Abstract
Ionizing radiation (IR) is of particular interest in biology because its exposure results in a severe oxidative stress to all the cell’s macromolecules. Many extremophiles are found to be resistant to IR, suggesting that radiation resistance is a fortuitous consequence of a high tolerance to other environmental stressors (e.g., desiccation). In that regard, IR-resistant organisms are true polyextremophiles. It is now established that proteins are the major targets of radiation and that protection against protein oxidation is an essential process for survival from IR exposure. The IR resistance found in the halophilic archaeon, Halobacterium salinarum, is attributed to high intracellular concentrations of Mn antioxidant complexes that protect proteins against IR-induced reactive oxygen species (ROS). The variety of Mn antioxidant complexes found so far, and the potential for compatible solutes from extremophiles to provide ROS-scavenging activity in the cell, suggests that the adaptations of extremophiles to their environments provide a tremendous reservoir for novel radioprotective molecules and antioxidants against the deleterious effect of IR.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Extremophiles and Radiation Resistance
Over the last few decades, studies of extremophiles have revealed an astonishing array of adaptations to the harsh environmental conditions to which those organisms are exposed (Cavicchioli et al., 2011). However, an extreme environment, from our point of view, is not extreme for the organisms that are specifically adapted to this environment. Hyperthermophilic organisms not only thrive at temperatures near the boiling point of water, but they also require those high temperatures for their cellular machinery to function. As an example, the glutamate dehydrogenase from Pyrococcus furiosus, a hyperthermophilic archaeon, does not function below 45 °C (Klump et al., 1992). Similarly, cells of the halophilic archaeon, Halobacterium salinarum, will lyse if the osmotic pressure of their aqueous environment decreases below 3 M salt (DasSarma and DasSarma, 2012).
An interesting case is that of radiation-resistant bacteria. They have garnered a great deal of attention from scientists seeking to expose the mechanisms underlying their incredible survival abilities. These microorganisms were most likely not exposed to extremes of ionizing radiation (IR) over geological times (Mattimore and Battista, 1996), raising the question of their adaptation to such high doses of radiation. Early work on the desiccation resistance of the extremely radiation-resistant bacterium, Deinococcus radiodurans, and more recent environmental studies have revealed that it is the adaptation to extremely dry environments and high tolerance to desiccation that impart IR resistance to these organisms (Mattimore and Battista, 1996; Fredrickson et al., 2008). In other words, the IR resistance in bacteria is an incidental mechanism evolved to resist the cellular damage induced by desiccation (Fredrickson et al., 2008). In that regard, IR-resistant organisms are true polyextremophiles.
The distribution of radiation-resistant organisms in the phylogenetic tree of life is not limited to bacteria (Fig. 1). Recent work has revealed the high level of IR resistance of several eukaryotes: the basidiomycete fungus Ustilago maydis (Holliday, 2004), the freshwater invertebrate animal Philodina roseola (Gladyshev and Meselson, 2008), the water bear Milnesium tardigradum (Horikawa et al., 2006), and the roundworm Caenorhabditis elegans (Johnson and Hartman, 1988). Among the Archaea, the halophilic archaeon H. salinarum, in addition to being adapted to high salt, also shows a high level of resistance to desiccation, high pressure, UV radiation, and IR (Kish et al., 2012; Robinson et al., 2011; Kottemann et al., 2005). This is also true for a number of thermophilic archaea, including the sulfate-reducing Archaeoglobus fulgidus, methanogens such as Methanocaldococcus jannaschii, and the hyperthermophiles P. furiosus, Thermococcus radiotolerans, and Thermococcus gammatolerans (Beblo et al., 2011; DiRuggiero et al., 1997; Jolivet et al., 2003, 2004). Despite the seeming prevalence of radiation-resistant thermophiles, it would be unjustified to assume this is true of all thermophiles; several have been shown to be radiation sensitive, such as the archaeon Sulfolobus solfataricus (Rolfsmeier et al., 2011). Table 1 lists radiation-resistant organisms with their D 10 value – the dose of radiation in gray (Gy) that reduces the survival of a population by 90 %. Radiation resistance is strongly linked to genome size and the number of DNA double-strand breaks (DSBs) resulting from exposure to IR, which is approximately 0.004–0.01 DSB/Gy/Mbp. Because of the large difference in genome size, at a dose of IR of 1 kGy, the roundworm C. elegans experiences 400 DNA DSBs, whereas the bacterium D. radiodurans faces only 158 DSBs at 12 kGy (Daly, 2012). As a result, the D 10 of eukaryotes is much lower than that of bacteria, but those organisms are considered to be highly resistant to IR.
While IR-resistant organisms are distributed across the three domains of life (Fig. 1), this distribution can vary dramatically between organisms of the same family and even between species. For example, Thermus thermophilus is as radiation sensitive as Escherichia coli (D 10 0.7 kGy) but belongs to the same clade as one of the most IR-resistant bacterium known to date, D. radiodurans (D 10 12 kGy) (Omelchenko et al., 2005; Table 1). This raises an important question regarding the evolution of radiation resistance and whether or not the mechanisms underlying IR resistance are shared between the three domains of life.
