Abstract
Oil reservoirs located deep within the earth crust represent one of the most challenging environments for life, usually providing combinations of high temperatures and pressures, as well as high concentrations of salts, heavy metals, and organic solvents. Organisms thriving in such environments, therefore, have to be truly poly-extremophiles, adapted to conditions otherwise very hostile to life. In spite of this, research carried out in many groups worldwide throughout the past decades has revealed that deep subsurface oil reservoirs indeed are populated by diverse consortia of poly-extremophilic Bacteria and Archaea. Numerous sites on all continents have been sampled in search for novel species and strains to describe and compare microbial consortia and to understand biological processes that might occur in response to and possibly interfere with an efficient oil production. In addition, the special adaptations of oil reservoir microbes to their extreme environments have rendered them highly attractive for bioprospecting approaches for novel enzymes and metabolites with potential industrial value.
In this chapter, we provide a current status overview of subsurface oil reservoir microbiology research, covering sites with in situ temperatures of 50 °C and higher. We also discuss the challenge of representative sampling and contamination issues affecting research results and derived conclusions. Further, the current understanding of metabolic capabilities predominant in oil reservoir communities is discussed, including the challenges these communities provide in oil production and their potential with respect to Biologically activated Enhanced Oil Recovery (Bio-EOR) and other industrial applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Microbial life has proven to be adapted to various extreme conditions on earth, including extremely cold and hot, acidic and alkaline, as well as high salt (Allen and Banfield, 2005; Podar and Reysenbach, 2006). Oil reservoirs located deep within the earth crust are providing not only very high temperatures but in addition often high pressure and high salt, heavy metal, and organic solvent concentrations (Youssef et al., 2009). Consequently, microorganisms tolerating and propagating under such conditions are truly poly-extremophiles, being both (hyper)thermophilic, piezotolerant, halophilic, and solventophilic (Kotlar, 2012). Oil reservoir microbial communities are interesting research objects, also due to their potential impact on oil production and their relevance for industrial bioprocess applications including approaches of Biologically activated Enhanced Oil Recovery (Bio-EOR) and bioprospecting for thermostable biocatalysts applicable in industrial bioprocesses. Due to the enormous commercial values associated with oil production, bio-probing for the purpose of reservoir monitoring and the development of complementary methods in search for new oil prospects also represents fields with potentially high impacts.
To date, numerous oil reservoirs worldwide have been studied with respect to their content of microorganisms (Fig. 1). These studies included (1) the description of new genera, species, and strains of both Bacteria and Archaea; (2) the specific enrichment and characterization of subpopulations like methanogens and sulfate-reducing bacteria (SRB), the latter being discussed to be related to reservoir and oil production problems like souring and corrosion; (3) cultivation-dependent and cultivation-independent studies aiming at characterizing the complexity of individual microbial communities, nowadays promoted by flourishing new cultivation-independent technologies like the meta’omics (metagenomics, metatranscriptomics, etc.); and (4) comparative studies covering multiple, distantly located reservoir sites. For such approaches, the access to representative, uncontaminated sample material is important, though often difficult to achieve and thus representing a major obstacle. Metagenome analysis and upcoming new technologies can be expected to revolutionize the view on oil reservoir microbial communities in the near future.
In this chapter, we follow the traditions of previous, excellent reviews, for example, by Magot and coworkers (Magot et al., 2000), focusing on microbiology in oil reservoirs with in situ temperatures of 50 °C or higher, including (hyper)thermophilic strain isolations and descriptions from such locations.
2 Microorganisms and Microbial Consortia of High-Temperature Oil Reservoirs
During the past three decades, many high-temperature oil reservoirs (≥50 °C in situ temperature) in Europe, North America, South America, Africa, Asia, and Australia have been subject to microbiological characterizations (see Fig. 1 for study types and references). The longest tradition of such studies exists in Europe and North America starting in the late 1980s, while Asian sites (particularly Chinese) have gained an increasing attention during the last few years. South American high-temperature reservoirs have only rarely been investigated so far, and a number of studies including some of the early consortia characterizations cover multiple sampling sites on different continents. The best surveyed region in this respect is the North Sea and the European part of the North Atlantic Ocean, both concerning consortium studies (mainly cultivation dependent) and related to the description of new species and strains. After 2006, obviously responding to the revolutionary technological developments in high throughput sequencing, culture-independent approaches have increased in number, mainly using samples from Asia but also from South America and Europe. Metagenomic (Kotlar et al., 2011) and other not yet applied meta’omic (e.g., metatranscriptomic, metaproteomic) approaches to study entire microbial communities, including the metabolically active fractions, can be expected to be applied in increasing frequency in the near future. Such studies will likely provide unprecedented insight into microbial in situ processes both in pristine reservoirs and reservoirs subjected to methods of enhanced oil recovery. Up to now, sampling has most frequently originated from production water or wellheads, and a large fraction of the reservoirs have also been flooded with production-associated water prior to sampling (see Fig. 1). This means that many of the strains or consortia described in the literature are likely to be contaminants relative to the original, untouched reservoir. Therefore, the significance of future microbial community studies of oil reservoirs will to a large degree depend on the quality of the sample material (closer discussed in Sect. 3), and some of the latest studies have already applied dedicated sampling devices that account for this type of challenge (Yamane et al., 2008; Kotlar et al., 2011).
2.1 New Microbial Isolates
A large number of both bacterial and archaeal species have for the first time been described as isolates from high-temperature oil reservoirs (see Fig. 2 for species names and references). Regularly, enrichment cultures have been grown, resulting in isolation of pure strain cultures, which subsequently have been characterized in detail to define their taxonomic placement in the microbial tree of life (Fig. 2). The Thermotogae, represented by the genera Petrotoga (6 species), Thermotoga (5 sp.), Geotoga (2 sp.), Kosmotoga, Oceanotoga, and Thermosipho, account for the highest number of new strain isolates from high-temperature oil reservoirs, followed by the Firmicutes with the genera Thermoanaerobacter (3 sp.), Geobacillus (2 sp.), Bacillus, Desulfotomaculum, Caldanaerobacter, and Mahella. Isolates from other bacterial groups represent the genera Deferribacter (Deferribacteres), Thermus (Deinococcus-Thermus), Anaerobaculum, and Thermovirga (both Synergistetes). Archaeal isolates from high-temperature oil reservoirs belong to the genera Methanoculleus and Methermicoccus (both Methanomicrobia), Methanothermobacter (Methanobacteria), Methanococcus (Methanococci), Archaeoglobus (Archaeoglobi), and Thermococcus (Thermococci).
Phylogenetic placement of microbes detected in oil reservoir samples. (#) Number of reported encounters of genera in the different studies explicitly referred to in Fig. 1. Bullet points/bold represent detailed strain descriptions. The current release no. 106 of the “All-Species Living Tree” Project (LTP) (Yarza et al., 2008) was used for the tree representation, modified using the ARB software (Ludwig et al., 2004).
It needs to be mentioned that it is widely accepted that only a very small fraction of microorganisms can be readily cultivated using established methods (Rappé and Giovannoni, 2003). In that sense, the listing of strain isolates in Fig. 2 (bold/bullet points) includes the strong bias of cultivability and therefore does not represent a valid picture of predominant species present in oil reservoirs. In addition, it cannot be ruled out that some of these strains are not indigenous to the sampled reservoir, but rather represent contaminations from oil production processes or sampling.
2.2 Cultivation-Dependent Consortial Studies
The majority of studies based on high-temperature oil reservoir derived sample material, including most of the strain isolations and descriptions mentioned above (Sect. 2.1), include enrichment culture prior to strain isolation or 16S rRNA gene amplification, library cloning, and sequence analysis (see Fig. 1 for references). Such enrichment steps are bound to introduce the potentially strong bias of cultivability in the analysis of a community composition within an oil reservoir (see comment at the end of Sect. 2.1), often on top of putative contaminations and biases originating from sampling and the way the respective original reservoir content has been altered by production processes. In spite of all these concerns, we found it interesting to relate all the reported organisms to the microbial tree of life, to see if some major trends could be observed (Fig. 2). The figure also includes the species names of the isolated strains (Sect. 2.1, bold/bullet points) and different genera detected in cultivation-independent studies (Sect. 2.3). It is striking from this figure that Proteobacteria are very frequently reported as being present based on consortium studies, while they seem to never have been identified as single novel isolates from oil reservoir samples growing at 50 °C or above. The reason for this discrepancy is not clear but could potentially be related, for example, to the conditions commonly used for strain purification, which may be in disfavor of this group of organisms. Another possible explanation might lie in contaminating DNA, leading to misinterpretations of obtained 16S rRNA gene sequence data. For the remaining parts of the tree, the correlation between individual isolates and community compositions is more consistent, and the Firmicutes and Thermotogae are heavily represented. The same accounts for various Archaea (like Methanomicrobia, Methanobacteria, and Thermococci). This indicates that members of these taxonomic groups indeed are typical indigenous inhabitants of hot oil reservoirs, a conclusion that also appears reasonable based on their biological properties.
