Abstract
Two modifications of the standard procedure for the isolation of humic acids (HA) from peat samples have been explored. The modifications comprise a Soxhlet extraction of the freeze-dried peat sample with an organic solvent and the use of organic acids for the precipitation of the HA fraction. The organic extraction effectively removes organo-soluble impurities (up to 12%) without obvious signs of alteration or loss of humic material (Meyer G, Klöcking R, Optimising the protocol for the isolation of humic substances with regards to their use in biological systems. Proceedings of the Arbeitstagung Torf und Huminstoffe, Zittau, Germany 28–30/09/11, 2011). The use of organic acids as precipitant aims at the halogen- and mineral acid-free preparation of HA. Here, the quality of HA obtained using the modified procedure is compared to those obtained by traditional HCl precipitation.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Motivation
During recent years, Saxonian fish farms have been plagued by a highly contagious fish disease that affects the gills and leads to death in 80–100% of the cases. This epidemic causes great financial losses and affects both food-producing fish farms and producers of much beloved ornamental fish like Koi carps (Fig. 1).
Koi carps (black and white version, by Stan Shebs, http://en.wikipedia.org/wiki/File:Six_koi.jpg)
Our interest was aroused when the pathogen was identified as a herpes virus, the cyprinid herpes virus 3 (Hedrick et al. 2000), and the disease was called Koi herpes virus infection (KHV-I, henceforth). From many years of experience in the field of humic acids (HA), we knew that these are potent agents against human pathogenic herpes viruses. Therefore it is intriguing to try whether they are active against KHV, too.
Biocompatibility of Humic Acids
During the last two decades, more and more sceptical reports regarding the use of HA for medical purposes have been published. It was recognised that HA make an important contribution to storage and distribution of environmental contaminants due to their high affinity towards many toxic heavy metals and aromatic organic compounds (Murphy and Zachara 1995). Moreover, HA are highly active compounds that can catalyse many reactions. In the past, these properties have lead to unpleasant surprises, e.g. the formation of toxic trichloromethane from HA upon treatment with chlorine during the preparation of drinking water (Adin et al. 1991). These problems prompted us to develop a protocol for the isolation of HA that focuses on minimising the incorporation of toxic organic and halogenated compounds into the isolated HA.
Materials and Methods
Chemicals
Methyl acetate and c-hexane were of synthesis grade. Water, used for GPC/HPLC, or HA isolations, was purified using a SG Reinstwassersystem Typ Clear UV. Organic solvents used for HPLC/GPC were of HPLC grade or better. Other chemicals were of analysis grade. All chemicals were used as received. Organic extraction: Freeze-dried peat was treated with methyl acetate/c-hexane (3:2) in a Soxhlet apparatus until the extract was colourless and dried in vacuum. Alkaline extraction: The residue was suspended in water at 30°C (10 mL/g wet peat), pH was adjusted to 9.0 with NaOH, and the mixture was stirred under pH control for 2 h. The sludge and floating particles were removed by centrifugation (2,800 g, 10 min) or by filtration, respectively. HA precipitation: 1.0 L of the extract was acidified with 0.33 L of 0.50 M oxalic acid (OA) or 10% HCl, respectively, at 30°C (pH 1.5–2.0). After stirring for 0.5 h, the precipitate was collected by centrifugation and washed with water (OA: 3–4x (HPLC); HCl: 1x).
Humate Synthesis
The crude HA were dissolved in water by pH adjustment to 7.0, centrifuged (16,500 g, 3 h) to remove insoluble material and freeze-dried. GPC/HPLC: A Varian Prostar HPLC system equipped with autosampler and diode array detector was used. GPC was performed on a PSS BIO MCX column (1,000 Å, 5 μm) using 283.9 mg Na2HPO4, 87.7 mg H4EDTA, and 467.5 mg NaCl in 1.00 L water/n-propanol (7:3) as eluent. The column was calibrated using polystyrene sulfonates (1–1,013 kDa) (PSS GmbH, Mainz, Germany), alizarin S red (320), p-aminosalicylic acid (153), and p-hydroxybenzoic acid (138) as standards. NaNO3 and [Fe(EDTA)]Cl were used as flow markers, respectively. Detection was performed at 254 nm. HPLC was performed on a LiChroCART® 250–4; RP-18 (5 μm) column (Merck) with 7.80 g NaH2PO4 in 1.00 L water at pH 2.8 as eluent. Detection was performed at 202 nm.