In this chapter, we first discuss the cellular effects of IR and the parallels with desiccation, we follow by the current concepts regarding radioprotection and damage repair and the role of Mn antioxidants in radiation resistance, and finally we discuss the mechanisms underlying the radiation resistance of polyextremophilic archaea.
2 Cellular Effects of Ionizing Radiation
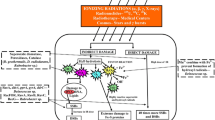
Ionizing radiation damages cellular components by direct and indirect effects (Riley, 1994). While direct ionization within the cell results in molecular damage, the vast majority of cellular insults under aqueous conditions are caused by indirect effects, through the actions of reactive oxygen species (ROS) formed by the radiolysis of water (Fig. 2) (Riley, 1994). Water radiolysis generates hydroxyl radicals (HO•), protons, and free electrons (Eq. 1).
Theoretical cellular reactions generating ROS following IR. Top: expected reactions resulting from the radiolysis of water and their rate constants. Bottom: cellular targets of ROS (From Daly 2009, reproduced with permission from Macmillan Publishers Ltd.).
Hydroxyl radicals react indiscriminately with all macromolecules in the cell and with each other to form hydrogen peroxide (H2O2) (Eq. 2), and free electrons react with dissolved oxygen to form superoxide (O2 •−) (Eq. 3). DNA-associated water molecules that undergo radiolysis become an immediate threat for nucleic acids, generating oxidized DNA bases and sugar moieties, abasic sites, strand breaks, and cross-links to proteins. These damages often produce complex clustered lesions resulting in DNA DSBs from attempted repair (Dianov et al., 2001; Regulus et al., 2007; Kish and DiRuggiero, 2008). DNA is further damaged through its association with free iron in the cells (Dianov et al., 2001; Ward, 1994). Proteins are attacked by hydroxyl radicals introducing carbonyl residues, amino acid radical chain reactions, cross-linking, and ultimately resulting in protein inactivation and denaturation (Daly, 2009). By analogy to chemical oxidative stress, it is hypothesized that the low reactivity and high specificity of superoxide and H2O2 for iron-sulfur and heme groups produce consequential damage to [4Fe-4S] clusters of labile dehydratases (Imlay, 2006). This results in the release of free Fe2+ in the cytoplasm and enzyme inactivation, failure of metabolic pathways, and the synthesis of aromatic and sulfur amino acids (Imlay, 2006, 2008). The released Fe2+ participates in the Fenton reaction (Eq. 4), which is the electron transfer from ferrous ion to H2O2 and the formation of superoxide (Imlay, 2008). The resulting HO• inflicts a barrage of oxidative damage upon all cellular components.
The mechanistic link between desiccation and IR resistance can be found in the formation of ROS resulting from both stresses (Nauser et al., 2005). During desiccation, loss of control of the electron transport chain, decrease membrane integrity compromising gas diffusion, impaired antioxidant systems, and macromolecule distortions as the result of volume changes contribute to the accumulation of ROS. This high level of ROS, in particular hydroxyl and peroxyl radicals, causes a major oxidative stress to the cell (Kranner, 2002; Kranner and Birtic, 2005). Thus, both desiccation and IR inflict severe oxidative damage to macromolecules in the cell that must either be prevented or repaired in order for the cell to survive.
3 Radioprotection and Damage Repair
In the 1960s, DNA was considered to be the principal target of radiation, and DNA damage was responsible for its lethal effects (Hutchinson, 1966). Scientists believed that IR-resistant microorganisms survived high-level radiation because they possessed unique and highly efficient DNA repair mechanisms. Several pathways for the repair of DNA DSBs after ionizing radiation have been proposed for bacteria, including homologous recombination; single-strand annealing; extended synthesis-dependent strand annealing, where cells need to contain another intact copy of the damaged DNA region; and nonhomologous end joining, which does not require a second homologous copy of DNA to join two contiguous fragments (Blasius et al., 2008; Confalonieri and Sommer, 2011; Slade and Radman, 2011). Recent work showed that the steps of DNA repair from IR damage were surprisingly ordinary in contrast to the extreme nature of the chromosome fragmentation and that the DNA repair proteins involved were not unique to IR-resistant organisms (Confalonieri and Sommer, 2011; Daly, 2012; Gutman et al., 1994).
In contrast to bacteria, DSB repair in the Archaea is less well characterized. In P. furiosus, DNA end processing is carried out by Rad50/Mre11 complexes that attach to the DNA ends and recruit nuclease and helicase proteins (NurA and HerA, respectively) to form 3´ overhangs (Hopfner et al., 2001). This in turn recruits RadA, a RecA homolog (Constantinesco et al., 2004; Hopfner et al., 2001). In H. salinarum, nurA and herA homologs are missing, and while Rad50/Mre11 proteins are present, Rad50 is not required for homologous recombination (Kish and DiRuggiero, 2008). Additionally, mutants of H. salinarum deficient in both Rad50 and Mre11 (∆rad50-∆mre11) are just as IR resistant as the wild type, though the repair of DNA DSBs occurs less efficiently (Kish and DiRuggiero, 2008). These facts together with the demonstration that IR-sensitive and IR-resistant organisms suffer the same number of DNA DSBs for an equivalent dose of IR (∼0.01 DSB/Gy/Mbp) depart from the dogma that DNA damage and in particular DNA DSBs are the most cytotoxic lesions resulting from exposure to IR. From early studies in 1940 (Dale, 1940), work from Gebicki’s group (Du and Gebicki, 2004; Nauser et al., 2005), and recent reports from Daly’s group (Daly, 2009; Daly et al., 2007), it is now established that proteins are the major targets for oxidation following exposure to IR. Thus, the idea that protein protection might govern radiation resistance has severely challenged the conventionally held view that DNA damage is paramount in radiation toxicity. The current model is that by protecting protein function from the damages inflicted by IR, DNA can be repaired by competent proteins, and the cell can survive (Fig. 3).
