Abstract
The aim of the research is to provide more detailed information about the nature and the associations of the organic matter (OM) in estuarine sediments from Galway Bay, Ireland. The work will help to determine the extents to which sediments are sinks for carbon (C) and also the effect the River Corrib (which flows into the estuary) is having on the OM present. Humic substances (HS) will be extracted and fractionated followed by spectroscopic analysis. The main focus will be on solid and liquid state nuclear magnetic resonance (NMR) spectroscopy which will give insights into the chemical compositions of the matter.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
International concerns about greenhouse gas (GHG) emissions and climate change are placing emphasis on the need to sequester carbon (C) from atmospheric carbon dioxide (CO2) arising from human activities. Oceanic sediments contain 150 Pg of organic matter (OM) (Ridgwell and Edwards 2007). A detailed awareness is needed of the processes that lead to the conservations of C in the environment and to understand better the structures and interactions of the organic components of sediments. Core samples are being studied from the transitional waters in Galway Bay. OM is washed into the bay from the River Corrib and its tributary streams, and it becomes a reservoir and important C sink in the sediments. A main focus is to study the effect that the estuary is having with respect to the OM in the sediments. Studies of the organic and inorganic colloidal components in the estuarine sediments can give indications of changes that have taken place over time to the compositions of the transported materials and give an insight into the compositions of the C sequestered in the sediments. The project is studying in detail the compositions of the HS at different depths and their associations with the inorganic colloids in the sediments.
Location
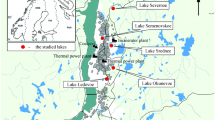
The River Corrib flows from Lough Corrib (area 165 km2, Ireland’s second largest lake) through Galway City and into the bay. The river has an annual mean discharge of c. 95 m3 s−1 (Goddijn and White 2006). The dominant current flow in Galway Bay is anticlockwise (Lei 1995). Using a vibrocorer (Geo-corer 6000, supplied by the Geological Survey of Ireland; Cruise No. CE09-04), four cores up to 6 m in depth were taken at increasing distances from the river inlet (Fig. 1). Surface grab samples were also taken, using a Day grab, near the core locations.
Materials and Methods
Sediment samples are H+ exchanged by standing for 4 h in 1 M HCl. Exhaustive extractions use methods of the Carbolea Research Group (www.carbolea.ul.ie). These involve uses of, first, 0.1 M NaOH (pH 12.6), followed by 0.1 M NaOH + 6 M urea, then water washing and drying, and finally DMSO + concentrated H2SO4 (6%). All extractions in base are carried out under N2. The HS are isolated using the XAD-8 resin procedure. Ultraviolet–visible (UV–vis) spectroscopy is employed to evaluate the E 4/E 6 ratios. Fourier transform infrared (FTIR) spectroscopy gives an initial indication of the compositions of the materials. Solid state CPMAS 13C NMR is employed to investigate the functionalities and compositions of the organic fractions. Fractionated humic samples are analysed using pyrolysis-gas chromatography mass spectrometry (Py-GCMS) to give indications of the complex components that make up the OM structures. The Walkley-Black procedure (Allison 1965) determines the OC in the sediments at different depths and in the different fractions. Elemental analyses determine the quantities of C, N, and O present. Shells in the sediments are dated using 14C accelerator mass spectrometry (AMS). The geochemistries of the sediment cores are analysed using the Itrax XRF core scanner to determine the elements present.
Results and Discussion
Results will be presented for analyses of all four grab samples and for core 1. OC determinations on the residues were carried out at each stage of the extraction to quantify the efficiency of the solvent. Fractionated humic samples are being analysed using various analytical techniques. FTIR spectroscopy was carried out on dry humic acid (HA) and fulvic acid (FA) fractions. UV–vis spectroscopy was carried out on HAs and FAs in solution at 465 and 665 nm to determine E 4/E 6 (465:665) ratios, related to the condensation of aromatic C (Kononova 1966). Humic samples including humin are being analysed using Py-GCMS, δ 13C, and solid state NMR spectroscopy.
Py-GCMS spectra have been obtained for each humic fraction from core 1. HAs, FAs, and humin contain numerous compounds including furan, phenol, and pyrrole compounds. The FA contains numerous carboxyl compounds related to their acidic compositions. Py-GCMS, using a 340 and 720°C temperature range, provided a more in-depth awareness of volatile compounds. Tetramethylammonium hydroxide (TMAH) analysis allowed the identification of less polar, lower molecular weight molecules.
