Summary
During leaf senescence and fruit ripening, chlorophyll is broken down to colorless linear tetrapyrroles, which are stored in the vacuoles of degreened cells. The pathway of chlorophyll degradation that is active in these developmental processes is fairly well known regarding its biochemistry and cell biology. It comprises at least six enzymatic and one non-enzymatic reaction and the chemical structures of several intermediary and final chlorophyll catabolites have been elucidated. In the last few years, genes coding for a number of chlorophyll catabolic enzymes have been characterized and mutants in these genes have been analyzed. This includes pheophorbide a oxygenase (PAO), the key enzyme of the pathway, which is responsible for opening of the chlorine macrocycle present in chlorophyll, thereby providing the characteristic structural basis of all further downstream breakdown products. The pathway is therefore nowadays termed the ‘PAO pathway’. This review summarizes information on the structures of chlorophyll breakdown products and the reactions involved in their formation. In addition cell biological and regulatory aspects of the PAO pathway are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Evolution of advanced life forms on Earth would probably not have occurred without the evolution of oxygenic photosynthesis some three billion years ago (Xiong and Bauer 2002). It allowed for cellular energy production through access to both indefinitely available H2O as a source of electrons and solar radiation as energy source. Chlorophyll (Chl) is essential for the absorption of sun light and thus for the conversion of solar energy to chemical energy during photosynthesis. Heterotrophic organisms depend on this source of energy. Besides its importance in photosynthesis, green-colored Chl appears to have positive effects on human psychological well-being (Pretty et al. 2007), and in urban environments, human health benefits from green infrastructure and urban green space systems (Tzoulas et al. 2007).
However, under adverse conditions, such as during stress or after the application of herbicides, the photosynthetic apparatus of plants can be overexcited. In these cases, Chl can act as a strong photosensitizer, generating reactive oxygen species (ROS) which in turn can cause cell damage and death (Apel and Hirt 2004). Likewise, defects in heme and Chl biosynthesis, which partly share the same pathway, as well as in Chl degradation, have been shown to result in cytotoxic effects, which are caused by the accumulation of photodynamic metabolic intermediates (Hu et al. 1998; Pružinská et al. 2003; Mochizuki et al. 2010).
These toxic effects explain the requirement for a tight regulation of Chl metabolism, well known in the case of Chl biosynthesis. Here transcriptional and posttranslational mechanisms as well as complex product feedback control at the level of aminolevulinic acid exist to prevent accumulation of biosynthesis intermediates (Cornah et al. 2003; Tanaka and Tanaka 2006, 2007). There is also evidence that Chl breakdown is subject to different levels of regulatory mechanisms with the aim of limiting the occurrence of photodynamic breakdown intermediates (Hörtensteiner 2006; Park et al. 2007).
It is believed that plants degrade Chl throughout their lifespan. Thus, breakdown occurs not only during leaf senescence and fruit ripening when Chl is massively degraded within short periods of time, but also as a response to many biotic and abiotic stress events and during post-harvest. Even at steady state, Chl turns over at a certain rate. Whether the mechanism of Chl breakdown under all these conditions is the same, remains unknown. To date, the best characterized mechanism is the ‘PAO pathway’ of Chl breakdown occurring during leaf senescence, and there is convincing evidence that Chl degradation in ripening fruits is (largely) identical. The pathway is named after a key enzyme, pheophorbide a oxygenase (PAO), which accounts for the open-tetrapyrrolic backbone structure of several key Chl catabolites found in senescent leaves and fruits. The first identification and structure determination of a nonfluorescent catabolite (NCC) from barley (Hordeum vulgare) in 1999 (Kräutler et al. 1991) marked a milestone in deciphering the fate of Chl, which before had been termed a biological enigma (Hendry et al. 1987).
The PAO pathway can be divided into two parts, i.e. (early) reactions affecting colored intermediates ending with the formation of a primary fluorescent Chl catabolite (pFCC) and (late) pFCC-modifying reactions, typically ending with the isomerization of modified FCCs (mFCCs) to their respective NCCs. These two parts of the pathway are also spatially separated in the cell: formation of pFCC occurs in plastids whereas subsequent modification and isomerization are localized in cytosol and vacuole, respectively.
This review summarizes our current knowledge of the PAO pathway of Chl breakdown during leaf senescence, including Chl catabolites, Chl catabolic reactions and their subcellular localization and pathway regulation. In addition, it aims to provide an overview of Chl turnover at the steady state, and finally discusses Chl breakdown in the context of stress responses.
II. Chlorophyll Turnover at Steady State
Labeling experiments using either radio-labeled CO2 or aminolevulinic acid have provided evidence that Chl turns over at the steady state in leaves, i.e. without net loss of overall Chl amount. The half-life of Chl has been calculated as between a few hours and several days (Stobart and Hendry 1984; Hendry and Stobart 1986), but interestingly, turnover seems mainly to affect Chl a (Feierabend and Dehne 1996; Beisel et al. 2010), thus correlating with the rapid turnover of the D1 polypeptide of photosystem II (PSII) (Melis 1999). Furthermore, long-term highlight acclimation accelerated rates of Chl turnover, indicating that under these conditions turnover also affected Chl bound to light harvesting complex (LHC) proteins of PSII (LHCII) (Beisel et al. 2010), the abundance of which decreases during high light acclimation (Ballottari et al. 2007).
Although Chl turnover occurs at steady state levels, the mechanism of turnover is largely unknown. 13C-labeling experiments in Synechocystis followed by pigment analysis using MALDI-TOF mass spectrometry indicated that Chl turnover involves hydrolysis of the phytol chain of Chl to yield chlorophyllide (Chlide) and re-esterification of Chlide to Chl (Vavilin and Vermaas 2007). As in plants, turnover in the cyanobacterium mainly was associated with PSII and was increased at high light intensities. Thus, Chl seems to be not degraded, but rather continuously recycled. The enzymes responsible for Chl de- and re-esterification are unknown. It remains to be shown whether a mechanism for Chl degradation beyond the level of Chlide is active in plants at steady state. The involvement of the PAO pathway is rather unlikely. For example, catabolites like FCCs or NCCs have never been observed before the initiation of senescence (Pružinská et al. 2005, 2007), and most of the Chl catabolic enzymes known to date are expressed in a senescence-related fashion (Pružinská et al. 2003; Kusaba et al. 2007; Park et al. 2007; Schelbert et al. 2009). In addition, turnover of D1 under normal illumination is not affected in a Festuca mutant that is deficient in the PAO pathway of Chl breakdown, but D1 exhibits an unusual stability during senescence in this mutant (Hilditch et al. 1986).
III. Chlorophyll Breakdown During Leaf Senescence
Until some 20 years ago, the fate of Chl during leaf senescence was enigmatic and the pigment seemed to disappear without leaving a trace (Hendry et al. 1987; Brown et al. 1991). Within the last two decades this situation has changed dramatically, starting with the pioneering identification of the first structure of a NCC from barley, Hv-NCC-1, (Kräutler et al. 1991). To date more than two dozen structures of different catabolites of Chl occurring transiently (‘intermediary’ catabolites) or permanently (‘final’ catabolites) during leaf senescence have been discovered. The identification of these catabolites was a key step for the elucidation of the sequence of reactions in the PAO pathway. Structures of final catabolites of Chl, i.e. FCCs and NCCs, will be presented in a separate section, while intermediary catabolites will be introduced along with the enzymes catalyzing their synthesis and further metabolism.
A. ‘Final’ Chlorophyll Catabolites
1. Nonfluorescent Chlorophyll Catabolites
When senescent leaves are extracted with methanolic buffers and the extracts subsequently analyzed by reversed-phase HPLC, NCCs can usually be identified by their typical UV absorption spectrum, exhibiting a characteristic peak maximum at around 315 nm. Depending on the plant species analyzed, patterns of NCCs can be complex, i.e. containing several different NCCs, such as in Arabidopsis thaliana (Arabidopsis), or simple with one major NCC, such as in the case of senescent leaves of the deciduous tree Cercidiphyllum japonicum. Furthermore, NCCs from different species are often unique and not found in other species. The reason for this complexity arises from the fact that although the open tetrapyrrole backbone of all NCCs is identical, they vary with regard to one or more of three side positions depicted as R1 to R3 in Fig. 16.1. In addition, the chiral center of C1 provides further variability in NCCs, because, depending on the plant species analyzed, the pyrrole ring A is present in the R or the S configuration. This results from the stereospecific action of red Chl catabolite reductases (RCCRs) from different plant species (Hörtensteiner et al. 2000; Pružinská et al. 2007), which are responsible for the introduction of the C1-stereocenter during the formation of pFCC from red Chl catabolite (RCC) (see below). Thus, two possible stereoisomers, pFCC and epi-pFCC, can occur and this ultimately results in C1-isomeric NCCs. With the exception of At-NCC-3 from Arabidopsis, all NCCs identified so far from plants have a methyl group at C7 and are therefore derived from Chl a (Kräutler 2003; Kräutler and Hörtensteiner 2006). It has been shown (see below) that conversion of Chl b to Chl a is a prerequisite for Chl breakdown through the PAO pathway, which explains this specificity. By contrast, RCC-like catabolites derived from both Chl a and Chl b have been identified in Auxenochlorella protothecoides when the green alga is forced to de-green under heterotrophic and N-limiting conditions in the dark (Iturraspe et al. 1994; Engel et al. 1996). At-NCC-3 has a hydroxymethyl group at C7, indicating that it derives from a slightly divergent, as yet unknown, PAO pathway (Müller et al. 2006). All major NCCs found so far carry a hydroxyl group at C82, indicating that this is an important and common modification of NCCs.
The PAO pathway of chlorophyll breakdown active in senescing leaves. Chemical structures of Chl and of Chl catabolites are shown along with the enzymes involved in the pathway. Enzymes, for which genes and mutants have been characterized in the past, are in bold. Note that dephytylation and Mg-dechelation were recently shown to proceed through Phein and that the alternative path involving CLH plays, if at all, a minor role. Note also that the PAO pathway is split at the later reactions, resulting in the production of either hFCCs or (ultimately) NCCs. Pyrrole rings (A-D), methane bridges (α-δ) and relevant carbon atoms are labeled in Chl a. R1-R4 in FCCs and NCCs indicate modifications as outlined in Table 16.1. For abbreviations see the text.
To date NCCs have been structurally characterized from senescent leaves of the following species (Table 16.1): Arabidopsis (5) (Pružinská et al. 2005; Müller et al. 2006), canola (Brassica napus; 4) (Mühlecker et al. 1993; Mühlecker and Kräutler 1996; Pružinská et al. 2005), maize (Zea mays; 2) (Berghold et al. 2006), C. japonicum (2) (Curty and Engel 1996; Oberhuber et al. 2003), barley (1) (Kräutler et al. 1991), Aztec tobacco (Nicotiana rustica; 2) (Berghold et al. 2004), Liquidambar orientalis (1) (Iturraspe et al. 1995), Liquidambar styraciflua (1) (Iturraspe et al. 1995), spinach (Spinacia oleacea; 5) (Berghold et al. 2002), peace lily (Spathiphyllum wallisii; 1) (Kräutler et al. 2010), pear (Pyrus communis; 2) (Müller et al. 2007). Despite the complexity of modifications occurring in NCCs, identical compounds were identified in several cases (Table 16.1). In particular Cj-NCC-1, which is derived from epi-pFCC and is hydroxylated at C82, is identical to NCCs isolated from three other plant species (Berghold et al. 2002; Müller et al. 2007).
The identification of NCCs as the major degradation end products of Chl brings into question the relevance of different types of (green) Chl catabolites that have been discussed as being genuine Chl breakdown intermediates. These include C132-hydroxylated Chls (Maunders et al. 1983; Schoch et al. 1984) and C132-decarboxymethylated (so-called ‘pyro’-) forms of pheophorbide or pheophytin (Schoch et al. 1981; Ziegler et al. 1988; Shimokawa et al. 1990). These intermediates might arise from unspecific oxidative reactions or be the result of artifacts of tissue extraction. For instance, the release of Mg2+ or the formation of pyro-forms readily takes place under acidic conditions (Amir-Shapira et al. 1987; Engel et al. 1996). In addition, the identification of pyropheophorbide as the ‘end’ product of in vitro Chl breakdown using enzyme preparations isolated from senescent leaves (Shioi et al. 1991) is possibly due to impropriate assay conditions that did not allow degradation beyond the level of pheophorbide (Pheide).