Model for death by protein damage in IR-sensitive cells and damage avoidance in IR-resistant cells (From Daly 2012, reproduced with permission from Elsevier).
4 Enzymatic Defense and IR
The major defenses of the cell against oxidative stress from exposure to H2O2 and redox cycling drugs – producing superoxide – are enzymatic (Imlay, 2008). Superoxide dismutases (SODs), catalases, and peroxidases are highly induced upon oxidative stress, and mutants of those proteins are greatly sensitive to chemical oxidants (Imlay, 2008). Surprisingly, SOD and catalase mutants of H. salinarum showed the same level of survival to IR as strains with active SODs and catalases (Fig. 4, Robinson et al., 2011), suggesting that those enzymes were not required for the survival of H. salinarum to IR; similar results were obtained with SOD and catalase mutants of D. radiodurans and E. coli (Markillie et al., 1999; Scott et al., 1989). This is quite paradoxical since the major stress from IR is oxidative stress (Daly, 2009) and both SOD and catalase are major ROS detoxification enzymes (Imlay, 2008). Furthermore, in bacterial systems and in H. salinarum, SODs and catalases were induced by several orders of magnitude in response to redox cycling drugs and H2O2 (Imlay, 2008; Kaur et al., 2010), but no increase in mRNA or protein levels for SODs, catalases, or peroxidases was detected in H. salinarum after IR (Whitehead et al., 2006). The question then is as follows: What protects the macromolecules of IR-resistant organisms since the enzymatic defenses of the cell against oxidative stress are not engaged by IR exposure?
Survival of H. salinarum Δura3 and mutant strains exposed to H2O2, paraquat, and IR. Survival was calculated as the average ratio (N/No) of surviving colony-forming units from treated (N) compared to untreated (No) cultures. (a) Doses of chemical oxydants: paraquat (PQ) and H2O2; (b) doses of ionizing radiation (From Robinson et al. 2011, reproduced with permission).
5 Manganese (Mn) Antioxidants
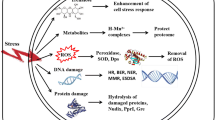
“Mn antioxidants” were first discovered in Lactobacillus plantarum where accumulation of millimolar concentrations of Mn suppressed oxidative stress and substituted for the lack of superoxide dismutase (SOD) (Archibald and Fridovich, 1981, 1982b). High levels of Mn also rescued E. coli and Saccharomyces cerevisiae SOD-deficient mutants (Al-Maghrebi et al., 2002; Chang and Kosman, 1989), and small molecule complexes of Mn have been shown to exhibit superoxide-scavenging activity in vitro (Archibald and Fridovich, 1982a; Barnese et al., 2008). Recently, the high Mn/Fe ratio found in IR-resistant bacteria and archaea revealed a direct link between Mn and protection of proteins from oxidative damage by ROS (Fredrickson et al., 2008; Daly, 2009; Kish et al., 2009). Work with D. radiodurans elegantly established the key role played by Mn-peptide complexes in the extreme radiation resistance of this organism (Daly et al., 2010), and in yeast, in vivo studies showed the important function of Mn-orthophosphate complexes in oxidative stress (McNaughton et al., 2010) (Fig. 5). In H. salinarum enzyme-free cell extracts rich in Mn, phosphate, amino acids, and peptides provided a great level of enzyme protection against the deleterious effect of IR (Robinson et al., 2011), and recent studies with Rubrobacter species showed that the association of Mn and trehalose was essential for the extreme radiation resistance observed in these organisms (Webb and DiRuggiero, 2012). High levels of intracellular concentration of trehalose were also reported in the IR-resistant cyanobacterium Chroococcidiopsis (Billi et al., 2000). Investigating the mechanisms of catalytic removal of superoxide by Mn compounds, Barnese et al. (2012) found that Mn phosphate and Mn carbonate, but not Mn pyrophosphate and citrate, can catalyze superoxide disproportionation in vitro at rates sufficient to mimic enzymatic SOD. They also noted that carboxylate and phosphate motifs, found in amino acids and nucleotides, are the most commonly available ligands for Mn in vivo (Fig. 5). In addition to its antioxidant activity, Mn may also act by functionally substituting for Fe in the Fe-S cluster of enzymes and thereby mitigating the deleterious effects of Fenton chemistry during oxidative stress (Sobota and Imalay, 2011).
Model for manganese antioxidants. Mn2+ in complex with orthophosphate (left), with free amino acids or peptides (center) and with nucleosides (right) catalytically scavenges superoxide radicals (O2 •−). Mn2+ in complex with free amino acids or peptide and orthophosphate catalytically decompose hydrogen peroxide (H2O2). Nucleosides, free amino acids, peptides, and other small organic metabolites scavenge hydroxyl radicals (HO•) (Daly et al., 2010).
