Abstract
Diisocyanate-induced asthma is difficult to diagnose since the immunopathological mechanisms and exposure determinants at the workplace are not well defined. The aim of this study was to evaluate the non-invasive methods of nasal lavage fluid (NALF) and induced sputum (IS) to enhance the diagnostic efficiency. Sixty-three diisocyanate-exposed workers with work-related shortness of breath underwent a standardized 4-steps-1-day-whole body exposure test with diisocyanates used at work up to 30 ppb. NALF and IS were collected before, 0.5, and 19 h after the end of exposure. Cellular composition and soluble inflammatory biomarkers were studied in the samples. In addition, ten controls with bronchial hyperresponsiveness, but without prior occupational diisocyanate exposure, were also examined. Twelve out of the 63 subjects (19 %) showed a significant asthmatic reaction (pulmonary responders) after challenge (FEV1 decrease >20 %). NALF samples did not demonstrate significant effects either on cellular composition or on mediator concentrations in the responders, non-responders, or controls at any time point. In contrast, in the IS samples of the pulmonary responders collected 19 h after challenge, the percentage of eosinophils was higher (p = 0.001) compared with baseline before challenge. Eosinophils were also increased 30 min and 19 h after challenge in IS samples of the responders compared with the non-responders or controls. In addition, 19 h after challenge the eosinophilic cationic protein (ECP) concentration was significantly higher in the responders than non-responders (p < 0.04) or controls (p < 0.002). In conclusion, positive asthmatic reactions to diisocyanates are accompanied by an influx of eosinophils into lower airways. Analysis of induced sputum should be implemented in the diagnostic procedure of diisocyanate-related airway diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Isocyanates and their products are important industrial chemicals related to occupational diseases. They are characterized by one or more isocyanate groups (–N = C = O) and exhibited strong chemical reactivity (Fisseler-Eckhoff et al. 2011; Wisnewski et al. 2006; Raulf-Heimsoth and Baur 1998). The most relevant commercial products are the monoisocyanate methyl isocyanate (MIC) and the diisocyanates 1,6-hexamethylene diisocyanate (HDI), 2,4- and 2,6-toluene diisocyanate (TDI), 1,5-naphthylene diisocyanate (NDI) and 4,4′-diphenylmethane diisocyanate (MDI) as well as oligomers and prepolymers of these isocyanates. Diisocyanates are widely used in the production of polyurethane foam, adhesives, plastic packaging material, laminates, polyurethane paints, and coatings. The compounds also are present in households, e.g. in paints or construction foam. Exposure to diisocyanates occurs mainly by inhalation and depends on the concentration and temperature during the manufacturing process. During recent years diisocyanates have been recognized as one of the main causes of occupational asthma (OA) induced by low-molecular-weight chemicals in Western countries (Paris et al. 2012; Vandenplas et al. 2011), but especially in the Asian-Pacific area (Wisnewski et al. 2006). The underlying mechanisms of diisocyanate-induced OA remain still unclear (Fisseler-Eckhoff et al. 2011; Raulf-Heimsoth and Baur 1998). At high concentrations, diisocyanates can have direct toxic effects on mucous membranes and at low concentrations they act as sensitizing agents after binding to different body proteins. In vitro studies supported a role of cell-mediated immune responses. The unequivocal diagnosis of diisocyanate asthma remains quite a challenge. Clinical history, questionnaires and physiological studies are frequently not definitive. Immunological tests have shown variable correlations with disease. Modern approaches in future development of laboratory tests are necessary (Palikhe et al. 2011). The prevalence of diisocyanate specific IgG- and IgE-antibodies among individuals with diisocyanate asthma is variable and not closely associated with the disease (Pronk et al. 2007). Specific inhalation challenge is considered the “gold standard” for diagnosis.
The aim of our study was to implement the non-invasive methods of the collection and analysis of nasal lavage fluid (NALF) and induced sputum (IS) in the diagnosis of diisocyanate-induced asthma.
2 Methods
2.1 Subjects and Protocol
The study was approved by the Ethics Committee of the Ruhr University Bochum and was conducted in accordance with the Declaration of Helsinki of the World Medical Association. All study participants gave written informed consent to the study protocol.
