Abstract
Mesenchymal stem cells (MSCs) are considered attractive therapeutic tools for the treatment of various diseases, including autoimmune disease. MSCs display many advantageous properties such as immunomodulation, capacity for differentiation and transdifferentiation, homing activity, and paracrine effects. Further, adipose tissue-derived MSCs (ASCs) are regarded an ideal source of these stem cells because of their plentiful supply, availability, non-immunogenic properties, and minimal ethical considerations as well as the fact that their capacity for proliferation and differentiation is less likely to be affected by aging. In the few preclinical studies and clinical trials that have been performed, treatment with ASCs have ameliorated the clinical symptoms of various autoimmune diseases by reducing inflammatory responses, improving Th1/Th2 balance, and inducing regulatory T cells. ASCs are also an ideal vehicle to deliver genes into target tissues for gene therapy because of their unique biological and immunological properties. Using ASCs as a vehicle to insert appropriate therapeutic genes into cells could prove a novel method for immune modulation in autoimmune disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mesenchymal Stem Cell
- Major Histocompatibility Complex Class
- Dextran Sulfate Sodium
- Human ASCs
- Chronic Experimental Autoimmune Encephalomyelitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Autoimmune disease occurs when the body tissues are attacked by its own immune system because of an inappropriate immune response directed toward self-antigens. Over 60 autoimmune diseases exist affecting about 6% of the population (Siatskas et al. 2006).
Current systemic therapies using steroids or immunosuppressant drugs are not always effective and are rarely curative for patients with severe autoimmune disease. Furthermore, they are associated with side effects and substantial toxicity (Jantunen and Myllykangas-Luosujärvi 2000). Recent advances in the understanding of the pathogenesis of autoimmune diseases have given rise to many targeted approaches for the treatment of these diseases, including the use of biological agents that target cytokines and immune cells, and stem cell therapy.
Mesenchymal stem cells (MSCs) are non-hematopoietic progenitor cells with multi-lineage differentiation potential. Recently, MSCs have been considered as natural immune modulators, and therefore, research has been directed at using MSCs for the treatment of immune-mediated diseases. In addition to immunomodulation, MSCs also hold potential for the repair of various defects because of a number of attractive properties, including differentiation capabilities, paracrine effects, and their capacity for directional migration (Wang et al. 2011). MSCs were first isolated from the bone marrow, and later from other tissues, including the placenta, muscle, cartilage, and fat. Bone marrow MSCs were the first to be used for therapy. However, collection from the bone marrow is a painful, invasive procedure that generates relatively low yields.
Adipose tissue is becoming an alternative source of MSCs, and it is readily available and easy to obtain. Research focusing on ASCs has intensified with many preclinical studies and clinical trials (Choi 2009). This review provides an overview on the etiology, classification, and experimental animal models of human autoimmune disease. It will highlight the advantageous properties of ASCs and the application of these cells in the treatment of autoimmune diseases.
Etiology and Classification of Autoimmune Diseases
The etiology of autoimmune diseases is unknown, but is thought to result from a combination of genetic factors, inappropriate immune regulations, and hormonal and environmental factors (Choi 2012). The underlying mechanism is generation of an adaptive immune response against self-antigens (Siatskas et al. 2006). This autoimmunity can be induced by many factors, including failure of clonal anergy or clonal suppression of self-reactive lymphocytes, release of sequestered antigens, molecular mimicry between microbial antigens and host proteins (self-antigen), inappropriate expression of major histocompatibility complex (MHC) class II molecules, cytokine imbalance, dysfunction of the idiotype network regulatory pathways, general regulatory T-cell defects, and polyclonal B cell activation due to infection by bacteria or viruses (Kuby 1994).
Autoimmune diseases can be broadly classified as organ-specific or systemic depending on the location of the target antigen and clinical features (Siatskas et al. 2006). Common examples of systemic autoimmune diseases include systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, ankylosing spondylitis, and polymyositis. Organ-specific autoimmune diseases include type 1 diabetes, Addison’s disease, Hashimoto thyroiditis, Graves’ disease, Sjögren’s syndrome, vitiligo, pernicious anemia, glomerulonephritis, myasthenia gravis, Goodpasture’s syndrome, autoimmune hemolytic anemia, idiopathic thrombocytopenia purpura, and pulmonary fibrosis (Kuby 1994).