2.3 Cultivation-Independent Studies
Many cultivation-dependent studies performed so far and discussed in Sect. 2.2 include approaches of amplification of ribosomal 16S rRNA genes followed by cloning and sequence determinations. Some of these studies follow experimental set-ups that in parts do not involve enrichment steps prior to 16S rRNA gene amplification (Orphan et al., 2000; Bonch-Osmolovskaya et al., 2003; Sette et al., 2007; Brakstad et al., 2008; Dahle et al., 2008; Korenblum et al., 2012). In addition, increasing numbers of studies in the most recent years, predominantly based on Asian reservoir samples, do not include enrichment steps at all, rendering them completely cultivation independent (see Fig. 1 for references). Such procedures should implicate a less biased picture of the microbial population in a given sample, particularly if contamination sources have also been minimized. 16S rRNA-based studies are limited with respect to the information that can be obtained, in the sense that the entire genetic make-up of the corresponding organisms remains unknown. To overcome these limitations, our group recently carried out a nontargeted metagenomic approach to sequence metagenomic DNA from an oil reservoir on the Norwegian Continental Shelf (Kotlar et al., 2011). In this case, we applied a pressurized sample methodology to a reservoir that had not been contaminated by sea water breakthrough. This study confirmed the presence of typical thermophilic bacteria (e.g., Thermotogales taxa) and Archaea (e.g., methanogens), but an apparently diverse group of Proteobacteria was also predicted from this study (Sect. 2.2). This may indicate that such bacteria are actually abundant in hot oil reservoirs but that they for some reason have not been cultivated as individual strains.
2.4 Studies Specifically Addressing Reservoir Problems
Some groups of microorganisms are seemingly related to specific reservoir conditions and problems like reservoir souring and corrosion which are discussed to be a consequence of microbial processes taking place during oil production (see Sect. 4.6), especially in oil reservoirs exploited by application of secondary recovery methods like the injection of sea or fresh water. As a consequence, a number of studies aim at enriching and describing the microbial species possibly involved in such reservoir problems, like for the souring case, sulfate-reducing bacteria (SRB), which in several studies have been enriched and analyzed with respect to taxonomy and/or metabolism (Rosnes et al., 1991; Leu et al., 1998, 1999; Rozanova et al., 2001a; Kaster et al., 2007). Though specific markers for SRB exist (e.g., the dsrAB and the aps genes (Teske et al., 2003)), they have obviously never been applied in the context of high-temperature oil reservoir studies. Other examples of enrichment and analysis of a specific subfraction of an oil reservoir microbial community are the analysis of methanogens by enrichment and sequencing of the mcrA and assA genes (Mbadinga et al., 2012) and organisms capable of alkane degradation/hydrocarbon oxidation using alkB gene enrichment (Shestakova et al., 2011).
3 Oil Reservoir Sample Recovery and Processing
Deeply buried oil reservoirs are particularly challenging to sample, at least if it is considered important to keep the level of contamination at a minimum. Localization, logistics, knowledge about sampling methodology, knowledge about reservoir history, as well as the dependence on extensive oil company collaboration are relevant factors influencing the sampling. Most of the studies reported in the literature are therefore not meeting the ideal requirements for understanding the composition and metabolic performance of the indigenous microbial communities. Since we believe that these problems are very important for the future progress in the field, we have chosen to elaborate more on these problems in the Sects. 3.1, 3.2, and 3.3 below.
3.1 Sampling Methodology
Oil reservoir samples for microbial studies can be collected in various ways, and the samples themselves can be very different, for example, originating from the oil phase (Pineda-Flores et al., 2004), from the water phase (Nilsen et al., 1996b), or from drilling cores (Spark et al., 2000). They can also be sampled from different parts of the technical infrastructure, like wellheads (l’Haridon et al., 2002; Bonch-Osmolovskaya et al., 2003), first separators (Leu et al., 1999; Brakstad et al., 2008), or tanks (Takahata et al., 2001). In addition, some of the reported samples are “mixed” (e.g., Rozanova et al., 2001a), containing material from more than one oil well, whereas others originate from single wells only (e.g., Li et al., 2006; Kotlar et al., 2011). Even if it can be seen as an oversimplification, one may probably assume that the more distant the sampling point is from the actual oil reservoir, the higher in general is the risk for contamination (discussed below).
In addition to differences in sampling sites, the methods used for sample withdrawal can also be very different. The overall impression from the relevant literature is that sampling methodology is often poorly described. Some studies use samples collected by tapping a pipeline at atmospheric pressure (e.g., Magot et al., 2004; Dahle et al., 2008; DiPippo et al., 2009), whereas other collect pressurized samples (e.g., Kotlar et al., 2011; Yamane et al., 2011) to avoid cell lysis (Sect. 3.2.2). Even if, to our knowledge, only applied in one study so far, there are technical possibilities for more advanced sample collection, where a sample is collected in situ in an oil reservoir well, enclosed, and transported up to the platform with a maintained high pressure (Yamane et al., 2011). Such a procedure further minimizes the loss of representativeness due to cell lysis and potential contamination of the sample from pipelines and other infrastructures, however, being extremely costly due to the advanced equipment used and a longer interruption of oil production from the well.
3.2 Special Challenges in Sample Collection
Sample collection from deeply buried oil reservoirs is virtually impossible without a close collaboration with the relevant oil company. Due to safety regulations for oil platforms and limited access to these areas, people with very different educational background are commonly involved at the different stages of the sample collection procedure, and samples are often collected by personnel lacking microbiological background, increasing the risk of contamination (see Sect. 3.2.2). Different challenges apply, dependent on the type of sample to be collected and the sampling methodology. Some of the main and particular pronounced aspects are described below.
3.2.1 Access to Reservoir Samples
Oil reservoirs are often remotely located, resulting in logistical challenges concerning sampling equipment and sampled material, leading to longer transportation times than preferred from a microbiological point of view. Due to these obstacles, oil reservoir samples are in practice restricted to selected research groups only, i.e., those that have managed to establish a collaboration agreement with an oil company. The process of sample collection from producing oil wells is often in conflict with a desirable continuous oil production and the associated enormous value generation. A stop in oil production for the purpose of sampling involves beside the direct economic loss often also substantial risks for the oil company (e.g., clogging of production pipes), rendering establishment of such collaboration a difficult task.
3.2.2 Representativeness of Sample Material
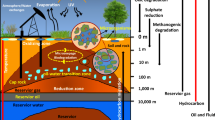
The question of whether or not microbes isolated/described/identified using oil reservoir samples are indigenous or not, is a constantly debated issue in the literature (e.g., Magot et al., 2000; Youssef et al., 2009). The first issue is the origin of the microbes analyzed, whether or not they originate from the actual original reservoir or if they have been introduced to the oil reservoir by processes like drilling or well treatment (Fig. 3). In addition, the exact origin of the sample material might be difficult to elucidate, since sediment particles and fluids might, apart from the actual reservoir site, originate from pipelines and platform infrastructures. Samples used for reported studies have also been found to be collected at different structures, and depending on the sampling site, non-indigenous microbes within the sample are often likely to occur. However, isolation of new strains and description of microbes from oil reservoir samples, indigenous or not, may still be valuable for certain types of studies, for example, within bioprospecting.
Most studies using oil reservoir samples are performed on samples subjected to a rapid pressure reduction (transportation of the oil sample to the platform followed by the release of sample pressure at the production liner), potentially resulting in substantial cell lysis within the sample (similar to the effect of a French pressure cell press). A few studies (Kotlar et al., 2011; Yamane et al., 2011) perform sampling into pressure flasks to maintain high pressure upon sampling. From these flasks, upon arrival in the laboratory, pressure is subsequently released by a procedure that is slow enough to presumably avoid extensive cell lysis. This approach does most likely result in the collection of a more representative sample with respect to the in situ microbial community. Therefore, such samples are preferred for total oil reservoir microbial community descriptions (Fig. 3).
