Abstract
Tetraiodothyroacetic acid (tetrac) is a naturally occurring derivative of thyroid hormone, T4. In the absence or presence of L-T4 or L-T3, tetrac has been found to disrupt a number of functions or events that are important to cancer cells via the known thyroid hormone-tetrac receptor on the plasma membrane integrin αvβ3. These functions include regulation of cell division, local stimulation of angiogenesis, chemo-resistance and resistance to radiation. It is desirable to reformulate tetrac as a nanoparticle whose activity is exclusively at the cell surface integrin. Nanotetrac has been designed to limit tetrac to the extracellular space on the basis of the size of the nanoparticle and to provide optimized exposure of the biphenyl structure and acetic acid side chain of its inner ring to the receptor site on αvβ3. Tetrac and its novel nanoparticulate formulation have anti-angiogenesis activity that transcends the inhibition of thyroid hormone-binding at the integrin. Restriction of nanotetrac to the extracellular space has been verified, and nanotetrac has been shown to be up to 10-fold more potent than unmodified tetrac at its integrin target. Nanotetrac formulations have potential applications as inhibitors of tumor-related angiogenesis and of angiogenesis that is unrelated to malignancy, including clinically significant disorders ranging from skin diseases to vascular proliferation in the retina and neovascularization associated with inflammatory states.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular Endothelial Growth Factor
- Thyroid Hormone
- Vascular Growth Factor
- Myeloid Cell Leukemia Sequence
- Nuclear Thyroid Hormone Receptor
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Biochemical History of Tetrac
Tetraiodothyroacetic acid (tetrac) is a naturally occurring derivative of thyroid hormone (L-thyroxine, T4). It accounts for less than 1 % of circulating thyroid hormone. Inside human cells, tetrac is a low-grade thyromimetic, that is, it has low-potency actions that resemble those of 3, 5, 3′-triiodo-L-thyronine (T3), the most active form of thyroid hormone [1]. For 30 or more years, tetrac has been known to be taken up by pituitary cells that secrete thyrotropin (TSH) and to inhibit endogenous human TSH release by the pituitary gland [2]. This action has been considered potentially useful in the clinic in the setting of TSH-dependent thyroid cancer.
Tetrac was also found to compete with T4 for thyroid hormone-binding sites on human serum pre-albumin (transthyretin, TTR) [3], but not for sites on human thyroxine-binding globulin (TBG), the principal transport protein for iodothyronines in human serum. These observations caused us in the 1980s to test tetrac for its ability to block the nongenomic actions of thyroid hormone that we demonstrated to exist in human mature red blood cell (RBC) membranes [4, 5] and intracellular membranes, such as those of the sarcoplasmic reticulum [6]. These actions included Ca2+ transport by calmodulin-responsive Ca2+-ATPase [7]. Such effects were ‘nongenomic’ because they were independent of the nuclear thyroid hormone receptor (TR) and gene transcription [8]. Tetrac indeed inhibited such actions of T4 and T3 and thus became a probe for certain actions of thyroid hormone that were initiated at the plasma membrane, although the cell surface receptor site for the hormone was not identified until 2005 [9].
Studies focused on the plasma membrane receptor for thyroid hormone on integrin αvβ3 revealed that this receptor mediated the pro-angiogenic action of T4 and T3, and that tetrac was anti-angiogenic in terms of its ability to block this action [10]. As will be discussed below, tetrac and a novel nanoparticulate formulation of the agent have anti-angiogenic activity that transcends the inhibition of thyroid hormone-binding at the integrin. Tetrac formulations also have anti-proliferative activity against tumor cells and have been shown to have chemosensitizing and radiosensitizing effects in cancer cells [10, 11].
Integrin αvβ3 Contains a Thyroid Hormone-Tetrac Receptor
J.J. Bergh et al. in 2005 [9] described the existence of a thyroid hormone-tetrac receptor on plasma membrane integrin αvβ3. This heterodimeric integrin is generously expressed on rapidly dividing blood vessel cells and by cancer cells, enabling scanning technology focused on the integrin to detect tumors [12]. The fit of unmodified tetrac into the hormone-binding groove in the extracellular domain [11, 13] of the integrin permits the agent to block the binding of T4 and T3 and inhibit actions of these agonist forms of thyroid hormone on cancer cell proliferation and cancer-related angiogenesis as discussed in Chap. 4. The integrin is found on virtually all cancer cells and reads the presence of specific extracellular matrix proteins that are relevant to tumor cell migration and tumor mass formation.