Recently, analysis of NCC structures has been extended to fruit ripening (Kräutler 2008). Different NCCs were isolated from the peels of ripening apples and pears and their constitution resolved (Müller et al. 2007). In addition, further, so far structurally uncharacterized, NCCs were identified in the peels of yellow bananas (Musa cavendish) (Moser et al. 2008a). The structures of the NCCs from fruits contain peripheral substituents like those found in NCCs isolated from leaves, and in the case of pear the same two NCCs were found in fruit peel and leaves (Müller et al. 2007). This indicates that the pathway of Chl breakdown is identical during leaf senescence and fruit ripening.
In most plant species analyzed so far, NCCs are the predominating final catabolites and FCCs are generally low in abundance. NCCs were shown to accumulate inside the vacuoles of senescing cells (Matile et al. 1988; Hinder et al. 1996) and for a long time their synthesis (from FCCs) was considered to occur before vacuolar import (Matile et al. 1996). However, experiments on Chl catabolite transport into isolated barley vacuoles (see below), indicated that FCCs rather than NCCs are imported into the vacuole (Hinder et al. 1996). This was corroborated by in vitro isomerization under acidic conditions of epi-pFCC to its corresponding NCC, which is identical in structure to Cj-NCC-2 (Oberhuber et al. 2003). Thus, after import into the vacuole, FCCs seem to be rapidly converted to NCCs by an acid-catalyzed isomerization. According to a mechanism proposed by Oberhuber et al. (2003), a free propionic acid side chain at C17 is required for this isomerization. This view is supported by the identification of persistent FCCs that are conjugated at C173 (see below). Furthermore, the FCC-to-NCC isomerization forms NCCs with a defined stereochemistry at C15, in which the sterically demanding functions at C132 and C15 are in trans configuration.
2. Fluorescent Chlorophyll Catabolites
As mentioned above, FCCs are generally low in abundance. However, several FCCs have been identified in senescent leaf extracts from different species (Ginsburg and Matile 1993; Bachmann et al. 1994; Pružinská et al. 2005) and the constitution of pFCC and some mFCCs have been resolved (Table 16.1). The fact that the modifications found in FCCs are identical to the ones of NCCs (Pružinská et al. 2005) supports the view that modification of these side positions occurs at the level of FCC rather than NCC. FCCs exhibit a characteristic UV absorption spectrum with maxima at 320 and 360 nm. In addition, they are blue fluorescing with a broad emission maximum around 430 nm. The fluorescence is due to the Schiff’s base configuration of the unsaturated γ-methine bridge linking pyrrole rings C and D, which is lost upon FCC-to-NCC isomerization.
In contrast to the low abundance of FCCs in senescent leaves of many species, it was recently shown that ripening bananas accumulate FCCs to rather high concentrations. These FCCs seem therefore not to occur transiently, but to persist to late ripening stages (Moser et al. 2008a, 2009). Subsequently, persistent FCCs have also been identified from senescent banana (Banala et al. 2010) and peace lily leaves (Kräutler et al. 2010). This new and unexpected finding is explained by the structures of these FCCs, which has been elucidated in some cases; in these FCCs, the C17 propionic acid group is conjugated with different moieties, including unusual moieties such as daucic acid and different C6-linked substituted pyranose units (Table 16.1). As a consequence of C17-modification, the normal acid-induced isomerization to NCCs does not occur and these FCCs have been termed ‘hypermodified’ (hFCC) (Moser et al. 2009). The identification of hFCCs indicates the existence of a second variant fate of Chl catabolites within the PAO pathway, i.e. C17-modified catabolites persist as FCCs, whereas unmodified ones are converted to NCCs. Interestingly, both variants, i.e. hFCCs and NCCs, occur simultaneously in banana and peace lily, pointing to the possibility that hFCCs might have some physiological role (Moser et al. 2009), e.g. in producing an optical effect protecting, for instance, against herbivores. Yet so far, hFCCs have been identified in only a few species, and large scale screening of senescent tissue for the presence of hFCC is needed in order to show how wide-spread hypermodification of FCCs is within the plant kingdom. This will be a prerequisite for investigating the possible physiological function of hFCCs.
3. Degradation Beyond FCCs/NCC?
In most plant species analyzed so far, abundance of NCCs increases with progression of senescence, in agreement with the idea that they are the final products of Chl breakdown. This is supported by calculations for C. japonicum (Curty and Engel 1996) and canola (Ginsburg and Matile 1993) that show that the amounts of NCCs account for almost all metabolized Chl. However, several further breakdown products have been identified, indicating a possible degradation beyond the NCC level. An urobilinogenoidic derivative of Hv-NCC-1 (Losey and Engel 2001) and monopyrrolic Chl catabolites were identified in barley (Suzuki and Shioi 1999). Furthermore, senescent leaves of C. japonicum contained small amounts of a yellow-colored Chl catabolite (YCC), an oxidation product of Cj-NCC-1 (Moser et al. 2008b). It remains to be shown whether any of these compounds are synthesized by enzymes as part of a defined pathway within intact senescing cells, or whether they are formed by unspecific oxidation events after tissue disintegration at late stages of senescence.
B. Biochemistry of the PAO Pathway of Chlorophyll Breakdown
FCCs and NCCs exhibit a common tetrapyrrolic backbone structure, which is the result of the activity of PAO. This points to the existence of a basic common pathway, which we nowadays call the PAO pathway. Analysis of mutants affected in different steps of Chl breakdown as well as inhibition and cell fractionation studies allowed the identification of further intermediates of Chl breakdown, in particular Pheide a. Furthermore, the establishment of in vitro enzyme assays and crucial efforts in chemical synthesis of potential intermediates (Kräutler et al. 1997; Oberhuber et al. 2008) provided the basis for the elucidation of most of the individual steps of the pathway and allowed the recent cloning of Chl catabolic genes. Mutants are available for all known enzymes involved in the pathway. The following sections summarize our current knowledge of the PAO pathway.
1. Chlorophyll b to Chlorophyll a Reduction
Apart from At-NCC-3 (Müller et al. 2006), all FCCs and NCCs identified so far from higher plants are derived from Chl a. In addition, barley leaf senescence in the presence of D2O partially labeled Hv-NCC-1, indicating it to derive in part from Chl b (Folley and Engel 1999). Furthermore, PAO was shown to accept Pheide a, but not Pheide b, as substrate (Hörtensteiner et al. 1995), and PAO mutants (see below) specifically accumulated Pheide a (Pružinská et al. 2003; Tanaka et al. 2003). Together these data demonstrated that conversion of Chl b to Chl a occurs before further degradation via PAO and upstream of Pheide formation. Biochemically, Chl b-to-Chl a conversion constitutes the reductive half of the so-called Chl cycle, which allows interconversion of Chl(ide) a and Chl(ide) b via C7-hydroxymethyl Chl(ide) (Rüdiger 2002). Substrate preferences of the enzymes involved indicate that the oxidative part is predominantly active towards Chlide (Oster et al. 2000), while the reductive part mainly uses phytylated pigments as substrate (Scheumann et al. 1999). The cycle is important to balance the ratio of a- and b-type pigments for adaptation to particular physiological conditions, for example changing light intensities.
Both oxygenation steps from Chlide a to Chlide b are catalyzed by Chlide a oxygenase, a Rieske-monooxygenase (Tanaka et al. 1998; Oster et al. 2000), which was recently shown to be regulated by a feedback mechanism (Sakuraba et al. 2007). Chl b modulates the stability of Chlide a oxygenase through the activity of the chloroplast Clp protease system (Sakuraba et al. 2009).
Chl b reduction was shown to require two different enzymes, Chl b reductase and hydroxymethyl Chl reductase (Ito et al. 1996; Scheumann et al. 1998). Whereas the latter is a stroma-localized, ferredoxin (Fd)-dependent enzyme whose molecular nature is not yet known, Chl b reductase has been identified in a forward genetic screen for stay-green mutants in rice (Kusaba et al. 2007). The protein identified, NON-YELLOW COLORING1 (NYC1), is a member of the family of short-chain dehydrogenases/reductases with three predicted transmembrane spanning domains. These features fit with the biochemically identified dependence on NADPH as electron source and the predicted localization of Chl b reductase activity at the thylakoid membrane (Scheumann et al. 1999). Furthermore, NYC1 gene expression correlated with leaf senescence (Kusaba et al. 2007), confirming the increase of Chl b reductase activity which was demonstrated in senescing barley leaves (Scheumann et al. 1999). The analysis of the rice nyc1 mutant, which showed particular retention of LHCII subunits and Chl b, favored the assumption that NYC1 encodes Chl b reductase (Kusaba et al. 2007). This was largely confirmed when analyzing Arabidopsis mutants defective in the ortholog of NYC1 (At4g13250) (Horie et al. 2009). However, in vitro activity could not be demonstrated for either rice or Arabidopsis NYC1 (Kusaba et al. 2007; Horie et al. 2009), possibly because of the hydrophobic nature of these proteins. In both rice and Arabidopsis a close homolog of NYC1, NYC1 LIKE (NOL), is present and both proteins were shown to exhibit Chl b reductase activity when expressed in E. coli (Horie et al. 2009; Sato et al. 2009). In addition, rice NYC1 and NOL physically interact, and in line with this, NOL, although predicted to be a soluble protein, co-purifies with the thylakoid membrane (Sato et al. 2009). Chl b reductase is an early (or the initial) step of Chl breakdown and NOL of Arabidopsis was shown to reduce Chl b to hydroxymethyl Chl when isolated trimeric LHCII complexes were used as substrate. In addition, this reaction was sufficient to release Chl from the complexes, indicating that Chl b reductase activity is an initial reaction required for both Chl breakdown and degradation of LHCII proteins (Horie et al. 2009).
2. Mg-Dechelation and Dephytylation
In Bf 993, a stay-green mutant of F. pratensis, Pheide a was shown to accumulate upon senescence induction (Vicentini et al. 1995a). Likewise, inhibition of Chl breakdown through addition of iron chelators, such as 2,2’ dipyridyl, caused Pheide a accumulation, indicating that Pheide a is a genuine intermediate of Chl breakdown (Langmeier et al. 1993; Vicentini et al. 1995a). The establishment of an in vitro assay that involves PAO and RCCR and converts Pheide a to pFCC (Hörtensteiner et al. 1995) corroborated this assumption. It demonstrated that removal of phytol and the central magnesium atom of Chl precedes the chlorine ring opening reaction through PAO. There was still a puzzling question concerning the order of these two reactions. Until recently, phytol removal was considered to precede Mg-chelation (Hörtensteiner 2006; Tanaka and Tanaka 2006), although in some instances, pheophytin (Phein) had been identified as an intermediate indicating the possibility of the reactions occurring in inverse order (Amir-Shapira et al. 1987; Heaton and Marangoni 1996).
It has been suggested that phytol, after hydrolysis from Chl, is re-utilized for the synthesis of α-tocopherol because its abundance increases during senescence (Peisker et al. 1989; Rise et al. 1989). This requires the activation of phytol to phytyl pyrophosphate, the co-substrate of condensation with homogentisic acid, and nucleotide-dependent phosphorylation of phytol was demonstrated in spinach chloroplast extracts (Soll et al. 1980). A phytol salvage pathway for tocopherol biosynthesis was recently established (Ischebeck et al. 2006). It involves two sequential phosphorylation steps, the first of which is catalyzed by VTE5 of Arabidopsis (Valentin et al. 2006) in a CTP-dependent reaction (Ischebeck et al. 2006). The second enzyme, phytylphosphate kinase, has not yet been cloned.
In addition to feeding Chl-derived phytol into α-tocopherol biosynthesis, substantial quantities of phytol are found during senescence or nitrogen deprivation in esterified form, mainly with various fatty acids (Ischebeck et al. 2006; Gaude et al. 2007). These fatty acid phytyl esters accumulate predominantly within plastoglobules, lipoprotein vesicles of plastids, whose abundance and size increases during senescence and which are considered to have an important role in lipid metabolism (Bréhélin et al. 2007).
Chlorophyllase hydrolyzing phytol from Chl was biochemically identified 100 years ago (Willstätter and Stoll 1913) and was believed to be active in Chl breakdown during senescence. The biochemical properties of chlorophyllase are intriguing because the enzyme was shown to be highly active at temperatures above 45°C and at high concentrations of acetone. In addition, it hydrolyzes a wide variety of hydrophobic substrates, such as Chl, Phein and fatty acid esters (McFeeters 1975; Arkus et al. 2005), and also catalyzes transesterification reactions (Fiedor et al. 1992). Chlorophyllase activity was shown to be present constitutively, but a relationship between chlorophyllase and senescence-related Chl breakdown was inferred from the latency of the enzyme and its proposed localization at the chloroplast envelope (Matile et al. 1997). Thus, in plant tissue extracts, chlorophyllase activity can only be measured after solubilization with solvents or detergents (Trebitsh et al. 1993; Matile et al. 1997). In addition, it was considered that chlorophyllase was able to act on Chl only after its release from Chl-binding proteins and shuttle to the chloroplast envelope, involving an unknown Chl carrier (Matile et al. 1999).