Sixty-three diisocyanate-exposed workers (85.7 % males, 34.9 % non-smokers and 23.8 % smokers; 27 % atopics) with work-related respiratory symptoms referred for a medical opinion by the accident insurance were examined at our institute. Ten subjects (30 % males, 50 % non-smokers, 30 % smokers, 70 % atopics) with bronchial hyperresponsiveness but without any prior occupational diisocyanate exposure were examined in the same way (controls). All subjects underwent a work-related challenge test with the dominant diisocyanate used at work. They were not exposed to diisocyanates for the last 5 days before the challenge with MDI (in 40 cases), TDI (in 6 cases), HDI (in 18 cases) and NDI (in 2 cases). The reference group was challenged with both TDI and MDI.
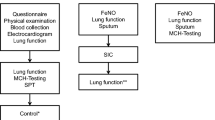
A standardized 4-step-1-day diisocyanate-exposure test was used. The diisocyanate atmospheres were generated in the air tide glass chamber as described previously (Marczynski et al. 2005; Merget et al. 2002). The concentration of diisocyanate vapor in the chamber was measured continuously with a calibrated tape monitor system. All participants in our study were continuously exposed to diisocyanates in four different concentrations, 5 ppb for 30 min, 10 ppb for 30, 90 min break; 20 ppb for 30, 90 min break; and 30 ppb for 30 min. The total diisocyanate exposure time was 2 h and the time from the beginning to the end of challenge was 5 h (Merget et al. 2004). Nasal lavage fluid and induced sputum were collected before, 30 min, and 19 h after exposure (Fig. 40.1).
2.2 Collection and Analysis of Nasal Lavage Fluid and Induced Sputum
Before, 30 min, and 19 h after challenge, nasal lavages were collected and analyzed as described earlier (Raulf-Heimsoth et al. 2011, 2000). Briefly, a syringe with 7 ml 0.9 % saline to which a nasal olive was added for close nostril fitting, was inserted into the left nasal cavity. The saline was passed slowly into the nasal cavity and back into the syringe five times. The recovered volume was recorded and stored at 4 °C.
After centrifugation, aliquots of the cell-free supernatants were stored at −80°C under argon protection until further analysis of soluble markers. The cell pellets were suspended in phosphate-buffered saline and the total cell number was determined in a Neubauer cell chamber.
IS was collected by inhalation of isotonic saline aerosol, generated by an ultrasonic nebulizer for 10 min. The subjects were motivated to actively cough, clear their throat, and expectorate sputum. The sputum processing started with selecting all viscid portions from the expectorated samples for minimizing contamination with saliva. The volume of IS was determined and an equal volume of 0.1 % sputulysin (dithiothreitol) was added. The samples were mixed gently by vortex mixer and incubated for 30 min at 37 °C. After centrifugation, the cell-free supernatants were aliquoted, stored at −80°C under argon protection until further analysis. The cell pellets were resuspended and the total cell number was determined.
For differential cell counts of NALF or sputum cells, slides were prepared by cytospin (Cytospin 2, Shandon Corp., Pittsburgh, PA) and stained with May-Grünwald-Giemsa. Three independent observers counted 200 cells on each slide by light microscopy. The results were expressed as the percentage of a total cell number and the absolute number of the cell population (without correction for squamous cells).
The inflammatory mediators were determined in the thawed cell-free supernatants of NALF and IS samples. All samples underwent only a single freeze-thaw cycle. The following soluble markers were measured: interleukins IL-5 and IL-8, NO derivatives, total protein, and eosinophilic cationic protein (ECP; using the ImmunoCAP system from Phadia, Uppsala, Sweden). IL-5 and IL-8 were measured with the OptEIATM ELISAs (BD Biosciences Pharmingen, Heidelberg, Germany) in a standard range of 2–500 pg/mL for IL-5 and 3–200 pg/mL for IL-8.
The amount of NO derivatives was measured by a colorimetric assay kit from AlexisTM (Cayman Chemicals; Grünberg, Germany) determining the total nitrate/nitrite concentration. The sensitivity of the assay was 5 μM and the standard range was between 0 and 35 μM. Total protein content was determined according to the method of Bradford with bovine serum albumin as standard solution (range 10–100 μg/mL) (Bradford 1976).
2.3 Statistical Analysis
Data were analysed with a GraphPad Prism vr. 5.01 for Windows (GraphPad Software, San Diego, CA). Values distribution was assessed using the D’Agostino & Pearson omnibus normality test. Values below the limit of quantification (LOQ) were set 2/3 of the LOQ. Comparisons of unpaired data were performed with a Mann-Whitney U test. For statistical analysis we used the adjusted mean ± SD. A-two-sided significance level of 0.05 was chosen for all tests.
