Abstract
China undertook a Natural Forest Protection Program (NFPP) between 1998 and 2010 to prevent soil erosion, desertification, and the decline of natural forest resources. This paper uses one of the most highly profiled species among the World’s Red List, the Amur tiger (Panthera tigris altaica) as an indicator species to demonstrate that conservation of biodiversity could be enhanced by managing the forest structures in northeastern China during and after NFPP. We first review the conceptual model of forest stand dynamics and show how stand structure changes can impact wildlife populations based on multiple cases from around the world. Second, we review the ongoing national NFPP and the past management and harvesting history of forests in Northeast China and Inner Mongolia. Third, we propose a new approach to the conservation of this tiger that complements other efforts as part of the next step in implementing NFPP and subsequent forest restoration programs. We hypothesize that open and savanna forest structures that have a documented positive impact on the amount and quality of forest floor vegetation and thus elevate the carrying capacity for ungulates herds also have a positive cascading effect on the tiger population. Therefore, the forest with a mixture of open and dense canopies will be the best since it overall provides the highest carrying capacity (food and shelter) for the prey of the tiger. Through estimating the Amur tiger’s carrying capacity and minimum home range in different forest structures and examining the current forest conditions in northeastern China, we conclude that the majority of the forests there are in the dense structure and the endangerment of the Amur tiger is caused in part by a shortage of forest stand structures suitable for its prey. Consequently, to enhance the Amur tiger’s population and other species, an additional step is to manage the forest to ensure that an appropriate diversity of stand structures are maintained to elevate the forest landscape carrying capacity of the tiger’s prey. Forests managed in this way may also reduce the human-tiger conflict caused by the tiger’s entering populated areas searching for domestic prey since wild prey may not be easily found in the current dense forests. Yet cautions need to be made because the proactive management may bring greater hunting opportunities and so must be accompanied by a strong commitment of controlling hunting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 China’s Natural Forest Protection Program
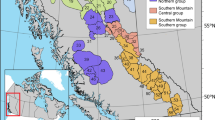
China began its Natural Forest Protection Program (NFPP) in 1998 to prevent soil erosion, desertification, and the decline of natural forest resources. NFPP is now being implemented in 17 of the 34 provinces (including municipalities, autonomous regions, and the special administrative regions) in China and has become one of the world’s largest ongoing ecological rehabilitation projects (NFPP SFA 2006). NFPP has been implemented in three broad geographical regions: the upper Yangtze River region, the upper and middle Yellow River region, and the key national forest region of the Northeast and Inner Mongolia including Xinjiang and Hainan provinces (Fig. 5.1). Different missions were emphasized in each of the three broad regions. In the regions of the upper Yangtze River and the upper and middle Yellow River, the programs cover 90 million ha of forest land and focus on restoring the vegetation and stopping soil erosion by ceasing all harvest of natural forests and accelerating afforestation of barren hills and fields. In the key national forest region of Northeast and Inner Mongolia, the program covers 34.2 million ha of forest land and focuses on reducing timber production, adjusting the forestry industry structure, and conserving biodiversity (NFPP SFA 2006).
This chapter is concerned with the NFPP in the Northeast and Inner Mongolia region, where much of China’s timber has been produced and a variety of wildlife lives, including the critically endangered species, the Amur tiger (Panthera tigris altaica; IUCN 2007). The program has brought high expectations for forest restoration and biodiversity conservation since protecting ecosystems and environments and conserving biodiversity are some of the major goals of NFPP in this area. To achieve these goals, all forests covered by the northeastern China NFPP were divided into three allocations to be treated differently (Table 5.1): 74.9% of the forest area was allocated either to preservation with no logging or to restricted cutting (limited logging) for ecological protection. The remaining 25.1% of the forest area was allocated to extensive plantations and forest harvesting for commercial purposes.
1.2 Endangerment, Population Fluctuation and Conservation of the Amur Tiger in Northeastern China
The Amur tiger is the northernmost subspecies of the five extant tigers. Historically it had a massive population distributed around all of northeastern China and the Russian Far East, but now it is critically endangered with no more than 600 individuals left in the wild. The wild Amur tiger population in China is estimated at less than 30 and is distributed in Jilin and Heilongjiang provinces adjacent to the border with Russia (Li et al. 2001; Nowell et al. 1996; Yu et al. 2000). The detailed population dynamics are shown in Fig. 5.2.