In Bacillus, spores resistant to wet and dry heat benefited from the accumulation of Mn coordinated with small molecules including dipicolinic acid (DPA), and possibly α-/β-type small, acid-soluble proteins (Ghosh et al., 2011). DPA also formed antioxidant complexes with Ca2+ and phosphate, indicating that other divalent metal ions may contribute to protection from IR (Granger et al., 2011). Mn-mycosporine complexes were also attributed to facilitating radiation and desiccation resistance in cyanobacteria (Oren and Gunde-Cimerman, 2007; Rastogi et al., 2010). Cellular accumulation of Mn together with a variety of organic and inorganic ligands may be a widespread mechanism to surviving oxidative stress, and there is evidence that this may extend also to simple animals such as rotifers (Krisko et al., 2012).
6 What Is the Basis for the Radiation Resistance of the Polyextremophile H. salinarum?
While H. salinarum is highly polyploidic, with 15–25 copies of its chromosome per cell (Breuert et al., 2006), no connection has been established between chromosome copy numbers and radiation resistance (Daly et al., 2004; Gladyshev and Meselson, 2008), nor does the presence of eukaryotic-like proteins involved in the repair of DNA DSBs account for the high level of survival of this organism to IR (Kish and DiRuggiero, 2008). However, recent work revealed the critical role played by nonenzymatic antioxidant processes in the resistance of H. salinarum to IR (Robinson et al., 2011; Kish et al., 2009). Scavenging of ROS by intracellular halides in H. salinarum resulted in increased protection against nucleotide modification and carbonylation of protein residues (Kish et al., 2009). Measurements of H. salinarum cell interior revealed a high Mn/Fe ratio similar to that of D. radiodurans and other radiation-resistant microorganisms, underlying the role of Mn in radiation resistance (Kish et al., 2009; Daly, 2009). Protein-free cell extracts from H. salinarum provided a high level of protection for protein activity against IR in vitro. Compared with cell extracts of radiation-sensitive bacteria, H. salinarum extracts were enriched in manganese-antioxidant complexes, supporting an essential role in ROS scavenging for those small molecules in vivo (Robinson et al., 2011).
To further elucidate the metabolic routes instrumental to this enhanced radiation resistance, IR-“super”-resistant mutants (IR+) of H. salinarum were evolved from the wild-type strain over multiple cycles of exposure to high doses of IR (Webb et al., 2013; DeVeaux et al., 2007). Proteomic analysis of IR+ mutants revealed overexpression of enzymes from central carbon metabolism, channeling a substantial flux of carbon into pyruvate and therefore the generation of energy and reducing equivalents (Webb et al., 2013). The corresponding IR+ mutants also had increased intracellular Mn concentration, compared to the wild type, supporting the case of an important role for Mn in central carbon metabolism, via strictly Mn-dependent enzymes or enzymes highly stimulated by Mn (Kehres and Maguire, 2003; Liedert et al., 2012; Ogunniyi et al., 2010). Maintenance of redox homeostasis was also activated by the overexpression of coenzyme biosynthesis pathways involved in redox reactions. These findings support the idea that increased IR tolerance is most likely achieved by a “metabolic route” and underscore the physiological importance in aerobic fitness of Mn antioxidants.
Recent studies regarding single-strand DNA-binding protein (SSB) in halophiles suggested a key role for these proteins in radiation resistance. Single-strand DNA-binding proteins (SSBs), also called replication protein A (RPAs), bind to ssDNA with high affinity and provide protection against nuclease and chemical attacks. These proteins are essential for DNA metabolism including DNA replication, recombination, and repair in all domains of life (Wold, 1997). The basic architecture of RPAs is based on the oligonucleotide-/oligosaccharide-binding (OB) fold, a five-stranded β-sheet coiled into a closed barrel, but the number of OB-folds present varies from species to species (Bochkarev et al., 1999). Unlike in bacteria and eukaryotes, there is a wide diversity in the architecture of RPAs present in archaea. Two operons, RPA1 (with the genes rfa2 and rfa7 in H. salinarum) and RPA3 (with the genes rfa3 and rfa8 in H. salinarum), and a single gene, rpa2 (rfa1 in H. salinarum), have been found to encode RPA proteins in the halophilic archaea (Fig. 6). H. salinarum IR+ mutants all showed overexpression of the RPA3 operon (Webb et al., 2013; DeVeaux et al., 2007). The same operon was upregulated in previous studies following irradiation of H. salinarum (Whitehead et al., 2006), and more recently two independent studies (Skowyra and Macneill, 2011; Stroud et al., 2012) reported hypersensitivity to DNA damaging agents of rpa3 mutants in Haloferax volcanii. Furthermore, H. volcanii constructs overexpressing the Rpa2 protein exhibited increased resistance to DNA damage (UV, MMS, and phleomycin) (Skowyra and MacNeill, 2011). These data clearly implicate RPA proteins in enhanced IR tolerance.
Operon organization and domain structures of H. volcanii and H. salinarum (gene names in parenthesis) of single-strand DNA-binding proteins (Adapted from Stroud et al. 2012, reproduced with permission).