The analysis and downstream handling of the reservoir samples might also influence whether or not indigenous microbes are analyzed (Fig. 3). Cultivation in general might be selective for nonindigenous microbes, since the in situ reservoir conditions might be difficult to mimic in the lab (e.g., Whitman et al., 1998; Reeder and Knight, 2009). Analysis of isolated DNA would therefore be a more accurate approach for the characterization of microbial communities (e.g., Amann et al., 1990; Reeder and Knight, 2009); however, contamination of the sample might still pose a problem, and DNA-based methods might also be biased towards more well-known microorganisms (e.g. primer design for 16R rRNA gene analysis). To date, a metagenomic approach (analyzing the total DNA content, i.e., the metagenome, of the sample) is believed to be the most accurate approach to environmental microbial community characterization (Cowan et al., 2005; Quince et al., 2008; Sleator et al., 2008).
3.3 Limitations and Restrictions on Sample Information and Dissemination of Analysis Results
Characterization of oil reservoir microbial communities usually requires (as described above) the collaboration with an oil company, which often raises the issues of confidentiality and Intellectual Property Rights (IPR). Academic groups are widely required to sharing research result by publication. However, due to the enormous investments in oil production and corresponding company secrecy policies, sharing of background information related to the sampled reservoir and the sample origin may be restricted. As a consequence of this, limited background information, for example, about the physicochemical characteristics of the reservoirs (even though existing), may complicate an interpretation of the results of analyses from these environments.
Also IPR issues might influence the research on oil reservoir microbial communities, since dissemination of results may be delayed due to patenting processes, or even be excluded from publication or patenting, and kept undisclosed. Findings in studies of oil reservoir microbes can be of high economical value for oil companies (or other companies), and hence, carefulness is indicated when sharing information from such findings. Since an in depth understanding of oil reservoirs as habitats for microbial life still is in its infancy, and in addition, such environments provide very special environmental conditions, new species with yet unknown but highly desired qualities or new enzymes with high industrial potential might be revealed in such studies.
4 Metabolic Capabilities of Oil Reservoir Microorganisms
As in many microbial habitats, the in situ microbial metabolic processes within oil reservoirs are likely to be very diverse. The processes are obviously dependent on the different groups of organisms present within the reservoirs at any given time, as well as on nutrients and other compounds available for consumption. These are parameters that differ from reservoir to reservoir and might also change within the same site due to natural processes and/or anthropogenic influences. In general, due to the absence of oxygen, microorganisms indigenous to oil reservoirs can be expected to be capable of anaerobic metabolism. In turn, strictly aerobic species unambiguously detected in reservoir samples are likely to be contaminants either from oil production processes or sampling. Due to the relatively limited knowledge of oil reservoir microbial communities, the understanding of the true in situ processes is also limited and will in most cases be rather speculative. However, by increasing knowledge on the composition of these microbial consortia and comparison with related (and currently better understood) habitats, some main metabolic processes of oil reservoirs can be predicted, and the main groups of microbes expected to be involved are discussed below.
4.1 Sulfate Reducers
Sulfate-reducing bacteria (SRB) constitute a group frequently isolated from oil reservoir samples (e.g., Rosnes et al., 1991; Tardy-Jacquenod et al., 1996; Leu et al., 1998, 1999; Kaster et al., 2007; Kotlar et al., 2011). SRB taxa are in many cases also likely to be able to reduce other sulfur-containing compounds (like sulfite or thiosulfate), and hence the name sulfate-reducing bacteria might in some cases be misleading. SRB recovered from oil reservoir samples do seemingly belong to different phyla; Proteobacteria, mainly Deltaproteobacteria as, for example, Desulfovibrionales and Desulfomonadales species (Beeder et al., 1995; Rees et al., 1995; Rozanova et al., 2001b), Firmicutes (Nilsen et al., 1996a, b; Magot et al., 2000; Dahle et al., 2008; Youssef et al., 2009; Kotlar et al., 2011), and Thermodesulfobacteria species (Beeder et al., 1995; Yamane et al., 2011). There are also several archaeal species recovered from oil reservoirs that exhibit sulfate-reducing capabilities, exemplified by Archaeoglobus fulgidus (Beeder et al., 1994), Archaeoglobus lithotrophicus (Stetter et al., 1993), and Thermococcus sp. (Slobodkin et al., 1999; Orphan et al., 2000).
SRB can partly or completely oxidize a broad variety of substrates, including aromatic and aliphatic constituents of petroleum oil, coupled to the reduction of sulfate to hydrogen sulfide (H2S) (Rosnes et al., 1991; Nilsen et al., 1996b; Rabus et al., 1996). H2S accumulation promoted by SRB activities might in some cases result in reservoir souring (Sect. 4.6.1). SRB strains normally grow by using sulfate, sulfite, or thiosulfate as electron acceptors; otherwise, their growth is linked to fermentative processes in the absence of these electron acceptors.
4.2 Methanogens
Methanogenesis is the final step in the complex processes involved in the anaerobic degradation of organic matter (such as petroleum oil), and methanogens are microbes able to convert hydrogen (H2), carbon dioxide (CO2), and fermentative substrates (e.g., acetate) into methane (Magot et al., 2000; Gray et al., 2009). These organisms do all belong to the archaeal domain and typically live in anaerobic habitats, often attributing extreme conditions. Methanogens are generally divided into three distinct groups depending on their substrates: hydrogenotrophs (H2/CO2), methylotrophs (methylated compounds), and acetoclasts (acetate) (Garcia et al., 2000).
There are numerous examples of methanogens recovered from, or identified in, oil reservoir samples, and several different genera have frequently been identified, such as Methanocalculus (Ollivier et al., 1998; Li et al., 2007a, 2012; Yamane et al., 2011; Mbadinga et al., 2012), Methanoculleus (Ollivier et al., 1998; Orphan et al., 2000; Li et al., 2007a, 2012; Brakstad et al., 2008; Cheng et al., 2008; Lan et al., 2011; Yamane et al., 2011; Mbadinga et al., 2012; Tang et al., 2012), Methanobacterium (Belyaev et al., 1983; Orphan et al., 2000; Bonch-Osmolovskaya et al., 2003; Li et al., 2007a, b, 2012; Ren et al., 2011; Yamane et al., 2011; Tang et al., 2012), Methanothermobacter (Bonch-Osmolovskaya et al., 2003; Nazina et al., 2006; Li et al., 2007a, b; Cheng et al., 2011; Lan et al., 2011; Ren et al., 2011; Yamane et al., 2011; Mbadinga et al., 2012; Tang et al., 2012), and Methanococcus (Nilsen and Torsvik, 1996; Orphan et al., 2000; Li et al., 2007a, b, 2012; Kaster et al., 2009; Kotlar et al., 2011). The different species seemingly use different substrates for growth but all produce methane, either by their own metabolism completely, but in many cases in syntrophic interactions with other microorganisms, for example, SRB and/or fermentative bacteria (Garcia et al., 2000; Scholten et al., 2007; Dar et al., 2008; Wintermute and Silver, 2010); see Sect. 4.5 below.
4.3 Fermentative Bacteria
Fermentative microorganisms constitute an important part of oil reservoir microbial communities. Several types of mesophilic fermentative bacteria have been isolated from low-temperature oil reservoirs (Magot et al., 2000), but for the thermophilic fermenters, the largest fraction of recovered species are members of the Thermotogae phylum (Davey et al., 1993; Jeanthon et al., 1995; Ravot et al., 1995; Fardeau et al., 1997; Lien et al., 1998; Orphan et al., 2000; l’Haridon et al., 2001, 2002; Takahata et al., 2001; Miranda-Tello et al., 2004, 2007; DiPippo et al., 2009; Youssef et al., 2009; Jayasinghearachchi and Lal, 2011; Mbadinga et al., 2012) or the family of Thermoanaerobiaceae (Fardeau et al., 1993, 2000; Cayol et al., 1995; l’Haridon et al., 1995; Leu et al., 1998; Li et al., 2007b).