In 1999, two groups independently succeeded in cloning chlorophyllase (CLH) genes from orange (Citrus sinensis) and white goosefoot (Chenopodium album) (Jakob-Wilk et al. 1999; Tsuchiya et al. 1999) based on amino acid sequence information obtained from purified protein fractions exhibiting in vitro chlorophyllase activity (Trebitsh et al. 1993; Tsuchiya et al. 1997). Since then, CLH genes have been described from a few other plant species, including Arabidopsis, broccoli (Brassica oleracea), Ginkgo biloba and wheat (Tsuchiya et al. 1999; Tang et al. 2004; Arkus et al. 2005; Chen et al. 2008). Surprisingly, prediction of subcellular localization revealed that some of the cloned CLHs might localize outside plastids, i.e. in the cytosol or the vacuole. CLHs were therefore considered to localize to different compartments, implying the existence of multiple pathways for Chl breakdown (Takamiya et al. 2000). Experimental analysis of subcellular CLH localization using different methods resulted in inconsistent conclusions. The two Arabidopsis CLHs, AtCLH1 and AtCLH2, localized to the cytosol when tagged with GFP (Schenk et al. 2007), while the N-terminus of G. biloba CLH targeted GFP to the chloroplast (Okazawa et al. 2006). Lemon (Citrus limon) CLH was shown by in situ immunofluorescence to reside inside the chloroplast in lemon flavedo tissue (Azoulay Shemer et al. 2008) and to co-purify with chloroplast membranes after heterologous expression in tobacco mesophyll protoplasts (Harpaz-Saad et al. 2007). Experiments aiming to investigate the involvement of CLHs in Chl breakdown produced similarly contradictory results: absence of one or both of AtCLH1 and AtCLH2 in the respective mutants (Schenk et al. 2007) as well as RNAi-based gene silencing of AtCLH1 (Kariola et al. 2005) or AtCLH2 (Liao et al. 2007) had little or no effect on Chl breakdown during leaf senescence. In addition, expression patterns of AtCLHs do not correlate with Chl breakdown (Zimmermann et al. 2004; Liao et al. 2007). This indicated that in Arabidopsis CLHs are not required for leaf senescence-related Chl breakdown. In contrast, investigations in some other species support an involvement of CLH in Chl breakdown. For example in broccoli, antisense-suppression of CLH delayed rates of postharvest head yellowing (Chen et al. 2008). Yet in the same tissue, expression of CLH genes does not correlate with progression of Chl breakdown (Büchert et al. 2011). During fruit ripening in Citrus species, CLH was convincingly shown to participate in Chl breakdown and also to promote Chl breakdown when expressed in squash (Cucurbita pepo) leaves or tobacco protoplasts (Harpaz-Saad et al. 2007; Azoulay Shemer et al. 2008).
The proposed non-involvement of CLHs in Arabidopsis leaf senescence prompted a search for alternative phytol-cleaving esterases. A candidate esterase was identified in three independent approaches, i.e. a functional genomics screen of the Arabidopsis proteome (Schelbert et al. 2009), a screen for genes co-expressed with NYE1/SGR in Arabidopsis (see below) (Ren et al. 2010) and a screen for stay-green mutants in rice (Morita et al. 2009). Yet only in one of these investigations, a functional analysis of the enzyme as a possible phytol hydrolase was performed (Schelbert et al. 2009). After heterologous expression, the Arabidopsis protein (At5g13800) exhibited esterase activity with Phein a or Phein b as substrate yielding the respective Pheide pigment. Surprisingly, the enzyme did not dephytylate Chl, pointing to an intriguing specificity towards metal-free pigments. The enzyme was therefore termed pheophytinase (PPH). This substrate specificity was consistent with accumulation of Phein a in senescent leaves of Arabidopsis pph mutants. pph exhibits a stay-green phenotype similar to the one observed in nyc1 mutants with high retention during senescence of thylakoid membrane structures, LHCII subunits and Chl (Morita et al. 2009; Schelbert et al. 2009; Ren et al. 2010). As expected for a Chl-dephytylating enzyme, PPH and its rice ortholog, NCY3, were shown to localize to the chloroplast. In addition, PPH/NYC3 mRNA increases with senescence, thus exhibiting high level of co-expression with other Chl catabolic genes such as PAO and NYE1/SGR (Ren et al. 2010). Similarly, PPH expression correlated with yellowing of broccoli heads, indicating that also during postharvest PPH but not CLH is active (Büchert et al. 2011).
In summary, there is increasing evidence that dephytylation occurs only after removal of Mg from Chl, and PPH/NYC3 is likely to be responsible for this reaction. This is certainly true for Arabidopsis and rice leaf senescence. However, for other systems, in particular fruit ripening, CLH could have a role (in addition?) and further investigations are required to solve this riddle. From a metabolic perspective it is understandable that Chl breakdown should proceed via PPH and not CLH, because then Chlide, the last precursor of Chl biosynthesis, is not simultaneously an intermediate of Chl breakdown. Hence, biosynthetic and catabolic reactions are entirely separated allowing better metabolic control of overall Chl metabolism.
The mechanism of Mg-dechelation has not been clear until now. Two types of activities have been described in the literature. In two cases, white goosefoot (Shioi et al. 1996a; Suzuki and Shioi 2002) and strawberry (Fragaria x ananassa) (Costa et al. 2002), heat-stable low-molecular weight compounds have been described that catalyze Mg-dechelation. These compounds were termed metal-chelating substance (MCS) and they have molecular weights of <400 Da (goosefoot) and 2,180 Da (strawberry). However, their molecular nature has so far not been determined. Inhibition studies indicated that MCS compounds may contain active SH-groups, pointing to a possible proteic nature (Shioi et al. 1996a; Costa et al. 2002). The speculation that MCS compounds merely represent prosthetic groups of Mg-dechelating proteins (Matile et al. 1996) has been refuted (Suzuki et al. 2005). The second type of Mg-dechelating activity was attributed to heat-labile proteins, termed Mg-releasing proteins (MRP) (Vicentini et al. 1995b; Suzuki and Shioi 2002). MRP activity has so far only been demonstrated using the artificial substrate chlorophyllin, i.e. alkali-hydrolyzed Chl, not with Chlide as substrate. This was interpreted as MCS being active in vivo (Kunieda et al. 2005). Considering the fact that, at least in some systems, Mg-dechelation occurs before dephytylation (Schelbert et al. 2009), it is possible that Chlide is not the natural substrate for Mg-dechelation. Hence, there is a need to re-examine MCS and MRP-like activities with Chl as substrate.
3. Macrocycle Ring Opening
The establishment of an in vitro assay that catalyzes conversion of Pheide a to pFCC (Ginsburg et al. 1994; Hörtensteiner et al. 1995) and the chemical synthesis of RCC (Kräutler et al. 1997), which is an intermediate of this reaction, were crucial for elucidating the mechanism of the two-step macrocycle ring opening reaction. A still puzzling aspect of this reaction is the fact that both individual steps, i.e. Pheide a-to-RCC conversion catalyzed by PAO and RCC-to-pFCC conversion catalyzed by RCCR, are inefficient on their own, and large amounts of pFCC can be produced only in the coupled reaction (Rodoni et al. 1997a). This biochemical property and the demonstration of physical interaction between PAO and RCCR using a bacterial two-hybrid system (Pružinská et al. 2007) strongly indicates that metabolic channeling takes place in the PAO/RCCR reaction.
PAO is an iron-dependent oxygenase, whose activity was shown to be inhibited by chelating substances such as 2,2¢-dipyridyl or o-phenanthroline (Ginsburg et al. 1994). In PAO, two iron centers, a Rieske iron-sulfur center and a mononuclear iron center, are present (Pružinská et al. 2003), the latter of which is responsible for the activation of molecular oxygen (Schmidt and Shaw 2001). pFCC-labeling experiments using 18O2 showed that PAO is a monooxygenase that specifically incorporates an oxygen atom derived from molecular oxygen at the formyl group attached to pyrrole ring B (Hörtensteiner et al. 1998). As for other Rieske type oxygenases, electrons required to drive the iron-redox cycle of PAO are supplied through reduced Fd (Ginsburg et al. 1994; Pružinská et al. 2003). Based on the distribution of its activity, PAO was believed to reside in the chloroplast envelope (Matile and Schellenberg 1996). PAO proteins do contain two C-terminally located transmembrane helices, and recent proteomics data indicate that PAO could also localize to thylakoid membranes (Joyard et al. 2009). A further C-terminal motif containing conserved cysteine residues was identified as a target of thioredoxin regulation, indicating redox-regulation of PAO function (Bartsch et al. 2008). As mentioned above, PAO exhibits an intriguing specificity for Pheide a, with Pheide b inhibiting the activity in a competitive manner. This specificity provides an explanation for the almost unique occurrence of Chl a-derived FCCs and NCCs.
Based on the biochemical properties of PAO, a functional genomics screen was performed in Arabidopsis to identify PAO at the molecular level (Pružinská et al. 2003). PAO turned out to be identical to ACCELERATED CELL DEATH (ACD) 1 (At3g44880) (Greenberg and Ausubel 1993), which after heterologous expression in E. coli exhibited PAO activity with properties similar to those of native PAO (Pružinská et al. 2003). In an independent approach using antisense silencing of candidate genes, ACD1 was also identified as likely to be PAO (Tanaka et al. 2003). PAO/ACD1 is the ortholog of LETHAL LEAF SPOT 1 in maize (Gray et al. 1997), and absence of these proteins in corresponding mutants or antisense lines from Arabidopsis, rice or tomato results in premature cell death phenotypes (Greenberg and Ausubel 1993; Gray et al. 2002; Spassieva and Hille 2002; Pružinská et al. 2003, 2005; Tanaka et al. 2003). In all cases investigated, Pheide a was shown to accumulate to high concentrations. The phototoxicity of Pheide was considered to trigger the observed cell death phenotype in a light-dependent manner. However, cell death in Arabidopsis PAO-antisense lines was recently shown to be light-independent and a cell death signaling mechanism involving Pheide a was proposed (Hirashima et al. 2009). Components of such a pathway have not yet been identified and it remains to be confirmed to what extent direct phototoxicity of Pheide a contributes to cell death.
RCCR is a soluble protein of about 30 kDa that catalyzes the C1/C20 reduction of RCC to pFCC. As in the case of PAO, electrons are supplied from reduced Fd, but the protein does not contain any known domain that would indicate the mechanism of the reaction. However, RCCR is distantly related to a family of Fd-dependent bilin reductases (Frankenberg et al. 2001). These include bilin reductases from algae and cyanobacteria required for phycobilin biosynthesis, such as phycoerythrobilin synthase (PebS) and phycocyanobilin:ferredoxin oxidoreductase (PcyA), as well as phytochromobilin synthase (HY2) from higher plants that catalyzes the final step in phytochrome chromophore biosynthesis (Kohchi et al. 2001). For PcyA and HY2, a reaction mechanism has been proposed that involves a radical intermediate resulting from direct transfer of an electron from Fd to a critical (conserved) glutamate residue in the enzymes (Tu et al. 2004, 2008). The recent elucidation of the crystal structures of Arabidopsis RCCR in the absence or presence of RCC (Sugishima et al. 2009, 2010) demonstrated a high degree of structural similarity to the 3D structures of PebS and PcyA (Hagiwara et al. 2006; Dammeyer et al. 2008), supporting the idea that in RCCR, a radical mechanism involving a glutamate residue (glutamate154 of Arabidopsis RCCR) is also active.
As mentioned above, formation of pFCC by RCCR is highly stereospecific, i.e. two possible C1-stereoisomers, pFCC or epi-pFCC, are formed. The constitution of both C1-epimers was verified by one- and two-dimensional NMR methods (Mühlecker et al. 1997, 2000). The source of RCCR defines this specificity, as shown by the analysis of more than 50 plant species (Hörtensteiner et al. 2000; Pružinská et al. 2007). In order to analyze this biochemically, chimeric proteins were produced in E. coli, in which parts of Arabidopsis RCCR (At4g37000) producing pFCC were replaced by the corresponding parts of tomato RCCR (specifically forming epi-pFCC). This attempt identified phenylalanine218, which when replaced by valine (present in tomato RCCR) switched Arabidopsis RCCR from pFCC to epi-pFCC production (Pružinská et al. 2007). Interestingly, this residue is located within the RCC binding pocket in the crystal structure (Sugishima et al. 2009), but 3D structure analysis of the phenylalanine218-to-valine variant did not convincingly explain the altered stereospecificity (Sugishima et al. 2010).
