades and good antibacterial properties, but the washing fastness of dyed fibers was poor.
Berberine has been similarly used as colorant and antimicrobial finish on nylon 66 fibers (Son et al. 2007). It imparted excellent bactericidal property to nylon fibers. It was concluded that finishing with berberine could be practical, eco-friendly, very simple and effective finish.
Das et al. (2008) carried out an interesting research on the dyeing of protein fibers using a natural dye. Wool and silk fibers were dyed with Rheum emodi extracted dye with/without Mn, Al, Fe metallic mordants and various shades from yellow to olive green were obtained. Chrysophanic acid, an anthraquinone derivative, is the main coloring matter of Rheum emodi whose structure is shown in Fig. 6.8. Interestingly, dyeing results exhibited no changes in the dye up-take of this dye in the pH range 4–8. Another finding was the unusual higher dye up-take, dye affinity, and dyeing rate of silk than wool fibers. The dyeing mechanism was shown corresponded to partition mechanism similar to those of disperse dyes.
It was concluded that those observations were related to typical structure of dye component behaving like disperse dyes in the dyeing process. Wash and light fastness of dyed fibers were moderate where wash fastness was further enhanced with iron mordant.
6.4.2.1.2 Seed
Seed of the plants could be a rich source of natural dyes/antimicrobuals (Table 6.2). Bixin is a natural dye which has been exploited in yellow to orange coloration of textiles. It is an apocarotenoid found in annatto, a natural food coloring obtained from the seeds of the achiote tree, Bixa orellana. Annatto seeds contain about 5 % pigments, which consist of 70–80 % bixin. The chemical structure of bixin is shown in Fig. 6.9. Bixin is chemically unstable when isolated and converts through isomerization into trans-bixin, double-bond isomer. This extract is soluble in fats but insoluble in water. Upon exposure to alkali, the methyl ester is hydrolyzed to produce the dicarboxylic acid norbixin, a water-soluble derivative.
Chemical structure of bixin found in annatto seed
Wool and silk have been dyed using bixin natural dye with/without metallic mordants (Das et al. 2007). Bixin colorant was extracted from the seeds of annato in alkali solution. Wool and silk in the fiber and fabric form, respectively, were first pre-treated, then pre-, post- and simultaneous mordanted, and finally dyed with (10 % on weight of fiber) annatto solution at 90 °C at wide range of pH 2–10. The shades obtained were from yellow to brown depending on the mordants employed.
Annato dye up-take was increased when dyeing bath pH decreased and maximum dye up-take and color strength was obtained at pH 4.5. Bixin with carboxylate groups can be considered as an acid dye (Fig. 6.9). It was discussed that with decreasing pH, the amino groups in protein fibers were protonated, ionic interaction of bixin carboxylate anions with amino cations increased, and as a result the affinity and dye up-take increased (Das et al. 2007). Interestingly, the color imparting ability of mordants for both wool and silk fibers was found to be in the descending order with bixin dye: ferrous sulphate > aluminium sulphate > magnesium sulphate > no salt. It was attributed to the ease and rapid salt formation of iron and aluminium with bixin compared to magnesium.
Generally low light fastness was reported for bixin on both wool and silk fibers. After mordants incorporation through pre- and post mordanting, light fastness was enhanced by one point scale. It was ascribed to the good complex formation ability of iron with tannin, associated with bixin, and subsequent deposition on the fibers. However, such improvement was not that much marked in simultaneous mordanting due to early combination of iron with dye in the solution before sorption with fibers. Magnesium did not improve the light fastness. Wash fastness data were in the range 2–3 without mordants. Wash fastness was improved by one point scale only in pre-mordanting condition (Das et al. 2007).
Popoola (2000) have studied bixin and curcumin in the coloration of cotton and compared their fastness properties. Bixin seeds and turmeric roots were used for extraction of coloring matter using soxhlet apparatus reflux system with n-hexane and methanol as extracting solvents, respectively. Afterwards, dyeing was performed without any mordant. The produced shades on cotton were yellow, orange and brown for bixin and yellow for curcumin. Both natural dyes had moderate light and alkaline wash fastness on cotton but, overall curcumin had higher light and alkaline wash fastness than bixin in identical conditions. It was attributed to the difference in chemical structure of coloring components, bixin with extended open-chain conjugated structure making it less stable against light and related photo-reactions compared to curcumin with two benzenoid rings, giving rise resonance stability, in its structure. Alkaline wash fatness data of both dyes were slightly higher than medium. But, similar to light fastness, curcumin dyed cotton had slightly higher wash fastness probably due to less solubility in equal alkaline condition.
In another report, Sathianarayanan et al. (2011) have tried to impart antibacterial finish on cotton fabrics using the bio-active ingredients extracted from ban-ajwain seeds, Thymus serpyllum. The extract was applied using different methods such as direct application, micro-encapsulation with acacia gum, crosslinking with resin, and the mixture of them. In direct application method, durability of finish against wash was very low. Wash fastness was enhanced with crosslinking and micro-encapsulation, however, some physical/mechanical properties were lost. Combined method gave the best fastness results so that the finish was durable up to 15 wash cycles.
6.4.2.1.3 Leaf
In some researches, leaves of different plants have been used as color resource (Iqbal et al. 2008; Ali et al. 2009; Giri Dev et al. 2009; Mongkholrattanasit et al. 2010, 2011) (Table 6.3). Henna, Lawsonia inermis, is a shrub or small tree frequently cultivated in India, Pakistan, Egypt, Yemen, Iran and Afghanistan. Henna has been used since ancient times to dye skin, hair, fingernails, leather, silk and wool. Henna’s coloring and antimicrobial properties are due to lawsone (2-hydroxy-1,4-naphthoquinone), an organic compound also known as hennotannic acid (Fig. 6.10). Lawsone is a red-orange dye primarily concentrated in the leaves, especially in the petioles of the leaf. Lawsone reacts chemically with the protein known as keratin in skin and hair, in a process known as Michael addition, resulting in a strong permanent stain that lasts until the skin or hair is shed. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning and sunscreens.
As an example of application, the extract of henna leaves has been used for dyeing of cotton fibers (Ali et al. 2009). The results revealed that extracts produced with alkaline extraction of henna leaves have moderate to good fastness properties and better color strength than the dye extracts obtained in distilled water.
In a similar study to develop an eco-friend, nontoxic and multifunctional finish on wool, Giri Dev et al. (2009) studied the role of chitosan treatment, individually and in combination with natural henna dye, on the antimicrobial property and dyeability of wool fabrics. They found that chitosan simultaneously showed bi-functional effects, i.e., imparted antimicrobial property against two common pathogenic bacteria, S. aureus and E. coli, and enhanced dye up-take. Fastness tests demonstrated good wash, light, and perspiration durability of finish on fabrics.
In another attempt to extend eco-friendly textiles processing techniques, Iqbal et al. (2008) have tried to improve the color strength and color fastness properties of henna dye on cotton fabric through UV irradiation treatment. They irradiated cotton and henna dye separately, extracted coloring matter using water, alum, and methanol, dyed irradiated cotton with the dye extract and subsequently compared the results with non-irradiated samples. They found that, generally, irradiated dyed samples demonstrate more color strength and deeper shades, and color fastness enhanced from medium to good level.
The observed behaviors were explained from one hand via enhanced affinity and facile sorption of hydrolytically degraded lawsone, decrease of insoluble impurities in the case of irradiated henna, and on the other hand via an increase of number and size of irradiated fiber pores and carboxylic acid groups produced upon UV photo-oxidation of cotton fiber (Iqbal et al. 2008). The industrial aspects of the process were discussed as well and the optimum dyeing condition were introduced as following: 65 °C dye bath temperature, 120 min dyeing time, 3 % NaCl, and 4 % copper sulphate (pre-mordanting).