3 Results
Twelve out of the 63 subjects (19 %) showed an FEV1 decrease >20 % after the challenge. Seven of these responders reacted to MDI (7/40; 18 %), two responded to TDI (2/6; 33 %) and one to HDI (1/18; 6 %). Two challenge tests were performed with NDI and both patients showed a positive response. Seven out of the 12 responders showed an early (immediate) type reaction (four of them challenged with MDI, one with HDI, and two with NDI) and three showed a dual reaction profile (all were challenged with MDI). Delayed type reactions were detected in two responders, both challenged with TDI. Fifty one diisocyanate-exposed subjects did not respond during or after the diisocyanate challenge test and were denominated as the non-responders. The characteristics of the three groups are summarized in Table 40.1. The age was similar in all groups, as was the smoking status. Forty two percent of the responders and only 23 % of the non-responders were atopics. According to our criteria of involving persons with bronchial hyperresponsiveness into the reference group, most of them were atopics (70 %). Median duration of occupational exposure to diisocyanate was different between the responders and non-responders; 8 and 20 years, respectively.
The cellular and mediator profiles of NALF and IS samples of 12 responders were compared with those of non-responders and 10 reference persons. Analysis of NALF, independent of the clinical reaction profile during and after the diisocyanate challenge test, showed no significant differences regarding either the cellular profile (especially eosinophil and neutrophil count) or the concentration of IL-4, IL-8, ECP, NO, or total protein (data not shown).
In contrast to NALF, in the IS samples of responders, the percentage of eosinophils significantly increased 19 h after challenge (p = 0.001) compared with baseline. In IS samples collected 30 min and 19 h after challenge, the percentages of eosinophils in the responders were significantly higher than in the non-responders and controls (both p < 0.001). No significant differences in the percentages of eosinophils were detected in IS samples before, 30 min, and 19 h after challenge between the non-responders and controls (Figs. 40.2 and 40.3). In 86 % of the IS samples of responders collected 19 h after challenge, more than 100 eosinophils/mL were detectable, whereas only in 11 % of the IS samples of non-responders and in no IS sample of controls this was the case. In addition, also the percentage of neutrophils increased after challenge in the responders (before: 6 %, 30 min: 12 %, 19 h: 20 % (median values)) and non-responders (before: 9 %, 30 min: 9 %, 19 h: 12 % (median values)), without reaching the significance level. No significant differences in the percentages of neutrophils were measured at any time point between the three groups.
The same analysis was performed for the ECP concentrations in IS: an increase of ECP concentrations after challenge in the responder group without similar increase in the non-responders and controls was detected. Nineteen hours after challenge significant differences in the level of ECP between the responder and non-responder groups (p < 0.04) and the responders and controls (p < 0.002) were measured. Thirty minutes after challenge no significant differences were observed between the three groups (Fig. 40.4).
Concentrations of IL-5 slightly increased 19 h after diisocyanate challenge in the responder group, but no significant differences between IL-5 levels in the non-responders and controls were detectable (Fig. 40.5). In 67 % of the IS samples of responders, the IL-5 levels were above 15 pg/mL (median: 19.6 pg/mL). 19 h after challenge, this was the case in only 20 % of non-responders (median: 5.6 pg/mL) and in 20 % of the controls (median: 6.7 pg/mL). Concentrations of IL-8 showed high inter-individual variability (range between <3.0 and 84,248.0 pg/mL), but in the IS samples of the responder group the IL-8 levels (median: 3,123.0 pg/mL) 19 h after challenge were higher than in the non-responders (median: 1,366.0 pg/mL) and controls (648.0 pg/mL), but without statistical significance.