RCCR has been cloned from barley based on amino acid sequence information obtained from the purified protein (Rodoni et al. 1997b; Wüthrich et al. 2000). The partial cDNA sequence obtained exhibited high homology to ACD2, which had been identified in a genetic screen for accelerated cell death in Arabidopsis and which had been considered to be a component of a cell death signaling pathway (Greenberg et al. 1994). Chloroplast-import experiments confirmed the proposed (Ginsburg et al. 1994; Rodoni et al. 1997a) chloroplast localization of RCCR (Wüthrich et al. 2000). However, ACD2/RCCR also partially localizes to mitochondria (Mach et al. 2001), in particular in response to stress conditions, such as pathogen infection or protoporphyrin IX treatment (Yao and Greenberg 2006). Mitochondrial localization of ACD2 was believed to have a possible function in preventing the cellular death that is observed in acd2 mutants. Cell death in acd2 is preceded by an early mitochondrial oxidative burst (Yao et al. 2004) and it was proposed that ACD2 could protect mitochondria from such an oxidative burst (Yao and Greenberg 2006), but the mechanism of this protection has not been resolved yet. This, together with the observation that cell death in acd2 also occurred in Chl-free root protoplasts, called into question the role of RCCR/ACD2 as a Chl catabolic enzyme (Yao and Greenberg 2006). However recently, the in vivo participation of RCCR in Chl breakdown during senescence has been demonstrated (see below) (Pružinská et al. 2007).
When analyzing acd2 with respect to Chl breakdown, it turned out that progression of cell death in the mutant was highly correlated with the accumulation of RCC and RCC-like pigments (Pružinská et al. 2007). Cell death was light-dependent and coincided with the production of singlet oxygen, indicating a phototoxic effect of RCCs. Whether RCCs are directly phototoxic, or whether cell death is the result of a death signaling pathway involving ROS, remains to be demonstrated. In the case of the Arabidopsis flu mutant, which accumulates high levels of protochlorophyllide (Meskauskiene et al. 2001), cell death does not occur because of direct phototoxicity of this Chl biosynthetic intermediate. Instead, it was shown that protochlorophyllide-dependent production of singlet oxygen (op den Camp et al. 2003) triggers a cell death pathway that involves EXECUTER 1 and EXECUTER 2, two novel chloroplast-localized proteins with so far unknown function (Wagner et al. 2004; Lee et al. 2007).
Surprisingly, acd2 mutants are not entirely blocked at the level of RCCR, because despite the accumulation of RCCs they still produce FCCs and NCCs (Pružinská et al. 2007). Interestingly, for several of these catabolites, including pFCC, the presence of both C1-epimers could be shown. This implied a loss of stereospecificity during RCC reduction, indicating that this reduction occurs through a stereo-unselective mechanism that is unknown so far. In electrochemical reduction experiments on RCC, pFCC and epi-pFCC are obtained in equal quantities (Oberhuber et al. 2008). This demonstrates that under appropriate conditions, which are possibly also present in chloroplasts, (stereo-unselective) RCC-to-pFCC reduction could occur without the involvement of an enzyme. Complementation of acd2 with versions of RCCR that exhibited different C1-specificities in vitro resulted in stereospecifically uniform patterns of catabolites, which corresponded to the specificity of the complementing enzyme (Pružinská et al. 2007). These experiments allowed it to be unambiguously conclude that RCCR is active in Chl breakdown.
4. Modifications of the Primary Fluorescent Chlorophyll Catabolite
The diversity of known FCC- and NCC-type catabolites indicates that additional reactions occur after the common formation of pFCC (or epi-pFCC). Overall, pFCC side group modification has so far been shown to be possible at four different positions. While hydroxylation at C82 appears to be a common reaction, other modifications occur in a species-specific manner. For example, although several identical NCCs are found in canola and Arabidopsis, Bn-NCC-1, carrying a malonyl group at C82, is absent from Arabidopsis. Enzymes catalyzing these modifications have so far not been identified at the molecular level and only for some reactions have biochemical activities been demonstrated.
C132-demethylated catabolites have been identified in Brassicaceae, but not in many other species such as barley (Table 16.1). This pattern of occurrence of demethylated forms of catabolites fits well with the presence in these species of an enzyme termed pheophorbidase (Suzuki et al. 2002). Pheophorbidase (PPD) is capable of hydrolyzing the C132-methylester of Pheide, but it does not accept metal-containing chlorins or Cj-NCC-1 as substrate, indicating a high degree of substrate specificity (Suzuki et al. 2006). The product of the reaction, C132-carboxyl pyropheophorbide, was shown to decarboxylate spontaneously to pyropheophorbide (Shioi et al. 1996b), a proposed product of Chl breakdown found mainly in algae and during post-harvest senescence (Ziegler et al. 1988; Aiamla-or et al. 2010). PPD was recently cloned from radish (Raphanus sativus) and was predicted to localize to the cytosol (Suzuki et al. 2006). It is arguable whether Pheide is the true substrate for C132-demethylation, because Pheide a formation and further metabolism occurs in plastids (Hörtensteiner 2006; Kräutler and Hörtensteiner 2006), hence PPD action on Pheide would require an unlikely export from, and re-import into, senescing chloroplasts. In addition, Arabidopsis pao1 does not accumulate C132-carboxyl pyropheophorbide or pyropheophorbide (Pružinská et al. 2005). PPD is a serine-type esterase and is highly homologous to the Arabidopsis methyl esterase (MES) protein family. The closest homolog of PPD, MES16, was shown to hydrolyze methyl esters of jasmonic acid and indole acetic acid (Yang et al. 2008).
An NCC that carries a malonyl group attached to C82 has been found in canola (Mühlecker et al. 1993). In this case, C82-hydroxylation by a (so far unknown) mechanism precedes malonylation (Hörtensteiner 1998). Likewise, Nr-NCC-2, a C82-glucosylated NCC from tobacco, was shown to have a malonylated counterpart, Nr-NCC-1 (Berghold et al. 2004). In both cases malonyltransferase reactions have been demonstrated that transfer the malonyl moiety from malonyl-coenzyme A to the NCC substrate. The activity isolated from canola was shown to be specific for Chl catabolites, but because of the presumed cytosolic localization of the transferase (Hörtensteiner 1998), FCCs rather than NCCs are the likely in vivo substrates.
C. Subcellular Localization of the Pathway and Catabolite Transport
Chl is localized in chloroplasts and the early catabolic reactions (at least up to pFCC) also localize to this organelle. This view is corroborated by the finding that isolated senescent chloroplasts are capable of pFCC formation and release (Matile et al. 1992). The site of pFCC-modifying activities is most probably located in the cytosol. This is deduced from the biochemical properties of the modifying enzymes characterized so far (Hörtensteiner 1998; Suzuki et al. 2006). A possible exception might be C82 hydroxylation. In addition to pFCC, isolated chloroplasts produce a second more polar FCC (Schellenberg et al. 1990; Ginsburg et al. 1994), which can serve as the substrate for malonylation, indicating it to be hydroxylated (own unpublished results).
Despite the undoubted plastidial localization of the Chl catabolic enzymes involved in the above-described PAO pathway (Wüthrich et al. 2000; Kusaba et al. 2007; Ren et al. 2007; Schelbert et al. 2009), several reports hint at extra-plastidial pathways for Chl catabolism (Takamiya et al. 2000). These include the identification of different types of chloroplast-derived vesicles, which participate in the breakdown of chloroplast constituents (Guiamét et al. 1999; Otegui et al. 2005; Martínez et al. 2008; see also Costa et al., Chap. 18), and a possible role for autophagic processes playing a role in chloroplast degradation during senescence (Ishida et al. 2008; Wada et al. 2009; see also Wada and Ishida, Chap. 19). However, to date none of these processes have demonstrated to involve Chl catabolism. The stay-green and cell death phenotypes of Chl catabolic mutants suggest that the majority of Chl is degraded via the PAO pathway inside senescing chloroplasts and that extra-plastidial pathways, may make – if at all – only a minor contribution.
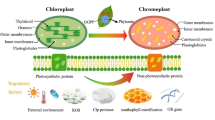
Different experimental approaches, such as fluorescent protein fusion analysis, proteomics and chloroplast-subfractionation studies, have been used to address the sub-chloroplast localization of the PAO pathway enzymes. The results are partially conflicting. Thus, NYC1 protein and Chl b reductase activity reside in the thylakoid membrane (Scheumann et al. 1999; Sato et al. 2009), while NOL, which was shown to interact with NYC1, localized to the envelope in a proteomics study (Joyard et al. 2009). Likewise, RCCR is a soluble protein localizing to the stroma (Rodoni et al. 1997b; Joyard et al. 2009), yet RCCR physically interacts with PAO (Pružinská et al. 2007), which was shown to localize to the envelope (Matile and Schellenberg 1996; Joyard et al. 2009). Finally, PPH was also localized to the stroma (Schelbert et al. 2009), but since from its activity towards Phein it has been inferred that it is likely to be attached to LHCs, localization in the thylakoid membrane would be expected. In summary, the data obtained so far imply that Chl catabolism may occur at both thylakoid and envelope membranes (Fig. 16.2). As a consequence a mechanism for shuttling Chl pigments from the thylakoid to the envelope has been postulated (Matile et al. 1999), but the nature of the Chl shuttle is unknown. Alternatively, the formation of contact between thylakoid and envelope membranes at sites of active Chl catabolism could overcome the spatial separation of enzymes of the PAO pathway.
Topographical model of the PAO pathway of Chl breakdown. The model updates the current state of knowledge about the pathway and incorporates the (presumed) subcellular localization of Chl catabolites and Chl catabolic enzymes. Putative steps are labeled with question marks. For abbreviations see the text.
Chl catabolites are deposited in the vacuoles of senescing cells (Matile et al. 1988; Hinder et al. 1996), indicating that two membranes, the chloroplast envelope and the tonoplast, need to be crossed (Fig. 16.2). In both cases, active transport processes are involved, but only for vacuolar import has the transport been shown to be primary active (Matile et al. 1992; Hinder et al. 1996). This implied the participation of members of the ATP binding cassette (ABC) transporter family, and for two of these transporters, ABCC2 and ABCC3, transport activity for Bn-NCC-1 has been demonstrated (Lu et al. 1998; Tommasini et al. 1998). However, the nature of the in vivo transporter(s) remains uncertain and an Arabidopsis ABCC2 mutant was only marginally affected in senescence (Frelet-Barrand et al. 2008). This might be due to functional redundancy of different transporters. Export from senescing chloroplasts required the (extra-plastidial) presence of a hydrolysable nucleotide, but ATP could be replaced by UTP (Matile et al. 1992). The nature of the transporter at the chloroplast envelope is unknown.
IV. Chlorophyll Breakdown and Its Relation to Stress Response
In addition to leaf senescence and fruit ripening, many biotic and abiotic stresses cause loss of Chl. However, the mechanism of Chl disappearance is not well understood and in only a few cases has a direct relation to the PAO pathway been demonstrated (Yang et al. 2004; Mur et al. 2010). Analysis of a large number of microarray studies investigating stress-related gene expression (Zimmermann et al. 2004) indicates that many Chl catabolic genes are regulated in response to different abiotic stress conditions, in particular drought and osmotic stress, as well as in response to challenge by several pathogens, including Pseudomonas syringae. For most abiotic stress conditions that result in Chl degradation, it remains to be established to what extent the PAO pathway contributes. In situations where stress results in cell death, Chl disappearance could be due to unspecific peroxidative or photooxidative pathways that become active after tissue death.
In contrast, a large body of evidence exists that relates Chl breakdown to pathogen infection as an active response process of plants. Thus, the Chl catabolic mutants of Arabidopsis, acd1 and acd2, were originally identified because of accelerated cell death in response to infection with P. syringae (Greenberg and Ausubel 1993; Greenberg et al. 1994). Furthermore, execution of the hypersensitive response (HR) in the Arabidopsis-P. syringae pathogenesis system is linked to light, i.e. a plant’s defense potential following an incompatible interaction that results in localized cell death is increased in the light compared to the dark (Zeier et al. 2004; Griebel and Zeier 2008). Among other mechanisms, imbalances in Chl metabolism were considered to trigger pathogen defense through ROS signaling. As shown for several lesion mimic mutants (Ishikawa et al. 2001; Mach et al. 2001; Meskauskiene et al. 2001; Pružinská et al. 2003) and transgenic plants de-regulated in Chl metabolism (Kruse et al. 1995; Tanaka et al. 2003), ROS production is linked to the photodynamic properties of many Chl metabolic intermediates. Recently, a stay-green mutant of Arabidopsis was shown to suppress HR-related cell death, and Pheide a played a crucial role in ROS-mediated establishment of the HR (Mur et al. 2010). This indicates that Chl breakdown could have an impact on cell death during the HR, explaining at least in part the known light-dependency of HR in Arabidopsis as elicited by some strains of P. syringae, but possibly also causing HR-like phenotypes in other instances of plant-pathogen and plant-herbivore interactions (Roberts and Paul 2006). However, it remains to be shown whether Chl catabolites are directly involved in the execution of cell death after pathogen attack.