Eucalyptus, Eucalyptus camaldulensis, is another example of natural dye resource. It is a rich source of natural polyphenols and tannins. Leaves contain 11 % quercetin as major component. Other components are rutin, gallic acid, ellagic acid, etc. that are very useful in dyeing yield and durability of substrate (Mongkholrattanasit et al. 2010, 2011). Chemical structures of these coloring matters are shown in Fig. 6.11.
Mongkholrattanasit et al. (2011) demonstrated that water extract of eucalyptus leaves could be effectively used in exhaustion coloration process of wool and silk. They used aluminium, iron, copper, and tin as mordants. The optimum dyeing condition was determined to be at pH 4, time 40 min, and temperature 90 °C. Wide variety of shades was achieved where wool fibers had higher color strength than silk fibers. Shades obtained were yellowish brown with aluminium and copper, dark grayish brown with iron and bright yellow with tin on both wool and silk substrates. Protection against UV was additional property achieved for both substrates. Both fibers showed good to very good wash fastness and medium to good light and rub fastness.
Tea plant, Camellia sinensis, is the rich resource of different beneficial natural components such as antioxidants, flavanols, flavonoids, polyphenols, and catechins. It is processed using various methods to produce variety kinds of teas: green, yellow, dark, white, oolong, and black. Aqueous extract of tea could also be used for textiles. Deo and Desai (1999) have studied the dyeing of cotton and jute with an aqueous extraction of black tea where the tannins were the main colorant. The mixture of black tea was stirred, heated, held at the boil for 30 min, allowed to stand for 15 min and then filtered and used as black pigment for dyeing of fabrics, where ferrous sulphate heptahydrate, alum and copper sulphate pentahydrate were used as mordants. The results showed deep color shad on jute fabrics, while good to excellent washing and light fastnesses were observed.
In a related study and with the aim to production of eco-friendly medical textiles, Syamili et al. (2012) have tried to apply extract of green tea leaves onto cotton to impart antibacterial properties. Various tests were performed to identify the effective ingredients of green tea. The ingredients were extracted using ethanol, chelated with copper to exploit intrinsic bactericidal property of copper, applied on cotton with and without citric acid as crosslinker agent. Significant inhibition against bacteria, S. aureus and E. coli, growth was obtained for finished fabrics.
The wash durability of antibacterial finish was tested and the results showed that after 10 wash cycles the bacterial inhibition rate was less than 25 % for fabrics finished directly with no crosslinker agent and more than 50 % for crosslinked finished fabrics. It was concluded that to increase the durability of antibacterial finish, extracted components should be chemically fixed onto substrate surface using proper crosslinker. This finish process was also introduced as an eco-safe, facile and economic process (Syamili et al. 2012).
Similarly, the leaves of deciduous plant with high content of tannin have been used as a natural dye sources for dyeing of wool (Raja and Thilagavathi 2008). The colorant agent of five different deciduous plants, namely, silver oak, flame of the forest, tanner’s senna, wattle and serviceberry, was extracted by boiling 250 g of the leaf material with 5 l of water for 1 h and, were used with mordants to dye wool. The authors have introduced the use of plant leaves more eco-friendly and economical than using their barks or other parts, showing a moderate to good fastness of the fibers dyed.
Symplocos spicata plant leaves extract have also been used in ultrasound coloration of cotton, wool, and silk with Al, Cu, Fe, Cr, Sn(II), Sn(IV) mordants (Vankar et al. 2008). An additional pretreatment with tannic acid was also done for cotton to provide carboxylic acid groups on the fiber surface for better mordanting. Dye up-take was markedly improved and became almost double in ultrasonic dyeing compared to common exhaustion dyeing process. Yellow to brown shades with high color fastness properties were obtained for all fibers. Lower required time, energy, and dye amount to obtain the same results of conventional dyeing process proved the superiority of ultrasonic natural dyeing technique.
6.4.2.1.4 Fruit
Fruits of the plants are another source of natural dyes and have been extensively used for textile dyeing (Table 6.4). Pomegranate is the fruit of deciduous shrub or small tree, Punica granatun. Fruit rind has been widely used in traditional coloration of textiles since ancient times. However, in open literature there are rare reports addressing the application of pomegranate rind on textiles scientifically except some recently published papers (Çalıs et al. 2009; Sathianarayanan et al. 2010; Kulkarni et al. 2011a; Rajendran et al. 2011). Rind of the pomegranate is a rich resource of tannins, phenolic contents, alkaloids, etc. Granatonine is the main coloring component found in the fruit rind in the form N-methyl granatonine (Fig. 6.12).
The extract of pomegranate rind has been used in environmentally friendly dyeing (Kulkarni et al. 2011a) and bactericidal treatment (Sathianarayanan et al. 2010; Rajendran et al. 2011) of cotton fabric. Wide range of shades such as yellow, brown, and black with good fastness properties were achieved using copper and iron mordants with different ratios (Kulkarni et al. 2011a).
As an example, pomegranate rind extract was used for bi-functional treatment of cotton, i.e., color and bactericidal finish (Rajendran et al. 2011). The dye was extracted using water and ethanol, separately. The dye extract showed good affinity for cotton, and was applied with alum and copper. Good wash, light, rub, perspiration fastnesses and excellent antibacterial property against common bacteria, S. Aureus and E. coli, was resulted. However, antibacterial property steadily decreased by successive wash cycles.
Pomegranate rind has also been utilized for dyeing of wool and silk with/without mordants (Das et al. 2006; Çalıs et al. 2009). The best dyeing pH was found to be four. Iron and aluminium were resulted in grey and yellow shades on fibers, respectively. Dye up-take, and light fastness were improved with mordants, however, wash fastness was not markedly changed (Das et al. 2006).
In a similar study, four common natural dyes were applied on wool and resulting antimicrobial effect was examined (Çalıs et al. 2009). Bacterial growth reduction of 4–80 % for pomegranate rind, Punica granatum, 53–86 % for onion, Allium cepa L., 32–52 % for madder, Rubia tinctorium L., and 28–91 % for peppermint, Mentha piperita L., was obtained against variety of common pathogenic bacteria used.
Guesmi et al. (2012) have extracted indicaxanthin from Opuntia ficus-indica fruit and used for dyeing of textile fabrics. Extraction method was to mix 50 g of juice from cactus pears with 100 ml of 80 % aqueous ethanol as solvent. This plant contains mainly two dyes, indicaxanthin and betanin (Fig. 6.13).
Extracted indicaxanthin was used for dyeing of wool fabrics with different mordants and the results showed that the fabrics dyed have good washing fastness, where KAl(SO4)2, MnSO4 and CoSO4 gave the best results.
In a similar work, indicaxanthin has been used for dyeing of modified acrylic fibers by both conventional and ultrasonic techniques (Guesmi et al. 2013). The authors have reported that sonicator dyeing with this orange-yellow natural dye showed marked improvement in dye uptake and fair to good fastness properties of the dyed fabrics.
Relevantly, Ali and El-Mohamedy (2011) have tried to extract the dyeing agent of red prickly pear plant, Opuntia lasiacantha Pfeiffer, and used it for dying of wool with different types of mordants. Betalain (Fig. 6.14), the red dye extracted from this plant, was used for dyeing of wool fabrics with ferrous sulfate, copper sulfate, potassium dichromate and tannic acid as mordants.
The fabrics dyed with betalain showed high bacteria inhibition activity and good fastness property.
Garcinia mangostana Linn, is a tropical evergreen tree which grows especially in Thailand, Indonesia, Malaysia, and Philippines. It is known for its best tasting fruits. Fruit shells contain various useful natural components such as mangosteen that is a red pigment. Chairat et al. (2007) have extracted mangosteen using an aqueous acetic acid solution where extract solution had dark red color. The extract solution was used in dyeing of silk and cotton with Al, Ca, Fe, Zn, and a natural mordant. Generally, color depth, wash and light fastnesses of dye were markedly improved using mordants and the best results obtained in post-mordanting method with Fe and Ca.