In the absence or presence of T4 and T3, however, tetrac has been found to disrupt via αvβ3 a number of other functions or events that are important to cancer cells. These functions include regulation of cell division, local stimulation of angiogenesis, chemoresistance and resistance to radiation [10, 14]. It was surprising to find that, acting at the cell surface, tetrac coherently interfered with expression of differentially regulated genes whose products included the cyclins and thrombospondin 1 [15]. The thrombospondin 1 gene is usually silent in tumor cells because it suppresses angiogenesis. Tetrac also blocked the actions of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [16], released by cancer cells to promote angiogenesis in an autocrine manner. The mechanism of tetrac involved here is thought to involve disorganization of crosstalk between the integrin and nearby receptors for VEGF [17] and bFGF [10, 18] and inhibition of local release of bFGF [18]. The hormone analogue was found to cause retention by cancer cells of traditional chemotherapeutic agents to which the cells previously showed resistance [19]. These agents included doxorubicin, etoposide, and cisplatin. Tetrac was also shown to inhibit the ability of cancer cells to repair double-strand DNA breaks that radiation induces [20, 21], thus radiosensitizing these cells. Finally, expression of genes for cytokines involved in inflammation and relevant to inflammation-associated cancer was shown to be blocked by tetrac [15].
The foregoing describes the anti-cancer and anti-angiogenic features of tetrac manifested at integrin αvβ3. However, unmodified tetrac is taken up by cells and has, within the cell, low-potency thyromimetic activity [1, 2]. This can include proliferative—rather than anti-proliferative—behavior (H.Y. Lin: unpublished). While the algebraic sum of these anti-proliferative and proliferative effects favors anti-cancer activity, it is desirable to reformulate tetrac as a nanoparticle whose activity is exclusively at the integrin (Section “Nanoparticulate Tetrac (Nanotetrac)”, below).
Nanoparticulate Tetrac (Nanotetrac)
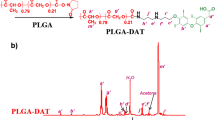
S.A. Mousa and co-workers have constructed an approximately 250 nm poly(lactic-co-glycolic acid) (PLGA) formulation in which a limited number of tetrac moieties are covalently bound to and protrude from a PLGA nanoparticle [22, 23] (Fig. 10.1). Binding of tetrac to the PLGA is via an ether bond at the hydroxyl on the outer ring of tetrac (position 4) to a linker that is amide-bonded to the PLGA. Nanotetrac was designed to limit tetrac to the extracellular space on the basis of the size of the nanoparticle and to provide optimized exposure of the biphenyl structure and acetic acid side chain of the inner ring to the receptor site on αvβ3. Restriction of the molecule to the extracellular space has been verified [24].
Chemical structures of unmodified tetraiodothyroacetic acid (tetrac) (a) and nanoparticulate tetrac (nanotetrac) (b). An ether bond involving the outer ring hydroxyl group joins tetrac to a linker molecule which, in turn, is attached by an imbedded amide bond to the nanoparticle. Multiple tetrac moieties are bonded to the surface of the PLGA, enabling access of tetrac to its receptor groove in the extracellular domain of integrin αvβ3
Interestingly, the reformulation of tetrac as a nanoparticle has conferred two other qualities on the molecule. First, nanotetrac is up to 10-fold more potent than unmodified tetrac. The basis for this may reflect interaction(s) of the polymer (PLGA) chain, away from the tetrac moieties, with the ‘legs’ of the extracellular domain of αvβ3, favoring ‘on’ kinetics. Another possibility is that the nanoparticulate tetrac ligand induces a long-lived change in the conformation of the αvβ3 that persists after ligand dissociation and modulates activity of the integrin [25]. Second, and acting at the integrin receptor, nanotetrac induces a pattern of cancer survival gene expression that is somewhat different from that of unmodified tetrac [15]. There is 80–85 % congruence of gene expression caused by nanotetrac and tetrac. Examples of the disparities are down-regulation by nanotetrac in cancer cells of expression of the epidermal growth factor receptor (EGFR) gene, down-regulation of the majority of the members of the Ras-oncogene family, and up-regulation of the apoptosis inhibitor MCL1 (myeloid cell leukemia sequence 1); these genes are unaffected by tetrac.
