Abstract
Cell fusion occurs when cells unit their membranes and share their cytoplasm. Cell fusion may occur between genetically identical or different cells. Gamete fusion is a short event of the organism life cycle but it is essential for all organisms that depend on sexual reproduction for the maintenance of the species. The fusion of gametes having inherited one half of the genetic material of their respective parents ensures the diversity of individuals within a population. This diversity is increased by the exchange of genetic material between homologous chromosomes during meiosis. Therefore gamete fusion is a critical biological step in the life cycle that has recently raised more interest due to the possibility of treating patients with a fertility defect by in vitro methods. Molecular mechanisms underlying the fusion process are far from evident since (1) gene-knock out technologies developed to test in vivo the data from in vitro fertilization (IVF) studies failed to confirm the essential role of most of previously candidate molecules and (2) the surface proteins involved are deprived of fusogen properties. Indeed, among the large number of in vivo tested proteins, only CD9 and CD81 tetraspanins on egg and Izumo on sperm have been shown to be essential in mammalian sperm-egg membrane fusion and none of these molecules contain a fusion peptide.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Fertilization and Gamete Membrane Interaction
Fertilization is defined as the process by which the sperm and the egg fuse to form a new individual, the zygote. Both gametes must undergo cellular and nuclear maturation before making contact and fusing. During meiotic maturation, the genome of diploid germ cells undergoes two rounds of division (first and second meiosis) resulting in haploid gametes which carry half of the genetic content of the original cell. The meiosis is a continuous process during spermiogenesis which begins at the time of puberty, whereas nuclear maturation of the oocyte starts during fetal life and undergoes two arrest points. At birth, the female germ cells are called primary oocytes and get arrested at diplotene stage of meiotic prophase I. This meiotic arrest lasts till puberty that marks the beginning of the menstrual cycles during which and following a Luteinizing Hormone (LH) peak, one primary oocyte (or several, depending on the species) grows and completes the first meiosis with extrusion of the first polar body. This is immediately followed by the initiation of the second meiosis arrested at the metaphase II stage. The mature secondary oocyte is ovulated. The second meiosis division will end at the time of fertilization triggered by the sperm penetration.
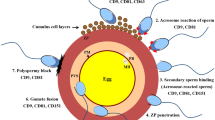
In mammals, male and female gametes are two physically separated cells. Their meeting takes place in the female oviduct. Depending on the species, semen is deposited in the vagina or directly in the uterus during coitus and only a tiny fraction of spermatozoa migrates successfully to the fertilization site. Ejaculated spermatozoa are capable of moving actively, yet they do not have the ability for fertilization. They acquire the competence to fuse with eggs through a maturation process, called capacitation, during the ascent of the female genital tract (Austin 1951; Ikawa et al. 2010). The capacitation is a biochemical event resulting in the destabilization of the sperm head plasma and acrosomal membranes rendering them more fusogenic. Mouse spermatozoa collected from epididymis for experimental purpose are capacitated by in vitro incubation in culture medium previous to their use for fertilization. Capacitating sperm that reach the oviductal ampulla readily penetrate the cumulus layer surrounding the ovulated mature oocyte (Fig. 9.1a.1). They bind, then, in a species-specific manner to the zona pellucida (ZP), the last barrier the spermatozoa must pass before fertilizing the egg (Fig. 9.1a.2). The ZP is an extracellular coat protecting the egg and the future embryo from physical damages. In mice, the most widely studied mammalian model, the ZP comprises three sulphate glycoproteins: ZP1 (181–200 kDa), ZP2 (120–140 kDa) and ZP3 (83 kDa) (Wassarman 1988) synthesized and secreted by the oocyte. In the human, a fourth glycoprotein has been identified, ZP4 (or ZPB), which is thought to be dysfunctional in the mouse (Lefievre et al. 2004). Ultrastructural evidence suggests that the mouse zona pellucida is composed of filaments constructed by head-to-tail association of globular proteins. ZP2–ZP3 heterodimers are the basic repeating units of the filament, with cross-linking of filaments mediated by dimeric ZP1 (Wassarman and Mortillo 1991). ZP3 is the primary ligand that binds to the plasma membrane over the acrosomal cap of acrosome intact sperm. ZP3 functions also as an acrosome-reaction inducer. The acrosome reaction consists in the fusion between sperm plasma membrane and outer acrosomal membrane leading to acrosomal content exocytosis (Fig. 9.1a.2). ZP2, in turn, has been postulated to serve as a secondary ligand of the inner acrosomal membrane of sperm, ensuring close contact between the ZP and the penetrating spermatozoon (Wassarman 2002). The fertilizing acrosome-reacted sperm reaches quickly the perivitelline space. The sperm head then binds and fuses with the egg plasma membrane (oolemma) (Fig. 9.1a.3). Early insights into gamete plasma membrane interaction came from scanning and transmission electron microscopy performed by R. Yanagimachi (for review (Yanagimachi 1994)). It appears that sperm interactions with the oolemma occur in a spatially restricted manner. Indeed, the egg membrane is covered with microvilli except in the region overlying the meiotic spindle and sperm egg fusion rarely occurs in this microvilli-free (amicrovillar) region (Runge et al. 2007). Furthermore, the fertilizing sperm makes the first contact with the oolemma via its inner acrosomal membrane, but binds and fuses with the egg microvilli via the plasma membrane of its equatorial region (Fig. 9.1b) (Yanagimachi 1994). If some acrosome-intact sperm are able to bind experimentally to oolemma of ZP-free eggs, they never fuse. The acrosome reaction is required to complete the fusion process. Following gamete fusion, the sperm tail movements decrease and stop within a few seconds. Electron microscopy analysis shows that the inner acrosomal membrane doesn’t fuse with the oolemma but is later engulfed by the oocyte (Fig. 9.1a.4). The sperm tail is also eventually incorporated in the egg cytoplasm. Gamete membrane fusion marks the end of the fertilization step and the beginning of the egg activation. The events associated with the egg activation include the initiation of oscillations in intracellular calcium concentration, the completion of the second meiosis with extrusion of a second polar body and the formation of a block to polyspermy via the release of ZP proteolysis enzyme from the egg’s cortical granules preventing further ZP crossing by other sperm.
(a) Schematic representation of the gamete fertilization. (1) Sperm penetration of the cumulus layer and binding to the zona pellucida (ZP). (2) Sperm acrosomal reaction during which the acrosome content is released. (3) Sperm passing through the ZP and entering the perivitelline space where it adheres to the oocyte and starts the fusion process. (4) Oocyte activation induced by sperm fusion and penetration leading to resumption of the second meiosis, expulsion of the second polar body and sperm head decondensation. (b) Schematic representation of the acrosome reaction. (1) Acrosome intact sperm. (2) Sperm head after complete acrosome reaction. Ac acrosome, PM plasma membrane, OAM outer acrosomal membrane, IAM inner acrosomal membrane, N nucleus, ER equatorial region
9.2 Sperm-Egg Fusion Candidate Molecules
9.2.1 Mouse Model
9.2.1.1 Essential Molecules
9.2.1.1.1 Tetraspanins
The first data suggesting the involvement of CD9 tetraspanin in fusogenic properties of the oolemma were provided by experiments performed in vitro using an anti-CD9 monoclonal antibody, JF9 (Chen et al. 1999). This work revealed (1) the presence of CD9 homogeneously distributed at the egg surface, with exception of the microvillar-free region and (2) the inhibition by JF9 mAb of sperm-egg binding and fusion, in a dose dependent manner. The essential role of CD9 was then firmly confirmed by three different teams who generated mutant mice in which the CD9 gene was disrupted (Le Naour et al. 2000; Miyado et al. 2000; Kaji et al. 2000). Homozygous adults CD9−/− were healthy, however, whereas CD9−/− male mice were normally fertile, CD9−/− female mice presented a severely reduced fertility. Only 50–60% of CD9−/− female mice produced litters after mating with fertile male mice with an extension of the delay to begin a successful pregnancy and a reduction of the litter size leading to 95% global reduction of fertility. CD9-deleted mice produced mature oocytes quantitatively and morphologically comparable to the wild type female, spontaneously as well as after hormones-induced superovulation (Fig. 9.2a–d). In vivo analysis revealed a normal mating behaviour of the CD9-deleted mice and the presence of numerous sperm in their oviducts. However, the mature eggs recovered in CD9−/− genital female tract 0.5 days after mating were not fertilized presenting several sperm into the perivitelline space and showing that the fusion didn’t occur resulting in persistence of ZP permeability to sperm (Fig. 9.2e, f). The fertilization tests performed in vitro confirmed the inability of the egg membrane to fuse with sperm (Miyado et al. 2000). For each team, the fertilization rate of zona free oocyte collected from CD9−/− female mice were equivalent, ranging from 0% to 4% versus 96% to 98% for wild type oocyte (Le Naour et al. 2000; Miyado et al. 2000; Kaji et al. 2000) despite a normal sperm binding to CD9-defective membrane. The development of rare but healthy pups suggested that CD9 would be strictly involved in fusion process. This was confirmed by intra-cytoplasmic injection of wild type sperm in CD9-deleted eggs which showed normal embryo development and uterus implantation with birth of healthy offspring (Miyado et al. 2000). Moreover, the fertilization defect of CD9-deficient eggs was reversed by exogenous mouse CD9 expressed by mRNA intra-cytoplasmic injection (Kaji et al. 2002). Interestingly, overexpression of human CD9 restored the fertilization rate of CD9−/− mouse oocytes to 90% indicating the absence of species specificity of the CD9 molecule, at least between mouse and human. Another tetraspanin, CD81 that shares more than 40% amino acid identities with CD9, is also expressed at the mouse egg surface. The pattern of labelling is different from CD9 since it partially colocalizes at cell surface where its level of expression is much lower and mAbs to CD81 also display a punctuated labelling of zona pellucida that is not observed in oocytes form CD81−/− mice (Fig. 9.3). CD81-deficient female mice were generated (Maecker et al. 1998) and have been shown to present an overall reduction of fertility of 40% (Rubinstein et al. 2006a). Indeed, 60% of CD81-mutant female produced litters after mating with a normal delay and a normal litter size, but an increased rate of postnatal mortality. This high level of dead animals in the first hours (Kelic et al. 2001; Rubinstein et al. 2006a) is still unexplained but could be due to a defect of maternal care. Surprisingly, only 10% of mature eggs recovered 0.5 day after CD81−/− female mouse mating presented a meiosis resumption. and non-fertilized oocytes had several sperm motile into the perivitelline space, indicating a defect of membrane fusion. There is no obvious explanation for the discrepancy between the residual fertility of CD81-deficient mouse (60%) and the low percentage of successful egg fertilization (10%) observed in vivo. One hypothesis could be an increase of the delay necessary for fusion after the crossing of ZP in the absence of CD81 so that the fusion had not occurred at 0.5 day but occurred later. The important role of CD81 in fertilization process appeared when both CD9 and CD81 gene were disrupted (Rubinstein et al. 2006a). Indeed, CD9−/− CD81−/− female mice were totally sterile (0% pregnancy after 2 months of mating). None of the CD9/CD81-deleted mature oocytes observed in vivo or in vitro after sperm contact was fertilized. This shows that CD9 and CD81 may play a complementary role in the fusion process. Interestingly, the increased defect of gamete fusion in double knockout mice and the ability of CD81 to partially compensate the CD9 function in CD9−/− mouse oocytes (50% fertilization upon overexpression of CD81) (Kaji et al. 2002) suggest a partially redundant role for these two tetraspanins. It has to be noticed that although CD151 may play a role in human gamete fusion (Ziyyat et al. 2006), CD151−/− mutant mice are normally fertile (Wright et al. 2004).