Emlica officialis G. fruit is a rich resource of natural tannins used as natural mordants. It contains mainly two hydrolysable tannins that on hydrolysis give gallic acid and ellagic acid. Prabhu et al. (2011) have used the ethanol isolated tannins individually and/or in mixture with copper sulphate for enhancement of color yield and color fastness properties in coloration of cotton and silk using natural dyes. Four natural dyes used were turmeric, Curcuma longa L., pomegranate rind, Punica granatum L., henna, Lawsonia inermis L., and indian madder, Rubia cordifolia L. Different shades were obtained using these dyes together with mordants showing good color fastness and color strength. Antibacterial test was also carried out to evaluate the bactericidal property of Emlica officialis G.on cotton and silk and it was found that cotton and silk treated with this natural mordant have good antibacterial activity which was durable up to 20 washes.
In a similar work, Kulkarni et al. (2011b) have tried to produce different shades of yellow color using the dye extracted from chili skin. The yellow pigment of Capsicum annum, that mainly contains oleoresin, was extracted by solvent extraction method and was used for dyeing of cotton fibers by mordants. Oleoresin is an oil soluble extract from the fruits of Capsicum annum Linn, and is primarily used as a coloring and/or flavouring in food products. It is composed of capsaicin, the main flavouring compound giving pungency in higher concentrations, and capsanthin and capsorubin, the main coloring compounds (Pérez-Gálvez et al. 2003) (Fig. 6.15).
Based on the results obtained, the authors have reported good light and rub fastness, and moderate wash fastness for the fabrics dyed with the green chili extract.
6.4.2.1.5 Bark
Some studies have reported the use of bark of various plants as natural dye resource (Table 6.5). Reuse of ash-tree bark for extraction of a natural dye for textile dyeing was the subject of an interesting research (Bechtold et al. 2007). A quercetin-rich beige olive pigment extracted with distilled water from ash-tree bark, Fraxinus excelsior L., was used for dyeing wool fabrics with different mordants. Good shade reproducibility and levelness of the dyed material along with the acceptable fastness to light and wash were obtained.
Again, the colorant extracted from the tree bark has been used by Vinod et al. (2010) for dyeing of textile fabrics. In this work, pigment from the bark of Macaranga peltata was extracted by methanol and, using spectral techniques, ellagic acid was identified as the main coloring agent. Then, this yellow pigment was used for dyeing silk with tannic acid and potash alum [Al(NH4)(SO4)2.12H2O] as mordant, and the results demonstrated good fastness of dyed fabrics.
Barberry plant, Berberis aristata DC., is another plant that its bark has been used in textile coloration (Pruthi et al. 2008). Silk was dyed with aqueous extract of barberry bark and post-mordanted with different combination of various mordants. Wide range of shades was obtained with high dye fastness properties. Based on the results several conclusions were drawn: best dyeing method for silk is simultaneous mordanting because of the highest dye up-take in this method; alum must be used in combination with other mordants due to its dye-up take improvement ability; the use of combination of mordants produce wide range of shades.
Similar attempt has been made to make use of Sticta coronata lichen as an abundant resource of natural dye in New Zealand in ultrasonic dyeing of silk (Mansour and Heffernan 2011). Catechu, Acacia catechu, a natural mordant and alum were used in dyeing process. Ultrasound energy improved dye up-take markedly. The dye showed good affinity and produced brilliant lilac color with good fastness on silk fibers.
In similar study, bark of Peepal tree, Ficus religiosa L., were used in natural dyeing of silk (Saravanan and Chandramohan 2011). The color component was extracted with water and then was used for dyeing of silk with metallic mordants such as Al, Cu, Cr, Ni, and Sn and natural mordants like myrobolan, Terminalia chebula, and cow dung. Myrobolan or harda is an important natural mordant with chebulinic acid as main ingredient whose chemical structure is shown in Fig. 6.16. The color fastness properties on fibers were overall acceptable.
Similarly, Samanta et al. (2007) have used the extraction of Jackfruit wood, Artocarpus heterophyllus Lam, in coloration of cotton and jut fibers with myrobolan, aluminium, iron, tin and EDTA mordants. The main coloring component of jackfruit is morol, a typical flavanol that imparted yellow to golden brown shade with various mordants on cotton and jut fibers. The optimum dyeing condition was, time 90 min, temperature 70–90 °C, 20–30 % mordant, 30–40 % dye with pre-mordanting. Good color fastness properties were reported for both dyed fibers.
After that, Samanta et al. (2008) in another study have used binary mixture of jackfruit extract with other natural dyes, manjistha, red sandal wood, marigold, sappan wood, and babool in dyeing of jute fibers with myrobolan and aluminium mordants. They also examined the compatibility of mixed dyes. After dyeing, additional treatment with various chemicals was performed to enhance wash and light fastness.
6.4.2.1.6 Flower
Natural dyes/antimicrobials can also be extracted from flower part of various plants (Table 6.6). Pigments extracted from marigold flowers, Tagetes, with a quite dark and saturated yellow to orange red color on textiles is another example of natural dyes/antimicrobials. The aqueous extraction of african marigold, Tagetes erecta L., that contains mainly lutein has been used for dyeing of cotton and silk fabrics with mordants (Jothi 2008). The author has reported good light fastness and good to excellent wash fastness for the fabrics dyed with this natural yellow pigment.
In a similar work, Guinot and co-workers (2008) have evaluated the dyeing potential of pigments from marigold flowers, Tagetes patula L. Flavonoids were obtained by water-ethanol mixture extraction technique, giving the highest extraction efficiency, and were used for dyeing of woven wool with mordants. Based on the results observed, the authors have introduced marigold flower as a potential dyeing plant.
A greenish yellow natural dye called luteolin was also extracted by methanol from leaves, stems and flowers of weld (Reseda luteola) (Cerrato et al. 2002). Then, it was used for dyeing of cotton and wool fibers with alum as mordant. A composite design experiment allowed the effects of particle size and liquid/solid ratio to be determined and the results demonstrated that luteolin concentrated extract could be used for home dyeing of fabrics of kits specially designed textiles.
Also, Vankar and Shanker (2009) have tried the pretreatment of silk fiber in an eco-friendly dyeing with flower extract. A bright reddish brown pigment, containing quercetin, was extracted by soaking the dried and ground flower sample, Delonix regia, in distilled water and then, was used for fiber dyeing with mordants.
Wool fabrics have also been dyed with catechu (Acacia catechu) extract by Bhattacharya and Shah (2000). The light brown pigment, containing mainly tannin, was used for dyeing of fabrics with different mordants. Different colors produced on wool fabric with catechu using various metal mordants. Moreover, the fastness properties were sufficiently good for practical dyeing.
Spathodea campanulata is a flowering tree that is planted extensively in tropical countries as an ornamental tree. It has very showy reddish-orange or crimson (rarely yellow), campanulate flowers. Kumaresan et al. (2012) have investigated the dyeing properties of cotton with dye extracted from Spathodea campanulata flowers without and with various binary mixtures of natural/metallic mordants. Extraction of dyed was carried out in boiling water and ethanol separately. Ethanol extract was good to obtain brown shades. Generally, in almost all mordant-dye-fiber cases, color fastness to wash, rub, and perspiration was quite high. It was inferred that this dye can be used as an eco-friendly natural dye in coloration of textiles.
On the other hands, antibacterial activity of fibers dyed with extracts from plants has been widely investigated by the scientists. The antimicrobial activity of five natural dyes, Acacia catechu, Kerria lacca, Quercus infectoria, Rubia cordifolia, Rumex maritimus, on wool fabrics has been shown by Singh et al. (2005). Moreover, tannin-rich extract of oak has been used as an antibacterial agent for textiles by Gupta and Laha (2007). In this work, the extract of Quercus infectoria plant in combination with alum, copper and ferrous mordants have been used as antibacterial agents for treatment of cotton fabrics. The results showed that the cotton textiles can be successfully treated with this extract to show antibacterial activities against both Gram-positive and Gram-negative bacteria.