Advantageous Properties of Mesenchymal Stem Cells
MSCs are considered promising therapeutic tools because of their capacity for differentiation and transdifferentiation and their paracrine effects. MSCs release paracrine factors, including trophic (anti-apoptotic, supportive, and angiogenic), chemoattractant, anti-scarring, and immunomodulatory factors. Ponte et al.’s study (2007) showed that BM MSCs express many receptors, including the tyrosine kinase receptors, platelet-derived growth factor receptor (PDGF-R)α, PDGF-Rβ, and insulin-like growth factor receptor (IGF-R), regulated upon activation, normal T-cell expressed and secreted receptor (RANTES) as well as the macrophage-derived chemokine (MDC) receptors, C-C motif receptor (CCR)2, CCR3, and CCR4, and the stroma-derived factor (SDF)-1 receptor, C-X-C chemokine receptor (CXCR)4. BM MSCs migrate in response to many chemotactic factors such as PDGF-AB, IGF-1, RANTES, MDCs, and SDF-1. Baek et al.’s study (2011) using flow cytometry and reverse-transcriptase polymerase chain reaction revealed that ASCs express CCR1, CCR7, CXCR4, CXCR5, CXCR6, epidermal growthfactor receptor (EGFR), fibroblast growth factor receptor 1 (FGFR1), transforming growth factor beta (TGF-ß) receptor 2, tumor necrosis factor (TNF) receptor superfamily member 1A, PDGF-Rα, and PDGF-Rβ. The authors also reported that the migration of ASCs is controlled by various growth factors and chemokines. Molecules that mediate immunomodulatory effects of MSCs include prostaglandin E2 (PGE2), transforming growth factor-β (tumor growth factor-β, TGF-β), hemoxygenase-1 (HO-1), hepatocyte growth factor (HGF), 2, 3-dioxygenase (IDO), interleukin (IL)-10, human leukocyte antigen G (HLA-G), and leukemia inhibitory factor (LIF). These molecules produce anti-proliferative effects on T cells or natural killer (NK) cells, PGE2 modulates the secretory profile of dendritic cells and macrophages, and HO-1 and LIF induces the generation of regulatory T cells (Meirelles Lda et al. 2009). Further, MSCs have low immunogenicity because they lack MHC class II or co-stimulatory molecules such as B7, CD40, and CD40L, and as such are poorly recognized by T cells. MSCs are, therefore, an attractive alternative source for treatment of tissue injury and immune-mediated diseases. As described above, ASCs are considered as an ideal source of stem cells for regenerative medicine because they are abundant, easily available, and non-immunogenic with minimal ethical objections to their clinical use. Further, several recent studies revealed that ASCs are less affected by aging and multiple passaging events compared to BM MSCs, as measured by p21 gene expression, telomerase activity, and senescence-associated β-galactosidase activity (Chen et al. 2012). Therefore, ASCs represent a promising autologous cell source for cell-based therapy and cell-based gene therapy in various patients, even in elderly patients.
Experimental Animal Models of Autoimmune Diseases
Animal models for autoimmune diseases have provided an understanding of the mechanism underlying autoimmunity and a valuable insight into potential treatments (Kuby 1994). Animal models for autoimmune diseases are broadly divided into spontaneous, experimentally induced, and genetically engineered animal models (Taneja and David 2001). Two major multigenic spontaneous mouse models of human autoimmune diseases are F1 hybrids of New Zealand Black and New Zealand White mice (NZB/W F1) and non-obese diabetic (NOD) mice. NZB/W F1 mice develop a spontaneous autoimmune disease with striking similarities to human systemic lupus erythematosus (SLE). In female NZB/W F1 mice, anti-nuclear antibodies, including anti-dsDNA antibodies, are produced, and develop severe immune complex-mediated glomerulonephritis. All mice die from renal failure by 10–12 months of age (Wang et al. 1996). Genetic analysis of these NZB/W F1 mice have provided very important information on the genetic mechanisms involved in the development of SLE (Taneja and David 2001). NOD mice spontaneously develop diabetes that resembles human type I diabetes mellitus (Type I DM). Human type I DM and NOD mice share a similar pathology and immunological basis. They have islet-reactive CD4+ T and CD8+ T cells and the predisposing MHC class II molecule (HLA-DQ8 and I-Ag7) genes (Taneja and David 2001). Other spontaneous animal models include the obese-strain chicken that resembles Hashimoto’s thyroiditis.