Oocyte DNA-tubulin labeling (Propidium iodide and mAb YL1/2 followed by FITC Goat antibody to mouse Ig on fixed oocytes). Oocytes from nonmated superovulated CD9−/− (a and b) or CD9+/+ (c and d) females are identically blocked in metaphase of meiosis II as confirmed Fig. 9.2 (continued) by the presence of a metaphase spindle (*) and a first polar body containing residual DNA-tubulin material (solid arrowheads). (e and f) An oocyte from a CD9−/− naturally ovulated mated female blocked in metaphase of meiosis II with sperm in the perivitelline space (open arrowheads). (g and h) An oocyte from a CD91/1 naturally ovulated mated female that has progressed to telophase and has extruded the second polar body (arrow). Inset shows partially decondensed sperm DNA in a different focal plane. Scale bar, 10 μm (Reproduced from Le Naour et al. 2000)
CD9 was not detected on sperm in early studies (Chen et al. 1999), however a recent work reported its presence at the sperm surface following acrosomal reaction (Ito et al. 2010). Nothing is known about its role in gamete membrane interaction; however, sperm CD9 is definitely not essential as CD9 null male mice are fully fertile (Le Naour et al. 2000).
9.2.1.1.2 GPI-Anchored Proteins
GPI-anchored proteins possess a covalently linked glycosylated phosphatidylinositol moiety which serves to attach the protein portion of the molecule to the cell surface lipid bilayer (Low and Saltiel 1988). They are thought to be preferentially located in the lipid rafts and known to be involved in a wide variety of cellular functions including T cell activation, hydrolysis of extracellular matrix proteins, transduction of extracellular stimuli, and cell–cell adhesion. GPI-anchored proteins can be released from the outer cell membrane by treatment with the highly specific enzyme phosphatidyinositol specific phospholipase C (PI-PLC) (Low and Finean 1978). Therefore, treatment of intact cells with PI-PLC has become a useful tool to characterize the released proteins and to investigate the role of GPI-anchored proteins in cell function. This strategy has been used to investigate the involvement of GPI-anchored proteins in fertilization process (Coonrod et al. 1999). Co-culture of mouse gametes in presence of PI-PLC led to a dramatic reduction of the fertilization rate (from 59.6% to 2.8%) despite a normal sperm zona binding. Interestingly, unfertilized mature eggs presented numerous motile sperm accumulated in the perivitelline space, suggesting a specific egg membrane fertilization defect. Zona free in vitro fertilization tests associated with alternative gamete pre incubation showed that PI-PLC pretreated sperm kept intact binding and fusion properties whereas pretreated eggs completely lost the ability to bind and fuse with sperm in a dose dependent manner (Coonrod et al. 1999). Electrophoresis analysis revealed that PI-PLC egg treatment induced the release of proteins with 70 kDA and 35–45 kDA apparent MW. Altogether, these observations support the hypothesis that one or more GPI-anchored egg surface proteins would be essential for sperm-egg binding and fusion. This was confirmed by the defect of gamete fusion in Pig-a deleted oocytes (Alfieri et al. 2003). The phosphatidylinositol glycan class-A (Pig-a) gene encodes a subunit of N-acetylglucosaminyltransferase that controls early steps of GPI anchor biosynthesis (Tiede et al. 2000). Since the deletion is embryonic lethal (Kawagoe et al. 1996), a conditional knockout with Cre/loxP recombinaison driven by the oocyte specific ZP3 promoter was used to obtain viable female mice that are infertile. None of the mature eggs recovered in vivo was fertilized despite the presence of perivitelline sperm. Zona-free in vitro fertilization tests confirmed the oolemma defect of fusion with a fertilization rate (percentage of fertilized eggs) eightfold lower and a fertilization index (number of fused sperm per fertilized egg) ninefold lower in GPI-AP −/− oocytes compared to the wild type. The 70 kDa component released from the egg surface after PI-PLC treatment could be the decay accelerating factor (DAF), also called CD55, since polyclonal goat anti-CD55 antibodies bind to wild type zona free egg surface. However, knockout of CD55 gene has no effect on female mice fertility (Sun et al. 1999), as well as the targeted deletion of CD59 gene (Holt et al. 2001), another GPI-anchored protein identified at the egg surface (Taylor and Johnson 1996).
9.2.1.1.3 Izumo
The protein Izumo 1 is the most recently sperm surface protein identified as an essential actor in sperm-egg binding and fusion. Izumo 1, named after a fertility shrine dedicated to marriage in the Shimane prefecture of Japan, is an Ig superfamily type 1 membrane protein expressed at the equatorial segment of acrosome reacted sperm (Fig. 9.4). Female mice with deleted Izumo 1 gene have normal fertility whereas male are completely sterile despite a normal mating behaviour and a normal sperm production (Inoue et al. 2005). Unfertilized eggs recovered in vivo after mating between wild type female and Izumo 1 −/− male showed sperm that had crossed the ZP but were accumulated in the perivitelline space unable to fuse with the oolemma. These results have confirmed initial data that have shown the inhibitory effect of OBF13, a monoclonal antibody raised against Izumo 1, on sperm-egg fusion (Okabe et al. 1988). Izumo 1 is exclusively involved in fusion since intracytoplasmic injection of Izumo 1 −/− sperm in wild type oocytes leads to normal egg activation and full development after the transfer in uterus of pseudo-pregnant female mice. Izumo 1 belongs to a protein family all having a so-called Izumo domain which form large complexes on sperm surface (Ellerman et al. 2009). The role of Izumo 1 in the fertilization process is still unknown even if these latter results raise the possibility that Izumo 1 could organize at the sperm surface protein complexes involved in membrane fusion machinery. Up to now, there are no reports describing a biochemical link between Izumo and egg tetraspanins.