The difference in potency of tetrac and nanotetrac is readily seen in the comparison of efficacy of the compounds in human cancer xenografts in the nude mouse. Within 3 days of initiation of treatment, the two formulations cause a greater than 50 % decrease in the vascular supply of the xenografts, but this anti-angiogenic effect can be obtained with a dose of nanotetrac that is 10-fold less than that of tetrac.
Triiodothyroacetic Acid (Triac)
Triac (3, 3′, 5-triiodothyroacetic acid), the deaminated derivative of T3, can reproduce the inhibition of actions of T4 and T3 at the αvβ3 thyroid hormone-tetrac receptor (H.Y. Lin: unpublished), but has not as yet been reformulated as a nanoparticle. These actions include inhibition of the pro-angiogenic activity of thyroid hormone studied in the chick chorioallantoic membrane (CAM) model. Inside the cell, triac is thyromimetic. It is thermogenic [26], has been shown in human subjects to have agonist thyroid hormone effects on liver and bone that are augmented compared to T4 [27], and has been postulated to be a primordial form of thyroid hormone [28].
The unmodified compound is mentioned here for several reasons. Triac may be purchased over-the-counter in several European countries as a dietary supplement (tiratricol). The availability of this iodothyronine raises the possibility that anti-angiogenic side effects may be encountered in users of the agent. Such effects have not been reported, but it is unlikely that they have been anticipated, e.g., in the setting of wound-healing.
Potential Applications of Tetrac and Nanotetrac in Angiogenesis
Tetrac and Anti-angiogenesis in the Setting of Cancer
The clinical desirability of establishing control of angiogenesis in and about tumors is obvious and the feasibility of such control became apparent with the work of Judah Folkman and colleagues [29, 30]. In the context of cancers, local angiogenesis that supports tumor biology may be the result of more than a single vascular growth factor. These growth factors include VEGF, bFGF and other proteins [31]. Erythropoietin (EPO) may also be included among factors that enhance tumor-relevant angiogenesis [32].
In contrast to several of the anti-angiogenic agents used clinically (see below) that are designed to inhibit actions of single, specific vascular growth factors, tetrac and nanotetrac have been shown, in the absence or presence of agonist thyroid hormone (T4 or T3), to antagonize actions of multiple growth factors. These include VEGF, bFGF [17, 33], PDGF (S.A. Mousa: unpublished), EGF (S.A. Mousa: unpublished) and EPO [34]. This plural effect explains, at least in part, the rapid decrease in vascularity so far encountered in human tumor xenografts in mice exposed to systemic tetrac and nanotetrac [35–38] and the resultant decrease in volume of xenografts.
There are multiple mechanisms by which thyroid hormone analogues inhibit (nanotetrac or tetrac) or enhance (T4 and T3) the activities of various vascular growth factors. First, vascular growth factor gene expression in tumor cells or endothelial cells may be enhanced by thyroid hormone [18]. Second, iodothyronines may increase release of the growth factor(s) by the secreting cell, e.g., bFGF [18]. Third, crosstalk between the integrin and adjacent vascular growth receptors is well-described. The crosstalk may involve signal transducing biochemistry within or immediately below the plasma membrane. For example, inhibition by tetrac of mitogen-activated protein kinase (MAPK) activity alters activity of bFGF and other factors. Hormonal effects may in other cases depend upon interactions of the extracellular domains of the receptor(s) and the integrin [39] that could involve, in the case of VEGFR, the inhibition by tetrac formulations of dimerization of the growth factor receptor or obscuring of one or more of its immunoglobulin (Ig)-like domains [39]. Fourth, tetrac decreases abundance of angiopoietin-2 (Ang-2) mRNA in endothelial cells, but does not affect accumulation of Ang-1 mRNA [17]. Ang-2 protein production in tumor vasculature anticipates or synergizes with vascular growth factor action to support tumor angiogenesis [40], whereas the Ang-1-Tie 2 system is a blood vessel-stabilizing pathway. This differential effect of tetrac with regard to angiogenesis is consistent with discrete, selective actions of tetrac or nanotetrac in cancer cells on elaboration of certain interleukins (see below) or of endogenous inhibitors or enhancers of apoptosis [15]. Fifth, tetrac can induce the expression of thrombospondin 1, an endogenous suppressor of angiogenesis that is almost invariably unexpressed in cancer cells [22]. Finally, T4 and T3 and tetrac may positively, in the case of the former, and negatively, in the case of tetrac, affect endothelial cell motility (S.A. Mousa: unpublished) that is important to neovascularization.