Results
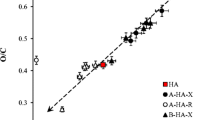
The modified isolation procedure for HA is outlined in Fig. 2. The peat was freeze-dried and treated with methyl acetate/c-hexane to remove organo-soluble impurities. Then the HA were extracted with NaOH at pH 9. A strong acid has to be used for precipitating HA because pH < 2 is necessary to ensure a complete precipitation of HA. Possible candidates are listed in Table 1. We did not want to use the very strong mineral acids as we plan to explore the possibility of a pH-dependent fractionation of HA in the future. Trifluoroacetic, trichloroacetic, and amidosulfonic acid would be very promising candidates but cannot be used due to the risk of halogen and nitrogen incorporation, respectively. Therefore, we tested the next most promising candidates, oxalic acid (OA) and citric acid, in the precipitation of HA isolated from peat of the Altteicher Moor and found them both capable of precipitating HA quantitatively (Kleiner et al. 2010). Furthermore, both acids have anions of low toxicity towards fish (e.g. sodium oxalate: LC50 (Danio rerio) = 630 mg/L, 96 h; citric acid: LC50 (Leuciscus idus melanotus) = 440 mg/L, 48 h, (MSDS 2012)) and are water-soluble and hence can be removed by washing the precipitated HA with water. Since OA is quantified by HPLC more easily, it was used further. Peat from the Altteicher Moor shows a high degree of decomposition. In order to explore how oxalic acid performs in the isolation of HA from less decomposed peat, we used peat from a second sampling site, the Dierhäger Moor (DM); HA were extracted as described above, precipitated using HCl or OA, respectively, thoroughly washed, and converted to the corresponding sodium salts. Analytical data obtained so far are summarised in Table 2. In all cases 71–91% of the material isolable via the HCl procedure could be also isolated by our newly developed procedure. The chemical composition is within the limits of HA reported in literature (Klavins 2010); however, the C/N ratio indicates that HA from the Dierhäger Moor have low N content. The molecular mass distribution, obtained by GPC, indicates that the developed method is biased against low molecular weight HA. This is in agreement with the higher absorption at 270 nm obtained for HA isolated using OA. The loss of material most likely occurs during the removal of the OA by washing as the later washings yield increasingly brown-coloured solutions. Further experiments to reduce loss of material are underway.
Conclusions/Discussion
A new method for the precipitation of humic acids (HA) from aqueous solution was tested using HA isolated from the Dierhäger Moor. Compared to hydrochloric acid, oxalic acid is capable of precipitating 71–91% of the HA. The analytical data of the obtained HA show a bias against low molecular weight HA.
References
Adin, A., et al. 1991. Trihalomethane formation in chlorinated drinking water: A kinetic model. Water Research 25: 797–805.
Hedrick, R.P., et al. 2000. A herpes virus associated with mass mortality of juvenile and adult Koi, a strain of common carp. Journal of Aquatic Animal Health 12: 44–57.
Klavins, M. 2010. Variations of humic acid properties within peat profiles in mires and peat, 175–197. Riga: University of Latvia Press.
Kleiner, C. et al. 2010. Halogen-free preparation and preliminary characterization of humic substances from different substrates. In Proceedings of the 15th Meeting of the IHSS, Spain, 27/6-2/7/10, vol. 3, pp. 325–328.
MSDS. 2012. MSDS from www.sigma-aldrich.com (29/2/12).
Murphy, E.M., and J.M. Zachara. 1995. The Role of Sorbed Humic Substances on the Distribution of Organic and Inorganic Contaminants in Groundwater. Geoderma 67: 103–124.
Acknowledgements
We thank the BMBF for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Zhejiang University Press and Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Meyer, G., Klöcking, R. (2013). Humic Acid Quality: Using Oxalic Acid as Precipitating Agent. In: Xu, J., Wu, J., He, Y. (eds) Functions of Natural Organic Matter in Changing Environment. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5634-2_195
Download citation
DOI: https://doi.org/10.1007/978-94-007-5634-2_195
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5633-5
Online ISBN: 978-94-007-5634-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)