Population dynamics of the Amur tiger in China, Russia, and the entire distribution range between 1890 and 2005 (After Tian et al. 2009). The small graph shows the population dynamics from 1930 to 2005 on an amplified scale
A massive population decline occurred before the 1940s, mainly because of a combination of poaching and the habitat destruction from intensive logging and continuous wars. Since the 1950s, the total Amur tiger wild population has fluctuated between 300 and 600, increasing in Russia and decreasing in China. It is suggested that the consistent population growth in Russia until the late 1980s was caused by the enforcement of hunting restrictions and the tiger migration from China (Matyushkin et al. 1996; Tian et al. 2009). Meanwhile, in China the tiger population kept declining because it was regarded as a menace to human life and so hunting was encouraged in 1950s and 1960s. Tiger hunting was banned in China in 1977, and dozens of reserves were established in the late 1970s and early 1980s. Timber production was also reduced in China (Fig. 5.4); however, the tiger population has continued to decline. In 1998, guns were confiscated from local people in northeastern China as a further effort to prevent hunting of the tiger.
The Far Eastern mixed deciduous-coniferous forest is generally known as the prime habitat for Amur tigers (Matyushkin et al. 1996; Stroganov 1969). Habitat loss and fragmentation are considered the primary threats to tiger survival (Ma 1998; Nowell et al. 1996). Because of the lack of more specific knowledge on how the forest characteristics are related to the Amur tiger’s habitat suitability, there is a dispute about what approaches should be taken to protect the Amur tiger and to promote its population recovery (Conniff 2009). The current conservation efforts are focusing on expanding forest preserves, reducing logging activities, and returning farmland to forest (Shi and Xu 2006). These efforts are based on the presumption that forest harvesting is innately deleterious without appreciating that a recently logged forest can be an important component of landscape conservation (Meijaard et al. 2005).
1.3 Importance of Forest Stand Structures in the NFPP
Forest harvesting – even clearcutting – differs from deforestation if the forest is allowed to regrow. A recently clearcut area is considered an “open” forest structure until it regrows, and a very heavily thinned forest is considered a “savanna” structure. Both of these are “open” structures that allow forest floor vegetation to grow vigorously and are similar to openings created by fires and windstorms (Oliver and O’Hara 2004). The open structures are some of the many structures in a forest, and different animals and plants depend on each structure (Oliver et al. 1998). If the tiger’s survival is hindered by an insufficient amount of open forest structures, then appropriate harvesting may increase this structure and the tiger’s survival. Current efforts of reforestation and protection of young growing forests may be inadvertently increasing the dense forest structures which may not be the most effective way to protect the tiger.
By managing forests in the different categories and reducing timber production, forest cover has been enhanced with implementation of the NFPP (Chen 2004), which was believed to favor the Amur tiger. Meanwhile, other policies beneficial to the tiger population’s recovery have been implemented or discussed, such as controlling human killing of the Amur tiger and its prey, aiding the tiger’s recolonization, and preserving sufficient total forest area for the tiger (Zhang et al. 2005). Besides these, we suggest that the forest stand structure distribution is another important element that is worth considering for Amur tiger and other biodiversity conservation when restoring the forest landscape. Forest stand structure distribution is the spatial occurrence and array of the forest stands of different densities, species, sizes, and distributions of trees. It is an important forest characteristic that changes with forest development. It has rarely been addressed by previous conservation plans but could play an important role in providing habitat for wildlife. In this article we hypothesize that open forest structures that have a documented positive impact on the amount and quality of forest floor vegetation and thus elevate the carrying capacity for ungulates herds also have a positive cascading effect on the tiger population and as such the protection of this endangered species. We further suggest that, for the next step of NFPP implementation and the forest restoration programs after it, some endangered species could be better protected and their population expansion could be enhanced when the forests are managed in appropriate, sustainable ways that assure all stand structures are well represented.
1.4 Objectives and Outline of This Study
This paper uses the Amur tiger as an indicator species to demonstrate that conservation of biodiversity could be enhanced by managing the forest structures in the northeastern China NFPP. We first review the conceptual model of forest stand dynamics and show how stand structure changes can impact wildlife populations based on multiple cases from around the world. Second, we review the ongoing national NFPP and the past management and harvesting history of forests in Northeast China and Inner Mongolia. Third, we use the Amur tiger as an indicator species, estimate its carrying capacity and minimum home range in different forest structures, examine the current forest conditions in northeastern China, and finally suggest how forests could be managed in this area to ensure an appropriate diversity of forest stand structures and thus further enhance the Amur tiger’s population during and after implementing the NFPP.