Experimentally induced animal models can be induced in various species by immunization with potential known autoantigens in complete Freund’s adjuvant. Well-known autoantigens include myelin basic protein, proteolipid protein, and myelin oligodendrocyte glycoprotein, which are used to induce autoimmune encephalomyelitis that resembles human multiple sclerosis. Other common autoantigens include thyroglobulin, type II collagen, acetylcholine receptor, and interphotoreceptor retinoid-binding protein that are used to induce models of Hashimoto’s thyroiditis, human rheumatoid arthritis, human myasthenia gravis, and human uveitis, respectively (Kuby 1994). The advantage of experimentally induced animal models is the control over the onset and progression of disease.
Genetically engineered animal models include MHC-transgenic models, TCR-transgenic models, cytokine knockout models with/without cytokine receptor, and TCR knockout models. For example, the HLA-DQ8 rat insulin promoter (RIP).B7-1-transgenic mice are an excellent model for human type I DM. HLA-DQ8 RIP.B7-1- transgenic mice are produced by crossing HLA-DQ8 (type 1 diabetes-predisposing MHC class II molecule) transgenic mice with RIP.B7-1 transgenic mice, which express the co-stimulatory molecule B7-1 in the β cells of islets (Wen et al. 2001). Crossing KRN mice that express rearranged specific TCR genes with a NOD strain (H-2Ag7) induces spontaneous systemic arthritis that resembles rheumatoid arthritis (Matsumoto et al. 1999). IL-2, IL-2R α, IL-10, and TCR-α knockout models develop spontaneous inflammatory bowel disease, resembling human Crohn’s disease, and IL-10 knockout models develop chronic enterocolitis that resembles human inflammatory bowel disease (Kühn et al. 1993).
Therapeutic Application of Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Animal Models of Autoimmune Diseases
The efficacy of ASCs has been studied in experimentally induced animal models of autoimmune diseases, including autoimmune encephalomyelitis, type II collagen-induced autoimmune arthritis, inflammatory bowel disease, and autoimmune thyroiditis.
Multiple sclerosis is a multifocal inflammatory disease of the central nervous system that leads to a broad spectrum of clinical signs. The main mechanism of the development of disease is the release of sequestered self-antigen such as myelin basic protein in oligodendrocytes caused by trauma to tissues following an accident or bacterial or viral infection (Kuby 1994). This leads to a lethal demyelinating attack, scarring, progression to physical and cognitive disability such as paralysis and sensation, and visual and sphincter problems. Activated T cells in the cerebrospinal fluid infiltrate the brain tissue and spinal cord, producing an inflammatory response and destruction of the myelin (Kuby 1994). Constantin et al. (2009) studied the effect of syngeneic ASC transplantation on experimental autoimmune encephalomyelitis. Chronic experimental autoimmune encephalomyelitis was induced in female C57BL/6 mice at 6–8 weeks of age by subcutaneous immunization with 200 μg of myelin oligodendrocyte glycoprotein (MOG33-35 peptide) in incomplete Freund’s adjuvant containing 0.8 mg/ml Mycobacterium tuberculosis. Pertussis toxin (50 ng) was also injected at the day of immunization and after 48 h. Intravenous administration of murine (syngeneic) ASCs before disease onset significantly reduced the severity of autoimmune encephalomyelitis and decreased spinal cord inflammation and demyelination. Intravenous administration of murine (syngeneic) ASCs in chronic experimental autoimmune encephalomyelitis also significantly reduced axonal loss and demyelination and induced Th2 cytokine shift. ASC inhibited MOG-specific T-cell proliferation; suppressed the production of IFN-γ, GM-CSF, IL-17, IL-4, and IL-5; and induced IL-10 production in vitro. Ex vivo analysis showed that peripheral lymph node cells isolated from mice treated with ASCs displayed suppressed MOG-specific T-cell proliferation and decreased production of both proinflammatory and anti-inflammatory cytokines (Constantin et al. 2009). Constantin et al.’s study showed that the migration of ASCs to the lymph nodes was significantly higher when the ASCs were injected shortly after the induction of the autoimmune response. It was also suggested that chronic inflammation and expression of VCAM-1 on the brain endothelium in mice with encephalomyelitis can recruit ASCs expressing α4 integrin from the blood.