Izumo1 distribution at sperm head surface. After 1 h of in vitro capacitation, live sperm have been exposed to a rabbit polyclonal antibody anti-mouse Izumo revealed by a FITC anti-rabbit antibody and analysed by epifluorescence. Izumo 1 is an acrosomal membrane protein which is exposed during the acrosome reaction and relocates from the acrosomal cap (b) to the equatorial region (d). (a and c): Dapi sperm DNA staining. The different regions of the sperm head are represented in d’ acrosomal cap (1); equatorial region (2); post-acrosomal cap (3). Scale bar, 2 μm
9.2.1.2 Non Essential Molecules
9.2.1.2.1 The Controversial ADAM2/α6β1 Couple
Before the discovery of Izumo protein, the best candidate egg ligand on sperm was an ADAM (A Disintegrin and Metalloprotease) family protein. ADAM2 was the first specific sperm ADAM identified in guinea pig, expressed at the equatorial region of the sperm head (Primakoff et al. 1987). ADAM2 is also present in other mammalian species such as mouse, macaque and human. In the mouse, antibodies and peptide of the disintegrin domain of ADAM2 (fertilin β) and ADAM3 (cyritestin), another testis specific ADAM, have been reported to inhibit sperm oocyte binding and fusion (Primakoff et al. 1987; Evans et al. 1998; Zhu et al. 2000; Yuan et al. 1997). Male mice with deletion of ADAM2 and ADAM3 genes are infertile (Cho et al. 1998; Shamsadin et al. 1999). Even though these experimental data explained the sterility by an impaired migration of sperm into female oviduct and a defect in zona pellucida binding of mutant sperm, they didn’t prove that ADAM2 and ADAM3 were also involved in membrane interaction. Since ADAM2 and ADAM3 proteins contain a tripetide sequence that mimics the Arg-Gly-Asp (RGD) and that controls sperm-oocyte membrane interactions (Blobel et al. 1992; Myles et al. 1994; Evans et al. 1995), this suggests that oolemma integrins are involved. Indeed, several integrins have been identified on the oolemma of mammalian eggs and in particular in mouse (Sengoku et al. 2004). The integrin α6β1 has been the primary candidate proposed to bind to sperm ADAM2 since (1) mouse sperm adhere to somatic cells when they express α6β1 and this binding is blocked by anti-mouse ADAM2 antibody (Almeida et al. 1995; Bigler et al. 2000), (2) soluble form of ADAM2 disintegrin domain binds to the egg microvillar surface in a distribution comparable to α6β1 (Evans et al. 1997; Bigler et al. 2000) and this adhesion is inhibited by the function blocking mAb for α6 subunit, GoH3 (Bigler et al. 2000). However, gene deletion experiments failed to confirm that α6β1 play a key role in sperm-egg membrane interaction. Indeed, α6 integrin null oocytes were normally fertilized in vitro by wild type sperm (Miller et al. 2000). Moreover, oocytes lacking all β1 integrins were competent to fuse with sperm in vivo as well as in vitro (He et al. 2003). The discrepancies between gene deletion and peptide/antibodies experiments found a possible explanation in a work which suggests that sperm integrins may also be involved (Barraud-Lange et al. 2007b). Acrosome reacted mouse sperm express α6β1 integrin in equatorial and post-acrosomal regions and treatment of sperm with α6 or β1 function blocking antibodies prior to egg insemination decreased cumulus intact fertilization rate by 50%. When antibodies were present in the insemination medium, thus blocking both sperm and egg integrins, the fertilization rate felt to 10%. Therefore, optimal conditions for fusion may require the presence of α6β1 on the two gamete membranes but the presence of α6β1 on only one of the gametes may be sufficient for fusion to occur. An example of such compensation is provided by β1 defective myoblasts where fusion is rescued by coculture with wild type myoblasts (Schwander et al. 2003). The analysis of fertilization performance of sperm and egg that are both inactivated for α6 or β1 is still lacking to validate this hypothesis.
The association of integrins and tetraspanins within the tetraspanin web (see below) suggesting that it could act as a mediator of the tetraspanins CD9 and CD81 effects (Berditchevski 2001; Boucheix and Rubinstein 2001; Charrin et al. 2009; Hemler 2005) reinforces the possibility of the involvement of α6β1 in sperm-egg membrane interaction.
9.2.1.2.2 Other Molecules
The Epididymal Cysteine-RIch secretory Proteins (CRISP) from seminal plasma adhere to sperm surface and one of them CRISP1 has been implicated in sperm-oolemma interaction (Cohen et al. 2007). Crips1 null mice have reduced sperm-oolemma fusion in vitro (Da Ros et al. 2008). CRISP2 is a sperm protein that seems to compete with CRISP1 for oolemma binding (Busso et al. 2007). Their ligands on oolemma are unknown.
Most of the other gamete surface proteins involved in gamete interaction act at the level of sperm-ZP interaction and don’t seem to participate in the fusion process (for review (Ikawa et al. 2010)).
9.2.2 Available Data in Human
Since human oocyte is an extremely rare material and the laws that authorize the human research programmes are very restrictive, there are very few experimental data available on fertilization process in human. However, based on what has been shown in other species, some molecules have been identified at the surface of human gametes and involved in in vitro sperm-egg membrane interaction.
9.2.2.1 Integrins and Their Ligands
Several integrin subunits have been identified in the human oolemma by immunostaining, particularly, α5β1, αvβ3 and α6β1 (Fusi et al. 1993; Campbell et al. 1995; Ji et al. 1998; Sengoku et al. 2004; Ziyyat et al. 2006). The involvement of the RGD-binding subfamily of integrins has been suggested by the inhibition of interaction of human sperm with zona-free hamster or human oocyte induced by RGD peptide (Bronson and Fusi 1990; Ji et al. 1998; Ziyyat et al. 2005). Furthermore, fibronectin and vitronectin, ligands of α5β1 and αvβ3 integrins respectively, via the RGD sequence, have been shown to be expressed by human sperm. If fibronectin is present at the head surface of capacitated human sperm, vitronectin is contained in the acrosomal vacuole, released in the extracellular medium following acrosome reaction and relocated to the equatorial segment (Fusi and Bronson 1992; Fusi et al. 1994). These data strengthened the hypothesis of a potential involvement of RGD-binding integrins in the recognition of human sperm and egg membranes. Interestingly, α5β1 and αvβ3 are also expressed at the human sperm surface, most of the time depending on sperm status (Fusi et al. 1996b). The presence of vitronectin receptor on both gametes leads to the hypothesis that vitronectin, released by human sperm during acrosome reaction, could participate in sperm/egg adhesion (Fusi et al. 1996a). The pair ADAM/α6β1 integrin was also proposed as a potential actor in human sperm/egg binding and fusion. On the one hand, a FEE containing peptide, mimicking the adhesion site of the disintegrin domain of human ADAM2, has been shown to inhibit or increase (depending on whether a linear or cyclic form was used) the fusion with human sperm to human zona-free eggs (Bronson et al. 1999; Ziyyat et al. 2005). On the other hand, the mAb for α6 subunit, GoH3, was shown to strongly inhibit human sperm-egg fusion in a dose dependent manner up to 96%, suggesting the involvement of α6β1 in the control of human gamete fusion (Ziyyat et al. 2006). Finally, α6β1 has been also described at the human sperm surface and proposed to be a potential clinical marker to evaluate sperm fertilizing ability in men (Reddy et al. 2003). Regardless of these experimental data, limited information is available concerning the involvement of integrins in human physiological fertilization conditions. Indeed, owing to bioethical laws, removal of the ZP is necessary to perform in vitro fertilization assays in human. This leads to the modification of integrin membrane distribution and to the binding and fusion of the oolemma with numerous sperm. This model of in vitro human gamete interaction could mask or bypass molecules necessary for membrane interaction (Ji et al. 1998; Sengoku et al. 2004).
9.2.2.2 Tetraspanins and Izumo
CD9, CD81 and CD151 tetraspanins have been identified on human egg surface (Ziyyat et al. 2006). These molecules are evenly distributed on zona intact oocyte. Surprisingly, on zona-free eggs, CD81 and CD151 form patches and co-localize while CD9 remains unchanged. CD151 and CD9 have been involved in human gamete membrane fusion since sperm and egg co-culture in presence of anti-CD151 and anti-CD9 monoclonal antibodies leads to a reduction of 50% and 78% of fusion, respectively (Ziyyat et al. 2006). No effect on sperm egg fusion was observed with the anti-human CD81 monoclonal antibodies (Ziyyat et al. 2006).
On the human sperm side, Izumo protein has been detected on sperm head surface after acrosome reaction as well as mouse sperm (Inoue et al. 2005). Using the hamster test, an heterologous system of human sperm insemination with zona free hamster egg, no fusion was observed in presence of the anti-human Izumo antibody, suggesting the involvement of Izumo in human sperm fertilization ability (Inoue et al. 2005).
Both molecules, egg CD9 tetraspanin and sperm Izumo protein, are expressed by human gametes and their function is supported by in vitro fertilization assays, suggesting that they take part in human fertilization mechanism. However, despite these experimental observations, no case of women and men infertility could be attributed to a genetic defect of CD9 and Izumo. The complete sequencing of the coding region of the CD9 gene in 87 women with unexplained infertility (Nishiyama et al. 2010) as well as the analysis of 9 exons encoding for the Izumo protein in 36 infertile men (Granados-Gonzalez et al. 2008) did not show any mutation. Nevertheless, the absence of mutations in the coding sequence does not exclude a defect in the regulation of the gene expression and when possible the levels of protein expression on sperm (Hayasaka et al. 2007) and oocytes should be monitored.
9.2.3 Other Species
The expression of CD9 on gametes of other mammalian species is compatible with its involvement in fertilization as confirmed by antibody (Li et al. 2004; Zhou et al. 2009) or soluble CD9 large extracellular loop inhibition of fertilization (Tang et al. 2008). Despite the fact that the C. elegans genome encodes at least 20 tetraspanins, none of them appear to be involved in gamete fusion (for review (Marcello and Singson 2010)). The explanation may rely on the observation that a phylogenetic comparison of mammalian tetraspanins with the multiple superfamily members found in C. elegans and drosophila showed that they are highly divergent and that no reliable orthologs of mammalian tetraspanins can be found in nematodes or insects with the exception of Tspan5/NET4 (Todres et al. 2000). Other proteins were shown to be involved in gamete fusion of non mammalian species. As shown in Table 9.1, they have variable structures, membrane topology and contain different protein motifs. In C. elegans, four sperm proteins and two oocyte proteins appear to be required for cell fusion. The two egg proteins are related and belong to the LDLR family of proteins (for reviews (Marcello and Singson 2010; Oren-Suissa and Podbilewicz 2010)). Also, an important ancestral gamete fusion apparatus is represented by the GCS1/HAP2 gene that has disappeared from the genome of recently diverged animals. The gene product of GCS1/HAP2, a type I membrane protein expressed on male gametocyte, is necessary for gamete fusion of plants like Arabidopsis thaliana (von Besser et al. 2006) and of two types of protists (Liu et al. 2008; Hirai et al. 2008; Liu et al. 2008).