In a similar work, the antibacterial activity of extracts from herb leaves, stem and flower has been studied (Sumithra and Raaja 2012). The methanol extracts of Ricinus communis, Senna auriculata and Euphorbia hirta were examined for their antimicrobial efficiency on the four variant of denim fabrics directly by using dip method and the findings demonstrated that the fabrics had good antibacterial activities. Similarly, the natural pigment from sappan was used for coloration of wool fabrics after treatment with the protease and transglutaminase (Zhang and Cai 2011). The pigment of sappan was extracted by dissolving the herb in distilled water and allowed to boil in a beaker kept over water bath for quick extraction. Then, this pigment was used for dyeing of wool fabrics with ferrous sulphate, potassium alum, and tannic acid as mordants. This process had a good anti-ultraviolet effect on the fibers while modification with protease led to some decrease in wet rub fastness, whereas transglutaminase had almost no influence on rub fastness. But, the treated fibers had good dry rub fastness.
6.4.2.1.7 Other Parts of Plants
Natural extracts of other parts of plants have also important dyeing/antimicrobial properties and their application on textile has been widely studied (Table 6.7).
A natural dye was extracted by ultrasonic method from a vegetable with botanic name Sargentodoxa cuneata, and its application on wool fabric was studied (Xinsheng et al. 2008). The authors have reported that the color of fabrics dyed with ultrasonic extraction solution is deeper.
In a similar work, the mordanted wool fabrics with alum, were dyed in 50 % Reseda luteola L. (weld), 20 % Rhamnus petiolaris Boiss (buckthorn) and 50 % Datisca cannabina L. (bastard hemp) dye-baths (Deveoglu et al. 2012). Extraction of yellow pigments, containing mainly flavonoid and anthraquinone, was carried out with a hydrogen chloride⁄methanol⁄water mixture. The authors have reported as a conclusion that, it would be possible to analyze and to identify the natural dyes present in historical textiles using a reverse-phase high-performance liquid chromatography.
Natural dye from Dahlia variabilis have been similarly used for dyeing of textile (Mishra et al. 2012). The flavonoids – anthocyanins rich colorant was extracted by ultra sound assisted technique and was used for dyeing of wool fibers with alum and milk of tartar as mordants. The authors have reported good fastness properties of dyed fibers as well as intrinsic potential of this dye to replace phenolphthalein as a pH indicator.
Cristea and Vilarem (2006) have also studied the light fastness of selected natural dyes from madder, weld and woad. Red, yellow and blue extracts of Rubia tinctoria, Reseda luteola L. and Isatis tinctoria, respectively, were used for dyeing of cotton with mordants. The authors have concluded that, in spite of poor fastness of fiber dyed with these three natural dyes, using UV absorbers or antioxidants improves the light fastness of dyed fabrics.
Samanta et al. (2011) have used aqueous extract of flower petals of tesu flowering tree, buteamonosperma frondosa, in coloration of jute fabric in alkaline medium to obtain various shades like creamish orange, yellowish orange, ochre brown, and reddish brown. The main color component in the yellowish red/orange extract was butein (Fig. 6.17). Good color fastness and color yield was reported for proper selected dyeing condition (dye-fiber-mordant system) and further improvements in light fastness were obtained with proper post-chemical treatment.
In an interesting and recent research work, Raja and Thilaghavati (2011) have tried to make use the inherent antibacterial characteristic of some natural dyes in bi-functional environmentally friendly treatment of textiles. In their work, four natural dyes, with in vitro antimicrobial activity against both gram positive and gram negative bacteria, were chosen. The effect of mordant and protease enzyme treatment of wool on the bactericidal performance of natural dyes were examined and compared with untreated wool results. Various test results showed higher dye up-take and antibacterial property in enzyme treated wool that was ascribed to increased dye diffusion and affinity due to hydrolysis of outer layer of wool. Moreover, although the color fastness properties of dyed wool were increased upon mordant treatment, but none of dyed fibers mordant treated showed antibacterial activity. Such behavior was attributed to the blocking of bio-active ingredients in natural dye through possible complex formation of metal ion with those components (Raja and Thilaghavati 2011).
6.4.2.2 Dyes/Antimicrobials from Microorganisms
The main disadvantage of natural dyes/antimicrobials from plants is the low efficiency of the extraction process (a few grams of pigment per kg of dried raw material). This makes their application limited to high-value-added natural-colored garments only (De Santis et al. 2005). To overcome this drawback, it was suggested to exploit the potentiality of microorganisms such as fungi, bacteria and algae that are fast growing and have the potential of being standardized commercially (Sharma et al. 2012).
There are several microorganisms which can produce pigments, which are one of the important classes of secondary metabolites and are often referred to as biopigments. Microorganisms produce various pigments including carotenoids, melanins, flavins, quinones, prodigiosins and more specifically monascins, violacein or indigo (Khanafari et al. 2006; Venil and Lakshmanaperumalsamy 2009). So, researchers have recently focused on the production, identification and application of these pigments on textiles and have studied the dyeing and antimicrobial characteristics of the dyed fibers (Table 6.8).
Chitosan is a linear polysaccharide produced commercially by deacetylation of chitin, the structural element in the crustaceans such as crabs and shrimp and cell walls of fungi.
Chitosan, N-deacetylated derivative of chitin that is a linear polysaccharide, is also an important natural antibacterial agent. It has been used in wool finishing, but its weak binding to the wool constitutes the main problems in its application. For instance, Ranjbar-Mohammadi and her co-workers (2010) have studied the grafting of chitosan onto wool fabric using anhydride bridge and its antibacterial property. The results showed that the wool fabrics treated by chitosan had a considerable anti-microbial and antifelting activities as well as good wash fastness.
In a similar works, chitosan has been used to impart antibacterial properties to the cotton yarns (Karolia and Mendapara 2007; Kavitha et al. 2007). It was observed that the chitosan finish provides better functionality to the fabrics with increasing tensile strength, flexural rigidity and shear strength as well as showing excellent activity of the cotton fabrics against bacteria.
Prodigiosin is a bright red pigment with a pyrrolylpyrromethane chemical structure that possesses antibacterial activity, too. Venil and Lakshmanaperumalsamy (2009) have studied the prodigiosin potential of Serratia and the effect of different factors influencing the production of this pigment. They have reported the optimum conditions for prodigiosin biosynthesis as well as the different techniques used for its extraction. It has been concluded that prodigiosin is a remarkable species for its diverse range of biological and dyeing effects, although much deeper insight into the mode of action of these compounds is required.
Gulani et al. (2012) have studied the effect of different parameters on the production of prodigiosin form Serratia marcescens. The effects of various media components and process parameters like carbon and nitrogen sources, temperature, pH, incubation period were investigated. The pigment was extracted by centrifugation and applied on wool, silk, nylon, cotton and polyester fibers. They have reported that the dyed fibers show good antibacterial, antifungal, and antioxidant activity where a good resistant to acid, alkali and detergents was observed.
This natural pigment has also been isolated from marine sediments by Alihosseini and her co-workers (2008). They have used this bright red pigment for dyeing wool, nylon, acrylics, and silk fibers and have reported that fabrics dyed with the microbial prodiginines demonstrate antibacterial activity.
Velmurugan et al. (2009) have studied the anti-bacterial activity and dyeing property of the pigments obtained from five fungal species. The fungi species were Monascus purpureus, Isaria farinosa, Emericella nidulans, Fusarium verticillioides, and Penicillium purpurogenum, producing red, pink, red, yellow and yellow pigments, respectively. The pigments were extracted by absorption on silica gel and used for dyeing of cotton fabric and processed leather with mordanting. The results showed that the dyed fibers have anti-microbial activity and good color fastness property.