Rheumatoid arthritis is characterized by chronic joint inflammation, subsequent cartilage destruction, and bone erosion. Rheumatoid arthritis develops following the activation and expansion of autoreactive Th1 and Th17 cells that modulate the release of proinflammatory cytokines and chemokines, promoting the activation and infiltration of neutrophils and macrophages. González et al. (2009) studied the effects of human and murine ASCs in DBA/1 mice with collagen-induced arthritis. In this study, collagen-induced arthritis was induced in DBA/1 mice at 7–10 weeks of age by subcutaneous immunization with 200 μg of chicken type II collagen emulsified in Freund’s complete adjuvant containing 200 μg of Mycobacterium tuberculosis. This was followed by a subcutaneous booster injection with 100 μg of chicken type II collagen emulsified in Freund’s complete adjuvant. Administration of human ASCs into mice with collagen-induced arthritis decreased Th1-mediated autoreactive response, Th17 production, and inflammation. The authors also reported significantly decreased concentrations of TNF-α and IL-1ß in sera as well as of TNF-α, IL-6, IL-12, IFN-γ, IL-1ß, IL-17, and RANTES in joint protein extracts. Increased levels of IL-10 were found in the joint protein extracts, and significantly greater numbers of CD4 + CD25 + FoxP3+ regulatory T cells were found in both draining lymph nodes and synovium. Intraperitoneal administration of 106 human ASCs at the onset of disease completely prevented the progression of arthritis, but the administration of human ASCs into mice with severe collagen-induced arthritis minutely attenuated the clinical signs. Both syngeneic (ASC from DBA/1 mice) and allogeneic (ASC from C57BL/6 mice) ASCs had beneficial effects on collagen-induced arthritis (González et al. 2009).
Inflammatory bowel disease is a group of inflammatory conditions and chronic idiopathic tissue destructive diseases of the colon and distal small intestine that is caused by dysfunctional mucosal T cells and cellular inflammation. The major types of inflammatory bowel disease are Crohn’s disease and ulcerative colitis. Activated Th1 cells promote the activation and infiltration of neutrophils and macrophages in both Crohn’s disease and experimental colitis. Cytokines and free radicals produced by infiltrating cells and resident macrophages play a pivotal role in tissue inflammation and destruction (Fiocchi 1998). Gonzalez-Rey et al. (2009) studied the effects of human and murine ASCs in C57BL/6 mice with experimental colitis. Colitis was induced in 7-week-old C57BL/6 mice by administering dextran sulfate sodium (DSS) in drinking water ad libitum. Acute colitis was induced by administering 5% DSS in drinking water from day 0 to day 7, and chronic colitis was induced by administering 3% DSS in drinking water in a cyclic manner (2 cycles of 7 days with DSS, followed by 10-day period without DSS supplementation). In their study, intraperitoneal administration of ASC significantly attenuated clinical signs, induced histological improvement, and prevented weight loss, diarrhea and inflammation. Treatment of ASCs decreased Th1-derived inflammatory response and the level of inflammatory cytokines in the colon. ASC treatment also increased IL-10 production in the colon and mesenteric lymph nodes, and the number of CD4 + CD25 + FoxP3+ regulatory T cells in the mesenteric lymph nodes. Both syngeneic (ASCs from C57BL/6 mice) and allogeneic (ASCs from BALB/c mice) ASCs had beneficial effects on experimental colitis (Gonzalez-Rey et al. 2009).