Most Thermotogales species isolated are able to grow on complex substrates (e.g., amino acids, sugars, and peptides), reducing sulfur and/or thiosulfate (Fardeau et al., 1997; Takahata et al., 2001). The Thermoanaerobacteriales ferment sugars (e.g., Cayol et al., 1995; Grassia et al., 1996), organic acids (e.g., Rees et al., 1997), and/or amino acids (e.g., Dahle and Birkeland, 2006) and typically reduce thiosulfate to sulfide or elemental sulfur, using electrons from carbohydrates or hydrogen (Magot et al., 2000). Common end products are H2, CO2, and acetate, compounds that are often used as substrates by other microbes within the habitat, indicating interactions and syntrophy involving fermentative microbes within the oil reservoirs (Sect. 4.5). There are also data indicating presence of fermentative archaeal species, like Thermococci and Pyrococci, within the oil reservoir habitats (e.g., l’Haridon et al., 1995; Miroshnichenko et al., 2001; Kaster et al., 2009; Kotlar et al., 2011; Lan et al., 2011; Ren et al., 2011; Lewin et al., 2013). When isolated and cultivated in the laboratory, such strains usually grow on complex carbon sources and reduce elemental sulfur to sulfide (Stetter et al., 1993).
4.4 Other Groups
Oil reservoir microbial communities may contain species belonging to other groups than those described above. Several studies have detected iron-reducing bacteria like Deferribacter thermophilus able to reduce iron or manganese (Greene et al., 1997; Orphan et al., 2000), Alteromonas/Shewanella species capable of iron, elemental sulfur, sulfite, and thiosulfate reduction (Magot et al., 2000; Brakstad et al., 2008), as well as nitrate-reducing bacteria (NRB), like different Geobacillus species (Nazina et al., 2001; Bonch-Osmolovskaya et al., 2003; Li et al., 2007b; Shestakova et al., 2011). However, due to the lack of data about availability of, for example, nitrate, iron, or manganese levels in oil reservoirs (as exists in higher extent for sulfate and methane), the relevance of metabolic processes related to these compounds is harder to evaluate and therefore becomes very speculative.
4.5 Syntrophic Interactions Within Oil Reservoir Microbial Consortia
Microbial processes within environmental habitats are usually not independent of each other, but rather connected, and many microbes are likely to form syntrophic interactions (Wintermute and Silver, 2010). The complexity of such interactions and their dependence on microbial growth and available nutrients make most discussions speculative. However, based on existing data and processes likely to occur, some syntrophic interactions can be expected within oil reservoir habitats. Furthermore, it has been shown that syntrophic processes in general result in low-energy yields and consequently slow growth of the microbes involved (McInerney et al., 2009). This is consistent with reports indicating slow growth of subsurface microbial communities (e.g., Price and Sowers, 2004; Jørgensen and D’Hondt, 2006; Morono et al., 2011), which is likely to also be true for microbes prevailing in oil reservoirs.
The presence of SRB and methanogens within the habitat and the indication of active methanogenic hydrocarbon metabolism within the oil fields (e.g., Jones et al., 2008) suggest the presence of syntrophic interactions between SRB and methanogens and possibly with additional microbial groups (Jones et al., 2008; Pernthaler et al., 2008; Gray et al., 2009; Mayumi et al., 2011). It has been shown that SRB and methanogens under some conditions compete for the same substrates (electrons and H2) produced by fermentative microbes (Dar et al., 2008). At high-sulfate levels, SRB growth will be favored (due to their thermodynamically favorable process), whereas at lower-sulfate concentrations, substrates will be used by methanogens. However, SRB do not compete for methylated substrates; hence, methylotrophic methanogens are then favored even at high-sulfate concentrations (Cetecioglu et al., 2009; Lazar et al., 2011).
Methanogens are capable of using different substrates; hence, methanogenesis is likely to occur under several conditions. In environments containing complex organic compounds, such as oil reservoirs, and with low levels of sulfate and nitrate, methanogens are reported to be linked to chemoheterotrophic bacteria for organic substrate degradation (Garcia et al., 2000). Polymers are microbiologically degraded, resulting in simpler organic compounds utilized for acidogenesis by fermentative bacteria, which in turn produce substrates for methanogens or for syntrophic bacteria. The resulting simple methylated compounds, acetate, alcohols, and H2/CO2 are then consumed by methanogenic Archaea in methanogenesis. The syntrophic interactions are dependent on a H2-consuming part in the interaction, keeping H2-levels low and hence making the whole reaction thermodynamically favorable (McInerney and Bryant, 1981).
4.6 Impact of Oil Reservoir Microbial Processes on Petroleum Oil and Oil Production
Metabolic processes attributed by oil reservoir microbial communities will, to a smaller or larger extent, have an impact on the petroleum oil and oil production from the reservoir. Different groups of microbes are proposed to have different effects and can affect reservoir souring, oil recovery, or corrosion of infrastructures (Youssef et al., 2009). Some processes do seemingly have negative effects on oil production, whereas others are suggested to aid oil recovery, normally referred to as Microbial Enhanced Oil Recovery (MEOR) or Biologically activated Enhanced Oil Recovery (Bio-EOR). Due to the constant need for increased oil recovery, progression within this field is naturally desirable. However, this is a complex and challenging research area. There are supposed differences between lab-scale experiments and actual oil field effects, as well as various challenges (e.g., long-term effects) connected to field studies, both being examples of factors complicating these studies.
4.6.1 Negative Effects of Microbial Processes in Oil Production
SRB (Sect. 4.1) are one of the most well-known microbial groups of oil reservoirs due to their possible different effects on petroleum oil and oil recovery. In situ growth of SRB using sulfate as electron acceptor can result in accumulation of H2S and consequently in reservoir souring (Bødtker et al., 2008). This might be a consequence of reservoir flooding, since seawater introduced into the reservoir usually is high in sulfate levels, promoting growth of SRB and consumption of H2 for H2S production over other processes (e.g., methanogenesis, see Sect. 4.5). However, not all flooded reservoirs are soured, and hence the level of reservoir souring also seems to depend on additional factors. Reservoir souring is often associated with plugging (due to accumulation of sulfide minerals), problems with corrosion of pipes, and platform structures and with risks associated with the toxicity of H2S (Cord-Ruwisch et al., 1987; Myhr et al., 2002; Duncan et al., 2009). Naturally, petroleum oil is rich in hydrocarbons and may hence be seen as microbial growth substrates within the reservoir. Several species of the microbial communities are able to degrade oil constituents and use them as carbon source. However, due to the expected lack of other nutrients, growth (and thereby oil consumption in situ) is limited. Different microorganisms and potential genes expected to play a role in oil degradation have been identified and analyzed (e.g., Head et al., 2006). One specific example is methanogens degrading petroleum hydrocarbons and producing methane gas (Jones et al., 2008; Gieg et al., 2010). SRB and Fe(III)-reducing bacteria are also expected to degrade hydrocarbons in oil reservoirs considering the presence of sulfate and Fe(III) oxides and the reported capacity of these microorganisms to degrade hydrocarbons (Van Hamme et al., 2003).
4.6.2 MEOR and Bio-EOR
Many oil fields are approaching tail production, and hence, various tertiary methods for enhanced oil recovery (EOR) are wished for and desirable to apply within these reservoirs. There are different microbial processes and products derived from microbial metabolism that are suggested to have positive effects on oil recovery from a reservoir site (MEOR/Bio-EOR processes). These involve the reduction in oil viscosity by production of solvents or gases, increase in oil mobilization by hydrocarbon metabolism, or production of emulsifiers (Belyaev et al., 2004; Sen, 2008). Potentially relevant EOR methods also include reservoir flooding using alkaline solutions or additives such as biologically derived polymers and surfactants. Several studies indicate promising results; however, actual in situ processes are often difficult to monitor, and field studies might be complex to perform and interpret, which makes this a very challenging research area.
A common problem in oil production is immobilized oil trapped within reservoir sediments. Several end products from microbial processes, like gases (CO2, H2), acids, and different solvents, have been proposed to reduce oil viscosity, dissolve deposits, and alter wettability, which might result in enhanced mobilization and transportation of oil and thus in increased oil recovery (Belyaev et al., 2004; Salehi et al., 2008; Youssef et al., 2009). Microbial groups potentially involved in such processes are mainly fermentative bacteria and methanogens. Oil mobilization is also believed to be aided by microbial production of biosurfactants (low molecular weight surface active agents with amphiphilic properties forming micelles) acting on the oil-water interphase and lowering the surface or interface tensions (e.g., Banat, 1995; Bordoloi and Konwar, 2009). Microbial species suggested to produce biosurfactants within oil reservoirs might be various, but main groups are Bacillus sp., Pseudomonas sp., and Rhodococcus sp. (Aburuwaida et al., 1991; Li et al., 2002; Mukherjee and Das, 2005; Youssef et al., 2009). Recent findings indicate that an increased recovery might be a combined effect of both reduced viscosity by scission of heavy molecules and a strong local biosurfactant production from activated microbial consortia. Additionally, oil biodegradation can promote the conversion of heavy (and “hard-to-recover”) oil fractions to lighter oil fractions, thus increasing oil mobilization, for example, by microorganisms able to degrade n-alkanes (Wentzel et al., 2007). Another oil production problem might be plugging of pipes by paraffin or by other deposits. These are often treated using chemical injections but might be removed by hydrocarbon degrading microorganisms injected together with or without nutrients (e.g., Lazar et al., 1999).