2 Background
2.1 Study Area
2.1.1 Ecological Characteristics of Northeastern China
The NFPP in the northeastern China region applies to nationally owned forests in the Jilin province, the Heilongjiang province, and the northeastern part of Inner Mongolia (41°22′–53°33′N, 119°31′–135°5′E). These areas overlap the major historic range of the Amur tiger. Northeastern China encompasses approximately 60 million ha of land with more than half of it covered by forest. It has been the primary timber producing region in China (Hao et al. 1999).
From west to east, this area covers the Daxinganling, Xiaoxinganling, Wandashan, and Changbaishan Mountains and the Argun, Nen, Amur, Wusuli, Songhua and Yalu Rivers. The area has a continental monsoon climate between the temperate and boreal zones. Rainfall is concentrated in summer (June to September), with annual precipitation ranging from 300 to 450 mm in the west of Inner Monogolia’s Daxinganling region to 600–1,000 mm in the east of Jilin’s Changbaishan area.
The region covers two major vegetation types: the boreal coniferous forest and the temperate deciduous-coniferous mixed forest. The boreal coniferous forests are in the Daxinganling mountain area and are dominated by Dahurian larch (Larix gmelinii). Mongolian Scots pine (Pinus svlvestris), dwarf Siberian pine (Pinus pumila) and Koyama spruce (Picea koraiensis) are also common in some areas. The major wildlife species inhabiting this vegetation type are ungulates such as moose (Alces alces), red deer (Cervus elaphus), Chinese river deer (Hydropotes inermis), and roe deer (Capreolus capreolus). The temperate deciduous-coniferous mixed forests are located in the Xiaoxinganling, Wandashan, and Changbaishan Mountain areas. They are dominated by Korean Pine (Pinus koraiensis) and broad-leaf deciduous species including basswood (Tilia spp.), Asian white birch (Betula platyphylla), Manchurian ash (Fraxinus mandshurica), Mongolian oak (Quercus mongolica), Mandshurica walnut (Juglans mandshurica), poplars (Populus spp.), and maples (Acer spp.). A variety of wildlife inhabit this vegetation type, including ungulates such as wild boar (Sus scrofa), red deer Chinese river deer, roe deer and sika deer (Cervus nippon). Carnivores include the Amur tiger (P. tigris altaica), Asiatic black bear (Selenarctos thibetanus), sable (Martes zibellina), and otter (Lutra lutra). Birds such as the northern goshawk (Accipiter gentilis), hazel grouse (Tetrastes bonasia), and black grouse (Lyrurus tetrix) are also present (Hao et al. 1999).
2.1.2 Historical Forest Harvesting and Management in Northeastern China
Historically, northeastern China was covered by natural forests with very little farming, grazing or fishing. The whole area was established as a reservation by the Qing Dynasty governor, who originated from here and conquered all of China in the seventeenth century. Very little outside immigration and development were allowed in the area at that time. The agricultural land began expanding into what were previously forests and wetlands after 1860, when the Qing Dynasty governor abolished the previous preserve policy and encouraged migration to northeastern China (Fig. 5.3; Yi and Ye 2004). Forests were harvested heavily without any management plans until the late twentieth century (Fig. 5.4).
Agriculture expansion during Qing Dynasty (1684–1911) (Data source: Li 1999)
The timber harvesting history in northeastern China passed through the following phases:
-
First Russia built more than ten railroads into the forests of this area beginning in 1897, either clearcutting or heavily selectively cutting trees in the easily accessible areas. For example, in 1915 Russia opened 22 forest sawmills along the major railroad, operating in 19.7 million ha of forest land (Li 2008; Wu and Huang 2007; Yao and Man 2004);
-
Japan began timber harvesting in 1905 after Russian-Japanese War. The forestland in Heilongjiang province declined 60% from 40 million ha of closed forest in 1896 to 16.7 million ha of closed forest in 1949 (Wu 2007);
-
After 1949, as New China took control of northeastern China’s forest resources, the natural forests were conserved for several years. Beginning in the late 1950s, however, the forests were extensively clearcut for development and construction purposes. This clearcutting lasted until the late 1970s when the cultural revolution ended (Li 2008; Wang 2003);
-
Since the 1980s, and especially after 1998 when NFPP was implemented in this area, the policies became concerned with the sustainability of the forest resources and promoted forest regeneration, plantation establishment, and afforestation.