A study on the effect of ASC transplantation in mice with experimental autoimmune thyroiditis is described in the next section “Genetically engineered ASCs for autoimmune disease.”
The efficacy of long-term serial human ASC transplantation was studied in NZB/W F1 mice (Fig. 2.1a), a spontaneous murine model of human SLE (Choi et al. 2012). Human ASCs (5 × 105) were intravenously administered every 2 weeks from the age of 6 weeks until 60 weeks [the total number of human ASCs injected to each surviving mouse of the human ASC-treated group at the end point was 1.4 × 107 (5 × 105 per injection, 28 times)]. Serial ASC transplantation before disease onset significantly improved survival rate (Fig. 2.1b), histopathology of kidneys (Fig. 2.1c), and immunologic abnormalities. ASC treatment also significantly decreased the concentrations of anti-dsDNA antibodies and blood urea nitrogen (BUN) as well as the incidence of proteinuria. Treatment of ASCs significantly increased the levels of GM-CSF, IL-4, and IL-10 in the serum and the proportion of CD4 + FoxP3+ cells in the spleen. Systemically infused ASCs labeled with red fluorescent tracker dye were mostly present in the spleen, with many also being evident in the kidney and liver (Fig. 2.1d). There was little evidence of fluorescent-labeled cells in the lung and heart (Fig. 2.1d).
Treatment of female F1 hybrids of New Zealand Black and New Zealand White (NZB/W F1) mice, a murine model of human systemic lupus erythematosus (SLE), with human adipose tissue-derived mesenchymal stem cells (ASCs). (a) Overview of experimental methods and serological, urological, and immunological results; (b) Survival in NZB/W F1 mice; (c) Hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) reagent, Masson’s trichrome staining, and immunofluorescence (using FITC-anti IgG and FITC-anti C3) of the kidneys obtained from the mice at the age of 40 weeks; (d) Confocal microscopy examination of CM-DiI-labeled ASCs (Choi et al. 2012)
Clinical Trials of Adipose Tissue-Derived Mesenchymal Stem Cells to Treat Autoimmune Diseases
Autologous ASC transplantations have been performed in patients with refractory autoimmune diseases (Ra et al. 2011). Patients were intravenously administered 1–5 × 108 ASCs around one to six times, with a multiple sclerosis patient receiving additional intrathecal injections (1 × 107 ASCs per injection, three times) and one of rheumatoid arthritis patients receiving additional intraarticular injections (1–1.5 × 108 ASCs per injection, two times). Immunological parameters measured from one patient with polymyositis, and three patients with rheumatoid arthritis showed decrease in inflammatory responses. Further, clinical signs were improved in all patients treated with autologous ASCs. A 19-year-old female patient with autoimmune inner-ear disease, who had severe progressive hearing loss, showed improved hearing, with the hearing in her right ear restored to normal. A 46-year-old woman with multiple sclerosis displayed improved clinical signs as measured by the “expanded disability status scale” (EDSS), which is a method of quantifying disability in multiple sclerosis. A 35-year-old woman with polymyositis, who was unable to walk slope, was able to climb stairs following this treatment. A 50-year-old woman with rheumatoid arthritis showed improved arthritis index, and two other female patients with rheumatoid arthritis, who were unable to stand or walk, were able to do both following ASC treatment; in these two cases, steroid treatment was also discontinued.