7 What About Thermophiles?
While halophilic archaea and bacteria are adapted to desiccating conditions, imparting resistance to IR, it is not the case for many thermophiles and hyperthermophiles found to be radiation resistant. In fact, no direct correlation was found between desiccation tolerance and radiation resistance among (hyper)-thermophilic archaea (Beblo et al., 2009, 2011).
Archaea are of particular interest because they synthesize unusual, low-molecular-weight organic compounds such as ß-amino acids, Nχ-acetyl-ß-lysine, mannosylglycerate (MG), and di-myo-inositol phosphate (DIP) known as compatible solutes (Fig. 7) (Martins et al., 1997; Santos and da Costa, 2002). These compounds are typically negatively charged in contrast to compatible solutes from mesophiles. Compounds such as DIP (found in Pyrococcus/Thermococcus, Archaeoglobus, and Aquifex species), di-glycerol-phosphate (in Archaeoglobus species), and MG (in Pyrococcus/Thermococcus and Archaeoglobus species) accumulate in the cell in response to supraoptimal growth temperature and osmotic shock, which are stress conditions likely to generate ROS (Müller et al., 2005). In addition, compounds such as DIP and MG have been shown to play a role in protein thermostabilization by protecting model enzymes against heat-induced denaturation, aggregation, and inactivation (Faria et al., 2004; Lamosa et al., 2003; Müller et al., 2005; Ramos et al., 1997; Scholz et al., 1992). The ability of such compounds to provide IR resistance is currently under investigation using P. furiosus and Thermococcus gammatolerans as model systems (Webb and DiRuggiero, unpublished), two hyperthermophiles highly resistant to IR (DiRuggiero et al., 1997; Jolivet et al., 2003; Table 1).
8 Relevance to Astrobiology
The understanding that adaptation to extreme environments might provide protection again high radiation levels is particularly relevant to the field of astrobiology. Lacking an atmosphere and magnetic shield to reduce the surface solar irradiance, microorganisms on the surface of Mars are exposed to far greater levels of UV-C (Cockell et al., 2000) and high-energy radiation than are microorganisms on Earth. Furthermore, there is evidence of evaporitic deposits containing high concentrations of chloride and bromide at both Meridiani Planum (Rieder et al., 2004) and Gusev crater (Haskin et al., 2005) gathered by the Mars Exploration Rovers and evidence of other evaporite deposits, possibly containing chloride, in the southern highlands of Mars reported by the Mars Odyssey Orbiter (Osterloo et al., 2008). The findings that the salt environment itself may be a protective factor for potential microbial life on the surface of Mars (Davila et al., 2008) indicate that chloride and bromide evaporite deposits showing water modification are excellent areas for surface investigations looking for evidence of life on Mars.
9 Conclusion
The study of extremophiles and how they meet the physical and chemical challenges found in the environmental extremes they inhabit lead to new insights on the mechanisms of stress response. Many extremophiles are found to be resistant to IR, suggesting that radiation resistance is a fortuitous consequence of a high tolerance to other environmental stressors (e.g., desiccation). Given the diversity of IR-resistant extremophiles and their natural environments, we do not know yet if there are universal features of IR resistance, such as high intracellular concentration of Mn (Daly et al., 2010; Robinson et al., 2011). The IR resistance found in H. salinarum is attributed to high intracellular concentrations of salts and Mn-antioxidant complexes that protect proteins from oxidative damage (Robinson et al., 2011; Kish et al., 2009). However, little is known regarding their physiology in the context of cellular adaptation to stress. The variety of Mn complexes found so far (Daly et al., 2010; Ghosh et al., 2011; Granger et al., 2011; Robinson et al., 2011) and the potential for compatible solutes from thermophiles to provide ROS-scavenging activity in the cell suggest that the adaptations of extremophiles to their environments provide a tremendous reservoir for novel radioprotective molecules and antioxidants against the deleterious effect of IR.