The harvesting of timber continued between the beginning of the last century and the late 1980s. Consequently, the majority of the forests in northeastern China are young or middle-aged (Lei 2005).
Because these forest harvesting and management histories have changed the forest cover, structures, and species of the tiger’s habitat, they have influenced the density and distribution of the Amur tiger and its prey. Another influence on the Amur tiger and its prey is the control of hunting. The nationwide prohibition of hunting began in 1977, and hunting guns were prohibited in 1998. Since then, illegal hunting has been reduced, and the population of the tiger’s prey such as wild boar has increased (Shi 2005).
2.2 The Importance of Forest Stand Structures in Maintaining Biodiversity
2.2.1 Shifting from the Steady-State to the Dynamic Ecological Paradigm
“The balance of nature” has been one of the most deeply embedded ecological concepts (Stevens 1990). This early ecological paradigm minimized the importance of disturbances in natural communities and assumed that natural ecosystems are closed systems and normally in a steady-state, or equilibrium. This condition was assumed to be the “climax” or “old growth” state, and all natural species were assumed to live well here. This perspective imagined the forest ecosystem as a potentially self-regulating “super organism” that could eventually remain in a stable state, under which the only disturbances would be the death of old trees and their replacement by younger ones (Oliver and Larson 1996). After a disturbance occurred – an “unnatural” event – the forests slowly regrew to the final, “mature” system – presumably characterized by the most stability, highest total biomass production, narrowest niche specialization, and greatest biodiversity both in terms of species richness and evenness (Odum 1969). Prior to 1990, this paradigm had been widely accepted by ecologists and wildlife conservationists. Those who held this perspective considered any human intervention as “deleterious by definition” (Stevens 1990). The forest equilibrium condition was presumably predictable and the goal of conservation had been to lead “nature” to that steady-state point by preservation or restoration.
Evidence accumulated during the past four decades suggests that the natural forest is much more dynamic, with continuing disturbances and fluctuation of species and stand structures (Oliver and O’Hara 2004). Restoration practices cannot guarantee that the historical landscapes will be reconstructed, as the steady-state theory predicts. On the contrary, restoration often produces unexpected outcomes that are different from the pre-disturbed state (Suding et al. 2004). Consequently, restoration and conservation plans need to take into account changes in the environment, and management goals need to be set on defined spatial, temporal, or organizational scales (Angermeier 2000; Hull et al. 2002). The ecological paradigm shift is recognized by scientists, but the dynamic paradigm has not been sufficiently conveyed to policy-makers (Wallington et al. 2005).
2.2.2 Forest Structure Changes with Development and Disturbances
The old “steady-state” ecological paradigm assumed all natural forests are in the old-growth stage with a “complex” structure, while the dynamic perspective recognizes that forest stand structures change following disturbances and growth (Oliver and Larson 1996). A major (stand-replacing) disturbance leads forests into an “open” structure where many trees and other plants first initiate from seeds, sprouts, or other mechanisms. With forest development, competition among trees and other plants becomes intense and the stands grow into the stem exclusion stage with a very “dense” structure. Later, as some old trees die, new trees invade but remain suppressed in the understory and the forest obtains the “understory” structure. Eventually, the younger trees grow up and generate multiple canopy layers and other features that lead to the old growth stage with a “complex” structure of diverse horizontal and vertical spatial patterns and relatively open canopies. Further, minor disturbances can partially eliminate trees from the dense and understory structures and quickly change them to the complex structure or to the “savanna” structure with few trees standing (Oliver and Larson 1996; Oliver et al. 1998).
2.2.3 The Value of Different Forest Structures in Maintaining Wildlife Populations
Forest structures play an important role in maintaining wildlife populations. Human hunting, lack of colonization, lack of food (prey or digestible vegetation), and lack of total forest area can cause the decline of a wildlife population. Different forest structures (i.e., open, savanna, dense, understory, and complex) provide different habitat types which support different plant and animal species. Although the steady-state paradigm embodies the assumption that the old-growth forest has the highest biodiversity, studies show that each structure supports a distinct suite of species. Forests in the savanna structure and open structure both contain very high biodiversity yet support different species than those found in the old-growth (complex) structure. The dense structure generally maintains the lowest biodiversity (Oliver et al. 1998).