The diversity of molecules at stake indicates that gamete fusion has evolved entirely different mechanisms during evolution to fulfill the aim of mixing haploid genomes in the process of reproduction.
9.3 Tetraspanins Function in Fertilization: Current Hypotheses
From a mechanistic point of view, cell fusion processes fall in two categories: the one for which an identified fusogenic protein is involved and the others for which proteins required for fusion, may (or may not) have been identified but underlying fusion mechanisms are completely unknown. Gamete fusion falls in the second category. In spite of the discovery of numerous molecules involved in gamete fusion and their structural diversity, the absence of fusogenic peptide renders elusive the way they permit fusion to occur. Despite the involvement of other tetraspanins in fertilization, CD9 prominent effects have attracted most of the studies and various mechanisms have been described and explored as contributing to the fusion process.
9.3.1 CD9 as a Sperm Receptor
In mouse macrophages, CD9 has been shown to be a receptor of PSG17 (Waterhouse et al. 2002). Pregnancy specific glycoproteins (PSGs) form a family of 14 proteins specifically secreted in the serum of pregnant female by the placenta. These secreted proteins belong to the Ig superfamily and to the carcinoembryonic antigen (CEA) subfamily. The high level of PSGs measured in the serum until term is necessary for successful pregnancy probably in modulating the maternal immune system (Ha et al. 2005). Considering the key role CD9 has in fertilization, the presence of a PSG17-related ligand on sperm has been investigated (Ellerman et al. 2003). Interestingly, convincing in vitro assays revealed that PSG17 bound specifically to the large extracellular loop (EC2) of CD9 and that the sequence SFQ, required for the functionality of CD9 in fertilization process (see below), was necessary for the recognition between PSG17 and CD9. Furthermore, the recombinant form of PSG17 adhered to the microvillar region of wild type eggs while the soluble protein did not bind to CD9 deleted eggs. Finally, PSG17 fixed at the egg surface inhibited sperm-egg fusion affecting fertilization rate and fertilization index. In light of these experimental data, the most attractive hypothesis is that CD9 may bind in trans to a PSG17-related protein on the sperm surface which still remains to be identified. A CEA protein called sperad/AH-20 has been identified on hamster sperm and has been implicated in sperm-egg fusion (Primakoff and Myles 1983; Allen and Green 1995; Ilayperuma 2002) but it remains to be shown if this protein is related to PSG17 (Ellerman et al. 2003).
In spite of the in vitro binding data, the preincubation of sperm with a recombinant protein corresponding to the EC2 domain of CD9 did not inhibit gamete fusion (Zhu et al. 2002). This experiment argues against a receptor function of CD9 but it is possible that a motif that would be critical for interaction of the EC2 domain with sperm is not presented in an adequate conformation.
Interestingly, CD81 EC2 has a phenylalanine residue F186 in a solvent-exposed, low polarity patch that is required for CD81 binding to the hepatitis C virus (Higginbottom et al. 2000). CD9 F174 is present in a corresponding head domain region and its mutation resulted in a fourfold reduction of the fusion rescue ability of CD9 mRNA injected into Cd9 null oocytes. The mutation of the tripeptide SFQ to AAA (encompassing the two F174 adjacent a.a.) resulted in an even greater inefficiency of the transcript to rescue the fusion despite a similar level of expression of CD9 at the surface of the oocyte and its recognition by all tested monoclonal antibodies (Zhu et al. 2002). The observation that the mutation of a sequence known to act in trans in a closely related tetraspanin supports a receptor function of CD9 in gamete fusion.
9.3.2 CD9 Positions a Partner Molecule Required for Fusion Within the Tetraspanin Web
9.3.2.1 Tetraspanins Organize a Network of Molecular Interactions at the Cell Surface
The key function attributed to tetraspanins is to organize extensive network of cis-partner proteins in the so-called Tetraspanin Web or Tetraspanin Enriched Microdomains (TEMs) within the plasma membrane (Berditchevski 2001; Boucheix and Rubinstein 2001; Charrin et al. 2009; Hemler 2005; Levy and Shoham 2005; Yanez-Mo et al. 2009). It has been proposed that within these microdomains each tetraspanin interact with their primary partners (like EWI2 for CD9) but heterotypic tetraspanin-tetraspanin interactions allow the assembly of higher molecular complexes that link molecules with diverse functions. For instance α6β1 integrin associates directly to the tetraspanin CD151 but indirectly to CD9 through a CD9-CD151 interaction. The loss of one of the structural components, in the present case CD9, may disorganize these membrane microdomains and deregulate the functions of associated proteins. Based on this view, the defect of fusion ability of Cd9-deleted oocyte may be due to the deregulation of one or more of the proteins which belong to the TEMs.
9.3.2.2 First Level Interactions: Primary Partners
Two primary partners of CD9, CD9P-1/EWI-F/CD315 and EWI2/CD316, were described in various cellular types (Charrin et al. 2001, 2003; Clark et al. 2001; Stipp et al. 2001, 2003). They are structurally related and, with EWI-3 and EWI-101, form a novel Ig domain subfamily that includes four members. EWI-2 and CD9P-1 are expressed on oocytes and as in other cellular types both molecules are primary CD9 partners (Rubinstein et al. 2006b; Runge et al. 2007; He et al. 2009; Glazar and Evans 2009). Luminescence assays revealed that the oocyte expression level of EWI2 was decreased by more than 90% in the absence of CD9 (He et al. 2009). The mechanism of this deregulation is unknown. It does not require palmitoylation of CD9 since Cd9 null mice positive for the depalmitoylated CD9 transgene (CD9plm Tg+ Cd9−/−) had oocytes which expressed normal level of CD9plm and EWI2 and were fully functional in sperm-egg fusion. Three studies have addressed the potential role of EWI-2. In one study, an anti-EWI-2 antibody showed a discrete inhibitory effect on sperm-egg binding but not on fusion (Glazar and Evans 2009). In another study, recombinant EWI-2 ectodomain did not result in an inhibition of sperm-egg binding and fusion despite the fact that it bound to the surface of 82% of acrosome-reacted sperm and to 22% of acrosome intact sperm (He et al. 2009). Finally, a recent work reporting the generation of EWI-2 deleted mice demonstrates surprisingly that EWI-2 is dispensable for sperm-egg fusion (Inoue et al. 2012). A compensatory mechanism during development can’t be excluded from these results but appears unlikely.
9.3.2.3 Second Level Interactions
The importance of these interactions in oocytes relied on the belief that sperm/egg adhesion was mediated by the binding of the disintegrin domain of ADAM2 to egg α6β1, a component of TEM (Chen et al. 1999; Zhu and Evans 2002). Moreover, CD9 was shown to control the lateral diffusion of α6β1 within the oolemma (Ziyyat et al. 2006) since (1) anti-CD9 monoclonal antibodies prevent the reorganization in patches of α6β1 induced by zona removal procedure on human oocytes (2) the patches induced by the antibody-mediated aggregation of α6 subunit were larger and more dispersed in absence of CD9 at the mouse egg surface. These observations led to the proposal that CD9 is necessary for the maintenance of a tetraspanin web to which the integrin α6β1 is linked through the tetraspanin CD151 (Serru et al. 1999), thus controlling its lateral mobility (Ziyyat et al. 2006). The cooperation of CD9 with egg membrane components was supported by another team which has generated a soluble form of the large extracellular loop of CD9 (EC2). The preincubation of eggs with the EC2 constructs, before insemination, significantly reduced the fertilization and index rates while sperm pre incubation had no effect (Zhu et al. 2000). This report strengthens the idea that CD9 function in gamete fusion is the consequence of its interaction with partners in cis on the egg surface.
9.3.3 CD9 Is Transferred from Egg to Sperm
Recent findings offer a new view on the potential role that CD9 would play in gamete membrane interaction. It originated from an unexpected observation describing a transfer of CD9 from oolemma to the head of sperm present in the perivitelline space of Cd81 null oocytes (Rubinstein et al. 2006a). This transfer of CD9 has been next confirmed between wild type gametes and proposed to be driven by an egg membrane fragment released during gamete contact prior to fusion (Barraud-Lange et al. 2007a). The authors proposed that this protein transfer might be trogocytosis-related. Trogocytosis is a phenomenon involved in lymphocyte activation, in which lymphocytes actively capture plasma membrane fragments of antigen-presenting cells containing MHC-peptide complexes (Joly and Hudrisier 2003). Another group showed transfer of oocyte material to the sperm using CD9-EGFP Tg+ CD9−/− oocytes (Miyado et al. 2008). The authors monitored IVF assays and showed that the fertilizing sperm which has reached the perivitelline space acquired CD9-EGFP by direct interaction with CD9-EGFP containing-material present in this space. Confocal and electron microscopy analyses allowed observation of the release from the oolemma of vesicles which contained CD9 and presented the morphological characteristics of exosomes. The functional significance of such a phenomenon is still an open question. Does this egg-derived material acquired by the fertilizing sperm contain information necessary for activation to sperm fusion ability? The required acquisition of CD9 by sperm before fusion in order to organize a multimolecular complex of fusion has been proposed (Barraud-Lange et al. 2007a) but is questioned by recent findings which proved that mature sperm express endogenous CD9 (Ito et al. 2010). Miyado’s group was able to restore Cd9 null oocytes fusion ability by CD9-containing vesicles recovered from wild type eggs. They proposed that sperm that had previously interacted with CD9-containing material released by wild type eggs became competent for Cd9 null egg fertilization. Based on these data, they concluded that egg CD9 bestows upon sperm its fusion ability. This model is questioned by others who failed to reproduce the CD9 null oocytes rescue experiments (Gupta et al. 2009; Lefevre et al. 2010). If the interaction of sperm with egg membrane material was confirmed by several groups, its requirement for fertilization needs to be proven.