Currently available clinically are pharmaceuticals that affect single vascular growth factors. Bevacizumab (Avastin®) and ranibizumab (Lucentis®) are monoclonal antibodies to VEGF, developed as anti-angiogenic agents. Administered parenterally—intravenously, or, in the case of eye disease, intra-ocularly—these humanized antibodies are unquestionably effective in clinical disease settings in which VEGF or VEGF-A are contributory pathophysiologic factors. There are several subtypes of VEGF; VEGF-A [41], for example, is a form frequently released locally by tumor cells and which induces a porous vasculature. It is clear that the application of bevacizumab to the cancers for which it is approved is primarily adjunctive [42, 43] and is not curative. It has not so far been practical to produce for clinical use multi-monoclonal antibody preparations that are directed at more than a single vascular growth factor.
Bevacizumab is applied with U.S. Food and Drug Administration (FDA) approval to management of several forms of cancer and used without FDA approval in settings of unwanted angiogenesis in the absence of cancer, e.g., diabetic retinopathy [44]. Ranibizumab is an anti-angiogenic drug marketed for management of a form of retinal macular degeneration that may lead to loss of vision [45]. Bevacizumab and ranibizumab are discussed in more detail in Chaps. 12 and 13.
Application of Tetrac/Nanotetrac to Clinical Conditions of Excessive Angiogenesis Not Associated with Malignancy
Skin Disorders
Skin redness (erythema) in specific settings such as acne rosacea [46] or psoriasis [47] may be VEGF-dependent. Systemic anti-VEGF treatment for cancer has been reported to induce remission of cutaneous manifestations of psoriasis coincident with the cancer [47]. Conventional treatments for both of these conditions are inconsistently effective and systemic bevacizumab (anti-VEGF) treatment is too expensive to consider in most patients with skin disease.
Topical application of tetrac in a vehicle that permits penetration of the agent to involved blood vessels in the dermis and limits systemic absorption of the hormone analogue has been proposed in management of rosacea and awaits clinical trial.
Retinopathy
Tetrac and nanotetrac have been tested for efficacy in the newborn mouse oxygen-induced retinopathy (OIR) model [34]. It was an effective intravitreal or intraperitoneal preventive intervention. Similar results in this model have been obtained by S.A. Mousa et al. (unpublished). Yoshida and co-workers also found that the effects of VEGF and erythropoietin (EPO) on retinal endothelial cells in vitro were blocked by tetrac and nanotetrac [34]. As noted earlier, EPO is another factor supporting angiogenesis whose activity is minimized by tetrac.
VEGF antibody administered by the intravitreal route has been examined for its effectiveness in the clinical setting of diabetic retinopathy [44, 48]. The substantial experience is largely favorable. The increased intravitreous and circulating levels of VEGF seen in proliferative retinopathy are both decreased by bevacizumab [49]. The agent may, however, increase the risk of retinal fibrosis [50].
Inflammation
Analysis of the gene signature of tetrac treatment in relatively chemoresistant human breast cancer cells [15] has revealed an important set of actions on inflammation-related genes [51]. For example, five of six differentially regulated interleukin genes—including IL-6 and IL-1α—are down-regulated by the compound and a suppressor of cytokine signaling (SOCS4) gene is up-regulated. Expression of interferon response pathway genes and chemokine genes is decreased. Such effects may be relevant to inflammation-associated cancers, as well as to other inflammatory states. The selectivity of the tetrac/nanotetrac effect on gene expression is shown in the case of IL-11, whose gene product is a proliferative factor for hematopoietic stem cells and whose expression is enhanced, rather than decreased, by nanotetrac and tetrac.
Additional Actions of Tetrac and Nanotetrac
Independently of their actions of angiogenesis, tetrac and nanotetrac inhibit proliferation of a variety of cancer cell lines in vitro, as noted above, and chemosensitize [19] and radiosensitize [20, 21] tumor cells. The mechanism of chemosensitization by tetrac is incompletely understood, but may involve acidification of the cancer cell by inhibition of the Na+/H+ exchanger [52]. Consequent increase in extracellular pH (pHe) or decrease in intracellular pH may be associated with decreased activity of the cancer cell P-glycoprotein (P-gp) or other multidrug resistance (MDR) pumps that export chemotherapeutic agents [53]. Tetrac may also interfere with stimulation of the cancer cell kinases relevant to MDR pump activation [54].