Studies have suggested that the biomass of ungulates is positively correlated with the average temperature and primary productivity (Jedrzejewska and Jedrzejewski 2005; McNaughton et al. 1989). “Bottom-up” and “top-down” controls regulate the populations of ungulates in two directions. Bottom-up control occurs when food is limited and so nutrient supply and productivity determine the ungulate abundance. When bottom-up control dominates the ecosystem, open and savanna structures would be critical for providing forage to maintain the ungulate population viability. Top-down control occurs when a higher trophic-level in the food chain such as large carnivores or human hunters limit the density of ungulates. When top-down control dominates the ecosystem structure, closed forests of dense, understory, and complex structures would be critical in providing hiding cover for carnivorous predators such as Amur tigers and their prey. In the past, presumption of the dominating importance of top-down control has led to conservation efforts that set aside protected areas to prevent hunting. If bottom-up control is occurring (i.e., lower trophic-level resources are the limiting factors of higher trophic-level animal populations such as forage to ungulates, or ungulates to tigers), the creation of diversified forest structures to increase the carrying capacity of ungulates in certain areas could increase the population of the tiger and its prey.
2.3 Examples of Biodiversity and Wildlife Population Changes with Forest Structure Changes
Different plant and animal species depend on different forest structures for their habitat; some animals utilize several structures. For example, spotted owls (Strix occidentalis caurina) utilize complex structures and have declined with the shortage of old growth forests in the northwestern United States (Gutierrez and Carey 1985); red-cockaded woodpeckers (Dendrocopos borealis) utilize pine savannas in the southeastern United States (U.S. Fish and Wildlife Service 1985); many butterflies have disappeared with the elimination of openings (Young 1992); elk (Cervus elaphus) in the northwestern United States survive best in openings which provide high quality food (Cook et al. 1998; Duncan 2000; Fisher and Wilkinson 2005); and the Florida panther (Puma concolor coryi), that had been assumed to be a forest obligate, actually utilizes openings as well (Gross 2005; Kostyack et al. 2006).
Wildlife populations can dramatically change when the forest structure alters. The deer population in the Allegheny Plateau in the northeastern United States had a dramatic expansion in the 1920s because of the increased open structure created by heavy forest cutting. It experienced a sharp drop in 1930s when the young secondary growth forests grew into the stem exclusion stage with dense structures (Marquis 1975).
Forestry management with appropriate silvicultural pathways could be used to balance the stand structures and enhance biodiversity conservation (Oliver 1998), while management without maintenance of certain structures could put the dependent species in danger. We suggest that, for the next step, some endangered species such as the Amur tiger could be even better protected than they are now if the forests are managed in ways that maintain an appropriate balance of stand structures during and after the northeastern China NFPP practices.
2.4 Diverse Forest Structures and the Amur Tiger’s Population
Although habitat loss along with poaching is considered as the primary threat to the Amur tiger by well-known conservation groups (Ma 1998; Nowell et al. 1996; WWF 2008), there is apparently no specific description of the habitat required for the Amur tiger in terms of forest type, structure, and species composition. Other factors that contribute to the suitability of habitat can be commercial poaching, population isolation, and prey depletion. To maintain and increase the population of the Amur tiger, besides preserving enough total forest area, the forest structures that provide suitable habitat need to be promoted. What forest structures are currently lacking that are needed to maintain the Amur tiger’s habitat?
The coincidence of Amur tiger population decline and the disappearance of the old growth coniferous-deciduous mixed forest during the past century have led to a widely accepted speculation, or hypothesis, that the intact old growth forest is the tiger’s best habitat. This “ideal,” old growth forest is characterized as mountainous coniferous – deciduous mixed forest with dense vegetation cover and minimum human disturbances (Matyushkin et al. 1996). In contrast to this frequently mentioned Amur tiger habitat hypothesis, a few old hunters and the early zoologists had described Amur tigers as living in areas with a mixed composition of dense vegetation and “open burns occupied by sparse forests or small growing oaks and filberts” (Kaplanov 1948a, b). The former old growth habitat hypothesis emphasized the importance of the old-growth forest stage with minimum external disturbances; while the latter, dynamic hypothesis emphasizes a disturbance-induced mosaic landscape with open structures and closed forests as well. The latter hypothesis is indirectly supported by recent studies of (i) prey as a determinant of the tiger’s population (Karanth and Stith 1999; Karanth et al. 2004; Miquelle et al. 1999); (ii) open habitats and forest edges being preferred by ungulates for forage and cover in North America (Cook et al. 1998; Duncan 2000); and, (iii) the fact that the major prey of Amur tigers (i.e., wild boar, roe deer, red deer, and sika deer) live in forest edges, shrubs, savannas, and open grasslands (Yan et al. 2006).