V. Regulation of Chlorophyll Breakdown
A. The Stay-Green Protein
In many species, stay-green mutants are known that upon induction of senescence show retention of greenness (as compared to the respective wild types). These have been categorized into two principal groups: functional and non-functional (also called cosmetic) stay-green mutants (Thomas and Howarth 2000). The difference between these categories is whether retention of greenness is coupled to loss (cosmetic stay-greens) or retention (functional stay-greens) of photosynthetic capacity. Thus, cosmetic stay-green mutants [categorized as type C (Thomas and Howarth 2000)] show normal senescence behavior, but retain green color, indicating them to be defective in Chl breakdown. As outlined above, mutations affecting some Chl catabolic enzymes such as PPH or NYC1 have been shown to fall into the type C category. However, most cosmetic stay-green mutants that have been identified from naturally occurring varieties of different species or during screening programs for stay-green phenotypes are defective in a different gene, termed STAY-GREEN (SGR). Plant varieties in which SGR deficiency has been proven include fruit ripening mutants of bell pepper (Capsicum annuum; chlorophyll retainer) (Efrati et al. 2005; Barry et al. 2008) and tomato (green flesh) (Barry et al. 2008) as well as Gregor Mendel’s famous I locus mutant of pea (Pisum sativum) (Armstead et al. 2007; Sato et al. 2007), Arabidopsis nonyellowing1 (nye1) (Ren et al. 2007), different rice mutants (Jiang et al. 2007; Park et al. 2007; Sato et al. 2007) and Bf 993 of F. pratensis (Armstead et al. 2006, 2007). It is to be expected that molecular defects in SGR are also present in other phenotypic type C mutants, such as soybean (Glycine max) d 1 d 2 (Guiamét et al. 1991) and Arabidopsis ore10 (Oh et al. 2003).
SGR proteins of different species share a high degree of sequence similarity (Hörtensteiner 2009), contain a C-terminally located cysteine-rich consensus sequence of unknown function (Aubry et al. 2008) and are targeted to the chloroplast (Park et al. 2007; Ren et al. 2007; Sato et al. 2007). However, a clear (biochemical) function for SGR remains elusive. For the Mendel’s I locus mutant and Bf 993 a link to PAO function was postulated, because the mutants had diminished PAO activity and accumulated Chlide and Pheide a (Vicentini et al. 1995a; Thomas et al. 1996). However, a recent detailed re-analysis of this hypothesis showed that SGR acts independently and upstream of PAO (Aubry et al. 2008). Interestingly, rice SGR was shown to interact with LHCII, but not LHCI, subunits, and this interaction was not compromised in a valine99 point mutation of rice SGR, which causes the stay-green phenotype (Park et al. 2007). Thus, it has been speculated that SGR could be important for destabilization of Chl-protein complexes as a prerequisite for subsequent Chl and apoprotein degradation, but the (point-) mutations shown to be present in several SGR mutants (Hörtensteiner 2009) could (in addition) affect an so far unknown enzyme activity or may affect binding of other factors required for Chl-apoprotein degradation (Park et al. 2007). This view is corroborated by the fact that during senescence mutants defective in SGR specifically retain LHCII subunits. In contrast, LHCI peptides are less affected and a corresponding activity that may control Chl-apoprotein degradation in PSI awaits detection. Interestingly, most plant species analyzed so far contain at least two SGR genes, but whether the respective paralogs could be involved in LHCI degradation remains to be shown. In the case of Arabidopsis, only SGR1 (At4g22920) deficiency causes the stay-green phenotype described; absence of SGR2 (At4g11910) does not affect Chl breakdown (Aubry et al. 2008).
In summary, SGR seems to function in destabilization of Chl-binding proteins. According to this view it is not itself a Chl catabolic enzyme, but may be required to allow the catabolic enzymes to access their substrates. Thus, SGR can be considered a regulator of both Chl and apoprotein degradation, which acts at the level of Chl-apoprotein complex stability. It is interesting to note that during senescence, Bf 993 accumulates N-terminally truncated fragments of LHCII subunits, indicating that absence of SGR prevents the membrane-embedded core part of the complexes from being proteolytically degraded (Thomas and Howarth 2000). SGR might work in addition to or in concert with NYC1/NOL. Stability of Chl-apoprotein complexes was shown to require a defined ratio of Chl a and Chl b (Horn and Paulsen 2004) and as a consequence Chl b-less mutants are pale and LHCII apoproteins are unstable in these mutants (Harrison et al. 1993).
B. Chlorophyll Breakdown and Its Relation to Nitrogen Metabolism
Remobilization of nutrients from leaves to storage organs or seeds is an intrinsic feature of leaf senescence. Nitrogen in particular is efficiently remobilized and the photosynthetic apparatus, containing some 20% of total cellular nitrogen (Peoples and Dalling 1988), contributes a major source. In contrast, Chl only accounts for about 2% of nitrogen in a leaf cell, a fraction which is lost when FCC/NCC-containing senescent leaves shed from the plant. Thus, Chl degradation is not aimed at recycling pigment-bound nitrogen, but Chl breakdown can be seen as an important detoxification process that is a prerequisite for the remobilization of nitrogen bound in Chl-apoproteins. Cosmetic stay-green mutants defective in SGR, NYC1 or PPH retain large fractions of these Chl-binding proteins, but so far the impact of this loss, e.g. for seed filling, has not been thoroughly investigated. It can be hypothesized that annual plants may be less affected than perennial species, such as for example deciduous trees. In addition, severe effects might only be seen under nitrogen-limiting growth conditions.
Except for D1 of the PSII reaction center, which is turned over by the joint activity of at least two types of proteases, DegP and FtsH (Adam et al. 2006; Sakamoto 2006), proteases responsible for the degradation of Chl-binding proteins are largely unknown, although members of the families of Lon, Clp and FtsH proteases have been implicated (Sakamoto 2006; Liu et al. 2008).
C. Transcriptional Control of the PAO Pathway
Chl breakdown is the visible symptom of senescence and the status of yellowing has been proposed as a biomarker of leaf senescence (Ougham et al. 2008). Thus, the phenotypic progression of senescence is mostly correlated with Chl breakdown. Nevertheless, the process of yellowing is integrated into fundamental metabolic changes that occur during leaf senescence and that are termed the ‘senescence syndrome’ (Smart 1994). These include structural changes, such as the chloroplast-to-gerontoplast transition, as well as biochemical processes largely aiming at the recycling of nutrients. Initiation and progression of the syndrome depend on a regulatory network, which is only partially understood so far (Lim et al. 2006). Regulation of Chl breakdown is integral to the regulation of leaf senescence. This is evident from the fact that, although specific sets of genes are up-regulated when different types of leaf senescence are compared, Chl catabolic genes belong to those genes that are up-regulated under all conditions tested (Buchanan-Wollaston et al. 2005; Van der Graaff et al. 2006). Yet in Arabidopsis, several Chl catabolic genes are, for example, also expressed in petals, which do not contain Chl (Zimmermann et al. 2004). This indicates that regulation of the PAO pathway could go beyond leaf senescence. In line with this is the observation that the genes of the PAO pathway that are known to be transcriptionally regulated, i.e. SGR, NYC1, PPH and PAO, are highly co-regulated (Ren et al. 2010) and consequently cluster closely together when performing gene network analyses (Fig. 16.3) (Obayashi et al. 2009). This suggests the possible involvement of specific transcription factors regulating the PAO pathway. Although several hundreds of transcription factors have been shown to be differentially regulated during leaf senescence (Balazadeh et al. 2008), factors that specifically target Chl catabolic genes are unknown. Interestingly, Arabidopsis RCCR is not co-regulated with the other known PAO pathway genes (Fig. 16.3), supporting the idea that RCCR may have additional functions (Yao et al. 2004; Yao and Greenberg 2006). Similarly, expression of Arabidopsis NOL clusters with RCCR rather than with the other genes. This is different from the situation in rice, where NOL and NYC1 both show senescence-related enhancement of expression (Sato et al. 2009).
Gene network of Chl catabolic genes in Arabidopsis thaliana. Coexpression analysis was performed with the NetworkDrawer tool of the ATTED2 platform (http://atted.jp/top_draw.shtml#NetworkDrawer) using as input the Chl catabolic genes shown in light gray shading (SGR1, NYE1, At4g22920; PPH, hydrolase, At5g13800; PAO, ACD1, At3g44880; NYC1, At4g13250; RCCR, ACD2, At4g37000; NOL, At5g04900). Thickness of lines connecting the different genes indicates relative extent of coexpression with the thickest lines denoting the highest levels of coexpression. Note that the dotted line between PAO (ACD1) and RCCR (ACD2) indicates that the two proteins were shown to physically interact, but there is no coexpression between the two respective genes. Transcription factor genes are shown with octagon-shaped symbols, whereas all other genes have circular symbols.
D. Metabolic Control of the PAO Pathway?
Chl catabolic mutants such as pph-1 and pao1 retain large quantities of Chl and accumulate only comparatively small quantities of the respective intermediates, Phein a and Pheide a (Pružinská et al. 2005; Schelbert et al. 2009). This indicates the existence of feedback control mechanisms that prevent further degradation of Chl if the pathway is blocked. In the case of PAO mutants, retention of greenness was shown to correlate with reduced abundance of SGR transcripts (Park et al. 2007). Thus, a retrograde signaling pathway seems to exist that regulates Chl catabolic gene expression in response to disturbances of the chloroplast-localized PAO pathway. The mechanism of signaling remains to be elucidated.
VI. Conclusions and Outlook
Since the first elucidation of an NCC structure 20 years ago (Kräutler et al. 1991), the ‘biological enigma’ of Chl breakdown has largely been solved. Most of the Chl catabolic enzymes of the PAO pathway are known along with their respective catabolic intermediates and end products. Despite this major progress, Chl breakdown remains mysterious in several aspects. The surprising recent identification of persistent hFCCs implies that the pathway is even more complex than the proposed linear conversion of Chl down to NCCs (Hörtensteiner and Kräutler 2011). It also raises the intriguing possibility that Chl catabolites might not only be by-products of Chl detoxification, but that they might have some biological role. For example, NCCs have a high antioxidative potential (Müller et al. 2007), and hFCCs and a C. japonicum YCC have been shown to contribute, respectively, to the optical appearance of fruits and to the fall colors of deciduous trees (Moser et al. 2008b; Kräutler et al. 2010). Regarding the biochemistry of the PAO pathway, none of the enzymes that convert pFCC to mFCCs or hFCCs has been identified at the molecular level. Cloning the genes encoding these enzymes will not be an easy task, in particular because several side group modifying reactions occur in a species-specific manner. Furthermore, the molecular mechanism of Mg-dechelation is unclear, and the steps involved in Chl turnover at the steady state also remain enigmatic.
Chl breakdown occurs massively during fruit ripening as well as during leaf senescence. Several lines of evidence indicate that the PAO pathway is active as well. FCCs and NCCs have been identified from fruit sources (Moser et al. 2008a, 2009), and PAO and RCCR have been isolated from bell pepper chromoplast membranes (Moser and Matile 1997). Uncertainty remains concerning dephytylation, i.e. there is a need to show whether PPH and/or CLH is active during fruit ripening.
On the basis of sequence homology of the Chl catabolic enzymes, Chl breakdown through the PAO pathway is a common occurrence in higher plants, but comparison of the proteins to the available genomic sequences of lower plants indicates the presence of RCCR-, PPH- and/or PAO-like proteins in algae and even in cyanobacteria (Gray et al. 2004; Pružinská et al. 2007; Schelbert et al. 2009; Thomas et al. 2009). It remains to be demonstrated whether any of these homologs does indeed encode catalytically active Chl breakdown enzymes.