9.3.4 CD9 Structures the Oocyte Membrane
CD9 has been proposed to be involved in the architecture of the oocyte plasma membrane, explaining the fertilization defect of Cd9 null eggs by the dimorphism of the oolemma since CD9 expression is restricted to the microvillar area where fusion occurs. By scanning electron microscopy (e.m.), it was shown that the microvilli of mutant Cd9 null oocytes appeared shorter, thicker, denser and more uniform than in wild type oocytes (Runge et al. 2007). The authors also showed by immunogold labeling that CD9 was congregated on the microvilli and not on the planar membrane regions. Interestingly, the loss of EWI2 consequent to the loss of CD9 renders this observation particularly relevant. Indeed, in somatic cells, EWI-2 has been shown to bind directly, through its N-terminal domain, to actin-linking ezrin-radixin-moesin (ERM) proteins (Sala-Valdes et al. 2006). ERM proteins bind in turn to the actin filaments in the microvillar core. Thus, a network composed by CD9-EWI-2-ERM-actin has been proposed to regulate the microvillar morphology. Based on these data, one can speculate that in the absence of CD9 and EWI-2, the link between the plasma membrane and the cytoskeleton is lost leading to the disruption of the microvillar structure and, consequently, rendering oolemma fusion incompetent.
There are some examples in which organ specific tetraspanins structure particular areas of the membrane like RDS/ROM for the rim of photoreceptors outer segment disk or uroplakins 1a and 1b for the urothelial plaques of the bladder. Also the tetraspanin CD151 is in epithelial cells a constituent of hemidesmosomes where it is targeted via its interaction with the integrin α6β4. These cellular structures may be considered as independent or as an extension of the tetraspanin web. No specific structure is seen with CD9 on oocytes apart from a pronounced membrane curvature at the tip of the microvilli. Although morphologically this part of the microvilli resembles the rim of photoreceptors outer segment disk, these two types of structures are not directly comparable since RDS/ROM have a particular mode of organization forming tetramers and intermolecular disulfide bonds and a restricted expression (Goldberg 2006), properties that are not shared by CD9.
9.3.5 Relationship Between Rafts and TEM in Gamete Fusion
Given the various observations showing that tetraspanins CD9 and CD81 are critical players in the gamete fusion, their function should also be considered in view of their involvement in the assembly of TEM. On the other hand the dramatic effect of GPI anchored protein depletion (see above) on gamete fusion suggests a role for lipid rafts. Until now these two types of membrane domains have been viewed as different based on biochemical (Le Naour et al. 2006) and dynamic membrane studies (single particle tracking or fluorescence recovery after photobleaching) (Barreiro et al. 2008; Espenel et al. 2008). Combining these observations leads to the hypothesis that cooperation between rafts and TEM is required for gamete fusion to occur. The requirement is to bring the gamete membranes in tight apposition (less than 10 nm) to overcome the energetic barrier that prevents spontaneous fusion to occur and allow lipid mixing between sperm and oocyte membranes. This could be achieved by creating patches on the membrane where repulsive forces are lowered and exposing these patches to the partner’s membrane, thus building a kind of fusion synapse.
Consequently, depending on associated membrane lipids and/or proteins, tetraspanins may either promote or inhibit cell fusion, explaining the contradictory effects of the CD9/CD81 double deletion on oocytes and monocytes/macrophages (see below).
Virus budding offers another example where lipid rafts and TEM may be cooperating. There is a colocalization of rafts with Gag and Env associated proteins during virus assembly (Holm et al. 2003; Ono and Freed 2005). Gag and TEM were also shown to colocalize (Jolly and Sattentau 2007; Mazurov et al. 2006; Nydegger et al. 2006). Furthermore rafts and TEM components are incorporated into viruses (Ott 2008). A GPI-anchored raft protein (BST2) inhibits virus assembly whereas the precise role of TEM associated proteins in virus assembly remains controversial (Chen et al. 2008; Grigorov et al. 2009; Krementsov et al. 2009; Ruiz-Mateos et al. 2008). A recent analysis of the dynamic relationship between viral components and these microdomains suggests that it is Gag assembly that creates a local microenvironment enriched in raft lipids and proteins and in which CD9 and presumably other tetraspanins are trapped. In these different types of microdomains, tetraspanins have reduced motility whereas raft markers diffuse freely (Krementsov et al. 2010). The ability of Gag to regroup membrane molecules localized in different microdomains raises the question of yet an unknown molecule at the surface of the oocyte that could play a similar role in building appropriate sites of fusion at the tip of microvilli.
9.3.6 Implication of Tetraspanins in Other Cell Fusion Processes
Instead of illuminating the mechanism of sperm-egg fusion, the involvement of tetraspanins in other cell fusion types has added another level of complexity. Indeed, it was reported that anti-CD9 or anti-CD81 antibodies delay the fusion of murine myoblastic cells to form multinucleated myotubes during muscle differentiation and that ectopic expression of CD9 increases the fusion of rhabdomyosarcoma cells (Tachibana and Hemler 1999). This observation is compatible with the properties of CD9 and CD81 in gamete fusion. The situation differs for osteoclasts and giant cells that are two types of multinucleated cells issued from the fusion of monocytes/macrophages and are involved in bone resumption and in the immune response respectively (Vignery 2005). Silencing of Tspan-5 and CD9 reduces the formation of giant multinuclear osteoclast-like cells by RANKL treated RAW264.7 cells, whereas silencing of Tspan-13/NET-6 has the opposite effect (Ishii et al. 2006; Iwai et al. 2007). However the CD9−/−/CD81−/− mice have greater numbers of osteoclasts associated with reduced bone-mineral density. In addition multinucleated giant cells are found spontaneously in the lung of these mice (Takeda et al. 2003). This in vivo observation was confirmed in vitro since the fusion of macrophages lacking these two tetraspanins is enhanced. Thus as in gamete fusion, the tetraspanins CD9 and CD81 play both a role in the fusion of macrophages, but in that case they regulate negatively the fusion process. Interestingly, tetraspanins CD9, CD63, CD81, CD82 and CD231 inhibit HIV-1 induced cell to cell fusion (Gordon-Alonso et al. 2006; Sato et al. 2008). It was further demonstrated that tetraspanins CD9 and CD63 inhibited cell fusion mediated by HIV1 Env protein, whereas CD82 effect was dependent on the coexpression of Gag (Weng et al. 2009). CD82 has also been shown to inhibit cell-cell fusion mediated by the envelope glycoprotein of human T-cell leukemia virus type 1, another retrovirus (Pique et al. 2000).
These findings indicate a complex role for tetraspanins in the fusion process where the same tetraspanin, depending on the cellular type and the molecular context may either promote or inhibit cell fusion, and where different tetraspanins in the same cellular type may have opposite effects on cell fusion. This suggests that the role of tetraspanins in cell fusion is dependent of their molecular environment within the Tetraspanin Web in which they exert a structuring and regulatory role.
9.4 Perspectives
Current knowledge on the mechanisms of cell fusion or more generally of membrane fusion have been of little help to understand the way two gametes unite to form a single cell. None of the gamete fusion proteins reported in the litterature are fusogenic. They don’t contain a fusion peptide and when expressed ectopically they don’t induce cell fusion as it is observed with FAST fusogens or C. elegans eff-1 and aff-1 (Oren-Suissa and Podbilewicz 2010). In addition, the formal proofs that they may interact with membrane proteins of the other gamete are still lacking. Most of the critical factors were discovered by genetic approach, either by systematic gene screening or by sheer luck with gene-manipulated animals that provided non anticipated phenotypes. Therefore, other surprises may be expected from additional genetic approaches, especially with the global effort of knocking out systematically all mouse genes. If the mouse gene deletion models have also eliminated some genes like α6β1 from the first line candidates, that doesn’t definitely rule out their implication in the process that may be more subtle. For instance, by increasing gametes binding rates, they may give a selection advantage that would appear after several generations of free competition mating (Sutovsky 2009). In addition, human biological mechanisms are not necessarily a copy of what happens in mice.
Ongoing and future studies will continue to focus on:
-
1.
Research of molecular candidates that fit with present models of membrane fusion
-
2.
Understanding of how membrane microdomains are critically involved
-
3.
Elaboration of new models of cell fusion that would modify or go beyond current paradigms and take into account the specific microdomain organization of the gamete membranes
-
4.
Development of new tools for studying gamete interaction with improvement of imaging and of biophysical techniques that would allow an easier appreciation of what occurs directly at the interaction/fusion site.
One of these new tools, called Biomembrane Force Probe (BFP), which allows the measurement of binding nano-forces involved in gamete membrane adhesion, was developed recently by a biophysics team (Jegou et al. 2008). They have also provided relevant information on the mechanical properties of the oolemma. Experiments using wild type cells revealed that some domains of the egg membrane presented an elastic deformation under sperm traction corresponding to strong interaction between gamete membranes (S-adhesion site). While others domains gave rise to a tether via a viscoelastic deformation corresponding to weak interaction (W-adhesion site). Applied to gametes from mutant mice, the BFP is of particular interest to decipher the role played by molecular candidates in gamete membrane fusion. Indeed, a very recent work revealed that the adhesion properties of CD9-deleted egg membrane are deeply modified (Jegou et al. 2011). Force measurement assays recorded sperm-egg adhesion events but showed a loss of membrane S-adhesion site in absence of CD9. Considering the organizer function often attributed to tetraspanins in many membrane cell types, the authors proposed that CD9 induced assembling of part of sperm receptors into multiprotein patches at the egg surface. A receptor involved in a patch is strongly anchored to the cytoskeleton and forms a S-adhesion site at the egg surface. Conversely, in absence of CD9, isolated receptor which is individually connected to the cytoskeleton, provide weak interaction. The authors proposed then that S-adhesion allows the tight sperm-egg contact necessary to induce fusion and finally that CD9 generates fusion competent adhesion sites on eggs. This model describes the fertilization as a direct consequence of CD9 controlled sperm-egg adhesion leading to fusion. To go further, it might be possible to investigate the nature of receptors anchoring to the egg membrane cytoskeleton as well as its involvement in fusion process.