Closed forests (i.e., dense, understory and complex structures) are important for providing shelter and cover for tigers and their prey. On the other hand, open, savanna, and complex structures are important for providing forage for ungulates. They enable sunlight to reach vegetation near the ground, promoting photosynthesis and increasing the abundance and nutrient quality of vegetation available for browsing near the ground.
In northeastern China, top-down control (i.e., an animal population controlled by higher trophic level predators) of the Amur tiger population was probably playing an important role before the 1980s, when the carnivores were plentiful. Hunting was advocated because the tiger was seen as a pest; however, currently and in the next few decades, bottom-up control (i.e., an animal population controlled by the availability of lower-trophic level forage/prey) probably is and will remain dominant because the large carnivores have become sparse, hunting in China has been effectively banned nation-wide since the confiscation of guns in 1998, and most forests are growing into the dense structure.
Habitats provide shelter, water, and food for wildlife. The availability of prey has been considered the primary determinant of tiger populations in recent studies (Karanth and Stith 1999; Karanth et al. 2004; Miquelle et al. 1999); therefore, forest structures that provide adequate forage for its prey are critical to promoting the Amur tiger’s conservation.
The Amur tiger preys mainly on ungulates such as wild boars and cervids including red deer, sika deer, and roe deer (Hornocker et al. 1998; Kerley 2003). Studies in northeastern China have shown that cervid habitats are openings, riverside shrub areas, forest edges, and savanna forests (Li 2003; Yan et al. 2006). Wild boars prefer to live in open structures consisting of dense shrubs or wet grassland, as well as in complex and savanna structures of broadleaf forests (Song et al. 2005). Wild boars also feed on the seeds of Korean pines that exist in closed structures, but produce the most seeds in the complex structure (Wang 1995). These studies show the tiger’s prey not only need forests in the dense structure for hiding cover, but also need forests in many other structures: savanna and open structures are needed by cervids, and complex and savanna structures are preferred by wild boars. Maintaining forests in diverse structures can ensure the density of ungulates and thus provide adequate prey density for the Amur tiger. The prey populations also depend on the distribution of these forest structures across the landscape. This study illustrates the importance of diverse structures, especially the open and savanna, for providing suitable habitat for the Amur tiger prey and hence for the Amur tiger’s population recovery.
The importance of diversifying the structures will be examined by using a simple model that estimates the forest’s carrying capacity for tiger prey and the tiger’s minimum home range in a forest in different stand structures.
3 Procedures
To examine the extent to which management of forest structures could enhance the conservation of Amur tigers in northeastern China’s NFPP management, we first use a model to estimate the Amur tiger’s possible habitat area requirements and its prey’s carrying capacity when the forest is in different stand structures. Then we examine the current status of forest structures in northeastern China by studying the historical harvesting pattern, official forestry statistical data, and land cover maps from publicly available interpreted satellite imagery. By comparing the estimated habitat area requirements under different stand structure regimes with the current forest stand structure distributions in northeastern China, we determine if the Amur tiger’s population could be enhanced markedly by altering the stand structure distribution.
3.1 Model Estimation of Amur Tiger Prey Carrying Capacity and Consequent Needed Tiger Home Range Under Different Stand Structures
3.1.1 Conceptual Model
Once the hunting and/or other human-caused depletion of an animal is curtailed and the animal is colonized in an area, the ability of the area to be habitat for the animal depends on the availability of suitable shelter, water, and food.
Shelter: Suitable shelter varies with the species and can be a dense, small thicket of trees; a small tree cavity with no other trees around; a large branch hidden by other branches; a cave; or another feature.
Water: Nearly all animals need water for drinking, and an area’s carrying capacity for a species is often related to how far and frequently the individuals of the species need to travel for water and how much water the individual needs.