Abbreviations
- ABC:
-
ATP binding cassette;
- ACD:
-
Accelerated cell death;
- Chl:
-
Chlorophyll;
- Chlide:
-
Chlorophyllide;
- CLH:
-
Chlorophyllase;
- Fd:
-
Ferredoxin;
- hFCC –:
-
Hypermodified fluorescent Chl catabolite;
- HMR:
-
Hydroxymethyl Chl reductase;
- LHC:
-
Light harvesting complex;
- MCS:
-
Metal chelating substance;
- mFCC –:
-
Modified fluorescent Chl catabolite;
- NCC:
-
Nonfluorescent Chl catabolite;
- NOL:
-
NYC1-like;
- NYC:
-
Non yellow coloring;
- NYE:
-
Non yellowing;
- PAO:
-
Pheophorbide a oxygenase;
- pFCC:
-
Primary fluorescent Chl catabolite;
- Pheide:
-
Pheophorbide;
- Phein:
-
Pheophytin;
- PPH:
-
Pheophytinase;
- PS:
-
Photosystem;
- RCC:
-
Red chl catabolite;
- RCCR:
-
Red Chl catabolite reductase;
- ROS:
-
Reactive oxygen species
References
Adam Z, Rudella A, van Wijk KJ (2006) Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr Opin Plant Biol 9:234–240
Aiamla-or S, Kaewsuksaeng S, Shigyo M, Yamauchi N (2010) Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) florets. Food Chem 120:645–651
Amir-Shapira D, Goldschmidt EE, Altman A (1987) Chlorophyll catabolism in senescing plant tissues: in vivo breakdown intermediates suggest different degradative pathways for Citrus fruit and parsley leaves. Proc Natl Acad Sci USA 84:1901–1905
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arkus KAJ, Cahoon EB, Jez JM (2005) Mechanistic analysis of wheat chlorophyllase. Arch Biochem Biophys 438:146–155
Armstead I, Donnison I, Aubry S, Harper J, Hörtensteiner S, James C, Mani J, Moffet M, Ougham H, Roberts L, Thomas A, Weeden N, Thomas H, King I (2006) From crop to model to crop: identifying the genetic basis of the staygreen mutation in the Lolium/Festuca forage and amenity grasses. New Phytol 172:592–597
Armstead I, Donnison I, Aubry S, Harper J, Hörtensteiner S, James C, Mani J, Moffet M, Ougham H, Roberts L, Thomas A, Weeden N, Thomas H, King I (2007) Cross-species identification of Mendel’s I locus. Science 315:73
Aubry S, Mani J, Hörtensteiner S (2008) Stay-green protein, defective in Mendel’s green cotyledon mutant, acts independent and upstream of pheophorbide a oxygenase in the chlorophyll catabolic pathway. Plant Mol Biol 67:243–256
Azoulay Shemer T, Harpaz-Saad S, Belausov E, Lovat N, Krokhin O, Spicer V, Standing KG, Goldschmidt EE, Eyal Y (2008) Citrus chlorophyllase dynamics at ethylene-induced fruit color-break; a study of chlorophyllase expression, post-translational processing kinetics and in-situ intracellular localization. Plant Physiol 148:108–118
Bachmann A, Fernández-López J, Ginsburg S, Thomas H, Bouwcamp JC, Solomos T, Matile P (1994) Stay-green genotypes of Phaseolus vulgaris L.: chloroplast proteins and chlorophyll catabolites during foliar senescence. New Phytol 126:593–600
Balazadeh S, Riaño-Pachón DM, Mueller-Roeber B (2008) Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol 10(suppl 1):63–75
Ballottari M, Dall’Osto L, Morosinotto T, Bassi R (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. J Biol Chem 282:8947–8958
Banala S, Moser S, Müller T, Kreutz CR, Holzinger A, Lütz C, Kräutler B (2010) Hypermodified fluorescent chlorophyll catabolites: source of blue luminescence in senescent leaves. Angew Chem Int Ed 49:5174–5177
Barry CS, McQuinn RP, Chung MY, Besuden A, Giovannoni JJ (2008) Amino acid substitutions in homologs of the STAY-GREEN protein are responsible for the green-flesh and chlorophyll retainer mutations of tomato and pepper. Plant Physiol 147:179–187
Bartsch S, Monnet J, Selbach K, Quigley F, Gray J, von Wettstein D, Reinbothe S, Reinbothe C (2008) Three thioredoxin targets in the inner envelope membrane of chloroplasts function in protein import and chlorophyll metabolism. Proc Natl Acad Sci USA 105:4933–4938
Beisel KG, Jahnke S, Hofmann D, Köppchen S, Schurr U, Matsubara S (2010) Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by 14CO2 pulse-chase labeling. Plant Physiol 152:2188–2199
Berghold J, Breuker K, Oberhuber M, Hörtensteiner S, Kräutler B (2002) Chlorophyll breakdown in spinach: on the structure of five nonfluorescent chlorophyll catabolites. Photosynth Res 74:109–119
Berghold J, Eichmüller C, Hörtensteiner S, Kräutler B (2004) Chlorophyll breakdown in tobacco: on the structure of two nonfluorescent chlorophyll catabolites. Chem Biodivers 1:657–668
Berghold J, Müller T, Ulrich M, Hörtensteiner S, Kräutler B (2006) Chlorophyll breakdown in maize: on the structure of two nonfluorescent chlorophyll catabolites. Monatsh Chem 137:751–763
Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12:260–266
Brown SB, Houghton JD, Hendry GAF (1991) Chlorophyll breakdown. In: Scheer H (ed) Chlorophylls. CRC Press, Boca Raton, pp 465–489
Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42:567–585
Büchert AM, Civello PM, Martínez GA (2011) Chlorophyllase versus pheophytinase as candidates for chlorophyll dephytilation during senescence of broccoli. J Plant Physiol 168:337–343
Chen LFO, Lin CH, Kelkar SM, Chang YM, Shaw JF (2008) Transgenic broccoli (Brassica oleracea var. italicia) with antisense chlorophyllase (BoCLH1) delays postharvest yellowing. Plant Sci 174:25–31
Cornah JE, Terry MJ, Smith AG (2003) Green or red: what stops the traffic in the tetrapyrrole pathway? Trends Plant Sci 8:224–230
Costa ML, Civello PM, Chaves AR, Martinez GA (2002) Characterization of Mg-dechelatase activity obtained from Fragaria x ananassa fruit. Plant Physiol Biochem 40:111–118
Curty C, Engel N (1996) Detection, isolation and structure elucidation of a chlorophyll a catabolite from autumnal senescent leaves of Cercidiphyllum japonicum. Phytochemistry 42:1531–1536
Dammeyer T, Hofmann E, Frankenberg-Dinkel N (2008) Phycoerythrobilin synthase (PebS) of a marine virus – crystal structures of the biliverdin complex and the substrate-free form. J Biol Chem 283:27547–27554
Efrati A, Eyal Y, Paran I (2005) Molecular mapping of the chlorophyll retainer (cl) mutation in pepper (Capsicum spp.) and screening for candidate genes using tomato ESTs homologous to structural genes of the chlorophyll catabolism pathway. Genome 48:347–351
Engel N, Curty C, Gossauer A (1996) Chlorophyll catabolism in Chlorella protothecoides. 8. Facts and artefacts. Plant Physiol Biochem 34:77–83
Feierabend J, Dehne S (1996) Fate of the porphyrin cofactors during the light-dependent turnover of catalase and of the photosystem II reaction-center protein D1 in mature rye leaves. Planta 198:413–422
Fiedor L, Rosenbach-Belkin V, Scherz A (1992) The stereospecific interaction between chlorophylls and chlorophyllase – possible implication for chlorophyll biosynthesis and degradation. J Biol Chem 267:22043–22047
Folley P, Engel N (1999) Chlorophyll b to chlorophyll a conversion precedes chlorophyll degradation in Hordeum vulgare L. J Biol Chem 274:21811–21816
Frankenberg N, Mukougawa K, Kohchi T, Lagarias JC (2001) Functional genomic analysis of the HY2 family of ferredoxin-dependent bilin reductases from oxygenic photosynthetic organisms. Plant Cell 13:965–978
Frelet-Barrand A, Kolukisaoglu HU, Plaza S, Rüffer M, Azevedo L, Hörtensteiner S, Marinova K, Weder B, Schulz B, Klein M (2008) Comparative mutant analysis of Arabidopsis ABCC-type ABC transporters: AtMRP2 contributes to detoxification, vacuolar organic anion transport and chlorophyll degradation. Plant Cell Physiol 49:557–569
Gaude N, Brehelin C, Tischendorf G, Kessler F, Dörmann P (2007) Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J 49:729–739
Ginsburg S, Matile P (1993) Identification of catabolites of chlorophyll porphyrin in senescent rape cotyledons. Plant Physiol 102:521–527
Ginsburg S, Schellenberg M, Matile P (1994) Cleavage of chlorophyll-porphyrin. Requirement for reduced ferredoxin and oxygen. Plant Physiol 105:545–554
Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell 89:25–31
Gray J, Janick-Bruckner D, Bruckner B, Close PS, Johal GS (2002) Light-dependent death of maize lls1 cells is mediated by mature chloroplasts. Plant Physiol 130:1894–1907
Gray J, Wardzala E, Yang M, Reinbothe S, Haller S, Pauli F (2004) A small family of LLS1–related non-heme oxygenases in plants with an origin amongst oxygenic photosynthesizers. Plant Mol Biol 54:39–54
Greenberg JT, Ausubel FM (1993) Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J 4:327–341
Greenberg JT, Guo A, Klessig DF, Ausubel FM (1994) Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell 77:551–563
Griebel T, Zeier J (2008) Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol 147:790–801
Guiamét JJ, Schwartz E, Pichersky E, Noodén LD (1991) Characterization of cytoplasmic and nuclear mutations affecting chlorophyll and chlorophyll-binding proteins during senescence in soybean. Plant Physiol 96:227–231
Guiamét JJ, Pichersky E, Noodén LD (1999) Mass exodus from senescing soybean chloroplasts. Plant Cell Physiol 40:986–992
Hagiwara Y, Sugishima M, Takahashi Y, Fukuyama K (2006) Crystal structure of phycocyanobilin: ferredoxin oxidoreductase in complex with biliverdin IXα, a key enzyme in the biosynthesis of phycocyanobilin. Proc Natl Acad Sci USA 103:27–32
Harpaz-Saad S, Azoulay T, Arazi T, Ben-Yaakov E, Mett A, Shiboleth YM, Hörtensteiner S, Gidoni D, Gal-On A, Goldschmidt EE, Eyal Y (2007) Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell 19:1007–1022
Harrison MA, Nemson JA, Melis A (1993) Assembly and composition of the chlorophyll a-b light-harvesting complex of barley (Hordeum vulgare L.): immunochemical analysis of chlorophyll b-less and chlorophyll b-deficient mutants. Photosynth Res 38:141–151
Heaton JW, Marangoni AG (1996) Chlorophyll degradation in processed foods and senescent plant tissues. Trends Food Sci Technol 7:8–15
Hendry GAF, Stobart AK (1986) Chlorophyll turnover in greening barley. Phytochemistry 25:2735–2737
Hendry GAF, Houghton JD, Brown SB (1987) The degradation of chlorophyll – a biological enigma. New Phytol 107:255–302
Hilditch P, Thomas H, Rogers LJ (1986) Two processes for the breakdown of the QB protein of chloroplasts. FEBS Lett 208:313–316
Hinder B, Schellenberg M, Rodoni S, Ginsburg S, Vogt E, Martinoia E, Matile P, Hörtensteiner S (1996) How plants dispose of chlorophyll catabolites. Directly energized uptake of tetrapyrrolic breakdown products into isolated vacuoles. J Biol Chem 271:27233–27236
Hirashima M, Tanaka R, Tanaka A (2009) Light-independent cell death induced by accumulation of pheophorbide a in Arabidopsis thaliana. Plant Cell Physiol 50:719–729
Horie Y, Ito H, Kusaba M, Tanaka R, Tanaka A (2009) Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b-protein complexes in Arabidopsis. J Biol Chem 284:17449–17456
Horn R, Paulsen H (2004) Early steps in the assembly of light-harvesting chlorophyll a/b complex – time-resolved fluorescence measurements. J Biol Chem 279:44400–44406
Hörtensteiner S (1998) NCC malonyltransferase catalyses the final step of chlorophyll breakdown in rape (Brassica napus). Phytochemistry 49:953–956
Hörtensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57:55–77
Hörtensteiner S (2009) Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci 14:155–162
Hörtensteiner S, Kräutler B (2011) Chlorophyll breakdown in higher plants. Biochem Biophys Acta 1807:977–988
Hörtensteiner S, Vicentini F, Matile P (1995) Chlorophyll breakdown in senescent cotyledons of rape, Brassica napus L.: enzymatic cleavage of phaeophorbide a in vitro. New Phytol 129:237–246
Hörtensteiner S, Wüthrich KL, Matile P, Ongania K-H, Kräutler B (1998) The key step in chlorophyll breakdown in higher plants. Cleavage of pheophorbide a macrocycle by a monooxygenase. J Biol Chem 273:15335–15339
Hörtensteiner S, Rodoni S, Schellenberg M, Vicentini F, Nandi OI, Qiu Y-L, Matile P (2000) Evolution of chlorophyll degradation: the significance of RCC reductase. Plant Biol 2:63–67