References
Aguilar PS, Engel A, Walter P (2007) The plasma membrane proteins prm1 and fig 1 ascertain fidelity of membrane fusion during yeast mating. Mol Biol Cell 18(2):547–556
Alfieri JA, Martin AD, Takeda J, Kondoh G, Myles DG, Primakoff P (2003) Infertility in female mice with an oocyte-specific knockout of gpi-anchored proteins. J Cell Sci 116(Pt 11):2149–2155
Allen CA, Green DP (1995) Monoclonal antibodies which recognize equatorial segment epitopes presented de novo following the a23187-induced acrosome reaction of guinea pig sperm. J Cell Sci 108(Pt 2):767–777
Almeida EA, Huovila AP, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG et al (1995) Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81(7):1095–1104
Austin CR (1951) Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B 4(4):581–596
Barraud-Lange V, Naud-Barriant N, Bomsel M, Wolf JP, Ziyyat A (2007a) Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J 21(13):3446–3449
Barraud-Lange V, Naud-Barriant N, Saffar L, Gattegno L, Ducot B, Drillet AS, Bomsel M, Wolf JP, Ziyyat A (2007b) Alpha6beta1 integrin expressed by sperm is determinant in mouse fertilization. BMC Dev Biol 7(1):102
Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F (2008) Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol 183(3):527–542
Berditchevski F (2001) Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114(Pt 23):4143–4151
Bigler D, Takahashi Y, Chen MS, Almeida EA, Osbourne L, White JM (2000) Sequence-specific interaction between the disintegrin domain of mouse adam 2 (fertilin beta) and murine eggs. Role of the alpha(6) integrin subunit. J Biol Chem 275(16):11576–11584
Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P, White JM (1992) A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356(6366):248–252
Boucheix C, Rubinstein E (2001) Tetraspanins. Cell Mol Life Sci 58(9):1189–1205
Bronson RA, Fusi F (1990) Sperm-oolemmal interaction: role of the arg-gly-asp (rgd) adhesion peptide. Fertil Steril 54(3):527–529
Bronson RA, Fusi FM, Calzi F, Doldi N, Ferrari A (1999) Evidence that a functional fertilin-like adam plays a role in human sperm-oolemmal interactions. Mol Hum Reprod 5(5):433–440
Busso D, Cohen DJ, Maldera JA, Dematteis A, Cuasnicu PS (2007) A novel function for crisp1 in rodent fertilization: involvement in sperm-zona pellucida interaction. Biol Reprod 77(5):848–854
Campbell S, Swann HR, Seif MW, Kimber SJ, Aplin JD (1995) Cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod 10(6):1571–1578
Charrin S, Le Naour F, Labas V, Billard M, Le Caer JP, Emile JF, Petit MA, Boucheix C, Rubinstein E (2003) Ewi-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem J 373(Pt 2):409–421
Charrin S, Le Naour F, Oualid M, Billard M, Faure G, Hanash SM, Boucheix C, Rubinstein E (2001) The major cd9 and cd81 molecular partner. Identification and characterization of the complexes. J Biol Chem 276(17):14329–14337
Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E (2009) Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J 420(2):133–154
Chatterjee I, Richmond A, Putiri E, Shakes DC, Singson A (2005) The caenorhabditis elegans spe-38 gene encodes a novel four-pass integral membrane protein required for sperm function at fertilization. Development 132(12):2795–2808
Chen H, Dziuba N, Friedrich B, von Lindern J, Murray JL, Rojo DR, Hodge TW, O’Brien WA, Ferguson MR (2008) A critical role for cd63 in hiv replication and infection of macrophages and cell lines. Virology 379(2):191–196
Chen MS, Tung KS, Coonrod SA, Takahashi Y, Bigler D, Chang A, Yamashita Y, Kincade PW, Herr JC, White JM (1999) Role of the integrin-associated protein cd9 in binding between sperm adam 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc Natl Acad Sci USA 96(21):11830–11835
Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG (1998) Fertilization defects in sperm from mice lacking fertilin beta. Science 281(5384):1857–1859
Clark KL, Zeng Z, Langford AL, Bowen SM, Todd SC (2001) Pgrl is a major cd81-associated protein on lymphocytes and distinguishes a new family of cell surface proteins. J Immunol 167(9):5115–5121
Cohen DJ, Da Ros VG, Busso D, Ellerman DA, Maldera JA, Goldweic N, Cuasnicu PS (2007) Participation of epididymal cysteine-rich secretory proteins in sperm-egg fusion and their potential use for male fertility regulation. Asian J Androl 9(4):528–532
Coonrod SA, Naaby-Hansen S, Shetty J, Shibahara H, Chen M, White JM, Herr JC (1999) Treatment of mouse oocytes with pi-plc releases 70-kda (pi 5) and 35- to 45-kda (pi 5.5) protein clusters from the egg surface and inhibits sperm-oolemma binding and fusion. Dev Biol 207(2):334–349
Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS (2008) Impaired sperm fertilizing ability in mice lacking cysteine-rich secretory protein 1 (crisp1). Dev Biol 320(1):12–18
Ellerman DA, Ha C, Primakoff P, Myles DG, Dveksler GS (2003) Direct binding of the ligand psg17 to cd9 requires a cd9 site essential for sperm-egg fusion. Mol Biol Cell 14(12):5098–5103
Ellerman DA, Pei J, Gupta S, Snell WJ, Myles D, Primakoff P (2009) Izumo is part of a multiprotein family whose members form large complexes on mammalian sperm. Mol Reprod Dev 76(12):1188–1199
Espenel C, Margeat E, Dosset P, Arduise C, Le Grimellec C, Royer CA, Boucheix C, Rubinstein E, Milhiet PE (2008) Single-molecule analysis of cd9 dynamics and partitioning reveals multiple modes of interaction in the tetraspanin web. J Cell Biol 182(4):765–776
Evans JP, Kopf GS, Schultz RM (1997) Characterization of the binding of recombinant mouse sperm fertilin beta subunit to mouse eggs: evidence for adhesive activity via an egg beta1 integrin-mediated interaction. Dev Biol 187(1):79–93
Evans JP, Schultz RM, Kopf GS (1995) Mouse sperm-egg plasma membrane interactions: analysis of roles of egg integrins and the mouse sperm homologue of ph-30 (fertilin) beta. J Cell Sci 108(Pt 10):3267–3278
Evans JP, Schultz RM, Kopf GS (1998) Roles of the disintegrin domains of mouse fertilins alpha and beta in fertilization. Biol Reprod 59(1):145–152
Fusi FM, Bernocchi N, Ferrari A, Bronson RA (1996a) Is vitronectin the velcro that binds the gametes together? Mol Hum Reprod 2(11):859–866
Fusi FM, Bronson RA (1992) Sperm surface fibronectin. Expression following capacitation. J Androl 13(1):28–35
Fusi FM, Lorenzetti I, Mangili F, Herr JC, Freemerman AJ, Gailit J, Bronson RA (1994) Vitronectin is an intrinsic protein of human spermatozoa released during the acrosome reaction. Mol Reprod Dev 39(3):337–343
Fusi FM, Tamburini C, Mangili F, Montesano M, Ferrari A, Bronson RA (1996b) The expression of alpha v, alpha 5, beta 1, and beta 3 integrin chains on ejaculated human spermatozoa varies with their functional state. SO -. Mol Hum Reprod 2(3):169–175
Fusi FM, Vignali M, Gailit J, Bronson RA (1993) Mammalian oocytes exhibit specific recognition of the rgd (arg-gly-asp) tripeptide and express oolemmal integrins. Mol Reprod Dev 36(2):212–219
Glazar AI, Evans JP (2009) Immunoglobulin superfamily member igsf8 (ewi-2) and cd9 in fertilisation: evidence of distinct functions for cd9 and a cd9-associated protein in mammalian sperm-egg interaction. Reprod Fertil Dev 21(2):293–303
Goldberg AF (2006) Role of peripherin/rds in vertebrate photoreceptor architecture and inherited retinal degenerations. Int Rev Cytol 253:131–175
Gordon-Alonso M, Yanez-Mo M, Barreiro O, Alvarez S, Munoz-Fernandez MA, Valenzuela-Fernandez A, Sanchez-Madrid F (2006) Tetraspanins cd9 and cd81 modulate hiv-1-induced membrane fusion. J Immunol 177(8):5129–5137
Granados-Gonzalez V, Aknin-Seifer I, Touraine RL, Chouteau J, Wolf JP, Levy R (2008) Preliminary study on the role of the human izumo gene in oocyte-spermatozoa fusion failure. Fertil Steril 90(4):1246–1248
Grigorov B, Attuil-Audenis V, Perugi F, Nedelec M, Watson S, Pique C, Darlix JL, Conjeaud H, Muriaux D (2009) A role for cd81 on the late steps of hiv-1 replication in a chronically infected t cell line. Retrovirology 6:28
Gupta S, Primakoff P, Myles DG (2009) Can the presence of wild-type oocytes during insemination rescue the fusion defect of cd9 null oocytes? Mol Reprod Dev 76(7):602
Ha CT, Waterhouse R, Wessells J, Wu JA, Dveksler GS (2005) Binding of pregnancy-specific glycoprotein 17 to cd9 on macrophages induces secretion of il-10, il-6, pge2, and tgf-beta1. J Leukoc Biol 77(6):948–957