Core 1 (0–100 cm) yielded HA and FA fractions from extractions using 0.1 M NaOH, 0.1 M NaOH +6 M urea, and humin from the DMSO + 6% H2SO4 extract. However, core 1 (435–535 cm) yielded negligible amounts of OM in the 0.1 M NaOH and 0.1 M NaOH + 6 M urea extracts, but humin was isolated at this depth. That implies that the HA and FA had been decomposed and the highly refractory humin was retained in the sediments over thousands of years.
Analysis of FTIR spectra allows initial identifications of functional groups. Results based on the E4/E6 ratios would suggest that the HAs and FAs in the base + urea extracts have higher molecular weights than those in the 0.1 M (pH 12.6) extract. Because humin is insoluble at all pH values, its E 4/E 6 data have little meaning.
NMR spectroscopy allows a more complete awareness of the compositional aspects of the materials isolated. The spectrum of the HA fraction of grab sample A1001 has different emphasis from those for HAs from soil (Fig. 2).
13C NMR spectrum of HA (grab sample A1001 (coring position 1, see Fig. 1)) extracted using 0.1 M NaOH solution
It shows strong terminal methyl and methylene signals, a pronounced signal at c. 55 ppm, indicative of methoxyl, possibly attributable to lignin residues, and supported by an O-aromatic resonance at 140–150 ppm. The resonance at 50–60 ppm could also be indicative of peptide. A strong resonance at 70–90 ppm indicative of carbohydrate is supported by a clear anomeric C resonance at 105 ppm. The symmetrical peak at 170–180 ppm indicates carboxyl/ester functionalities. The resonance at 130–140 ppm, indicative of aromaticity, is definite, though not strong. That would suggest that the carboxyl (or ester) signal is from fatty acids/waxes, represented also in the methylene signal.
Radiocarbon dating on shells from 0 to 100 cm of core 1 suggests that the material is 535 years old. Two dates obtained for the bottom of core 1 (435–535 cm) indicate that the material is between 8,452 and 8,662 years old. The difference in the age accounts for the significant changes in the HS retention and prompts further investigations of the effects of time on the HSs.
Further extractions of core samples along with integration of the results from XRF and palaeoecology data will give better insights into the compositions of the materials with time and of the changes that have taken place. Differences in terrestrial and marine OM will also be investigated.
Conclusion
New insights into the chemical compositions of HS retained in sediments over time will be acquired through this research. The novel investigations of humin (the least studied and most refractory OM fraction) at depth, in comparison with the estuarine HAs and FAs, will indicate how changes in the compositions and distributions of these fractions have taken place over time. Also, studies of the same fractions in recent freshwater sediments in the river Corrib will aid our understanding of changes that have taken place in the terrestrial OM as the result of its residence in the estuary. The study will add to the pool of knowledge on C sinks.
References
Allison, E. 1965. Organic carbon. In Method of soil properties, ed. C.A. Black et al., 1367–1370. Madison: American Society of Agronomy, Inc.
Goddijn, L.M., and M. White. 2006. Digital camera measurements of water quality parameters in Galway Bay, Ireland. Estuarine, Coastal and Shelf Science 66(3–4): 429–436.
Kononova, M.M. 1966. Soil organic matter, 400–404. Oxford: Pergamon Press.
Lei, W. 1995. Three dimensional hydrodynamic modelling in Galway Bay, PhD thesis. Dublin: National University of Ireland.
Ridgwell, A., and U. Edwards. 2007. Geological carbon sinks. In Greenhouse gas sinks, ed. D. Raey, C.N. Hewitt, K. Smith, and J. Grace. Wallingford: CABI Publishing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Zhejiang University Press and Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Mylotte, R., Byrne, C.M.P., Chang, R.R., Dalton, C., Hayes, M.H.B. (2013). Studies of Humic Substances from Sediments in Galway Bay, Ireland. In: Xu, J., Wu, J., He, Y. (eds) Functions of Natural Organic Matter in Changing Environment. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5634-2_23
Download citation
DOI: https://doi.org/10.1007/978-94-007-5634-2_23
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5633-5
Online ISBN: 978-94-007-5634-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)