Hu G, Yalpani N, Briggs SP, Johal GS (1998) A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize. Plant Cell 10:1095–1105
Ischebeck T, Zbierzak AM, Kanwischer M, Dörmann P (2006) A salvage pathway for phytol metabolism in Arabidopsis. J Biol Chem 281:2470–2477
Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Ohsumi Y, Hanson MR, Mae T (2008) Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol 148:142–155
Ishikawa A, Okamoto H, Iwasaki Y, Asahi T (2001) A deficiency of coproporphyrinogen III oxidase causes lesion formation in Arabidopsis. Plant J 27:89–99
Ito H, Ohysuka T, Tanaka A (1996) Conversion of chlorophyll b to chlorophyll a via 7-hydroxymethyl chlorophyll. J Biol Chem 271:1475–1479
Iturraspe J, Engel N, Gossauer A (1994) Chlorophyll catabolism. Isolation and structure elucidation of chlorophyll b catabolites in Chlorella protothecoides. Phytochemistry 35:1387–1390
Iturraspe J, Moyano N, Frydman B (1995) A new 5-formylbilinone as the major chlorophyll a catabolite in tree senescent leaves. J Org Chem 60:6664–6665
Jakob-Wilk D, Holland D, Goldschmidt EE, Riov J, Eyal Y (1999) Chlorophyll breakdown by chlorophyllase: isolation and functional expression of the Chlase1 gene from ethylene-treated Citrus fruit and its regulation during development. Plant J 20:653–661
Jiang H, Li M, Liang N, Yan H, Wei Y, Xu X, Liu J, Xu Z, Chen F, Wu G (2007) Molecular cloning and function analysis of the stay green gene in rice. Plant J 52:197–209
Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2009) Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol Plant 2:1154–1180
Kariola T, Brader G, Li J, Palva ET (2005) Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17:282–294
Kohchi T, Mukougawa K, Frankenberg N, Masuda M, Yokota A, Lagarias JC (2001) The Arabidopsis HY2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. Plant Cell 13:425–436
Kräutler B (2003) Chlorophyll breakdown and chlorophyll catabolites. In: Kadish KM, Smith KM, Guilard R (eds) The porphyrin handbook. Elsevier Science, Amsterdam, pp 183–209
Kräutler B (2008) Chlorophyll breakdown and chlorophyll catabolites in leaves and fruit. Photochem Photobiol Sci 7:1114–1120
Kräutler B, Hörtensteiner S (2006) Chlorophyll catabolites and the biochemistry of chlorophyll breakdown. In: Grimm B, Porra R, Rüdiger W, Scheer H (eds) Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. Springer, Dordrecht, pp 237–260
Kräutler B, Jaun B, Bortlik K-H, Schellenberg M, Matile P (1991) On the enigma of chlorophyll degradation: the constitution of a secoporphinoid catabolite. Angew Chem Int Ed Engl 30:1315–1318
Kräutler B, Mühlecker W, Anderl M, Gerlach B (1997) Breakdown of chlorophyll: partial synthesis of a putative intermediary catabolite. Helv Chim Acta 80:1355–1362
Kräutler B, Banala S, Moser S, Vergeiner C, Müller T, Lütz C, Holzinger A (2010) A novel blue fluorescent chlorophyll catabolite accumulates in senescent leaves of the peace lily (Spathiphyllum wallisii) and indicates a divergent path of chlorophyll breakdown. FEBS Lett 584:4215–4221
Kruse E, Mock HP, Grimm B (1995) Reduction of coproporphyrinogen oxidase level by antisense RNA synthesis leads to deregulated gene expression of plastid proteins and affects the oxidative defense system. EMBO J 14:3712–3720
Kunieda T, Amano T, Shioi Y (2005) Search for chlorophyll degradation enzyme, Mg-dechelatase, from extracts of Chenopodium album with native and artificial substrates. Plant Sci 169:177–183
Kusaba M, Ito H, Morita R, Iida S, Sato Y, Fujimoto M, Kawasaki S, Tanaka R, Hirochika H, Nishimura M, Tanaka A (2007) Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19:1362–1375
Langmeier M, Ginsburg S, Matile P (1993) Chlorophyll breakdown in senescent leaves: demonstration of Mg-dechelatase activity. Physiol Plant 89:347–353
Lee KP, Kim C, Landgraf F, Apel K (2007) EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc Natl Acad Sci USA 104:10270–10275
Liao Y, An K, Zhou X, Chen W-J, Kuai B-K (2007) AtCLH2, a typical but possibly distinctive chlorophyllase gene in Arabidopsis. J Integr Plant Biol 49:531–539
Lim PO, Kim HJ, Nam HG (2006) Leaf senescence. Annu Rev Plant Biol 58:115–136
Liu J, Wu YH, Yan JJ, Liu YD, Shen FF (2008) Protein degradation and nitrogen remobilization during leaf senescence. J Plant Biol 51:11–19
Losey FG, Engel N (2001) Isolation and characterization of a urobilinogenoidic chlorophyll catabolite from Hordeum vulgare L. J Biol Chem 276:27233–27236
Lu Y-P, Li Z-S, Drozdowicz Y-M, Hörtensteiner S, Martinoia E, Rea PA (1998) AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10:267–282
Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98:771–776
Martínez DE, Costa ML, Gomez FM, Otegui MS, Guiamet JJ (2008) ‘Senescence-associated vacuoles’ are involved in the degradation of chloroplast proteins in tobacco leaves. Plant J 56:196–206
Matile P, Schellenberg M (1996) The cleavage of pheophorbide a is located in the envelope of barley gerontoplasts. Plant Physiol Biochem 34:55–59
Matile P, Ginsburg S, Schellenberg M, Thomas H (1988) Catabolites of chlorophyll in senescing barley leaves are localized in the vacuoles of mesophyll cells. Proc Natl Acad Sci USA 85:9529–9532
Matile P, Schellenberg M, Peisker C (1992) Production and release of a chlorophyll catabolite in isolated senescent chloroplasts. Planta 187:230–235
Matile P, Hörtensteiner S, Thomas H, Kräutler B (1996) Chlorophyll breakdown in senescent leaves. Plant Physiol 112:1403–1409
Matile P, Schellenberg M, Vicentini F (1997) Localization of chlorophyllase in the chloroplast envelope. Planta 201:96–99
Matile P, Hörtensteiner S, Thomas H (1999) Chlorophyll degradation. Annu Rev Plant Physiol Plant Mol Biol 50:67–95
Maunders MJ, Brown SB, Woolhouse HW (1983) The appearance of chlorophyll derivatives in senescing tissue. Phytochemistry 22:2443–2446
McFeeters RF (1975) Substrate specificity of chlorophyllase. Plant Physiol 55:377–381
Melis A (1999) Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4:130–135
Meskauskiene R, Nater M, Goslings D, Kessler F, op den Camp R, Apel K (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 98:12826–12831
Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci 15:488–498
Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M (2009) Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J 59:940–952
Moser D, Matile P (1997) Chlorophyll breakdown in ripening fruits of Capsicum annuum. J Plant Physiol 150:759–761
Moser S, Müller T, Ebert MO, Jockusch S, Turro NJ, Kräutler B (2008a) Blue luminescence of ripening bananas. Angew Chem Int Ed 47:8954–8957
Moser S, Ulrich M, Müller T, Kräutler B (2008b) A yellow chlorophyll catabolite is a pigment of the fall colours. Photochem Photobiol Sci 7:1577–1581
Moser S, Müller T, Holzinger A, Lutz C, Jockusch S, Turro NJ, Kräutler B (2009) Fluorescent chlorophyll catabolites in bananas light up blue halos of cell death. Proc Natl Acad Sci USA 106:15538–15543
Mühlecker W, Kräutler B (1996) Breakdown of chlorophyll: constitution of nonfluorescing chlorophyll-catabolites from senescent cotyledons of the dicot rape. Plant Physiol Biochem 34:61–75
Mühlecker W, Kräutler B, Ginsburg S, Matile P (1993) Breakdown of chlorophyll: a tetrapyrrolic chlorophyll catabolite from senescent rape leaves. Helv Chim Acta 76:2976–2980
Mühlecker W, Ongania K-H, Kräutler B, Matile P, Hörtensteiner S (1997) Tracking down chlorophyll breakdown in plants: elucidation of the constitution of a ‘fluorescent’ chlorophyll catabolite. Angew Chem Int Ed Engl 36:401–404
Mühlecker W, Kräutler B, Moser D, Matile P, Hörtensteiner S (2000) Breakdown of chlorophyll: a fluorescent chlorophyll catabolite from sweet pepper (Capsicum annuum). Helv Chim Acta 83:278–286
Müller T, Moser S, Ongania K-H, Pružinská A, Hörtensteiner S, Kräutler B (2006) A divergent path of chlorophyll breakdown in the model plant Arabidopsis thaliana. Chembiochem 7:40–42
Müller T, Ulrich M, Ongania KH, Kräutler B (2007) Colorless tetrapyrrolic chlorophyll catabolites found in ripening fruit are effective antioxidants. Angew Chem Int Ed 46:8699–8702
Mur LAJ, Aubry S, Mondhe M, Kingston-Smith A, Gallagher J, Timms-Taravella E, James C, Papp I, Hörtensteiner S, Thomas H, Ougham H (2010) Accumulation of chlorophyll catabolites photosensitizes the hypersensitive response elicited by Pseudomonas syringae in Arabidopsis. New Phytol 188:161–174
Obayashi T, Hayashi S, Saeki M, Ohta H, Kinoshita K (2009) ATTED-II provides coexpressed gene networks for Arabidopsis. Nucleic Acids Res 37:D987–D991
Oberhuber M, Berghold J, Mühlecker W, Hörtensteiner S, Kräutler B (2001) Chlorophyll breakdown – on a nonfluorescent chlorophyll catabolite from spinach. Helv Chim Acta 84:2615–2627
Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B (2003) Breakdown of chlorophyll: a nonenzymatic reaction accounts for the formation of the colorless “nonfluorescent” chlorophyll catabolites. Proc Natl Acad Sci USA 100:6910–6915
Oberhuber M, Berghold J, Kräutler B (2008) Chlorophyll breakdown by a biomimetic route. Angew Chem Int Ed 47:3057–3061
Oh MH, Moon YH, Lee CH (2003) Increased stability of LHCII by aggregate formation during dark-induced leaf senescence in the Arabidopsis mutant, ore10. Plant Cell Physiol 44:1368–1377
Okazawa A, Tang L, Itoh Y, Fukusaki E, Kobayashi A (2006) Characterization and subcellular localization of chlorophyllase from Ginkgo biloba. Z Naturforsch C 61:111–117
op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, Nater M, Apel K (2003) Rapid induction of distinct stress responses after release of singlet oxygen in Arabidopsis. Plant Cell 15:2320–2332
Oster U, Tanaka R, Tanaka A, Rüdiger W (2000) Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J 21:305–310
Otegui MS, Noh YS, Martinez DE, Vila Petroff MG, Andrew Staehelin L, Amasino RM, Guiamet JJ (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J 41:831–844
Ougham H, Hörtensteiner S, Armstead I, Donnison I, King I, Thomas H, Mur L (2008) The control of chlorophyll catabolism and the status of yellowing as a biomarker of leaf senescence. Plant Biol 10(suppl 1):4–14
Park S-Y, Yu J-W, Park J-S, Li J, Yoo S-C, Lee N-Y, Lee S-K, Jeong S-W, Seo HS, Koh H-J, Jeon J-S, Park Y-I, Paek N-C (2007) The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell 19:1649–1664
Peisker C, Düggelin T, Rentsch D, Matile P (1989) Phytol and the breakdown of chlorophyll in senescent leaves. J Plant Physiol 135:428–432
Peoples MB, Dalling MJ (1988) The interplay between proteolysis and amino acid metabolism during senescence and nitrogen allocation. In: Noodén LD, Leopold AC (eds) Senescence and aging in plants. Academic, San Diego, pp 181–217
Pretty J, Peakock J, Hine R, Sellens M, South N, Griffin M (2007) Green exercise in the UK countryside: effects on health and physiological well-being, and implications for policy and planning. J Environ Plan Manag 50:211–231
Pružinská A, Anders I, Tanner G, Roca M, Hörtensteiner S (2003) Chlorophyll breakdown: pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc Natl Acad Sci USA 100:15259–15264
Pružinská A, Tanner G, Aubry S, Anders I, Moser S, Müller T, Ongania K-H, Kräutler B, Youn J-Y, Liljegren SJ, Hörtensteiner S (2005) Chlorophyll breakdown in senescent Arabidopsis leaves: characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol 139:52–63
Pružinská A, Anders I, Aubry S, Schenk N, Tapernoux-Lüthi E, Müller T, Kräutler B, Hörtensteiner S (2007) In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 19:369–387
Ren G, An K, Liao Y, Zhou X, Cao Y, Zhao H, Ge X, Kuai B (2007) Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiol 144:1429–1441