Hayasaka S, Terada Y, Inoue N, Okabe M, Yaegashi N, Okamura K (2007) Positive expression of the immunoglobulin superfamily protein izumo on human sperm of severely infertile male patients. Fertil Steril 88(1):214–216
He ZY, Brakebusch C, Fassler R, Kreidberg JA, Primakoff P, Myles DG (2003) None of the integrins known to be present on the mouse egg or to be adam receptors are essential for sperm-egg binding and fusion. Dev Biol 254(2):226–237
He ZY, Gupta S, Myles D, Primakoff P (2009) Loss of surface ewi-2 on cd9 null oocytes. Mol Reprod Dev 76(7):629–636
Hemler ME (2005) Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 6(10):801–811
Higginbottom A, Quinn ER, Kuo CC, Flint M, Wilson LH, Bianchi E, Nicosia A, Monk PN, McKeating JA, Levy S (2000) Identification of amino acid residues in cd81 critical for interaction with hepatitis c virus envelope glycoprotein e2. J Virol 74(8):3642–3649
Hirai M, Arai M, Mori T, Miyagishima SY, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H (2008) Male fertility of malaria parasites is determined by gcs1, a plant-type reproduction factor. Curr Biol 18(8):607–613
Holm K, Weclewicz K, Hewson R, Suomalainen M (2003) Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55(gag) associates with membrane domains that are largely resistant to brij98 but sensitive to triton x-100. J Virol 77(8):4805–4817
Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP (2001) Targeted deletion of the cd59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood 98(2):442–449
Ikawa M, Inoue N, Benham AM, Okabe M (2010) Fertilization: a sperm’s journey to and interaction with the oocyte. J Clin Invest 120(4):984–994
Ilayperuma I (2002) Identification of the 48-kda g11 protein from guinea pig testes as sperad. J Exp Zool 293(6):617–623
Inoue N, Ikawa M, Isotani A, Okabe M (2005) The immunoglobulin superfamily protein izumo is required for sperm to fuse with eggs. Nature 434(7030):234–238
Inoue N, Nishikawa T, Ikawa M, Okabe M (2012) Tetraspanin-interacting protein igsf8 is dispensable for mouse fertility. Fertil Steril 98(2):465–470
Ishii M, Iwai K, Koike M, Ohshima S, Kudo-Tanaka E, Ishii T, Mima T, Katada Y, Miyatake K, Uchiyama Y, Saeki Y (2006) Rankl-induced expression of tetraspanin cd9 in lipid raft membrane microdomain is essential for cell fusion during osteoclastogenesis. J Bone Miner Res 21(6):965–976
Ito C, Yamatoya K, Yoshida K, Maekawa M, Miyado K, Toshimori K (2010) Tetraspanin family protein cd9 in the mouse sperm: unique localization, appearance, behavior and fate during fertilization. Cell Tissue Res 340(3):583–594
Iwai K, Ishii M, Ohshima S, Miyatake K, Saeki Y (2007) Expression and function of transmembrane-4 superfamily (tetraspanin) proteins in osteoclasts: reciprocal roles of tspan-5 and net-6 during osteoclastogenesis. Allergol Int 56(4):457–463
Jegou A, Pincet F, Perez E, Wolf JP, Ziyyat A, Gourier C (2008) Mapping mouse gamete interaction forces reveal several oocyte membrane regions with different mechanical and adhesive properties. Langmuir 24(4):1451–1458
Jegou A, Ziyyat A, Barraud-Lange V, Perez E, Wolf JP, Pincet F, Gourier C (2011) Cd9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc Natl Acad Sci USA 108(27):10946–10951
Ji YZ, Wolf JP, Jouannet P, Bomsel M (1998) Human gamete fusion can bypass beta1 integrin requirement. Hum Reprod 13(3):682–689
Jin H, Carlile C, Nolan S, Grote E (2004) Prm1 prevents contact-dependent lysis of yeast mating pairs. Eukaryot Cell 3(6):1664–1673
Jolly C, Sattentau QJ (2007) Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in t cells take place in tetraspanin-enriched plasma membrane domains. J Virol 81(15):7873–7884
Joly E, Hudrisier D (2003) What is trogocytosis and what is its purpose? Nat Immunol 4(9):815
Kadandale P, Stewart-Michaelis A, Gordon S, Rubin J, Klancer R, Schweinsberg P, Grant BD, Singson A (2005) The egg surface ldl receptor repeat-containing proteins egg-1 and egg-2 are required for fertilization in caenorhabditis elegans. Curr Biol 15(24):2222–2229
Kahn-Kirby AH, Bargmann CI (2006) Trp channels in c. Elegans. Annu Rev Physiol 68:719–736
Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A (2000) The gamete fusion process is defective in eggs of cd9-deficient mice. Nat Genet 24(3):279–282
Kaji K, Oda S, Miyazaki S, Kudo A (2002) Infertility of cd9-deficient mouse eggs is reversed by mouse cd9, human cd9, or mouse cd81; polyadenylated mrna injection developed for molecular analysis of sperm-egg fusion. Dev Biol 247(2):327–334
Kawagoe K, Kitamura D, Okabe M, Taniuchi I, Ikawa M, Watanabe T, Kinoshita T, Takeda J (1996) Glycosylphosphatidylinositol-anchor-deficient mice: implications for clonal dominance of mutant cells in paroxysmal nocturnal hemoglobinuria. Blood 87(9):3600–3606
Kelic S, Levy S, Suarez C, Weinstein DE (2001) Cd81 regulates neuron-induced astrocyte cell-cycle exit. Mol Cell Neurosci 17(3):551–560
Krementsov DN, Weng J, Lambele M, Roy NH, Thali M (2009) Tetraspanins regulate cell-to-cell transmission of hiv-1. Retrovirology 6:64
Krementsov DN, Rassam P, Margeat E, Roy NH, Schneider-Schaulies J, Milhiet PE, Thali M (2010) Hiv-1 assembly differentially alters dynamics and partitioning of tetraspanins and raft components. Traffic 11(11):1401–1414
Kroft TL, Gleason EJ, L’Hernault SW (2005) The spe-42 gene is required for sperm-egg interactions during c. Elegans fertilization and encodes a sperm-specific transmembrane protein. Dev Biol 286(1):169–181
Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C (2000) Severely reduced female fertility in cd9-deficient mice. Science 287(5451):319–321
Le Naour F, Andre M, Boucheix C, Rubinstein E (2006) Membrane microdomains and proteomics: lessons from tetraspanin microdomains and comparison with lipid rafts. Proteomics 6(24):6447–6454
Lefevre B, Wolf JP, Ziyyat A (2010) Sperm-egg interaction: is there a link between tetraspanin(s) and gpi-anchored protein(s)? Bioessays 32(2):143–152
Lefievre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, Hughes DC, Barratt CL (2004) Four zona pellucida glycoproteins are expressed in the human. Hum Reprod 19(7):1580–1586
Levy S, Shoham T (2005) The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol 5(2):136–148
Li YH, Hou Y, Ma W, Yuan JX, Zhang D, Sun QY, Wang WH (2004) Localization of cd9 in pig oocytes and its effects on sperm-egg interaction. Reproduction 127(2):151–157
Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O (2008) The conserved plant sterility gene hap2 functions after attachment of fusogenic membranes in chlamydomonas and plasmodium gametes. Genes Dev 22(8):1051–1068
Low MG, Finean JB (1978) Specific release of plasma membrane enzymes by a phosphatidylinositol-specific phospholipase c. Biochim Biophys Acta 508(3):565–570
Low MG, Saltiel AR (1988) Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science 239(4837):268–275
Maecker HT, Do MS, Levy S (1998) Cd81 on b cells promotes interleukin 4 secretion and antibody production during t helper type 2 immune responses. Proc Natl Acad Sci USA 95(5):2458–2462
Marcello MR, Singson A (2010) Fertilization and the oocyte-to-embryo transition in c. Elegans. BMB Rep 43(6):389–399
Mazurov D, Heidecker G, Derse D (2006) Htlv-1 gag protein associates with cd82 tetraspanin microdomains at the plasma membrane. Virology 346(1):194–204
Miller BJ, Georges-Labouesse E, Primakoff P, Myles DG (2000) Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is cd9-dependent. J Cell Biol 149(6):1289–1296
Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E (2000) Requirement of cd9 on the egg plasma membrane for fertilization. Science 287(5451):321–324
Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A (2008) The fusing ability of sperm is bestowed by cd9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 105(35):12921–12926
Myles DG, Kimmel LH, Blobel CP, White JM, Primakoff P (1994) Identification of a binding site in the disintegrin domain of fertilin required for sperm-egg fusion. Proc Natl Acad Sci USA 91(10):4195–4198
Nishiyama S, Kishi T, Kato T, Suzuki M, Nishizawa H, Pryor-Koishi K, Sawada T, Nishiyama Y, Iwata N, Udagawa Y, Kurahashi H (2010) Cd9 gene variations are not associated with female infertility in humans. Gynecol Obstet Invest 69(2):116–121
Nydegger S, Khurana S, Krementsov DN, Foti M, Thali M (2006) Mapping of tetraspanin-enriched microdomains that can function as gateways for hiv-1. J Cell Biol 173(5):795–807
Okabe M, Yagasaki M, Oda H, Matzno S, Kohama Y, Mimura T (1988) Effect of a monoclonal anti-mouse sperm antibody (obf13) on the interaction of mouse sperm with zona-free mouse and hamster eggs. J Reprod Immunol 13(3):211–219