References
Al-Maghrebi M, Fridovich I, Benov L (2002) Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch Biochem Biophys 402:104–109
Archibald FS, Fridovich I (1981) Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol 146:928–936
Archibald FS, Fridovich I (1982a) Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys 215:589–596
Archibald FS, Fridovich I (1982b) The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys 214:452–463
Barnese K, Gralla EB, Cabelli DE, Valentine JS (2008) Manganous phosphate acts as a superoxide dismutase. J Am Chem Soc 130:4604–4606
Barnese K, Gralla EB, Valentine JS, Cabelli DE (2012) Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc Natl Acad Sci U S A 109:6892–6897
Beblo K, Rabbow E, Rachel R, Huber H, Rettberg P (2009) Tolerance of thermophilic and hyperthermophilic microorganisms to desiccation. Extremophiles 13:521–531
Beblo K, Douki T, Schmalz G, Rachel R, Wirth R, Huber H, Reitz G, Rettberg P (2011) Survival of thermophilic and hyperthermophilic microorganisms after exposure to UV-C, ionizing radiation and desiccation. Arch Microbiol 193:797–809
Billi D, Friedmann EI, Hofer KG, Caiola MG, Ocampo-Friedmann R (2000) Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl Environ Microbiol 66:1489–1492
Blasius M, Sommer S, Hubscher U (2008) Deinococcus radiodurans: what belongs to the survival kit? Crit Rev Biochem Mol Biol 43:221–238
Bochkarev A, Bochkareva E, Frappier L, Edwards AM (1999) The crystal structure of the complex of replication protein A subunits RPA32 and RPA14 reveals a mechanism for single-stranded DNA binding. EMBO J 18:4498–4504
Breuert S, Allers T, Spohn G, Soppa J (2006) Regulated polyploidy in halophilic archaea. PLoS One 1:e92
Carreto L, Moore E, Nobre MF, Wait R, Riley PW, Sharp RJ, da Costa M (1996) Rubrobacter xylanophilus sp. nov., a new thermophilic species isolated from a thermally polluted effluent. Int J Syst Bacteriol 46:460–465
Cavicchioli R, Amils R, Wagner D, McGenity T (2011) Life and applications of extremophiles. Environ Microbiol 13:1903–1907
Chang EC, Kosman DJ (1989) Intracellular Mn (II)-associated superoxide scavenging activity protects Cu, Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem 264:12172–12178
Cockell CS, Catling DC, Davis WL, Snook K, Lee P, McKay CP (2000) The ultraviolet environment of Mars: biological implications past, present, and future. Icarus 146:343–359
Confalonieri F, Sommer S (2011) Bacterial and archaeal resistance to ionizing radiation. J Phys 261:012005
Constantinesco F, Forterre P, Koonin EV, Aravind L, Elie C (2004) A bipolar DNA helicase gene, herA, clusters with rad50, mre11 and nurA genes in thermophilic archaea. Nucleic Acids Res 32:1439–1447
Dale WM (1940) The effect of X-rays on enzymes. Biochem J 34:1367–1373
Daly MJ (2009) A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol 7:237–245
Daly MJ (2012) Death by protein damage in irradiated cells. DNA Repair 11:12–21
Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D (2004) Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028
Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, Lai B, Ravel B, Li SMW, Kemmer KM, Fredrickson JK (2007) Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol 5:e92
Daly MJ, Gaidamakova EK, Matrosova VY, Kiang JG, Fukumoto R, Lee DY, Wehr NB, Viteri GA, Berlett BS, Levine RL (2010) Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS One 5:e12570
DasSarma S, DasSarma P (2012) Halophiles. In: Encyclopedia of life sciences. Wiley, Chichester. doi:10.1002/9780470015902.a0000394.pub3
Davila AF, Gomez-Silva B, de los Rios A, Ascaso C, Olivares H, McKay CP, Wierzchos J (2008) Facilitation of endolithic microbial survival in the hyperarid core of the Atacama Desert by mineral deliquescence. J Geophys Res 113:GO1028
Delmas S, Shunburne L, Ngo HP, Allers T (2009) Mre11-Rad50 promotes rapid repair of DNA damage in the polyploid archaeon Haloferax volcanii by restraining homologous recombination. PLoS Genet 5:e1000552
DeVeaux LC, Muller JA, Smith J, Petrisko J, Wells DP, DasSarma S (2007) Extremely radiation-resistant mutants of a halophilic archaeon with increased single-stranded DNA-binding protein (RPA) gene expression. Radiat Res 168:507–514
Dianov GL, O’Neill P, Goodhead DT (2001) Securing genome stability by orchestrating DNA repair: removal of radiation-induced clustered lesions in DNA. Bioessays 23:745–749
DiRuggiero J, Santangelo N, Nackerdien Z, Ravel J, Robb FT (1997) Repair of extensive ionizing-radiation DNA damage at 95°C in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 179:4643–4645
Du J, Gebicki JM (2004) Proteins are major initial cell targets of hydroxyl free radicals. Int J Biochem Cell Biol 36:2334–2343
Faria TQ, Lima JC, Bastos M, Maçanita AL, Santos H (2004) Protein stabilization by osmolytes from hyperthermophiles: effect of mannosylglycerate on the thermal unfolding of recombinant nuclease a from Staphylococcus aureus studied by picosecond time-resolved fluorescence and calorimetry. J Biol Chem 47:48680–48691
Fredrickson JK, Li SM, Gaidamakova EK, Matrosova VY, Zhai M, Sulloway HM, Scholten JC, Brown MG, Balkwill DL, Daly MJ (2008) Protein oxidation: key to bacterial desiccation resistance? ISME J 2:393–403