Food: Assuming suitable shelter and water are available, food availability is directly dependent on the structure of the vegetation, its growth rate, and the population sizes of other species of the same level in the food web.
The maximum number of animals of a given species that can be supported in an area is known as the area’s “carrying capacity.” Most mammals confine their daily activities to a specific area and utilize the shelter, water and food in the vicinity. This area is known as the animal’s “home range.” For some species, an individual or a group possesses an area exclusively and defends this area by aggressive territorial behaviors. This area is known as the animal’s “territory” (Feldhamer 2007). Reproducing tigers are territorial; therefore, their home range and territory size are equivalent (Sunquist 1981).
The size of the home range is shown as a function of body size and food habits (Mcnab 1963), which suggests that the home range size of the most mammals is determined by the food supply (Feldhamer 2007; Gittleman and Harvey 1982; Herfindal et al. 2005). Much data shows that carnivore density is positively correlated with prey density (Fuller and Sievert 2001). This is especially true for the pure carnivores such as felids, including tigers, whose dentitions do not allow diet from plant material supplements (Shaw 2010). Previous studies in Nepal also revealed that the territory size of female tigers depends on the availability of prey (Smith et al. 1987; Sunquist 1981). Therefore, it is reasonable to presume that the home range for the Amur tiger, a solitary animal, is associated with the availability of prey.
Vegetation, ungulates as herbivores as well as prey, and tigers as predators make up three trophic levels in the food chain. Ungulate density varies with the availability of vegetation as forage in different forest structures when adequate shelter and water are not limiting. The density of ungulates further determines the food availability for Amur tigers. Thus, when shelter and water are sufficient for tigers in certain areas, the Amur tiger’s home range would vary with the ungulate density provided by the different forest structures comprising the area.
Although previous studies have portrayed the relationships of tiger home range or spatial distribution and the vegetation type, habitat physical attributes, prey density, and the human impacts (Carroll and Miquelle 2006; Karanth et al. 2004; Miquelle et al. 1999; Smith et al. 1987, 1998; Sunquist 1981), there is no study to date directly measuring ungulate carrying capacities in different forest structures or how each structure influences the Amur tiger’s home range in northeastern China and the Russian Far East. Using the literature, we quantitatively estimate ungulate carrying capacity (U), tiger carrying capacity (T) (number/km2), and predict tiger minimum home range H (km2) in the forests of open, dense, and complex structures with a simplified mechanistic model (Fig. 5.5). This approach is unlike most traditional mammalian home range analyses which were conducted with statistical models based on the empirical home range observation data. Instead, the model is based on the mechanistic approach used in rangeland management and ecology (Heady and Child 1994), with modifications described below.
This model is tenable when we simplify the plant-prey-predator relations to a single food chain of forage-ungulate-tiger, and assume only bottom-up control is occurring; i.e., the ungulate abundance is determined only by the availability of forage, and the tiger’s abundance is determined only by the availability of prey. The following conditions are assumed:
-
(i)
Suitable shelter and water are available for the Amur tigers. Human killing or lack of colonization is not restricting the number of Amur tigers. Prey is the only limiting factor that determines its home range. These assumptions are reasonable because recent studies suggest prey is the primary determinant of the tiger’ population (Karanth and Stith 1999; Karanth et al. 2004; Miquelle et al. 1999).
-
(ii)
Suitable shelter and water are available for ungulates as tiger prey, and abundance of forage is presently the only determining factor regulating the ungulates’ carrying capacity. These assumptions are reasonable because of the high vegetation density in northeastern China’s forest and the restriction of hunting since 1998.
-
(iii)
No animals except the Amur tiger hunt the prey.