4 Discussion
Collection of NALF and IS and analysis of specific cellular and soluble biomarkers are useful non-invasive tools to assess upper and lower airway inflammation. The major result presented in this study is the detection of eosinophilic inflammation in the lower airways in a group of workers responding with a FEV1-decrease >20.0 % to the diisocyanate challenge. In the IS samples, the percentages of eosinophils as well as ECP- and IL-5 concentrations of subjects with diisocyanate-induced positive reactions were increased 30 min and/or 19 h after challenge compared to baseline. Comparison between the IS profile of responders, non-responders, and controls indicated that the increase of eosinophils after challenge is the most sensitive sputum parameter for the responder allocation. Although our study is limited in the number of responders, the increase of eosinophils in the IS samples of responders was nearly independent of the type of asthmatic reaction (early, late, or dual) and independent of the inducing diisocyanate. In the case of occupational asthma induced by high molecular weight substances, the majority of subjects developed an eosinophilic airway inflammation after exposure, e.g., during a specific inhalation challenge with the relevant culprit. In the EAACI Task Force Consensus Paper (Quirce et al. 2010) the authors concluded that an increase in sputum eosinophil counts greater than 3 % after specific inhalation challenge often precedes the occurrence of functional changes on subsequent exposure. In 85 % of the IS obtained from the responders after diisocyanate challenge in our study eosinophil counts greater than 3 % were detected. In contrast, in only 11 % of the non-responders and 13 % of the controls the eosinophil percentages were greater than 3 % after challenge. The characteristics of eosinophilic inflammation were also supported by an increase of soluble factors like IL-5, a protein produced by several different cells including CD4+ T cells, mast cells and eosinophils, is involved in the development, survival, and activation of eosinophils and the eosinophil granule-derived cationic protein (ECP). Therefore, it is possible to conclude that positive reactions to diisocyanates are accompanied by an influx of eosinophils in the lower airways, documented by induced sputum analysis. Lemière et al. (2002) also showed in their study with 12 diisocyanate-exposed patients and IS analysis an acute inflammatory response, but with only a moderate increase of eosinophils and a dominant influx of neutrophils instead. In our study, the percentage of neutrophils also increased, but without significant differences between the responders and non-responders. In addition, differences in the study design (lower diisocyanate concentration during challenge test and induction of sputum with hypertonic saline solution) by Lemière et al. (2002) may explain the differences in the outcome compared to our study. In contrast to our study, Park et al. (1999) found that activated neutrophils may contribute to bronchoconstriction induced by TDI which may be associated with IL-8 release. In that study, the authors focused on neutrophil activation with the parameter chemotaxis, myeloperoxidase, and IL-8 analyzed in IS at the last time point of 7 h after challenge. The differences (neutrophilic vs. eosinophilic inflammation) may be caused by kinetics of cell influx: starting with a quick, but more or less unspecific, increase of neutrophils after challenge and only in responders, the eosinophils increased at later time points (e.g., the next day, 19 h after challenge) as a sign of (sub)-chronic inflammation.
In contrast to high molecular weight occupational allergens like latex (Raulf-Heimsoth et al. 2000), diisocyanates did not induce detectable cellular or mediator changes in the upper airways, documented by analysis of NALF. NALF has been previously described as an accepted non-invasive method to objectively measure nasal inflammation (Quirce et al. 2010). Therefore, these results indicate that although the nasal cavity is the primary route of entry for inhaled air and the first region of the respiratory tract to be in contact with the diisocyanate, the upper airways are not significantly affected by diisocyanate.
In conclusion, positive asthmatic reactions to diisocyanates are accompanied by an influx of eosinophils to the lower airways. Therefore, in the diagnostic procedure of diisocyanate-related airway diseases the analysis of induced sputum with focus on eosinophilic inflammatory markers should be implemented.
References
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Fisseler-Eckhoff, A., Bartsch, H., Zinsky, R., & Schirren, J. (2011). Environmental isocyanate-induced asthma: morphologic and pathogenetic aspects of an increasing occupational disease. International Journal of Environmental Research and Public Health, 8, 3672–3687.
Lemière, C., Romeo, P., Chabboillez, S., Tremblay, C., & Malo, J. L. (2002). Airway inflammation and functional changes after exposure to different concentrations of isocyanates. The Journal of Allergy and Clinical Immunology, 110, 641–646.
Marczynski, B., Merget, R., Mensing, T., Rabstein, S., Kappler, M., Bracht, A., Haufs, M. G., Käfferlein, H. U., & Brüning, T. (2005). DNA strand breaks in the lymphocytes of workers exposed to diisocyanates: Indications of individual differences in susceptibility after low-dose and short-term exposure. Archives of Toxicology, 79, 355–362.