Ghosh S, Ramirez-Peralta A, Gaidamakova E, Zhang P, Li YQ, Daly MJ, Setlow P (2011) Effects of Mn levels on resistance of Bacillus megaterium spores to heat, radiation and hydrogen peroxide. J Appl Microbiol 111:663–670
Gladyshev E, Meselson M (2008) Extreme resistance of bdelloid rotifers to ionizing radiation. Proc Natl Acad Sci U S A 105:5139–5144
Granger AC, Gaidamakova EK, Matrosova VY, Daly MJ, Setlow P (2011) Effects of levels of Mn and Fe on Bacillus subtilis spore resistance, and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on resistance of a protein to ionizing radiation. Appl Environ Microbiol 77:32–40
Gutman PD, Fuchs P, Minton KW (1994) Restoration of the DNA damage resistance of Deinococcus radiodurans DNA polymerase mutants by Escherichia coli DNA polymerase I and Klenow fragment. Mutat Res 314:87–97
Haskin LA, Wang A, Jolliff BL, McSween HY, Clark BC, Des Marais DJ, McLennan SM, Tosca NJ, Hurowitz JA, Farmer JD, Yen A, Squyres SW, Arvidson RE, Klingelhöfer G, Schröder C, De Souza PA Jr, Ming DW, Gellert R, Zipfel J, Brückner J, Bell JF III, Herkenhoff K, Christensen PR, Ruff S, Blaney D, Gorevan S, Cabrol NA, Crumpler L, Grant J, Soderblom L (2005) Water alteration of rocks and soils on Mars at the Spirit rover site in Gusev crater. Nature 436:66–69
Holliday R (2004) Early studies on recombination and DNA repair in Ustilago maydis. DNA Repair 6:671–682
Hopfner KP, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA (2001) Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell 105:473–485
Horikawa DD, Sakashita T, Katagiri C, Watanabe M, Kikawada T, Nakahara Y, Hamada N, Wada S, Funayama T, Higashi S, Kobayashi Y, Okuda T, Kuwabara M (2006) Radiation tolerance in the tardigrade Milnesium tardigradum. Int J Radiat Biol 82:843–848
Hutchinson F (1966) The molecular basis for radiation effects on cells. Cancer Res 26:2045–2052
Imlay JA (2006) Iron-sulphur clusters and the problem with oxygen. Mol Microbiol 59:1073–1082
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776
Johnson TE, Hartman PS (1988) Radiation effects on life span in Caenorhabditis elegans. J Gerontol 43:B137–B141
Jolivet E, L’Haridon S, Corre E, Forterre P, Prieur D (2003) Thermococcus gammatolerans sp. nov., a hyperthermophilic archaeon from a deep-sea hydrothermal vent that resists ionizing radiation. Int J Syst Evol Microbiol 53:847–851
Jolivet E, Corre E, L’Haridon S, Forterre P, Prieur D (2004) Thermococcus marinus sp. nov. and Thermococcus radiotolerans sp. nov., two hyperthermophilic archaea from deep-sea hydrothermal vents that resist ionizing radiation. Extremophiles 8:219–227
Kaur A, Robinson C, van PT, Busch C, Robinson CK, Pan M, Pang WL, Reiss DJ, DiRuggiero J, Baliga NS (2010) Coordination of frontline defense mechanisms under severe oxidative stress. Mol Syst Biol 6:393
Kehres DG, Maguire ME (2003) Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27:263–290
Kish A, DiRuggiero J (2008) Rad50 is not essential for the Mre11-dependent repair of DNA double strand breaks in Halobacterium sp. str. NRC-1. J Bacteriol 190:5210–5216
Kish A, Kirkali G, Robinson C, Rosenblatt R, Jaruga P, Dizdaroglu M, DiRuggiero J (2009) Salt shield: intracellular salts provide protection against ionizing radiation in the halophilic archaeon, Halobacterium salinarum NRC-1. Environ Microbiol 11:1066–1078
Kish A, Griffin PL, Rogers KL, Fogel ML, Hemley RJ, Steele A (2012) High-pressure tolerance in Halobacterium salinarum NRC-1 and other non-piezophilic prokaryotes. Extremophiles 16:355–361
Klump H, DiRuggiero J, Kessel M, Park JB, Adams MWW, Robb FT (1992) Glutamate dehydrogenase from the hyperthermophile Pyroccocus furiosus: thermal denaturation and activation. J Biol Chem 267:22681–22685
Kottemann M, Kish A, Iloanusi C, Bjork S, Diruggiero J (2005) Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation. Extremophiles 9:219–227
Kranner I (2002) Glutathione status correlates with different degrees of desiccation tolerance in three lichens. New Phytol 154:451–460
Kranner I, Birtic S (2005) A modulating role for antioxidants in desiccation tolerance. Integr Comp Biol 45:734–740
Krisko A, Leroy M, Radman M, Meselson M (2012) Extreme anti-oxidant protection against ionizing radiation in bdelloid rotifers. Proc Natl Acad Sci U S A 109:2354–2357
Lamosa P, Turner D, Ventura R, Maycock C, Santos H (2003) Protein stabilization by compatible solutes. Effect of diglycerol phosphate on the dynamics of Desulfovibrio gigas rubredoxin studied by NMR. Eur J Biochem 270:4606–4614
Liedert C, Peltola M, Bernhardt J, Neubauer P, Salkinoja-Salonen M (2012) Physiology of resistant Deinococcus geothermalis bacterium aerobically cultivated in low-manganese medium. J Bacteriol 194:1552–1561
Markillie LM, Varnum SM, Hradecky P, Wong KK (1999) Targeted mutagenesis by duplication insertion in the radioresistant bacterium Deinococcus radiodurans: radiation sensitivities of catalase (katA) and superoxide dismutase (sodA) mutants. J Bacteriol 181:666–669
Martins LO, Huber R, Stetter KO, da Costa MS, Santos H (1997) Organic solutes in hyperthermophilic archaea. Appl Environ Microbiol 63:896–902
Mattimore V, Battista JR (1996) Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol 178:633–637