In a similar work, dyeing properties of pigment from fungi Monascus purpureus has been investigated by De Santis and co-workers (2005). Red to orange pigment has been extracted by ethanol and along with alum or stannic chloride as mordants applied for dyeing wool fibers. The authors have reported good dye fastness for the dyed fibers and introduced this process as a cost-effective production.
Kim et al. (2005) have used the protease extracted from bacteria “bacillus” for improvement of dyeing quality in wool and silk. The enzyme was extracted by dilution and incubation of the soil sample containing the bacteria and was used for dyeing the fibers. Their results showed that by increasing the protease treatment, the dyeing characteristics of the fibers are enhanced, and as a result of soil removal, the fibers become smoother.
Extraction, characterization and use of a pigment produced by bacteria, Serratia sakuensis has been tried by Vaidyanathan et al. (2012). This novel red pigment was produced by solid-state cultivation of a bacterial isolate obtained from garden soil. It was a prodigiosin-like pigment that shows properties similar to prodigiosin. Then, the pigment was used for dyeing of silk, wool and cotton fabrics and the results showed that the dyed fabrics have good color fastness, high color strength values and dye uptake as well as antibacterial activity.
Similarly an attempt has also been made to apply the dyes obtained from fungus, Trichoderma virens, Alternaria alternate, and Curvularia lunata to the fibers by Sharma et al. (2012). Wool and silk fibers were dyed unmordanted and the results obtained show very good wash and rub fastnesses as well as antifungal activity.
Velmurugan et al. (2010) have also studied the dyeing of fibers with soluble pigments of selected fungi. They have used water soluble fungal pigments obtained from Monascus purpureus, Isaria farinose, Emericella nidulans, Fusariumverticillioides, and Penicillium purpurogenum species. These red pink, reddish brown, and yellow pigments extracted by ethanol were used for dyeing of cotton fibers accompanying mordanting. The authors have reported that a strong variation in shade and color depth could be achieved by using alum and ferrous sulfate that gives good wash fastness properties to the fabrics.
6.4.2.3 Reusing and Recycling of Natural Dyes
Some researchers have focused on the economical aspects of the application of natural dyes for textile such as reusing or recycling (Table 6.9). Mirjalili et al. (2011) have studied the dyeing of wool fibers with the extract of weld and concluded that in the case of dyeing with this extract, and keeping the good light fastness, the exhaustion rate for the extracted dye increased by 49 % compared to the raw dye.
In a similar work, Meksi and co-workers (2012) have used the olive mill wastewater as a potential source of natural dyes for wool dyeing. This wastewater was filtered and the coloring agents such as pectines, mucilages and tannins were extracted with hexane. Along with the darker shades and with generally good fastnesses, the results proved that mordanting give deeper shades and enhanced fastness properties.
In another work, plant waste materials including Terminalia catappa, Artocarpus heterophyllus, Tectona grandis and Morinda citrofolia were extracted by methanol and used for dyeing of silk fibers (Prusty et al. 2010). The fibers dyed with the extracts showed excellent antimicrobial activity and good durable fastness properties.
Another example for recycling of useless by-products of natural materials in sustainable treatment of textiles is the study that has been accomplished by Ahmad et al. (2011). They extracted color component from Gluta aptera wood (Rengas) saw dust and used it in environmentally friendly dyeing of cotton and silk. Water at boil and methanol at room temperature were used as extracting solvents. Rice husk ash and Al were used as natural and metallic mordants, respectively. Different brownish shades with good color fastness properties were obtained for both substrates.
In another similar work, Lee (2007) has tried to use the coffee sludge extract for dyeing of textile. The natural colorant was extracted from Coffea arabica L., using water as extractant and was applied for dyeing of cotton, silk, and wool with a variety of metallic salts as mordants. The author has reported that the fabrics dyed show good deodorization performance as well as excellent light fastness with mordanting.
Interestingly, Shams-Nateri (2011) has tried to reuse the dyebath containing madder extract for dyeing of wool fibers. The dyebath contained mainly alizarin, purpurin, and water, was reconstructed by adding madder. The author has reported that dye fastness of the samples dyed in reconstructed dyebath is the same as initial wool dyeing while the economic analysis showed that the reusing wastewater causes 19.91 % cost saving in wool dyeing with madder.
In exploring to find an alternative to decrease environmental pollution from burning of useless by product of banana fields, the studies led to find out the coloring components present in banana peel (Salah 2011). Natural dye extracted from banana peel wastes, Musa cavendish, by alkaline extraction was used for coloration of cotton with iron mordant. In addition to good color imparted, dyed cotton showed excellent antibacterial activity and high UV protection properties.
To develop an eco-friendly and economical alternative for production of medical cotton and polyester/cotton, the extracts of pomegranate rind, Punica granatum, and leaves of tulsi, Ocimum sanctum (Sathianarayanan et al. 2010), neem, Azadirachta indica, and mexican daisy (Thilagavathi and Krishna Bala 2007) have been applied on cotton through variety of methods such as direct application, pad-dry-cure, micro-encapsulation, resin crosslinking and their combinations. Excellent antibacterial property was obtained in all methods with good wash durability except for direct application of extracts where wash fastness was poor (Joshi et al. 2007).
Interestingly, Kiumarsi et al. (2009) have recently optimized the coloration process of yarrow, Achillea millefolium, on wool using Taguchi statistical method. Achillea millefolium is a pharmaceutical plant known as a powerful healing herb containing flavoniods such as luteolin and apigenin as natural dyes (Fig. 6.18).
The dye aqueous extract was used for dyeing of wool with aluminium, tin, and copper mordants. Interesting variety of shades from pale yellow to dark green was obtained with very good wash and light fastness for all dyed samples. The process was claimed to be economical due to saving time and energy that could also be commercialized.
In a related and most recent work, Mortazavi et al. (2012) introduced saffron, Crocus sativus L., as a potent natural dye resource. They used saffron flower petals and sepals extract, a rich resource of anthocyanidin natural dye previously discarded as useless wastes, in coloration of wool. They used different metallic mordants such as tin, copper, aluminium, iron, and chrome together with oxalic and tartaric acid to obtain a variety of shades as well as to enhance fastness properties of natural dye on wool. Although the color of fresh petals and sepals was light purple, but the shade range obtained on wool varied from yellow to brown with different mordants. Wash and light fastnesses of wool fibers were excellent with iron and chrome, moderate with copper and aluminium, and poor with tin mordants.
Dyeing of poly(ethylene terphthalate) fabrics with an aqueous extract of an indeciduous tree, Caesalpinia sappan L., was also investigated by Park and co-workers (2008). The results showed that the pretreatment with chitosan and/or plasma is better than a metal mordant in terms of the dye uptake and shortening of the dyeing time.
6.5 Conclusion
Due to the increasing awareness of the high risk impact associated with the synthetic colorants used for textiles, the application of natural or green extracts as colorants or antimicrobials has been these days increasingly considered. Review of the related published papers revealed that the non-reproducibility, time-consuming extraction methods and poor fastness properties are the main problems ahead the dyers and finishers to use these products in textile industry and consequently, must be the cores of scientific researches in this field.