As noted earlier, the mechanism of radiosensitization of cancer cells involves inhibition of repair of double-strand DNA breaks induced by radiation [20]. Under normal conditions, this repair process is highly efficient in tumor cells.
Conclusions
Acting at the thyroid hormone-tetrac receptor on the plasma membrane, tetrac and nanotetrac have potentially important anti-angiogenic effects. The receptor is located on integrin αvβ3, a highly plastic protein that is capable of transducing interactions of its extracellular domain with extracellular matrix proteins and small molecules, like thyroid hormone, into important intracellular events. Tetrac and its nanoparticulate formulation disrupt the communication between the integrin and nearby receptors for VEGF, bFGF, and other polypeptide factors important to neovascularization. That is, these thyroid hormone derivatives affect the activity of a number of pro-angiogenic proteins, in addition to blocking the angiogenic activity of agonist thyroid hormones, T4 and T3. Tetrac and nanotetrac also stimulate expression of the endogenous angiogenic suppressor gene, thrombospondin 1. The actions of tetrac and nanotetrac are generally coherent, that is, the effects that they have on expression of multiple genes, on crosstalk between integrin αvβ3 and receptors such as VEGFR and bFGFR, and on vascular growth factor release by tumor cells fit an anti-angiogenic pattern. That the agents distinguish between endothelial cell Ang-1 and Ang-2 also supports coherence of the anti-angiogenic pharmacology of tetrac and nanotetrac.
These agents have potential applications as inhibitors of tumor-related angiogenesis and of angiogenesis that is not associated with malignancy, but contributes to clinically significant skin disorders, to vascular proliferation of the retina, and neovascularization associated with inflammatory states.
References
Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F (2008) Metabolic effects of thyroid hormone derivatives. Thyroid 18(2):239–253
Burger AG, Engler D, Sakaloff C, Staeheli V (1979) The effects of tetraiodothyroacetic and triiodothyroacetic acids on thyroid function in euthyroid and hyperthyroid subjects. Acta Endocrinol (Copenhagen) 92(3):455–467
Davis PJ, Handwerger BS, Gregerman RI (1972) Thyroid hormone binding by human serum prealbumin (TBPA). Electrophoretic studies of triiodothyronine-TBPA interaction. J Clin Invest 51(3):515–521
Davis FB, Cody V, Davis PJ, Borzynski LJ, Blas SD (1983) Stimulation by thyroid hormone analogues of red blood cell Ca2+-ATPase activity in vitro. Correlation between hormone structure and biologic activity in a human cell system. J Biol Chem 258(20):12373–12377
Davis PJ, Davis FB, Lawrence WD, Blas SD (1989) Thyroid hormone regulation of membrane Ca(2+)-ATPase activity. Endocr Res 15(4):651–682
Warnick PR, Davis PJ, Davis FB, Cody V, Galindo J Jr, Blas SD (1993) Rabbit skeletal muscle sarcoplasmic reticulum Ca(2+)-ATPase activity: stimulation in vitro by thyroid hormone analogues and bipyridines. Biochim Biophys Acta 1153(2):184–190
Nieman LK, Davis FB, Davis PJ, Cunningham EE, Gutman S, Blas SD, Schoenl M (1983) Effect of end-stage renal disease on responsiveness to calmodulin and thyroid hormone of calcium-ATPase in human red blood cells. Kidney Int Suppl 16:S167–S170
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31(2):139–170
Bergh JJ, Lin HY, Lansing L, Mohamed SN, Davis FB, Mousa S, Davis PJ (2005) Integrin alphavbeta3 contains a cell surface receptor for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 146(7):2864–2871
Davis PJ, Davis FB, Mousa SA, Luidens MK, Lin HY (2011) Membrane receptor for thyroid hormone: physiologic and pharmacologic implications. Annu Rev Pharmacol Toxicol 51:99–115
Lin HY, Cody V, Davis FB, Hercbergs AA, Luidens MK, Mousa SA, Davis PJ (2011) Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discov Med 11(59):337–347