As mentioned (Sect. 4.6.1), SRB can in some cases cause problems in oil production, and one strategy that has been used to counteract their effects is to stimulate nitrate-reducing bacteria (NRB, Sect. 4.4) in situ. This can be done by introduction of nitrate and potentially of NRB (Bødtker et al., 2009; Lysnes et al., 2009). NRB might then compete with SRB for electron donors, oxidize undesirable high levels of sulfide, and increase the redox potential in the habitat, leading to inhibition of SRB growth (Jenneman et al., 1986; Telang et al., 1997; Nemati et al., 2001; Myhr et al., 2002; Voordouw et al., 2009). Hence, NRB stimulation might then outcompete SRB growth in situ, with reduced reservoir souring as a positive effect (Grigoryan and Voordouw, 2008).
5 Future Perspectives and Biotechnological Exploitation of High-Temperature Oil Reservoir Microbiology Research
The highest reported temperature supporting life of microorganisms is very close to 120 °C (Takai et al., 2008), and in the deep biosphere, within sediments buried 200–500 million years ago, extraordinary new types of organisms may be found. These microbes are truly poly-extremophiles, being highly thermophilic, halophilic, piezotolerant, and solventophilic. Studies of these (belonging to both the bacterial and the archaeal domain) can provide new knowledge and understanding of various oil reservoir-associated mechanisms and characteristics, as well as reveal very exciting properties since the genetic materials of these microbes may encode biocatalysts with potentially highly relevant industrial implications. Gene mining and bioprospecting for a variety of new properties in proteins and metabolites may lead to new industrial applications, including those for enhanced oil recovery: Bio-EOR (Sect. 4.6.2). The use of extremophiles in various biocatalytic processes has already provided a new wave in the biotech industry, with bioprocesses performable at temperature and pressure conditions never before considered possible, and enzymes isolated from organisms originating from oil reservoirs might furnish new incentives for the development of entirely new processes. In addition, the genetic information of these microbes also has the potential to be developed into new tools for searching for new oil deposits in sensitive areas (like the Arctic, Antarctica, or jungle areas) using novel detection systems.
Obtaining new reserves of oil answers the increasing need of oil products and ensures a sustainable development of oil companies. Obtaining new reserves implies either to discover and develop new oil and gas fields by exploration or to increase the recovery rate of existing fields. One important research aim today is to develop biotechnological methods to enhance oil recovery (EOR). Two-thirds of the world’s extractable fossil fuels lay within the category of heavy to extra heavy oil, and the world’s average recovery rate from this type of oil reservoirs is only at about 7 %. Therefore, technologies that could boost these recoveries would have a tremendous economic impact. Today, different process technologies exist to extract these oils. However, these are all high-cost, high-energy, and high-emission technologies, and they are also associated with other environmental concerns. In addition to limitations in recovery of heavy oils, there are other concerns in oil recovery from present reservoir sites, including oil immobilization and plugging (Sect. 4.6.2), issues also being targets for combinations of conventional methods, and Bio-EOR processes. Further and deepened characterization of oil reservoir microbial communities is therefore very important. Not only are there numerous applicable features (suitable for both oil and biotechnology industry) to discover but also a need for an increased understanding of these communities. In order to access high quality samples for such studies, extensive collaborations with the oil industry are crucial and require well-designed and accurate sampling methods, limiting the risks of contamination and loss of sample representativeness to an absolute minimum.
References
Aburuwaida AS, Banat IM, Haditirto S, Salem A, Kadri M (1991) Isolation of biosurfactant-producing bacteria product characterization, and evaluation. Acta Biotechnol 11:315–324
Allen EE, Banfield JF (2005) Community genomics in microbial ecology and evolution. Nat Rev Microbiol 3:489–498
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Banat IM (1995) Characterization of biosurfactants and their use in pollution removal state of the art. Acta Biotechnol 15:251–267
Beeder J, Nilsen RK, Rosnes JT, Torsvik T, Lien T (1994) Archaeoglobus fulgidus isolated from hot North Sea oil field waters. Appl Environ Microbiol 60:1227–1231
Beeder J, Torsvik T, Lien T (1995) Thermodesulforhabdus norvegicus gen. nov., sp. nov., a novel thermophilic sulfate-reducing bacterium from oil field water. Arch Microbiol 164:331–336
Belyaev SS, Wolkin R, Kenealy WR, Deniro MJ, Epstein S, Zeikus JG (1983) Methanogenic bacteria from the bondyuzhskoe oil field: general characterization and analysis of stable-carbon isotopic fractionation. Appl Environ Microbiol 45:691–697
Belyaev SS, Borzenkov IA, Nazina TN, Rozanova EP, Glumov IF, Ibatullin RR, Ivanov MV (2004) Use of microorganisms in the biotechnology for the enhancement of oil recovery. Microbiology 73:590–598
Bødtker G, Thorstenson T, Lillebø BL, Thorbjørnsen BE, Ulvøen RH, Sunde E, Torsvik T (2008) The effect of long-term nitrate treatment on SRB activity, corrosion rate and bacterial community composition in offshore water injection systems. J Ind Microbiol Biotechnol 35:1625–1636
Bødtker G, Lysnes K, Torsvik T, Bjørnestad EO, Sunde E (2009) Microbial analysis of backflowed injection water from a nitrate-treated North Sea oil reservoir. J Ind Microbiol Biotechnol 36:439–450
Bonch-Osmolovskaya EA, Miroshnichenko ML, Lebedinsky AV, Chernyh NA, Nazina TN, Ivoilov VS, Belyaev SS, Boulygina ES, Lysov YP, Perov AN, Mirzabekov AD, Hippe H, Stackebrandt E, L’Haridon S, Jeanthon C (2003) Radioisotopic, culture-based, and oligonucleotide microchip analyses of thermophilic microbial communities in a continental high-temperature petroleum reservoir. Appl Environ Microbiol 69:6143–6151
Bordoloi NK, Konwar BK (2009) Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J Hazard Mater 170:495–505
Brakstad OG, Kotlar HK, Markussen S (2008) Microbial communities of a complex high-temperature offshore petroleum reservoir. Int J Oil Gas Coal Technol 1:211–228
Cayol JL, Ollivier B, Patel BK, Ravot G, Magot M, Ageron E, Grimont PA, Garcia JL (1995) Description of Thermoanaerobacter brockii subsp. lactiethylicus subsp. nov., isolated from a deep subsurface French oil well, a proposal to reclassify Thermoanaerobacter finnii as Thermoanaerobacter brockii subsp. finnii comb. nov., and an emended description of Thermoanaerobacter brockii. Int J Syst Bacteriol 45:783–789
Cetecioglu Z, Ince BK, Kolukirik M, Ince O (2009) Biogeographical distribution and diversity of bacterial and archaeal communities within highly polluted anoxic marine sediments from the Marmara Sea. Mar Pollut Bull 58:384–395
Cheng L, Qiu TL, Yin XB, Wu XL, Hu GQ, Deng Y, Zhang H (2007) Methermicoccus shengliensis gen. nov., sp. nov., a thermophilic, methylotrophic methanogen isolated from oil-production water, and proposal of Methermicoccaceae fam. nov. Int J Syst Evol Microbiol 57:2964–2969