Ren GD, Zhou Q, Wu SX, Zhang YF, Zhang LG, Huang JR, Sun ZF, Kuai BK (2010) Reverse genetic identification of CRN1 and its distinctive role in chlorophyll degradation in Arabidopsis. J Integr Plant Biol 52:496–504
Rise M, Cojocaru M, Gottlieb HE, Goldschmidt EE (1989) Accumulation of α-tocopherol in senescing organs as related to chlorophyll degradation. Plant Physiol 89:1028–1030
Roberts MR, Paul ND (2006) Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol 170:677–699
Rodoni S, Mühlecker W, Anderl M, Kräutler B, Moser D, Thomas H, Matile P, Hörtensteiner S (1997a) Chlorophyll breakdown in senescent chloroplasts. Cleavage of pheophorbide a in two enzymic steps. Plant Physiol 115:669–676
Rodoni S, Vicentini F, Schellenberg M, Matile P, Hörtensteiner S (1997b) Partial purification and characterization of red chlorophyll catabolite reductase, a stroma protein involved in chlorophyll breakdown. Plant Physiol 115:677–682
Rüdiger W (2002) Biosynthesis of chlorophyll b and the chlorophyll cycle. Photosynth Res 74:187–193
Sakamoto W (2006) Protein degradation machineries in plastids. Annu Rev Plant Biol 57:599–621
Sakuraba Y, Yamasato A, Tanaka R, Tanaka A (2007) Functional analysis of N-terminal domains of Arabidopsis chlorophyllide a oxygenase. Plant Physiol Biochem 45:740–749
Sakuraba Y, Tanaka R, Yamasato A, Tanaka A (2009) Determination of a chloroplast degron in the regulatory domain of chlorophyllide a oxygenase. J Biol Chem 284:36689–36699
Sato Y, Morita R, Nishimura M, Yamaguchi H, Kusaba M (2007) Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc Natl Acad Sci USA 104:14169–14174
Sato Y, Moria R, Katsuma S, Nishimura M, Tanaka A, Kusaba M (2009) Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. Plant J 57:120–131
Schelbert S, Aubry S, Burla B, Agne B, Kessler F, Krupinska K, Hörtensteiner S (2009) Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 21:767–785
Schellenberg M, Matile P, Thomas H (1990) Breakdown of chlorophyll in chloroplasts of senescent barley leaves depends on ATP. J Plant Physiol 136:564–568
Schenk N, Schelbert S, Kanwischer M, Goldschmidt EE, Dörmann P, Hörtensteiner S (2007) The chlorophyllases AtCLH1 and AtCLH2 are not essential for senescence-related chlorophyll breakdown in Arabidopsis thaliana. FEBS Lett 581:5517–5525
Scheumann V, Schoch S, Rüdiger W (1998) Chlorophyll a formation in the chlorophyll b reductase reaction requires reduced ferredoxin. J Biol Chem 273:35102–35108
Scheumann V, Schoch S, Rüdiger W (1999) Chlorophyll b reduction during senescence of barley seedlings. Planta 209:364–370
Schmidt CL, Shaw L (2001) A comprehensive phylogenetic analysis of Rieske and Rieske-type iron-sulfur proteins. J Bioenerg Biomembr 33:9–26
Schoch S, Scheer H, Schiff JA, Rüdiger W, Siegelman HW (1981) Pyropheophytin a accompanies pheophytin a in darkened light grown cells of Euglena. Z Naturforsch C 36:827–833
Schoch S, Rüdiger W, Lüthy B, Matile P (1984) 132_hydroxychlorophyll a, the first product of the reaction of chlorophyll-oxidase. J Plant Physiol 115:85–89
Shimokawa K, Hashizume A, Shioi Y (1990) Pyropheophorbide a, a catabolite of ethylene-induced chlorophyll a degradation. Phytochemistry 29:2105–2106
Shioi Y, Tatsumi Y, Shimokawa K (1991) Enzymatic degradation of chlorophyll in Chenopodium album. Plant Cell Physiol 32:87–93
Shioi Y, Tomita N, Tsuchiya T, Takamiya K (1996a) Conversion of chlorophyllide to pheophorbide by Mg-dechelating substance in extracts of Chenopodium album. Plant Physiol Biochem 34:41–47
Shioi Y, Watanabe K, Takamiya K (1996b) Enzymatic conversion of pheophorbide a to a precursor of pyropheophorbide a in leaves of Chenopodium album. Plant Cell Physiol 37:1143–1149
Smart CM (1994) Gene expression during leaf senescence. New Phytol 126:419–448
Soll J, Kemmerling M, Schultz G (1980) Tocopherol and plastoquinone synthesis in spinach chloroplast subfractions. Arch Biochem Biophys 204:544–550
Spassieva S, Hille J (2002) A lesion mimic phenotype in tomato obtained by isolating and silencing an Lls1 homologue. Plant Sci 162:543–549
Stobart AK, Hendry GAF (1984) The turnover of chlorophyll in greening wheat leaves. Phytochemistry 23:27–30
Sugishima M, Kitamori Y, Noguchi M, Kohchi T, Fukuyama K (2009) Crystal structure of red chlorophyll catabolite reductase: enlargement of the ferredoxin-dependent bilin reductase family. J Mol Biol 389:376–387
Sugishima M, Okamoto Y, Noguchi M, Kohchi T, Tamiaki H, Fukuyama K (2010) Crystal structures of the substrate-bound forms of red chlorophyll catabolite reductase: implications for site-specific and stereospecific reaction. J Mol Biol 402:879–891
Suzuki Y, Shioi Y (1999) Detection of chlorophyll breakdown products in the senescent leaves of higher plants. Plant Cell Physiol 40:909–915
Suzuki T, Shioi Y (2002) Re-examination of Mg-dechelation reaction in the degradation of chlorophylls using chlorophyllin a as substrate. Photosynth Res 74:217–223
Suzuki Y, Doi M, Shioi Y (2002) Two enzymatic reaction pathways in the formation of pyropheophorbide a. Photosynth Res 74:225–233
Suzuki T, Kunieda T, Murai F, Morioka S, Shioi Y (2005) Mg-dechelation activity in radish cotyledons with artificial and native substrates, Mg-chlorophyllin a and chlorophyllide a. Plant Physiol Biochem 43:459–464
Suzuki Y, Amano T, Shioi Y (2006) Characterization and cloning of the chlorophyll-degrading enzyme pheophorbidase from cotyledons of radish. Plant Physiol 140:716–725
Takamiya K, Tsuchiya T, Ohta H (2000) Degradation pathway(s) of chlorophyll: what has gene cloning revealed? Trends Plant Sci 5:426–431
Tanaka A, Tanaka R (2006) Chlorophyll metabolism. Curr Opin Plant Biol 9:248–255
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346
Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA 95:12719–12723
Tanaka R, Hirashima M, Satoh S, Tanaka A (2003) The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: inhibition of pheophorbide a oxygenase activity does not lead to the “stay-green” phenotype in Arabidopsis. Plant Cell Physiol 44:1266–1274
Tang L, Okazawa A, Itoh Y, Fukusaki E, Kobayashi A (2004) Expression of chlorophyllase is not induced during autumnal yellowing in Ginkgo biloba. Z Naturforsch C 59:415–420
Thomas H, Howarth CJ (2000) Five ways to stay green. J Exp Bot 51:329–337
Thomas H, Schellenberg M, Vicentini F, Matile P (1996) Gregor Mendel’s green and yellow pea seeds. Bot Acta 109:3–4
Thomas H, Huang L, Young M, Ougham H (2009) Evolution of plant senescence. BMC Evol Biol 9:163
Tommasini R, Vogt E, Fromenteau M, Hörtensteiner S, Matile P, Amrhein N, Martinoia E (1998) An ABC transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J 13:773–780
Trebitsh T, Goldschmidt EE, Riov J (1993) Ethylene induces de novo synthesis of chlorophyllase, a chlorophyll degrading enzyme, in Citrus fruit peel. Proc Natl Acad Sci USA 90:9441–9445
Tsuchiya T, Ohta H, Masuda T, Mikami B, Kita N, Shioi Y, Takamiya K (1997) Purification and characterization of two isozymes of chlorophyllase from mature leaves of Chenopodium album. Plant Cell Physiol 38:1026–1031
Tsuchiya T, Ohta H, Okawa K, Iwamatsu A, Shimada H, Masuda T, Takamiya K (1999) Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: finding of a lipase motif and the induction by methyl jasmonate. Proc Natl Acad Sci USA 96:15362–15367
Tu SL, Gunn A, Toney MD, Britt RD, Lagarias JC (2004) Biliverdin reduction by cyanobacterial phycocyanobilin: ferredoxin oxidoreductase (PcyA) proceeds via linear tetrapyrrole radical intermediates. J Am Chem Soc 126:8682–8693
Tu SL, Chen HC, Ku LW (2008) Mechanistic studies of the phytochromobilin synthase HY2 from Arabidopsis. J Biol Chem 283:27555–27564
Tzoulas K, Korpela K, Venn S, Yli-Pelkonen V, Kazmierczak A, Niemela J, James P (2007) Promoting ecosystem and human health in urban areas using Green Infrastructure: a literature review. Landsc Urban Plan 81:167–178
Valentin HE, Lincoln K, Moshiri F, Jensen PK, Qi Q, Venkatesh TV, Karunanandaa B, Baszis SR, Norris SR, Savidge B, Gruys KJ, Last RL (2006) The arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 18:212–224
Van der Graaff E, Schwacke R, Schneider A, Desimone M, Flugge UI, Kunze R (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141:776–792
Vavilin D, Vermaas W (2007) Continuous chlorophyll degradation accompanied by chlorophyllide and phytol reutilization for chlorophyll synthesis in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1767:920–929
Vicentini F, Hörtensteiner S, Schellenberg M, Thomas H, Matile P (1995a) Chlorophyll breakdown in senescent leaves: identification of the biochemical lesion in a stay-green genotype of Festuca pratensis Huds. New Phytol 129:247–252
Vicentini F, Iten F, Matile P (1995b) Development of an assay for Mg-dechelatase of oilseed rape cotyledons, using chlorophyllin as the substrate. Physiol Plant 94:57–63
Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, Makino A (2009) Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol 149:885–893
Wagner D, Przybyla D, op den Camp R, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K (2004) The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science 306:1183–1185
Willstätter R, Stoll A (1913) Die Wirkungen der Chlorophyllase. In: Willstätter R, Stoll A (eds) Untersuchungen über Chlorophyll. Verlag Julius Springer, Berlin, pp 172–187
Wüthrich KL, Bovet L, Hunziker PE, Donnison IS, Hörtensteiner S (2000) Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J 21:189–198
Xiong J, Bauer CE (2002) Complex evolution of photosynthesis. Annu Rev Plant Biol 53:503–521
Yang M, Wardzala E, Johal GS, Gray J (2004) The wound-inducible Lls1 gene from maize is an orthologue of the Arabidopsis Acd1 gene, and the LLS1 protein is present in non-photosynthetic tissues. Plant Mol Biol 54:175–191
Yang Y, Xu R, Ma CJ, Vlot AC, Klessig DF, Pichersky E (2008) Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiol 147:1034–1045
Yao N, Greenberg JT (2006) Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 18:397–411
Yao N, Eisfelder BJ, Marvin J, Greenberg JT (2004) The mitochondrion – an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40:596–610
Zeier J, Pink B, Mueller MJ, Berger S (2004) Light conditions influence specific defence responses in incompatible plant-pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 219:673–683
Ziegler R, Blaheta A, Guha N, Schönegge B (1988) Enzymatic formation of pheophorbide and pyropheophorbide during chlorophyll degradation in a mutant of Chlorella fusca SHIRIA et KRAUS. J Plant Physiol 132:327–332
Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136:2621–2632
Acknowledgments
I would like to thank Bernhard Kräutler for many stimulating discussions and fruitful long-term collaboration. Many thanks also to my present and former group members for their important contributions in the area of chlorophyll breakdown. I thank Helen Ougham for critical reading and language editing of the manuscript. My work is financially supported by grants from the Swiss National Science Foundation and by the National Center of Competence in Research Plant Survival, a research program of the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Hörtensteiner, S. (2013). The Pathway of Chlorophyll Degradation: Catabolites, Enzymes and Pathway Regulation. In: Biswal, B., Krupinska, K., Biswal, U. (eds) Plastid Development in Leaves during Growth and Senescence. Advances in Photosynthesis and Respiration, vol 36. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5724-0_16
Download citation
DOI: https://doi.org/10.1007/978-94-007-5724-0_16
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5723-3
Online ISBN: 978-94-007-5724-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)