The papers published in this field show that most of natural dyes have inherently antimicrobial properties and could consequently possess high medicinal activity. They are extracted from the different types of microorganisms as well as various parts of the plants such as bark, leaf, root, seed, fruit, and flower containing coloring materials like tannin, flavonoids, quinonoids, etc. Natural sources can offer not only a rich and varied source of dyestuffs, but also could be considered as safe, environmentally friendly and low cost treatments with additional benefit of coloring in a single stage. There have also been undeniable developments in extraction techniques of natural dyes/antimicrobials such as the invention of ultrasonic or enzyme assisted ultrasonic extraction methods where they prove high extraction efficiency over the conventional methods. But, there are still some important issues to solve. Most particularly, the application of metallic mordants for better fastness purpose is one of the problems concerning the environmental impact of natural dyes. The result of this survey showed that the shade and colorfastness of the fiber dyed depend not only on the natural colorant but also on the mordant and mordant assistants used. While dyes from natural sources are eco-friendlier than synthetic ones, the level of mordants used is normally higher than the permitted official concentration and poses environmental problem. It is expected that mordants will be replaced by modern environmentally friendlier technologies like plasma treatment. Moreover, studying and enlightening different processes involving natural dyes will be helpful in identification and analyzing color characteristics of historic/old textiles.
Real effort has been put to include all the relevant referred publications on the exploiting natural dyes/antimicrobials in green treatments of textiles in this review. However, the limitation of our resources and the sheer number of publications in this field may have prevented the comprehensiveness of this report. Our sincere apologies are extended to any and all authors whose works are not included in this report.
References
Ahmad WYW, Rahim R, Ahmad MR, Abdul Kadir MI, Misnon MI (2011) The application of Gluta Aptera wood (Rengas) as natural dye on silk and cotton fabrics. Universal J Environ Res Technol 1(4):545–551
Ali NF, El-Mohamedy RSR (2011) Eco-friendly and protective natural dye from red prickly pear (Opuntia Lasiacantha Pfeiffer) plant. J Saudi Chem Soc 15:257–261
Ali S, Hussain T, Nawaz R (2009) Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J Clean Prod 17:61–66
Alihosseini F, Ju K, Lango J, Hammock BD, Sun G (2008) Antibacterial colorants: characterization of prodiginines and their applications on textile materials. Biotechnol Prog 24(3):742–747
Ammayappan L, Moses JJ (2009) Study of antimicrobial activity of aloevera, chitosan, and curcumin on cotton, wool, and rabbit hair. Fiber Polym 10(2):161–166
Arora A, Gulrajani ML, Gupta D (2009) Identification and characterization of Ratanjot (Arnebia nobilis Reichb.f.). Nat Prod Radiance 8(2):142–145
Arora A, Gupta D, Rastogi D, Gulrajanii ML (2012a) Kinetics and thermodynamics of dye extracted from Arnebia nobilis Rech.f on wool. Indian J Fibre Text Res 37:178–182
Arora A, Rastogi D, Gupta D, Gulrajanii ML (2012b) Dyeing parameters of hydroxynaphthoquinones extracted from Arnebia nobilis Rech.f. Indian J Fibre Text Res 37:91–97
Bechtold T, Mahmud-Ali A, Mussak RAM (2007) Reuse of ash-tree (Fraxinus excelsior L.) bark as natural dyes for textile dyeing: process conditions and process stability. Color Technol 123:271–279
Bhattacharya SD, Shah AK (2000) Metal ion effect on dyeing of wool fabric with catechu. J Soc Dyers Colour 116:10–12
Borges ME, Tejera RL, Díaz L, Esparza P, Ibáñez E (2012) Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem 132:1855–1860
Çalıs A, Yuvalı Çelik G, Katırcıoglu H (2009) Antimicrobial effect of natural dyes on some pathogenic bacteria. Afr J Biotechnol 8(2):291–293
Cerrato A, De Santis D, Moresi M (2002) Production of luteolin extracts from Reseda luteola and assessment of their dyeing properties. J Sci Food Agric 82:1189–1199
Chairat M, Bremner JB, Chantrapromma K (2007) Dyeing of cotton and silk yarn with the extracted dye from the fruit hulls of mangosteen, Garcinia mangostana linn. Fiber Polym 8(6):613–619
Chan EWC, Lim Y, Wong S, Lim K, Tan S, Lianto F, Yong M (2009) Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem 113(1):166–172
Chen C, Chang W (2007) Antimicrobial activity of cotton fabric pretreated by microwave plasma and dyed with onion skin and onion pulp. Indian J Fibre Text Res 32:122–125
Choo CKK, Lee YE (2002) Analysis of dyeings produced by traditional Korean methods using colorants from plant extracts. Color Technol 118:35–42
Cristea D, Vilarem G (2006) Improving light fastness of natural dyes on cotton yarn. Dyes Pigm 70:238–245
Das D, Bhattacharya SC, Maulik SR (2006) Dyeing of wool and silk with Punica granatum. Indian J Fibre Text Res 31:559–564
Das D, Maulik SR, Bhattacharya SC (2007) Dyeing of wool and silk with Bixa orellana. Indian J Fibre Text Res 32:366–372
Das D, Maulik SR, Bhattacharya SC (2008) Colouration of wool and silk with Rheum emodi. Indian J Fibre Text Res 33:163–170
Dawson TL (2008) It must be green: meeting society’s environmental concerns. Color Technol 124:67–78
Dawson TL (2009) Biosynthesis and synthesis of natural colours. Color Technol 125:61–73
De Santis D, Moresi M, Gallo AM, Petruccioli M (2005) Assessment of the dyeing properties of pigments from Monascus purpureus. J Chem Technol Biotechnol 80:1072–1079
Deo HT, Desai BK (1999) Dyeing of cotton and jute with tea as a natural dye. J Soc Dyers Colour 115:224–227
Deveoglu O, Torgan E, Karadag R (2012) High-performance liquid chromatography of some natural dyes: analysis of plant extracts and dyed textiles. Color Technol 128:133–138
Farizadeh K, Montazer M, Yazdanshenas ME, Rashidi A, Mohammad Ali Malek R (2009) Extraction, identification and sorption studies of dyes from madder on wool. J Appl Polym Sci 113:3799–3808
Ghoranneviss M, Shahidi S, Anvari A, Motaghi Z, Wiener J, Šlamborová I (2010) Influence of plasma sputtering treatment on natural dyeing and antibacterial activity of wool fabrics. Prog Org Coat 70(4):388–393
Giri Dev VR, Venugopal J, Sudha S, Deepika G, Ramakrishna S (2009) Dyeing and antimicrobial characteristics of chitosan treated wool fabrics with henna dye. Carbohydr Polym 75:646–650
Guesmi A, Ben Hamadi N, Ladhari L, Sakli F (2012) Dyeing properties and colour fastness of wool dyed with indicaxanthin natural dye. Ind Crop Prod 37:493–499
Guesmi A, Ben Hamadi N, Ladhari N, Sakli F (2013) Sonicator dyeing of modified acrylic fabrics with indicaxanthin natural dye. Ind Crop Prod 42:63–69
Guinot P, Roge A, Gargadennec A, Garcia M, Dupont D, Lecoeur E, Candelier L, Andary C (2006) Dyeing plants screening: an approach to combine past heritage and present development. Color Technol 122:93–101
Guinot P, Gargadennec A, Valette G, Fruchier A, Andary C (2008) Primary flavonoids in marigold dye: extraction, structure and involvement in the dyeing process. Phytochem Anal 19:46–51