Cheng L, Qiu TL, Li X, Wang WD, Deng Y, Yin XB, Zhang H (2008) Isolation and characterization of Methanoculleus receptaculi sp. nov. from Shengli oil field, China. FEMS Microbiol Lett 285:65–71
Cheng L, Dai L, Li X, Zhang H, Lu Y (2011) Isolation and characterization of Methanothermobacter crinale sp. nov., a novel hydrogenotrophic methanogen from the Shengli oil field. Appl Environ Microbiol 77:5212–5219
Christensen B, Torsvik T, Lien T (1992) Immunomagnetically captured thermophilic sulfate-reducing bacteria from North Sea oil field waters. Appl Environ Microbiol 58:1244–1248
Coohrane WJ, Jones PS, Sanders PF, HoIt DM, Mosley MJ (1988) Studies on the thermophilic sulfate-reducing bacteria from a souring North Sea oil field. SPE European petroleum conference, London, October 16–19, 1988, SPE 18368
Cord-Ruwisch R, Kleinitz W, Widdel F (1987) Sulfate-reducing bacteria and their activities in oil production. J Petrol Technol 39:97–106
Cowan D, Meyer Q, Stafford W, Muyanga S, Cameron R, Wittwer P (2005) Metagenomic gene discovery: past, present and future. Trends Biotechnol 23:321–329
Dahle H, Birkeland NK (2006) Thermovirga lienii gen. nov., sp. nov., a novel moderately thermophilic, anaerobic, amino-acid-degrading bacterium isolated from a North Sea oil well. Int J Syst Evol Microbiol 56:1539–1545
Dahle H, Garshol F, Madsen M, Birkeland NK (2008) Microbial community structure analysis of produced water from a high-temperature North Sea oil-field. Antonie van Leeuwenhoek 93:37–49
Dar SA, Kleerebezem R, Stams AJ, Kuenen JG, Muyzer G (2008) Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl Microbiol Biotechnol 78:1045–1055
Davey ME, Wood WA, Key R, Nakamura K, Stahl DA (1993) Isolation of three species of Geotoga and Petrotoga: two new genera, representing a new lineage in the bacterial line of descent distantly related to the “Thermotogales”. Syst Appl Microbiol 16:191–200
DiPippo JL, Nesbo CL, Dahle H, Doolittle WF, Birkland NK, Noll KM (2009) Kosmotoga olearia gen. nov., sp. nov., a thermophilic, anaerobic heterotroph isolated from an oil production fluid. Int J Syst Evol Microbiol 59:2991–3000
Duncan KE, Gieg LM, Parisi VA, Tanner RS, Tringe SG, Bristow J, Suflita JM (2009) Biocorrosive thermophilic microbial communities in Alaskan north slope oil facilities. Environ Sci Technol 43:7977–7984
Fardeau ML, Cayol JL, Magot M, Ollivier B (1993) H2 oxidation in the presence of thiosulfate, by a Thermoanaerobacter strain isolated from an oil-producing well. FEMS Microbiol Lett 113:327–332
Fardeau ML, Ollivier B, Patel BK, Magot M, Thomas P, Rimbault A, Rocchiccioli F, Garcia JL (1997) Thermotoga hypogea sp. nov., a xylanolytic, thermophilic bacterium from an oil-producing well. Int J Syst Bacteriol 47:1013–1019
Fardeau ML, Magot M, Patel BK, Thomas P, Garcia JL, Ollivier B (2000) Thermoanaerobacter subterraneus sp. nov., a novel thermophile isolated from oilfield water. Int J Syst Evol Microbiol 50:2141–2149
Fardeau ML, Bonilla Salinas M, L’Haridon S, Jeanthon C, Verhe F, Cayol JL, Patel BK, Garcia JL, Ollivier B (2004) Isolation from oil reservoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment of T. subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum to Caldanaerobacter subterraneus gen. nov., sp. nov., comb. nov. as four novel subspecies. Int J Syst Evol Microbiol 54:467–474
Garcia JL, Patel BKC, Ollivier B (2000) Taxonomic phylogenetic and ecological diversity of methanogenic Archaea. Anaerobe 6:205–226
Gieg LM, Davidova IA, Duncan KE, Suflita JM (2010) Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ Microbiol 12:3074–3086
Grassia GS, McLean KM, Glénat P, Bauld J, Sheehy AJ (1996) A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol Ecol 21:47–58
Gray ND, Sherry A, Larter SR, Erdmann M, Leyris J, Liengen T, Beeder J, Head IM (2009) Biogenic methane production in formation waters from a large gas field in the North Sea. Extremophiles 13:511–519
Greene AC, Patel BK, Sheehy AJ (1997) Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol 47:505–509
Grigoryan A, Voordouw G (2008) Microbiology to help solve our energy needs: methanogenesis from oil and the impact of nitrate on the oil-field sulfur cycle. Ann N Y Acad Sci 1125:345–352
Hao R, Lu A, Wang G (2004) Crude-oil-degrading thermophilic bacterium isolated from an oil field. Can J Microbiol 50:175–182
Head IM, Jones DM, Roling WFM (2006) Marine microorganisms make a meal of oil. Nat Rev Microbiol 4:173–182
Illias RMD, Wei OS, Idris AK, Rahman WAWA (2001) Isolation and characterization of halotolerant aerobic bacteria from oil reservoir. Jurnal Teknologi 35:1–10
Jayasinghearachchi HS, Lal B (2011) Oceanotoga teriensis gen. nov., sp. nov., a thermophilic bacterium isolated from offshore oil-producing wells. Int J Syst Evol Microbiol 61:554–560
Jeanthon C, Reysenbach AL, l’Haridon S, Gambacorta A, Pace NR, Glenat P, Prieur D (1995) Thermotoga subterranea sp. nov., a new thermophilic bacterium isolated from a continental oil reservoir. Arch Microbiol 164:91–97
Jenneman GE, Mcinerney MJ, Knapp RM (1986) Effect of nitrate on biogenic sulfide production. Appl Environ Microbiol 51:1205–1211
Jones DM, Head IM, Gray ND, Adams JJ, Rowan AK, Aitken CM, Bennett B, Huang H, Brown A, Bowler BF, Oldenburg T, Erdmann M, Larter SR (2008) Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 451:176–180
Jørgensen BB, D’Hondt S (2006) Ecology. A starving majority deep beneath the seafloor. Science 314:932–934
Kaster KM, Grigoriyan A, Jenneman G, Voordouw G (2007) Effect of nitrate and nitrite on sulfide production by two thermophilic, sulfate-reducing enrichments from an oil field in the North Sea. Appl Microbiol Biotechnol 75:195–203
Kaster KM, Bonaunet K, Berland H, Kjeilen-Eilertsen G, Brakstad OG (2009) Characterisation of culture-independent and -dependent microbial communities in a high-temperature offshore chalk petroleum reservoir. Antonie van Leeuwenhoek 96:423–439
Korenblum E, Souza DB, Penna M, Seldin L (2012) Molecular analysis of the bacterial communities in crude oil samples from two Brazilian offshore petroleum platforms. Int J Microbiol 2012:156537
Kotlar HK (2012) Extreme to the 4th power! Oil-, high temperature-, salt- and pressure-tolerant microorganisms in oil reservoirs. What secrets can they reveal? In: Anitori RP (ed) Extremophiles. Microbiology and biotechnology. Caister Academic Press, Norfolk, pp 159–182
Kotlar HK, Lewin A, Johansen J, Throne-Holst M, Haverkamp T, Markussen S, Winnberg A, Ringrose P, Aakvik T, Ryeng E, Jakobsen K, Drabløs F, Valla S (2011) High coverage sequencing of DNA from microorganisms living in an oil reservoir 2.5 kilometres subsurface. Environ Microbiol Rep 3:674–681
L’Haridon S, Reysenbacht AL, Glenat P, Prieur D, Jeanthon C (1995) Hot subterranean biosphere in a continental oil reservoir. Nature 377:223–224
L’Haridon SL, Miroshnichenko ML, Hippe H, Fardeau ML, Bonch-Osmolovskaya E, Stackebrandt E, Jeanthon C (2001) Thermosipho geolei sp. nov., a thermophilic bacterium isolated from a continental petroleum reservoir in Western Siberia. Int J Syst Evol Microbiol 51:1327–1334
L’Haridon S, Miroshnichenko ML, Hippe H, Fardeau ML, Bonch-Osmolovskaya EA, Stackebrandt E, Jeanthon C (2002) Petrotoga olearia sp. nov. and Petrotoga sibirica sp. nov., two thermophilic bacteria isolated from a continental petroleum reservoir in Western Siberia. Int J Syst Evol Microbiol 52:1715–1722
Lan G, Li Z, Zhang H, Zou C, Qiao D, Cao Y (2011) Enrichment and diversity analysis of the thermophilic microbes in a high temperature petroleum reservoir. Afr J Microbiol Res 5:1850–1857