Genetically Engineered Adipose Tissue-Derived Mesenchymal Stem Cells for Autoimmune Diseases
Genetically engineered MSCs can further improve the therapeutic effects of MSCs by the overexpression or suppression of the target genes and by providing additional control over disease progression or relapse. MSCs are an ideal vehicle to deliver genes into the target tissues because of their unique biological and immunological properties such as homing activity and immunoprivileged status (Baksh et al. 2004). MSCs have been modified by various non-viral or viral transduction methods. When permanent expression of the therapeutic gene is required, integrating viruses such as lentivirus or retrovirus are preferred because of their capacity for long-term expression. On the other hand, when transient expression of the therapeutic gene is preferred, a non-viral gene delivery system or non-integrating vectors such as adenovirus are the method of choice (Park et al. 2003). Lentiviral systems are advantageous in the treatment of autoimmune diseases by using cell-based gene therapy. First, lentiviral systems can efficiently be applied to a broad range of cell types, including non-dividing, senescent, and terminally differentiated cells (Naldini 1998). Second, lentiviral system has a capacity for long-term expression as described above. Further, transduction with lentiviral vector did not adversely affect the morphology, viability, and differentiation potential of MSCs (McGinley et al. 2011).
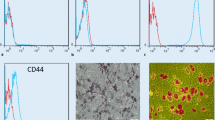
As adipose tissues are an alternative source of MSCs, treatment with genetically engineered ASCs that can modulate immune responses was also attempted in experimental animals with autoimmune disease. Choi et al. (2011) studied the effect of ASCs overexpressing CTLA4Ig on the development of experimental autoimmune thyroiditis by using a lentiviral system. Antigenic stimulation of T cells generally requires the presence of two signals provided by an antigen-presenting cell (APC) and cytokines (Gimmi et al. 1993). The first signal is mediated via the T cell receptor/CD3 complex and an antigenic peptide presented by a MHC molecule, and the second signal is mediated via B7:CD28 co-stimulation that induces the proliferation of T cells. Blockade of the B7:CD28 co-stimulatory interactions with soluble CTLA4Ig fusion protein has been shown to inhibit humoral immunity (Linsley et al. 1992), graft rejection (Lenschow et al. 1992), and graft-versus-host disease (Blazar et al. 1994), and to ameliorate autoimmune diseases (Finck et al. 1994; Choi et al. 2008). Transplantation of ASCs or CTLA4Ig gene-transduced ASCs reduced inflammatory immune response and improved Th1/Th2 balance in experimental autoimmune thyroiditis. Moreover, CTLA4Ig-ASC transplantation showed superior results in reducing the concentration of serum anti-thyroglobulin autoantibodies compared to treatment with ASCs only. Transduction with lentiviral vector in ASCs did not significantly affect the immunophenotype of ASCs, which showed long-term expression of therapeutic gene (Fig. 2.2), These findings suggest that target gene transduction using lentiviral vector system is well matched with ASCs and can elicit therapeutic potential without significantly changing the innate characteristic of ASCs (Choi et al. 2011).
Construction and confirmation of human adipose tissue-derived mesenchymal stem cells transduced with mouse CTLA4Ig. (a) Therapeutic gene was composed of the extracellular domain of mouse CTLA4 (V: NM_009 843, 258-629) to inhibit the B7:CD28 co-stimulatory signal and the hinge and CH2-CH3 domains of the human immunoglobulin gamma 1 constant region (H-CH2-CH3: J00228, 503, 892-936, 1055-1384, and 1481-1803) to prolong the half-life of the therapeutic protein in vivo. The human oncostatin M signal sequence (SP: NM_020 530, 53-127) for the secretion to body fluid was ligated to the therapeutic gene. Mouse CTLA4Ig gene transduction into MSC was conducted by ViraPowerTM Lentiviral Expression Systems (Invitrogen). The ViraPower packaging mix and Mouse CTLA4Ig-pLenti6/V5 TOPO expression plasmid were co-transfected into the 293FT cell line by using Lipofectamine 2000. Virus particles were collected and transfected to human adipose tissue-derived MSCs. Selection was performed using blasticidin (5 μg/ml). (b) To confirm whether CTLA4Ig-transduced ASCs expressed CTLA4 and secreted CTLA4 effectively into the extracellular space, cell culture supernatants were collected to determine the concentration of mouse CTLA4 by ELISA (Choi et al. 2011)
Insertion of inducible regulatory genes within ASCs by non-toxic and effective new gene transfer systems may hold potential as a novel method for immune modulation in autoimmune diseases. By determining the appropriate gene targets for the treatment of autoimmune diseases, stem cell-based gene therapy will be able to facilitate the synergic effect of gene therapy and stem cell therapy. Before application in clinical settings, the genetically engineered cells will have to be extensively tested at the genetic, molecular, and cellular levels.