Gaertner FC, Schwaiger M, Beer AJ (2010) Molecular imaging of αvβ3 expression in cancer patients. Q J Nucl Med Mol Imaging 54(3):309–326
Freindorf M, Furlani TR, Kong J, Cody V, Davis FB, Davis PJ (2012) Combined QM/MM study of thyroid and steroid hormone analogue interactions with αvβ3 integrin. J Biomed Biotechnol Article ID 959057, doi:10.1155/2012/959057
Davis PJ, Davis FB, Lin HY, Mousa SA, Zhou M, Luidens MK (2009) Translational implications of nongenomic actions of thyroid hormone initiated at its integrin receptor. Am J Physiol Endocrinol Metab 297(6):E1238–E1246
Glinskii AB, Glinsky GV, Lin HY, Tang HY, Sun M, Davis FB, Luidens MK, Mousa SA, Hercbergs AH, Davis PJ (2009) Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 8(21):3554–3562
Somananth PR, Malinin NL, Byzova TV (2009) Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis 12(2):177–185
Mousa SA, Bergh JJ, Dier E, Rebbaa A, O’Connor LJ, Yalcin M, Aljada A, Dyskin E, Davis FB, Lin HY, Davis PJ (2008) Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis 11(2):183–190
Davis FB, Mousa SA, O’Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ (2004) Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94(11):1500–1506
Rebbaa A, Chu P, Davis FB, Davis PJ, Mousa SA (2008) Novel function of the thyroid hormone analog tetraiodothyroacetic acid: a cancer chemosensitizing and anti-cancer agent. Angiogenesis 11(5):269–276
Hercbergs A, Davis PJ, Davis FB, Ciesielski MJ, Leith JT (2009) Radiosensitization of GL261 glioma cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 8(16):2586–2591
Hercbergs AH, Lin HY, Davis FB, Davis PJ, Leith JT (2011) Radiosensitization and production of DNA double-strand breaks in U87MG brain tumor cells induced by tetraiodothyroacetic acid (tetrac). Cell Cycle 10(2):352–357
Bridoux A, Cui H, Dyskin E, Schmitzer AR, Yalcin M, Mousa SA (2010) Semisynthesis and pharmacological activities of thyroxine analogs: development of new angiogenesis modulators. Bioorg Med Chem Lett 20(11):3394–3398
Bharali DJ, Yalcin M, Davis PJ, Mousa SA (2012) Tetraiodothyroacetic acid (Tetrac) conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer. Nanomedicine (in press), 2013. doi:10.2217/nnm.12.200
Yalcin M, Dyskin E, Lansing L, Bharali DJ, Mousa SS, Bridoux A, Hercbergs AH, Lin HY, Davis FB, Glinsky GV, Glinskii AB, Ma J, Davis PJ, Mousa SA (2010) Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J Clin Endocrinol Metab 95(4):1972–1980
Zolotarjova NI, Hollis GF, Wynn R (2001) Unusually stable and long-lived ligand-induced conformations of integrins. J Biol Chem 276(20):17063–17068
Medina-Gomez G, Calvo RM, Obregon MJ (2008) Thermogenic effect of triiodothyroacetic acid at low doses in rat adipose tissue without adverse effects in the thyroid axis. Am J Physiol Endocrinol Metab 294(4):E688–E697
Sherman SI, Ringel MD, Smith MJ, Kopelen HA, Zoghbi WA, Ladenson PW (1997) Augmented hepatic and skeletal thyromimetic effects of tiratricol in comparison with levothyroxine. J Clin Endocrinol Metab 82(7):2153–2158
Klootwijk W, Friesema EC, Visser TJ (2004) A nonselenoprotein from amphioxus deiodinates triac but not T3: is triac the primordial bioactive thyroid hormone? Endocrinology 152(8):3259–3267
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1(1):27–31
Folkman J (2007) Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6(4):273–286
Jensen RL (1998) Growth factor-mediated angiogenesis in malignant progression of glial tumors: a review. Surg Neurol 49(2):189–195
Ribbati D (2010) Erythropoietin and tumor angiogenesis. Stem Cells Dev 19(1):1–4
Mousa SA, Davis FB, Mohamed S, Davis PJ, Feng X (2006) Pro-angiogenesis action of thyroid hormone and analogs in a three-dimensional in vitro microvascular endothelial sprouting model. Int Angiol 25(4):407–413
Yoshida T, Gong J, Xu Z, Wei Y, Duh EJ (2012) Inhibition of pathological angiogenesis by the integrin αvβ3 antagonist tetraiodothyroacetic acid (tetrac). Exp Eye Res 94(1):41–48