Guinot P, Gargadennec A, La Fisca P, Fruchier A, Andary C, Mondolot L (2009) Serratula tinctoria, a source of natural dye: flavonoid pattern and histolocalization. Ind Crop Prod 29:320–325
Gulani C, Bhattacharya S, Das A (2012) Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potentials. Malays J Microbiol 8(2):116–122
Gupta D, Bhaumik S (2007) Antimicrobial treatments for textiles. Indian J Fibre Text Res 32:254–263
Gupta D, Laha A (2007) Antimicrobial activity of cotton fabric treated with Quercus infectoria extract. Indian J Fibre Text Res 32:88–92
Gupta D, Kumari S, Gulrajani M (2001a) Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 2: Dyeing of nylon and polyester with nordamncanthal. Color Technol 117:333–336
Gupta D, Kumari S, Gulrajani M (2001b) Dyeing studies with hydroxyanthraquinones extracted from Indian madder. Part 1: Dyeing of nylon with purpurin. Color Technol 117:328–332
Gupta D, Gulrajani M, Kumari S (2004a) Light fastness of naturally occurring anthraquinone dyes on nylon. Color Technol 120:205–212
Gupta D, Khareb SK, Lahaa A (2004b) Antimicrobial properties of natural dyes against Gram-negative bacteria. Color Technol 120:167–171
Han S, Yang Y (2005) Antimicrobial activity of wool fabric treated with curcumin. Dyes Pigm 64:157–161
Hill DJ (1997) Is there a future for natural dyes? Rev Prog Color 27:18–25
Iqbal J, Bhatti IA, Adeel S (2008) Effect of UV radiation on dyeing of cotton fabric with extract of henna leaves. Indian J Fibre Text Res 33:157–162
Joshi M, Wazed Ali S, Rajendran S (2007) Antibacterial finishing of polyester/cotton blend fabrics using neem (Azadirachta indica): a natural bioactive agent. J Appl Polym Sci 106:793–800
Jothi D (2008) Extraction of natural dyes from African marigold flower (Tagetes ereectal) for textile coloration. Autex Res J 8(2):49–53
Kamel MM, El-Shishtawy RM, Yussef BM, Mashaly H (2005) Ultrasonic assisted dyeing III. Dyeing of wool with lac as a natural dye. Dyes Pigm 65:103–110
Kamel MM, El-Shishtawy RM, Youssef BM, Mashaly H (2007) Ultrasonic assisted dyeing. IV. Dyeing of cationised cotton with lac natural dye. Dyes Pigm 73(3):279–284
Karolia A, Mendapara S (2007) Imparting antimicrobial and fragrance finish on cotton using chitosan with silicon softener. Indian J Fibre Text Res 32:99–104
Kasiri MB, Khataee AR (2011) Photooxidative decolorization of two organic dyes with different chemical structures by UV/H2O2 process: experimental design. Desalination 270:151–159
Kasiri MB, Aleboyeh H, Aleboyeh A (2008) Degradation of acid blue 74 using Fe-ZSM5 zeolite as a heterogeneous photo-Fenton catalyst. Appl Catal B 84:9–15
Kavitha T, Padmashwini R, Swarna A, Giri Dev VR, Neelakandan R (2007) Effect of chitosan treatment on the properties of turmeric dyed cotton yarn. Indian J Fibre Text Res 32:53–56
Ke G, Yu W, Xu W (2006) Color evaluation of wool fabric dyed with Rhizoma coptidis extract. J Appl Polym Sci 101:3376–3380
Khanafari A, Assadi MM, Fakhr FA (2006) Review of prodigiosin, pigmentation in Serratia marcescens. Online J Biol Sci 6(1):1–13
Kim T, Son Y (2005) Effect of reactive anionic agent on dyeing of cellulosic fibers with a Berberine colorant – part 2: anionic agent treatment and antimicrobial activity of a Berberine dyeing. Dyes Pigm 64(1):85–89
Kim T, Yoon S, Son Y (2004) Effect of reactive anionic agent on dyeing of cellulosic fibers with a Berberine colorant. Dyes Pigm 60(2):121–127
Kim S, Cha M, Oh ET, Kang S, So J, Kwon Y (2005) Use of protease produced by Bacillus sp. SJ-121 for improvement of dyeing quality in wool and silk. Biotechnol Bioprocess Eng 10:186–191
Kiumarsi A, Abomahboub R, Rashedi SM, Parvinzadeh M (2009) Achillea millefolium, a new source of natural dye for wool dyeing. Prog Color Colorants Coat 2:87–93
Kulkarni SS, Gokhale AV, Bodake UM, Pathade GR (2011a) Cotton dyeing with natural dye extracted from pomegranate (Punica granatum) peel. Universal J Environ Res Technol 1(2):135–139
Kulkarni SS, Bodake UM, Pathade GR (2011b) Extraction of natural dye from chili (Capsicum annum) for textile coloration. Universal J Environ Res Technol 1:58–63
Kumaresan M, Palanisamy PN, Kumar PE (2012) Application of eco-friendly natural dye on cotton using combination of mordants. Indian J Fibre Text Res 37:194–198
Lee YH (2007) Dyeing, fastness, and deodorizing properties of cotton, silk, and wool fabrics dyed with coffee sludge (Coffea arabica L.) extract. J Appl Polym Sci 103:251–257
Mansour HF, Heffernan S (2011) Environmental aspects on dyeing silk fabric with sticta coronata lichen using ultrasonic energy and mild mordants. Clean Technol Environ Policy 13:207–213
McCarthy BJ (1997) Biotechnology and coloration. Rev Prog Color 27:26–31
Meksi N, Haddar W, Hammami S, Mhenni MF (2012) Olive mill wastewater: a potential source of natural dyes for textile dyeing. Ind Crop Prod 40:103–109
Mirjalili M, Nazarpoor K, Karimi L (2011) Eco-friendly dyeing of wool using natural dye from weld as co-partner with synthetic dye. J Clean Prod 19:1045–1051
Mishra PK, Singh K, Gupta KK, Tiwari H, Srivastave P (2012) Extraction of natural dye from Dahlia variabilis using ultrasound. Indian J Fibre Text Res 37:83–86
Mongkholrattanasit R, Krystufek J, Wiener J (2010) Dyeing and fastness properties of natural dyes extracted from Ecalyptus leaves using padding techniques. Fiber Polym 11(3):346–350
Mongkholrattanasit R, Kryštůfek J, Wiener J, Viková M (2011) Dyeing, fastness, and UV protection properties of silk and wool fabrics dyed with eucalyptus leaf extract by the exhaustion process. Fibres Text East Eur 19(3):94–99
Montazer M, Parvinzadeh M (2004) Effect of ammonia on madder-dyed natural protein fiber. J Appl Polym Sci 93:2704–2710
Montazer M, Parvinzadeh M, Kiumarsi A (2004) Colorimetric properties of wool dyed with natural dyes after treatment with ammonia. Color Technol 120:161–166
Montazer M, Taghavi FA, Toliyat T, Moghadam MB (2007) Optimization of dyeing of wool with madder and liposomes by central composite design. J Appl Polym Sci 106:1614–1621
Mortazavi SM, Kamali Moghaddam M, Safi S, Salehi R (2012) Saffron petals, a by-product for dyeing of wool fibers. Prog Color Colorants Coat 5:75–84
Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC (1999) The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew Chem Int Ed 38(3):270–300
Park Y, Koo K, Kim S, Choe J (2008) Improving the color fastness of poly (ethylene tetraphthalate) fabrics with the natural dye of Caesalpinia sappan L. wood extract and the effect of chitosan and low-temperature plasma. J Appl Polym Sci 109:160–166
Pérez-Gálvez A, Martin HD, Sies H, Stahl W (2003) Incorporation of carotenoids from paprika oleoresin into human chylomicrons. Br J Nutr 89(6):787–793
Popoola AV (2000) Comparative fastness assessment performance of cellulosic fibers dyed using natural colorants. J Appl Polym Sci 77:752–755
Prabhu KH, Teli MD, Waghmare NG (2011) Eco-friendly dyeing using natural mordant extracted from Emblica officinalis G. fruit on cotton and silk fabrics with antibacterial activity. Fiber Polym 12(6):753–759
Prusty AK, Das T, Nayak A, Das NB (2010) Colourimetric analysis and antimicrobial study of natural dyes and dyed silk. J Clean Prod 18:1750–1756
Pruthi N, Chawla GD, Yadav S (2008) Dyeing of silk with barberry bark dye using mordant combination. Nat Prod Radiance 7(1):40–44