Ono A, Freed EO (2005) Role of lipid rafts in virus replication. Adv Virus Res 64:311–358
Oren-Suissa M, Podbilewicz B (2010) Evolution of programmed cell fusion: common mechanisms and distinct functions. Dev Dyn 239(5):1515–1528
Ott DE (2008) Cellular proteins detected in hiv-1. Rev Med Virol 18(3):159–175
Pique C, Lagaudriere-Gesbert C, Delamarre L, Rosenberg AR, Conjeaud H, Dokhelar MC (2000) Interaction of cd82 tetraspanin proteins with htlv-1 envelope glycoproteins inhibits cell-to-cell fusion and virus transmission. Virology 276(2):455–465
Primakoff P, Myles DG (1983) A map of the guinea pig sperm surface constructed with monoclonal antibodies. Dev Biol 98(2):417–428
Primakoff P, Hyatt H, Tredick-Kline J (1987) Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol 104(1):141–149
Putiri E, Zannoni S, Kadandale P, Singson A (2004) Functional domains and temperature-sensitive mutations in spe-9, an egf repeat-containing protein required for fertility in caenorhabditis elegans. Dev Biol 272(2):448–459
Reddy VR, Rajeev SK, Gupta V (2003) Alpha 6 beta 1 integrin is a potential clinical marker for evaluating sperm quality in men. SO Fertil Steril 79(Suppl 3):1590–1596
Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, Le Naour F, Boucheix C (2006a) Reduced fertility of female mice lacking cd81. Dev Biol 290(2):351–358
Rubinstein E, Ziyyat A, Wolf JP, Le Naour F, Boucheix C (2006b) The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol 17(2):254–263
Ruiz-Mateos E, Pelchen-Matthews A, Deneka M, Marsh M (2008) Cd63 is not required for production of infectious human immunodeficiency virus type 1 in human macrophages. J Virol 82(10):4751–4761
Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG (2007) Oocyte cd9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 304(1):317–325
Sala-Valdes M, Ursa A, Charrin S, Rubinstein E, Hemler ME, Sanchez-Madrid F, Yanez-Mo M (2006) Ewi-2 and ewi-f link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem 281(28):19665–19675
Sato K, Aoki J, Misawa N, Daikoku E, Sano K, Tanaka Y, Koyanagi Y (2008) Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J Virol 82(2):1021–1033
Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U (2003) Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell 4(5):673–685
Sengoku K, Takuma N, Miyamoto T, Horikawa M, Ishikawa M (2004) Integrins are not involved in the process of human sperm-oolemmal fusion. Hum Reprod 19(3):639–644
Serru V, Le Naour F, Billard M, Azorsa DO, Lanza F, Boucheix C, Rubinstein E (1999) Selective tetraspan-integrin complexes (cd81/alpha4beta1, cd151/alpha3beta1, cd151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem J 340(Pt 1):103–111
Shamsadin R, Adham IM, Nayernia K, Heinlein UA, Oberwinkler H, Engel W (1999) Male mice deficient for germ-cell cyritestin are infertile. Biol Reprod 61(6):1445–1451
Singson A, Mercer KB, L’Hernault SW (1998) The c. Elegans spe-9 gene encodes a sperm transmembrane protein that contains egf-like repeats and is required for fertilization. Cell 93(1):71–79
Stipp CS, Kolesnikova TV, Hemler ME (2001) Ewi-2 is a major cd9 and cd81 partner and member of a novel ig protein subfamily. J Biol Chem 276(44):40545–40554
Stipp CS, Kolesnikova TV, Hemler ME (2003) Ewi-2 regulates alpha3beta1 integrin-dependent cell functions on laminin-5. J Cell Biol 163(5):1167–1177
Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song WC (1999) Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci USA 96(2):628–633
Sutovsky P (2009) Sperm-egg adhesion and fusion in mammals. Expert Rev Mol Med 11:e11
Tachibana I, Hemler ME (1999) Role of transmembrane 4 superfamily (tm4sf) proteins cd9 and cd81 in muscle cell fusion and myotube maintenance. J Cell Biol 146(4):893–904
Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, Kimura H, Yamane H, Saito Y, Goto H, Yoneda T, Yoshida M, Kumagai T, Osaki T, Hayashi S, Kawase I, Mekada E (2003) Tetraspanins cd9 and cd81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol 161(5):945–956
Tang Y, Tan XM, Yue CW, Li CX, Fan ZX, Zhang YZ (2008) Cloning, sequence, and function analyses of giant panda (ailuropoda melanoleuca) cd9 gene. Mol Reprod Dev 75(9):1418–1425
Taylor CT, Johnson PM (1996) Complement-binding proteins are strongly expressed by human preimplantation blastocysts and cumulus cells as well as gametes. Mol Hum Reprod 2(1):52–59
Tiede A, Nischan C, Schubert J, Schmidt RE (2000) Characterisation of the enzymatic complex for the first step in glycosylphosphatidylinositol biosynthesis. Int J Biochem Cell Biol 32(3):339–350
Todres E, Nardi JB, Robertson HM (2000) The tetraspanin superfamily in insects. Insect Mol Biol 9(6):581–590
Vignery A (2005) Macrophage fusion: are somatic and cancer cells possible partners? Trends Cell Biol 15(4):188–193
von Besser K, Frank AC, Johnson MA, Preuss D (2006) Arabidopsis hap2 (gcs1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133(23):4761–4769
Wassarman PM (1988) Zona pellucida glycoproteins. Annu Rev Biochem 57:415–442
Wassarman PM (2002) Sperm receptors and fertilization in mammals. Mt Sinai J Med 69(3):148–155
Wassarman PM, Mortillo S (1991) Structure of the mouse egg extracellular coat, the zona pellucida. Int Rev Cytol 130:85–110
Waterhouse R, Ha C, Dveksler GS (2002) Murine cd9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med 195(2):277–282
Weng J, Krementsov DN, Khurana S, Roy NH, Thali M (2009) Formation of syncytia is repressed by tetraspanins in human immunodeficiency virus type 1-producing cells. J Virol 83(15):7467–7474
Wright MD, Geary SM, Fitter S, Moseley GW, Lau LM, Sheng KC, Apostolopoulos V, Stanley EG, Jackson DE, Ashman LK (2004) Characterization of mice lacking the tetraspanin superfamily member cd151. Mol Cell Biol 24(13):5978–5988
Xu XZ, Sternberg PW (2003) A c. Elegans sperm trp protein required for sperm-egg interactions during fertilization. Cell 114(3):285–297
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction, Secondth edn. Raven, New York, pp 189–317
Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F (2009) Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol 19(9):434–446
Yuan R, Primakoff P, Myles DG (1997) A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the adam family, in mouse sperm-egg plasma membrane adhesion and fusion. J Cell Biol 137(1):105–112
Zannoni S, L’Hernault SW, Singson AW (2003) Dynamic localization of spe-9 in sperm: a protein required for sperm-oocyte interactions in caenorhabditis elegans. BMC Dev Biol 3:10
Zhou GB, Liu GS, Meng QG, Liu Y, Hou YP, Wang XX, Li N, Zhu SE (2009) Tetraspanin cd9 in bovine oocytes and its role in fertilization. J Reprod Dev 55(3):305–308
Zhu X, Evans JP (2002) Analysis of the roles of rgd-binding integrins, alpha(4)/alpha(9) integrins, alpha(6) integrins, and cd9 in the interaction of the fertilin beta (adam2) disintegrin domain with the mouse egg membrane. Biol Reprod 66(4):1193–1202
Zhu X, Bansal NP, Evans JP (2000) Identification of key functional amino acids of the mouse fertilin beta (adam2) disintegrin loop for cell-cell adhesion during fertilization. J Biol Chem 275(11):7677–7683
Zhu GZ, Miller BJ, Boucheix C, Rubinstein E, Liu CC, Hynes RO, Myles DG, Primakoff P (2002) Residues sfq (173–175) in the large extracellular loop of cd9 are required for gamete fusion. Development 129(8):1995–2002
Ziyyat A, Naud-Barriant N, Barraud-Lange V, Chevalier F, Kulski O, Lemkecher T, Bomsel M, Wolf JP (2005) Cyclic fee peptide increases human gamete fusion and potentiates its rgd-induced inhibition. Hum Reprod 20(12):3452–3458
Ziyyat A, Rubinstein E, Monier-Gavelle F, Barraud V, Kulski O, Prenant M, Boucheix C, Bomsel M, Wolf JP (2006) Cd9 controls the formation of clusters that contain tetraspanins and the integrin {alpha}6{beta}1, which are involved in human and mouse gamete fusion. J Cell Sci 119(Pt 3):416–424
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Barraud-Lange, V., Boucheix, C. (2013). The Role of Tetraspanin Complexes in Egg-Sperm Fusion. In: Berditchevski, F., Rubinstein, E. (eds) Tetraspanins. Proteins and Cell Regulation, vol 9. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6070-7_9
Download citation
DOI: https://doi.org/10.1007/978-94-007-6070-7_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6069-1
Online ISBN: 978-94-007-6070-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)