References
Baek SJ, Kang SK, Ra JC (2011) In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med 43:596–603
Baksh D, Song L, Tuan RS (2004) Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med 8:301–316
Blazar BR, Taylor PA, Linsley PS, Vallera DA (1994) In vivo blockade of CD28/CTLA4:B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood 83:3815–3825
Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, Wang GJ, Kang L, Chang JK (2012) Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med 16:582–593
Choi EW (2009) Adult Stem Cell Therapy for Autoimmune Disease. Int J Stem Cells 2:122–128
Choi EW (2012) New Therapeutic Challenges in Autoimmune Diseases. In: Chan J (ed) Autoimmune Diseases: Contributing factors, specific cases of autoimmune diseases, and stem cell and other therapies. INTECH, Rijeka, pp 253–280
Choi EW, Shin IS, Lee CW, Youn HY (2008) The effect of gene therapy using CTLA4Ig/silica-nanoparticles on canine experimental autoimmune thyroiditis. J Gene Med 10:795–804
Choi EW, Shin IS, Lee HW, Park SY, Park JH, Nam MH, Kim JS, Woo SK, Yoon EJ, Kang SK, Ra JC, Youn HY, Hong SH (2011) Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. J Gene Med 13:3–16
Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC, Hong SH (2012) Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum 64:243–253
Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galiè M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B (2009) Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 27:2624–2635
Finck BK, Linsley PS, Wofsy D (1994) Treatment of murine lupus with CTLA4Ig. Science 265:1225–1227
Fiocchi C (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182–205
Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM (1993) Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci U S A 90:6586–6590
González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M (2009) Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 60:1006–1019
Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M (2009) Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58:929–939
Jantunen E, Myllykangas-Luosujärvi R (2000) Stem cell transplantation for treatment of severe autoimmune diseases: current status and future perspectives. Bone Marrow Transplant 25:351–356
Kuby J (1994) Autoimmunity. In: Kuby J (ed) Immunology, 2nd edn. W. H. Freeman and Company, New York, pp 445–467
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274
Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA (1992) Longterm survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science 257:789–792
Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA (1992) Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257:792–795
Matsumoto I, Staub A, Benoist C, Mathis D (1999) Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 286:1732–1735
McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, O’Brien T (2011) Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther 2:12
Meirelles Lda S, Fontes AM, Covas DT, Caplan AI (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20:419–427
Naldini L (1998) Lentiviruses as gene transfer agents for delivery to non-dividing cells. Curr Opin Biotechnol 9:457–463
Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, Neukam FW, Schneider H (2003) Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther 10:1089–1098
Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25:1737–1745
Ra JC, Kang SK, Shin IS, Park HG, Joo SA, Kim JG, Kang BC, Lee YS, Nakama K, Piao M, Sohl B, Kurtz A (2011) Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J Transl Med 9:181
Siatskas C, Chan J, Field J, Murphy K, Nasa Z, Toh BH, Alderuccio F (2006) Gene therapy strategies towards immune tolerance to treat the autoimmune diseases. Curr Gene Ther 6:45–58
Taneja V, David CS (2001) Lessons from animal models for human autoimmune diseases. Nat Immunol 2:781–784
Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA (1996) Amelioration of lupus-like autoimmune disease in NZB/WF1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci U S A 93:8563–8568
Wang J, Liao L, Tan J (2011) Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther 11:893–909
Wen L, Chen NY, Tang J, Sherwin R, Wong FS (2001) The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest 107:871–880
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Choi, E.W. (2013). Experimental (Preclinical) Studies and Clinical Trials of Adipose Tissue-Derived Mesenchymal Stem Cells for Autoimmune Diseases. In: Hayat, M. (eds) Stem Cells and Cancer Stem Cells, Volume 10. Stem Cells and Cancer Stem Cells, vol 10. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6262-6_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-6262-6_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6261-9
Online ISBN: 978-94-007-6262-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)