Raja ASM, Thilagavathi G (2008) Dyes from the leaves of deciduous plants with a high tannin content for wool. Color Technol 124:285–289
Raja ASM, Thilagavathi G (2011) Influence of enzyme and mordant treatments on the antimicrobial efficiency of natural dyes on wool materials. Asian J Text 1(3):138–144
Rajendran R, Balakumar C, Kalaivani J, Sivakumar R (2011) Dyeing and antimicrobial properties of cotton fabrics finished with Punica Granatum extracts. J Text Apparel Technol Manage 7(2):1–12
Ranjbar-Mohammadi M, Arami M, Bahrami H, Mazaheri F, Mahmoodi NM (2010) Grafting of chitosan as a biopolymer onto wool fabric using anhydride bridge and its antibacterial property. Colloids Surf B 76:397–403
Rattanaphani S, Chairat M, Bremner JB, Rattanaphani V (2007) An adsorption and thermodynamic study of lac dyeing on cotton pretreated with chitosan. Dyes Pigm 72(1):88–96
Safapour S, Seyed-Esfahani M, Auriemma F, Ruiz de Ballesteros O, Vollaro P, Di Girolamo R, De Rosa C, Khosroshahi A (2010) Reactive blending as a tool for obtaining poly (ethylene terephthalate)-based engineering materials with tailored properties. Polymer 51(19):4340–4350
Salah SM (2011) Antibacterial activity and ultraviolet (UV) protection property of some Egyptian cotton fabrics treated with aqueous extract from banana peel. Afr J Agric Res 6(20):4746–4752
Samanta AK, Agarwal P (2009) Application of natural dyes on textiles. Indian J Fibre Text Res 34:384–399
Samanta AK, Konar A (2011) Dyeing of textiles with natural dyes, 3rd chapter. In: Kumbasar EPA (ed) Natural dyes. InTech, Rijeka
Samanta AK, Agarwal P, Datta S (2007) Dyeing of jute and cotton fabrics using jackfruit wood extract: Part I – effects of mordanting and dyeing process variables on colour yield and colour fastness properties. Indian J Fibre Text Res 32:466–476
Samanta AK, Agarwal P, Datta S (2008) Dyeing of jute with binary mixture of jackfruit wood and other natural dyes – study on colour performance and dye compatibility. Indian J Fibre Text Res 33:171–180
Samanta AK, Konar A, Chakraborti S (2011) Dyeing of jute fabric with tesu extract: Part 1 – effects of different mordants and dyeing process variables. Indian J Fibre Text Res 36:63–73
Saravanan P, Chandramohan G (2011) Dyeing of silk with ecofriendly natural dye obtained from barks of Ficus Religiosa.L. Universal J Environ Res Technol 1(3):268–273
Sathianarayanan MP, Bhat NV, Kokate SS, Walunj VE (2010) Antimicrobial finish for cotton fabrics from herbal products. Indian J Fibre Text Res 35:50–58
Sathianarayanan MP, Chaudhari BM, Bhat NV (2011) Development of durable antibacterial agent from ban-ajwain seed (Thymus serpyllum) for cotton fabric. Indian J Fibre Text Res 36:234–241
Shams-Nateri A (2011) Reusing wastewater of madder natural dye for wool dyeing. J Clean Prod 19:775–781
Sharma D, Gupta C, Aggarwal S, Nagpal N (2012) Pigment extraction from fungus for textile dyeing. Indian J Fibre Text Res 37:68–73
Singh R, Jain A, Panwar S, Gupta D, Khare SK (2005) Antimicrobial activity of some natural dyes. Dyes Pigm 66:99–102
Sinha K, Saha PD, Datta S (2012) Extraction of natural dye from petals of flame of forest (Butea monosperma) flower: process optimization using response surface methodology (RSM). Dyes Pigm 94:212–216
Sivakumar V, Vijaeeswarri J, Anna JL (2011) Effective natural dye extraction from different plant materials using ultrasound. Ind Crop Prod 33:116–122
Son Y, Kim B, Ravikumar K, Kim T (2007) Berberine finishing for developing antimicrobial nylon 66 fibers: % exhaustion, colorimetric analysis, antimicrobial study, and empirical modeling. J Appl Polym Sci 103:1175–1182
Son Y, Ravikumar K, Kim B (2008) Development of berberine attraction sites onto cellulosic substrates modified by reactive bridging agent: statistical optimization and analysis. Colloids Surf A 325(3):120–126
Sumithra M, Raaja NV (2012) Antibacterial efficacy analysis of Ricinus communis, Senna auriculata and Euphorbia hirta extract treated on the four variant of denim fabric against Escherichia coli and Staphylococcus aureus. J Text Sci Eng 2(3):1–4
Syamili E, Elayarajah B, Kulanthaivelu, Rajendran R, Venkatrajah B, Ajith Kumar P (2012) Antimicrobial cotton finish using green tea leaf extracts interacted with copper. Asian J Text 2(1):6–12
Thilagavathi G, Krishna Bala S (2007) Microencapsulation of herbal extracts for microbial resistance in healthcare textiles. Indian J Fibre Text Res 32:351–354
Tiwari HC, Singh P, Mishra PK, Srivastava P (2010) Evaluation of various techniques for extraction of natural colorants from pomegranate rind – ultrasound and enzyme assisted extraction. Indian J Fibre Text Res 35:272–276
Vaidyanathan J, Bhathena-Langdana Z, Adivarekar RV, Nerurkar M (2012) Production, partial characterization, and use of a red biochrome produced by Serratia sakuensis subsp. nov strain KRED for dyeing natural fibers. Appl Biochem Biotechnol 166:321–335
Vankar PS, Shanker R (2009) Eco-friendly pretreatment of silk fabric for dyeing with Delonix regia extract. Color Technol 125:155–160
Vankar PS, Shanker R, Srivastava J (2007) Ultrasonic dyeing of cotton fabric with aqueous extract of Eclipta alba. Dyes Pigm 72(1):33–37
Vankar PS, Shanker R, Dixit S, Mahanta D, Tiwari SC (2008) Sonicator dyeing of natural polymers with Symplocos spicata by metal chelation. Fiber Polym 9(2):121–127
Velmurugan P, Chae J, Lakshmanaperumalsamy P, Yun B, Lee K, Oh B (2009) Assessment of the dyeing properties of pigments from five fungi and anti-bacterial activity of dyed cotton fabric and leather. Color Technol 125:334–341
Velmurugan P, Kim M, Park J, Karthikeyan K, Lakshmanaperumalsamy P, Lee K, Park Y, Oh B (2010) Dyeing of cotton yarn with five water soluble fungal pigments obtained from five fungi. Fiber Polym 11(4):598–605
Venil CK, Lakshmanaperumalsamy P (2009) An insightful overview on microbial pigment, prodigiosin. Electron J Biol 5(3):49–61
Vinod KN, Puttaswamy, Gowda KNN, Sudhakar R (2010) Natural colorant from the bark of Macaranga peltata: kinetic and adsorption studies on silk. Color Technol 126:48–53
Xinsheng X, Lu W, Shunhua J, Qicheng Z (2008) Extraction of coloring matter from Sargentodoxa cuneata by ultrasonic technique and its application on wool fabric. Indian J Fibre Text Res 33:426–430
Zarkogianni M, Mikropoulou E, Varella E, Tsatsaroni E (2010) Colour and fastness of natural dyes: revival of traditional dyeing techniques. Color Technol 127:18–27
Zhang R, Cai Z (2011) Study on the natural dyeing of wool modified with enzyme. Fiber Polym 12(4):478–483
Acknowledgments
The authors are grateful to Tabriz Islamic Art University, Tabriz, Iran for the all supports.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kasiri, M.B., Safapour, S. (2013). Natural Dyes and Antimicrobials for Textiles. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (eds) Green Materials for Energy, Products and Depollution. Environmental Chemistry for a Sustainable World, vol 3. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6836-9_6
Download citation
DOI: https://doi.org/10.1007/978-94-007-6836-9_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6835-2
Online ISBN: 978-94-007-6836-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)