McNaughton RL, Reddi AR, Clement MHS, Sharma A, Barnese K, Rosenfeld L, Gralla EB, Valentine JS, Culotta VC, Hoffman BC (2010) Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci U S A 107:15335–15339
Müller V, Spanheimer R, Santos H (2005) Stress response by solute accumulation in archaea. Curr Opin Microbiol 8:729–736
Nauser T, Koppenol WH, Gebicki JM (2005) The kinetics of oxidation of GSH by protein radicals. Biochem J 392:693–701
Ogunniyi AD, Mahdi LK, Jennings MP, McEwan AG, McDevitt CA, van der Hoek MB, Bagley CJ, Hoffmann P, Gould KA, Paton JC (2010) Central role of manganese in regulation of stress responses, physiology, and metabolism in Streptococcus pneumoniae. J Bacteriol 192:4489–4497
Omelchenko MV, Wolf YI, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Daly MJ, Koonin EV, Makarova KS (2005) Comparative genomics of Thermus thermophilus and Deinococcus radiodurans: divergent routes of adaptation to thermophily and radiation resistance. BMC Evol Biol 5:57
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269:1–10
Osterloo MM, Hamilton VE, Bandfield JL, Glotch TD, Baldridge AM, Christensen PR, Tornabene LL, Anderson FS (2008) Chloride bearing materials in the southern highlands of Mars. Science 319:1651–1654
Ramos A, Raven NDH, Sharp RJ, Bartolucci S, Rossi M, Cannio R, Lebbink J, van der Oost J, de Vos WM, Santos H (1997) Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microbiol 63:4020–4025
Rastogi RP, Richa, Sinha RP, Singh SP, Häder DP (2010) Photoprotective compounds from marine organisms. J Ind Microbiol Biotechnol 37:537–558
Regulus P, Duroux B, Bayle P, Favier A, Cadet J, Ravanat JL (2007) Oxidation of the sugar moiety of DNA by ionizing radiation or bleomycin could induce the formation of a cluster DNA lesion. Proc Natl Acad Sci U S A 104:14032–14037
Rieder R, Gellert R, Anderson RC, Bruckner J, Clark BC, Dreibus G, Economou T, Kingelhöfer G, Lugmair GW, Ming DW, Squyres SW, d’Uston C, Wänke H, Yen A, Zipfel J (2004) Chemistry of rocks and soils at Meridiani Planum from the alpha particle X-ray spectrometer. Science 306:1746–1749
Riley PA (1994) Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65:27–33
Robinson CK, Webb K, Kaur A, Jaruga P, Dizdaroglu M, Baliga NS, Place A, DiRuggiero J (2011) A major role for nonenzymatic antioxidant processes in the radioresistance of Halobacterium salinarum. J Bacteriol 193:1653–1662
Rolfsmeier ML, Laughery MF, Haseltine CA (2011) Repair of DNA double-strand breaks induced by ionizing radiation damage correlates with upregulation of homologous recombination genes in Sulfolobus solfataricus. J Mol Biol 414:485–498
Santos H, da Costa MS (2002) Compatible solutes of organisms that live in hot saline environments. Environ Microbiol 4:501–509
Scholz S, Sonnenbichler J, Schäfer W, Hense R (1992) Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS J 306:239–242
Scott MD, Meshnick SR, Eaton JW (1989) Superoxide dismutase amplifies organismal sensitivity to ionizing radiation. J Biol Chem 264:2498–2501
Skowyra A, MacNeill SA (2011) Identification of essential and non-essential single-stranded DNA-binding proteins in a model archaeal organism. Nucleic Acids Res 40:1077–1090
Slade D, Radman M (2011) Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev 75:133–191
Sobota JM, Imalay JA (2011) Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A 108:5402–5407
Stroud A, Liddell S, Allers T (2012) Genetic and biochemical identification of a novel single-stranded DNA-Binding complex in Haloferax volcanii. Front Microbiol 3:224
Suzuki K, Collins MD, Iijima E, Komagata K (1988) Chemotaxonomic characterization of a radiotolerant bacterium, Arthrobacter radiotolerans: Description of Rubrobacter radiotolerans gen. nov., comb. nov. FEMS Microbiol Lett 52:33–40
Ward JF (1994) The complexity of DNA damage: relevance to biological consequences. Int J Radiat Biol 66:427–432
Webb KM, DiRuggiero J (2012) Role of Mn2+ and compatible solutes in the radiation resistance of thermophilic bacteria and archaea. Archaea, Article ID 845756
Webb K, Wu J, Robinson CK, Tomiya N, Lee Y, DiRuggiero J (2013) Effects of intracellular Mn on the radiation resistance of the halophilic archaeon Halobacterium salinarum. Extremophiles, doi:10.1007/s00792-013-0533-9
Whitehead K, Kish A, Pan M, Kaur A, Reiss DJ, King N, Hohmann L, DiRuggiero J, Baliga NS (2006) An integrated systems approach for understanding cellular responses to gamma radiation. Mol Syst Biol 2:47–53
Wold MS (1997) Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem 66:61–92
Acknowledgment
This work was possible with the support of AFOSR (grant FA95500710158) to J.D.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Webb, K.M., DiRuggiero, J. (2013). Radiation Resistance in Extremophiles: Fending Off Multiple Attacks. In: Seckbach, J., Oren, A., Stan-Lotter, H. (eds) Polyextremophiles. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 27. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6488-0_10
Download citation
DOI: https://doi.org/10.1007/978-94-007-6488-0_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6487-3
Online ISBN: 978-94-007-6488-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)