Yalcin M, Bharali DJ, Dyskin E, Dier E, Lansing L, Mousa SS, Davis FB, Davis PJ, Mousa SA (2010) Tetraiodothyroacetic acid and tetraiodothyroacetic acid nanoparticle effectively inhibit the growth of human follicular thyroid cell carcinoma. Thyroid 20(3):281–286
Yalcin M, Bharali DJ, Lansing L, Dyskin E, Mousa SS, Hercbergs A, Davis FB, Davis PJ, Mousa SA (2009) Tetraiodothyroacetic acid (tetrac) and tetrac nanoparticles inhibit growth of human renal cell carcinoma xenografts. Anticancer Res 29(10):3825–3831
Mousa SA, Yalcin M, Bharali DJ, Meng R, Tang HY, Lin HY, Davis FB, Davis PJ (2012) Tetraiodothyroacetic acid and its nanoformulation inhibit thyroid hormone stimulation of non-small cell lung cancer cells in vitro and its growth in xenografts. Lung Cancer 76(1):39–45
Borges E, Jan Y, Ruoslahti E (2000) Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta3 integrin through its extracellular domain. J Biol Chem 275(51):39867–39873
Stuttfeld E, Ballmer-Hofer K (2009) Critical review. Structure and function of VEGF receptors. Life 61(9):915–922
Thomas M, Augustin HG (2009) The role of the angiopoietins in vascular morphogenesis. Angiogenesis 12(2):125–137
Nagy JA, Dvorak AM, Dvorak HF (2007) VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol 2:251–275
Shojaei F (2012) Antiangiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett 320(2):130–137
Ranpura V, Hapani S, Wu S (2011) Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA 305(5):487–494
Salam A, Mathew R, Sivaprasad S (2011) Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmol 89(5):405–411
The IVAN Study Investigators Writing Committee, Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN Randomized Trial. Ophthalmology 119:1399–1411
Smith JR, Lanier VB, Braziel RM, Falkenhagen KM, White C, Rosenbaum JT (2007) Expression of vascular endothelial growth factor and its receptors in rosacea. Br J Ophthalmol 91(2):226–229
Canavese M, Altruda F, Ruzicka T, Schauber J (2010) Vascular endothelial growth factor (VEGF) in the pathogenesis of psoriasis—a possible target for novel therapies. J Dermatol Sci 58(3):171–176
Arevalo JF, Sanchez JG, Lasave AF, Wu L, Maia M, Bonafonte S, Brito M, Alezzandrini AA, Restrepo N, Berrocal MH, Saravia M, Farah ME, Fromow-Guerra J, Morales-Canton V (2010) Intravitreal bevacizumab (Avastin®) for diabetic retinopathy at 24-months: The 2008 Juan Verdaguer-Planas Lecture. Curr Diabetes Rev 6(5):313–322
Ma Y, Zhang Y, Zhao T, Jiang YR (2012) Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol 153(2):307–313
Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, Klaassen I, Schlingermann RO (2012) A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol 96(4):587–590
Lin HY, Glinsky GV, Glinskii AB, Davis FB, Mousa SA, Luidens MK, Hercbergs A, Davis PJ (2012) Tetraiodothyroacetic acid (tetrac) acts at a plasma membrane receptor to modulate expression of inflammation-related genes in tumor cells.In: 94th Annual meeting of The Endocrine Society, Houston, TX, 23–26 June, abstract 852243
D’Arezzo S, Incerpi S, Davis FB, Acconcia F, Marino M, Farias RN, Davis PJ (2004) Rapid nongenomic effects of 3, 5, 3′-triiodo-L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology 145(12):5694–5703
Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ (2011) Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm 8(6):2032–2038
Hait WN, Aftab DT (1992) Rational design and pre-clinical pharmacology of drugs for reversing multidrug resistance. Biochem Pharmacol 43(1):103–107
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Davis, P.J., Davis, F.B., Luidens, M.K., Lin, HY., Mousa, S.A. (2013). Tetraiodothyroacetic Acid (Tetrac), Nanotetrac and Anti-angiogenesis. In: Mousa, S., Davis, P. (eds) Angiogenesis Modulations in Health and Disease. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6467-5_10
Download citation
DOI: https://doi.org/10.1007/978-94-007-6467-5_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6466-8
Online ISBN: 978-94-007-6467-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)