Lazar I, Voicu A, Nicolescu C, Mucenica D, Dobrota S, Petrisor IG, Stefanescu M, Sandulescu L (1999) The use of naturally occurring selectively isolated bacteria for inhibiting paraffin deposition. J Petrol Sci Eng 22:161–169
Lazar CS, Parkes RJ, Cragg BA, L’Haridon S, Toffin L (2011) Methanogenic diversity and activity in hypersaline sediments of the centre of the Napoli mud volcano, Eastern Mediterranean Sea. Environ Microbiol 13:2078–2091
Leu JY, McGovern-Traa CP, Porter AJ, Harris WJ, Hamilton WA (1998) Identification and phylogenetic analysis of thermophilic sulfate-reducing bacteria in oil field samples by 16S rDNA gene cloning and sequencing. Anaerobe 4:165–174
Leu JY, McGovern-Traa CP, Porter AJ, Hamilton WA (1999) The same species of sulphate-reducing Desulfomicrobium occur in different oil field environments in the north sea. Lett Appl Microbiol 29:246–252
Lewin A, Johansen J, Wentzel A, Kotlar HK, Drabløs F, Valla S (2013) The microbial communities of two apparently physically separated deep sub-surface oil reservoirs show extensive DNA sequence similarities. Environ Microbiol Rep, submitted for publication
Li D, Hendry P (2008) Microbial diversity in petroleum reservoirs. Microbiol Aust 29:25–27
Li QX, Kang CB, Wang H, Liu CD, Zhang CK (2002) Application of microbial enhanced oil recovery technique to Daqing Oilfield. Biochem Eng J 11:197–199
Li H, Yang SZ, Mu BZ, Rong ZF, Zhang J (2006) Molecular analysis of the bacterial community in a continental high-temperature and water-flooded petroleum reservoir. FEMS Microbiol Lett 257:92–98
Li H, Yang SZ, Mu BZ (2007a) Phylogenetic diversity of the archaeal community in a continental high-temperature, water-flooded petroleum reservoir. Curr Microbiol 55:382–388
Li H, Yang SZ, Mu BZ, Rong ZF, Zhang J (2007b) Molecular phylogenetic diversity of the microbial community associated with a high-temperature petroleum reservoir at an offshore oilfield. FEMS Microbiol Ecol 60:74–84
Li D, Midgley DJ, Ross JP, Oytam Y, Abell GC, Volk H, Daud WA, Hendry P (2012) Microbial biodiversity in a Malaysian oil field and a systematic comparison with oil reservoirs worldwide. Arch Microbiol 194:513–523
Lien T, Madsen M, Rainey FA, Birkeland NK (1998) Petrotoga mobilis sp. nov., from a North Sea oil-production well. Int J Syst Bacteriol 48:1007–1013
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Lysnes K, Bødtker G, Torsvik T, Bjørnestad EO, Sunde E (2009) Microbial response to reinjection of produced water in an oil reservoir. Appl Microbiol Biotechnol 83:1143–1157
Magot M, Ollivier B, Patel BK (2000) Microbiology of petroleum reservoirs. Antonie van Leeuwenhoek 77:103–116
Magot M, Basso O, Tardy-Jacquenod C, Caumette P (2004) Desulfovibrio bastinii sp. nov. and Desulfovibrio gracilis sp. nov., moderately halophilic, sulfate-reducing bacteria isolated from deep subsurface oilfield water. Int J Syst Evol Microbiol 54:1693–1697
Mayumi D, Mochimaru H, Yoshioka H, Sakata S, Maeda H, Miyagawa Y, Ikarashi M, Takeuchi M, Kamagata Y (2011) Evidence for syntrophic acetate oxidation coupled to hydrogenotrophic methanogenesis in the high-temperature petroleum reservoir of Yabase oil field (Japan). Environ Microbiol 13:1995–2006
Mbadinga SM, Li KP, Zhou L, Wang LY, Yang SZ, Liu JF, Gu JD, Mu BZ (2012) Analysis of alkane-dependent methanogenic community derived from production water of a high-temperature petroleum reservoir. Appl Microbiol Biotechnol 96:531–542
McInerney MJ, Bryant MP (1981) Anaerobic degradation of lactate by syntrophic associations of Methanosarcina barkeri and Desulfovibrio species and effect of H2 on acetate degradation. Appl Environ Microb 41:346–354
McInerney MJ, Sieber JR, Gunsalus RP (2009) Synthropy in anaerobic global carbon cycles. Curr Opin Biotechnol 20:623–632
Miranda-Tello E, Fardeau ML, Thomas P, Ramirez F, Casalot L, Cayol JL, Garcia JL, Ollivier B (2004) Petrotoga mexicana sp. nov., a novel thermophilic, anaerobic and xylanolytic bacterium isolated from an oil-producing well in the Gulf of Mexico. Int J Syst Evol Microbiol 54:169–174
Miranda-Tello E, Fardeau ML, Joulian C, Magot M, Thomas P, Tholozan JL, Ollivier B (2007) Petrotoga halophila sp. nov., a thermophilic, moderately halophilic, fermentative bacterium isolated from an offshore oil well in Congo. Int J Syst Evol Microbiol 57:40–44
Miroshnichenko ML, Hippe H, Stackebrandt E, Kostrikina NA, Chernyh NA, Jeanthon C, Nazina TN, Belyaev SS, Bonch-Osmolovskaya EA (2001) Isolation and characterization of Thermococcus sibiricus sp. nov. from a Western Siberia high-temperature oil reservoir. Extremophiles 5:85–91
Morono Y, Terada T, Nishizawa M, Ito M, Hillion F, Takahata N, Sano Y, Inagaki F (2011) Carbon and nitrogen assimilation in deep subseafloor microbial cells. Proc Natl Acad Sci U S A 108:18295–18300
Mukherjee AK, Das K (2005) Correlation between diverse cyclic lipopeptides production and regulation of growth and substrate utilization by Bacillus subtilis strains in a particular habitat. FEMS Microbiol Ecol 54:479–489
Myhr S, Lillebo BL, Sunde E, Beeder J, Torsvik T (2002) Inhibition of microbial H2S production in an oil reservoir model column by nitrate injection. Appl Microbiol Biotechnol 58:400–408
Nazina TN, Tourova TP, Poltaraus AB, Novikova EV, Grigoryan AA, Ivanova AE, Lysenko AM, Petrunyaka VV, Osipov GA, Belyaev SS, Ivanov MV (2001) Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int J Syst Evol Microbiol 51:433–446
Nazina TN, Shestakova NM, Grigor’ian AA, Mikhailova EM, Turova TP, Poltaraus AB, Feng C, Ni F, Beliaev SS (2006) Phylogenetic diversity and activity of anaerobic microorganisms of high-temperature horizons of the Dagang Oilfield (China). Microbiology (Russia) 75:70–81
Nemati M, Mazutinec TJ, Jenneman GE, Voordouw G (2001) Control of biogenic H2S production with nitrite and molybdate. J Ind Microbiol Biotechnol 26:350–355
Nilsen RK, Torsvik T (1996) Methanococcus thermolithotrophicus isolated from North Sea oil field reservoir water. Appl Environ Microbiol 62:728–731
Nilsen RK, Torsvik T, Lien T (1996a) Desulfotomaculum thermocisternum sp. nov., a sulfate reducer isolated from a hot north sea oil reservoir. Int J Syst Bacteriol 46:397–402
Nilsen RK, Beeder J, Thorstenson T, Torsvik T (1996b) Distribution of thermophilic marine sulfate reducers in North Sea oil field waters and oil reservoirs. Appl Environ Microbiol 62:1793–1798
Ollivier B, Fardeau ML, Cayol JL, Magot M, Patel BK, Prensier G, Garcia JL (1998) Methanocalculus halotolerans gen. nov., sp. nov., isolated from an oil-producing well. Int J Syst Bacteriol 48:821–828
Orphan VJ, Taylor LT, Hafenbradl D, Delong EF (2000) Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl Environ Microbiol 66:700–711
Pernthaler A, Dekas AE, Brown CT, Goffredi SK, Embaye T, Orphan VJ (2008) Diverse syntrophic partnerships from deep-sea methane vents revealed by direct cell capture and metagenomics. Proc Natl Acad Sci U S A 105:7052–7057
Pineda-Flores G, Boll-Arguello G, Lira-Galeana C, Mesta-Howard AM (2004) A microbial consortium isolated from a crude oil sample that uses asphaltenes as a carbon and energy source. Biodegradation 15:145–151
Podar M, Reysenbach AL (2006) New opportunities revealed by biotechnological explorations of extremophiles. Curr Opin Biotechnol 17:250–255
Price PB, Sowers T (2004) Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc Natl Acad Sci U S A 101:4631–4636
Quince C, Curtis TP, Sloan WT (2008) The rational exploration of microbial diversity. ISME J 2:997–1006