Merget, R., Marczynski, B., Chen, Z., Remberger, K., Raulf-Heimsoth, M., Willrot, P. O., & Baur, X. (2002). Haemorrhagic hypersensitivity pneumonitis due to naphthylene-1,5-diisocyanate. European Respiratory Journal, 19, 377–380.
Merget, R., Heinze, E., Korn, M., Raulf-Heimsoth, M., Marczynski, B., & Brüning, T. (2004). Diagnostische Validität und Sicherheit eines 4-Stufen-1-Tagesexpositionstests mit Diisocyanaten. Atemwegs- und Lungenkrankheiten, 30, 380–381.
Palikhe, N. S., Kim, J. H., & Park, H. S. (2011). Biomarkers predicting isocyanate-induces asthma. Allergy, Asthma & Immunology Research, 3, 21–26.
Paris, C., Ngatchou-Wandji, J., Luc, A., McNamee, R., Bensefa-Colas, L., Larabi, L., Telle-Lamberton, M., Herin, F., Bergeret, A., Bonneterre, V., Brochard, P., Choudat, D., Dupas, D., Garnier, R., Pairon, J. C., Agius, R. M., Ameille, J., & Members of the RNV3P. (2012). Work-related asthma in France: Recent trends for the period 2001–2009. Occupational and Environmental Medicine, 69, 391–397.
Park, H., Jung, K., Kim, H., Nahm, D., & Kang, K. (1999). Neutrophil activation following TDI bronchial challenges to the airway secretion from subjects with TDI-induced asthma. Clinical and Experimental Allergy, 29, 1395–1401.
Pronk, A., Preller, L., Raulf-Heimsoth, M., Jonkers, I. C., Lammers, J. W., Wouters, I. M., Doekes, G., Wisnewski, A. V., & Heederik, D. (2007). Respiratory symptoms, sensitization, and exposure response relationships in spray painters exposed to isocyanates. American Journal of Respiratory and Critical Care Medicine, 176, 1090–1097.
Quirce, S., Lemière, C., de Blay, F., Del Pozo, V., Gerth Van Wijk, R., Maestrelli, P., Pauli, G., Pignatti, P., Raulf-Heimsoth, M., Sastre, J., Storaas, T., & Moscato, G. (2010). Noninvasive methods for assessment of airway inflammation in occupational settings. Allergy, 65, 445–458.
Raulf-Heimsoth, M., & Baur, X. (1998). Pathomechanisms and pathophysiology of isocyanate-induced diseases – Summary of present knowledge. American Journal of Industrial Medicine, 34, 137–143.
Raulf-Heimsoth, M., Wirtz, C., Papenfuss, F., & Baur, X. (2000). Nasal lavage mediator profile and cellular composition of nasal brushing material during latex challenge tests. Clinical and Experimental Allergy, 30, 110–121.
Raulf-Heimsoth, M., Pesch, B., Kendzia, B., Spickenheuer, A., Bramer, R., Marczynski, B., Merget, R., & Brüning, T. (2011). Irritative effects of vapours and aerosols of bitumen on the airways assessed by non-invasive methods. Archives of Toxicology, 85, S41–S52.
Vandenplas, O., Lantin, A. C., D’Alpaos, V., Larbanois, A., Hoet, P., Vandeweerdt, M., Thimpont, J., & Speybroeck, N. (2011). Time trends in occupational asthma in Belgium. Respiratory Medicine, 105, 1364–1372.
Wisnewski, A. V., Redlich, C. A., Mapp, C. E., & Bernstein, D. I. (2006). Polyisocyanates and their prepolymers. In I. L. Bernstein, M. Chan-Yeung, J.-L. Malo, & D. I. Bernstein (Eds.), Asthma in the workplace (3rd ed., pp. 481–504). New York: Taylor & Francis Group, LLC.
Acknowledgements
This study is part of a doctor thesis of R. Liebig and part of the IPA-projects All/Imm-D and Med-D funded by the DGUV (German Social Accident Insurance).
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Raulf-Heimsoth, M. et al. (2013). Implementation of Non-invasive Methods in the Diagnosis of Diisocyanate-Induced Asthma. In: Pokorski, M. (eds) Neurobiology of Respiration. Advances in Experimental Medicine and Biology, vol 788. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6627-3_40
Download citation
DOI: https://doi.org/10.1007/978-94-007-6627-3_40
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6626-6
Online ISBN: 978-94-007-6627-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)