Rabus R, Fukui M, Wilkes H, Widdel F (1996) Degradative capacities and 16S rRNA-targeted whole-cell hybridization of sulphate-reducing bacteria in an anaerobic enrichment culture utilizing alkylbenzenes from crude oil. Appl Environ Microbiol 62:3605–3613
Rappé MS, Giovannoni SJ (2003) The uncultured microbial majority. Annu Rev Microbiol 57:369–394
Ravot G, Magot M, Fardeau ML, Patel BK, Prensier G, Egan A, Garcia JL, Ollivier B (1995) Thermotoga elfii sp. nov., a novel thermophilic bacterium from an African oil-producing well. Int J Syst Bacteriol 45:308–314
Reeder J, Knight R (2009) The ‘rare biosphere’: a reality check. Nat Methods 6:636–637
Rees GN, Grassia GS, Sheehy AJ, Dwivedi PP, Patel BKC (1995) Desulfacinum infernum gen. nov., sp. nov., a thermophilic sulfate-reducing bacterium from petroleum reservoir. Int J Syst Bacteriol 45:85–89
Rees GN, Patel BK, Grassia GS, Sheehy AJ (1997) Anaerobaculum thermoterrenum gen. nov., sp. nov., a novel, thermophilic bacterium which ferments citrate. Int J Syst Bacteriol 47:150–154
Ren HY, Zhang XJ, Song ZY, Rupert W, Gao GJ, Guo SX, Zhao LP (2011) Comparison of microbial community compositions of injection and production well samples in a long-term water-flooded petroleum reservoir. PLoS One 6:e23258
Rosnes JT, Torsvik T, Lien T (1991) Spore-forming thermophilic sulfate-reducing bacteria isolated from North Sea oil field waters. Appl Environ Microbiol 57:2302–2307
Rozanova EP, Borzenkov IA, Tarasov AL, Suntsova LA, Dong CL, Belyaev SS, Ivanov MV (2001a) Microbiological processes in a high-temperature oil field. Microbiology (Russia) 70:102–110
Rozanova EP, Tourova TP, Kolganova TV, Lysenko AM, Mityushina LL, Yusupov SK, Belyaev SS (2001b) Desulfacinum subterraneum sp nov., a new thermophilic sulfate-reducing bacterium isolated from a high-temperature oil field. Microbiology 70:466–471
Salehi M, Johnson SJ, Liang JT (2008) Mechanistic study of wettability alteration using surfactants with applications in naturally fractured reservoirs. Langmuir 24:14099–14107
Salinas MB, Fardeau ML, Thomas P, Cayol JL, Patel BK, Ollivier B (2004) Mahella australiensis gen. nov., sp. nov., a moderately thermophilic anaerobic bacterium isolated from an Australian oil well. Int J Syst Evol Microbiol 54:2169–2173
Scholten JC, Culley DE, Brockman FJ, Wu G, Zhang W (2007) Evolution of the syntrophic interaction between Desulfovibrio vulgaris and Methanosarcina barkeri: involvement of an ancient horizontal gene transfer. Biochem Biophys Res Commun 352:48–54
Sen R (2008) Biotechnology in petroleum recovery: the microbial EOR. Prog Energy Combust Sci 34:714–724
Sette LD, Simioni KC, Vasconcellos SP, Dussan LJ, Neto EV, Oliveira VM (2007) Analysis of the composition of bacterial communities in oil reservoirs from a southern offshore Brazilian basin. Antonie van Leeuwenhoek 91:253–266
Shestakova N, Korshunova A, Mikhailova E, Sokolova D, Tourova T, Belyaev S, Poltaraus A, Nazina T (2011) Characterization of the aerobic hydrocarbon-oxidizing enrichments from a high-temperature petroleum reservoir by comparative analysis of DNA- and RNA-derived clone libraries. Microbiology 80:60–69
Sleator RD, Shortall C, Hill C (2008) Metagenomics. Lett Appl Microbiol 47:361–366
Slobodkin AI, Jeanthon C, L’Haridon S, Nazina T, Miroshnichenko M, Bonch-Osmolovskaya E (1999) Dissimilatory reduction of Fe(III) by thermophilic bacteria and archaea in deep subsurface petroleum reservoirs of Western Siberia. Curr Microbiol 39:99–102
Spark I, Patey I, Duncan B, Hamilton A, Devine C, McGovern-Traa C (2000) The effects of indigenous and introduced microbes on deeply buried hydrocarbon reservoirs, North Sea. Clay Miner 35:5–12
Stetter KO, Huber R, Blöchl E, Kurr M, Eden RD, Fielder M, Cash H, Vance I (1993) Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 365:743–745
Takahata Y, Nishijima M, Hoaki T, Maruyama T (2000) Distribution and physiological characteristics of hyperthermophiles in the Kubiki oil reservoir in Niigata, Japan. Appl Environ Microbiol 66:73–79
Takahata Y, Nishijima M, Hoaki T, Maruyama T (2001) Thermotoga petrophila sp. nov. and Thermotoga naphthophila sp. nov., two hyperthermophilic bacteria from the Kubiki oil reservoir in Niigata, Japan. Int J Syst Evol Microbiol 51:1901–1909
Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, Hirayama H, Nakagawa S, Nunoura T, Horikoshi K (2008) Cell proliferation at 122 °C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci U S A 105:10949–10954
Tang YQ, Li Y, Zhao JY, Chi CQ, Huang LX, Dong HP, Wu XL (2012) Microbial communities in long-term, water-flooded petroleum reservoirs with different in situ temperatures in the Huabei Oilfield, China. PLoS One 7:e33535
Tardy-Jacquenod C, Caumette P, Matheron R, Lanau C, Arnauld O, Magot M (1996) Characterization of sulfate-reducing bacteria isolated from oil-field waters. Can J Microbiol 42:259–266
Telang AJ, Ebert S, Foght JM, Westlake DWS, Jenneman GE, Gevertz D, Voordouw G (1997) Effect of nitrate injection on the microbial community in an oil field as monitored by reverse sample genome probing. Appl Environ Microbiol 63:1785–1793
Teske A, Dhillon A, Sogin ML (2003) Genomic markers of ancient anaerobic microbial pathways: sulfate reduction, methanogenesis, and methane oxidation. Biol Bull 204:186–191
Van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67:503–549
Voordouw G, Grigoryan AA, Lambo A, Lin S, Park HS, Jack TR, Coombe D, Clay B, Zhang F, Ertmoed R, Miner K, Arensdorf JJ (2009) Sulfide remediation by pulsed injection of nitrate into a low temperature Canadian heavy oil reservoir. Environ Sci Technol 43:9512–9518
Wang L, Tang Y, Wang S, Liu RL, Liu MZ, Zhang Y, Liang FL, Feng L (2006) Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles 10:347–356
Wentzel A, Ellingsen TE, Kotlar HK, Zotchev SB, Throne-Holst M (2007) Bacterial metabolism of long-chain n-alkanes. Appl Microbiol Biotechnol 76:1209–1221
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 95:6578–6583
Wintermute EH, Silver PA (2010) Emergent cooperation in microbial metabolism. Mol Syst Biol 6:407
Yamane K, Maki H, Nakayama T, Nakajima T, Nomura N, Uchiyama H, Kitaoka M (2008) Diversity and similarity of microbial communities in petroleum crude oils produced in Asia. Biosci Biotechnol Biochem 72:2831–2839
Yamane K, Hattori Y, Ohtagaki H, Fujiwara K (2011) Microbial diversity with dominance of 16S rRNA gene sequences with high GC contents at 74 and 98 °C subsurface crude oil deposits in Japan. CORD Conf Proc 76:220–235
Yarza P, Richter M, Peplies J, Euzéby J, Amann R, Schleifer KH, Ludwig W, Glockner FO, Rossello-Mora R (2008) The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol 31:241–250
Youssef N, Elshahed MS, McInerney MJ (2009) Microbial processes in oil fields: culprits, problems, and opportunities. Adv Appl Microbiol 66:141–251
Acknowledgements
This work was supported by the Research Council of Norway (grant numbers 187317/S30 and 208541/O10) and Statoil ASA.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wentzel, A., Lewin, A., Cervantes, F.J., Valla, S., Kotlar, H.K. (2013). Deep Subsurface Oil Reservoirs as Poly-extreme Habitats for Microbial Life. A Current Review. In: Seckbach, J., Oren, A., Stan-Lotter, H. (eds) Polyextremophiles. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 27. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6488-0_19
Download citation
DOI: https://doi.org/10.1007/978-94-007-6488-0_19
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6487-3
Online ISBN: 978-94-007-6488-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)