The conceptual description of this simplified model is shown in Fig. 5.5, and the detailed model is described step by step below:
3.1.2 Estimation of Ground Vegetation Productivity in Different Forest Stand Structures
In most forests, light is enough of a limiting factor so that the overstory density (crown closure) strongly influences the forest’s structure (Oliver and Larson 1996); and forestry management with appropriate silvicultural pathways change the stands’ structures. Canopy cover has usually been used by empirical studies as a factor in classifying forest stand structures (Johnson and O’Neil 2001; O’Hara et al. 1996). For this model, the amount of sunlight reaching the forest floor is linearly related to the canopy cover; and the percentage of canopy cover is a characteristic of the forest structure. There is no canopy in the open structure, a little in the savanna structure, a medium canopy in the complex structure, and quite closed canopies in the dense and understory structures. To simplify the model, we included only the dense structure, complex structure and open structures in this estimation. Based on O’Hara et al. (1996) and Johnson and O’Neil (2001), the average canopy cover is approximately 85% in the dense structure, 65% in the complex structure, and 0% in the open structure. We made the simplified assumption that the percentage of sunlight reaching the ground is directly related to canopy cover. That is, the percentage of sunlight reaching the forest floor or ground is approximately 100% for open structures, 15% for dense structures, and 35% for complex structures. The percentage of sunlight is then assumed to be transferred to the same proportion of vegetation productivity on the forest floor in these structures, because the plant’s rate of photosynthesis increases near linearly as the light intensity increases before it levels off at the saturation point of the photosynthetic system. This level-off point of light intensity is consistent with the light availability of the environment that the species have evolved and adapted to (Begon et al. 1996; Molles 2002).
That is, when the ground vegetation productivity of the open structure is P(open), the ground vegetation productivity of the complex structure is P(complex) = 0.35P(open); and the ground vegetation productivity of the dense structure is P(dense) = 0.15P(open).
3.1.3 Ungulate Carrying Capacity Estimation
Forest floor vegetation provides forage for ungulates. Studies have suggested that biomass of ungulates is positively correlated with the average temperature and primary productivity (Jedrzejewska and Jedrzejewski 2005; McNaughton et al. 1989). Thus, we made the simple analogy between the vegetation productivity and forage availability (i.e., P α U), which leads to U(complex) = 0.35U(open), and U(dense) = 0.15U(open), where U is ungulate carrying capacity. Note that because the different ungulate species have forage preferences for different plant species or growth forms, the analogy does not always match precisely with reality (and the real relationship of vegetation productivity and forest availability cannot be portrayed precisely without quantitative field experiments); however, we feel this simplification is acceptable for the rough estimates of this model.
Following the convention of rangeland ecology and management, we used animal unit month (AUM) as a standardized measure to indicate the amount of forage available on the given land area. An animal unit is a mother and one progeny, and a mature cow and her sucking calf are generally counted as one animal unit. AUM in an area unit (in our case AUM/km2) indicates the ungulate carrying capacity of an area with different forest stand structure, when the abundance of forage is the only determining factor regulating the ungulates’ population (i.e., suitable shelter and water are available for ungulates as tiger prey).
We used Cervus spp. to represent ungulates. The Cervus spp. are generally both browsers and grazers (Hofmann 1989), and so are the major deer species of the Amur tiger’s prey in northeastern China (i.e., Cervus elaphus, Cervus nippon, and Capreolus capreolus) (Yan et al. 2006). We assumed the diet habits of elk (Cervus elaphus) in North America and Cervus spp. in northeastern China (Cervus elaphus or C. nippon) are similar, because they are either the same species, or in the same genus. Studies in Bighorn Creek Valley, Canada (Morgantini et al. 1994) show that an open field of 73.1 ha provides forage for 44.5 elk cow and calf pairs for a month. That is, the forage in 73.1 ha of open field provides 44.5 elk AUM’s. A mature cow and her sucking calf are generally counted as one animal unit, and an elk is counted as 0.67 animal unit equivalent (Ruyle and Ogden 1993). We used these numbers to estimate the carrying capacity of the open structure for ungulates: U(open) = 44.5 × 0.67AUM/73.1 ha = 40.8 AUM/km2. Therefore, U(complex) = 0.35U(open) = 14.3 AUM/km2, and U(dense) = 0.15U(open) = 6.1 AUM/km2.
For the above estimation, we used the existing data only for grazing, because similar data for browsing was not available. However, low browse buds, foliage, etc. behave physiologically similarly to grasses; i.e., more shade means less browse (as well as less graze). This is the best estimate based on the available data. Further refinement of this equation and procedure would be helpful in the future.
5.3.1.4 Amur Tiger Carrying Capacity Estimation
The mechanism for the tiger carrying capacity estimation of our model follows Karanth et al. (2004). That is, when prey density is the limiting factor for the tiger’s population in a certain area, tiger carrying capacity (T) is determined only by the abundance of prey (U), prey needed for the tiger to survive (p), and the ability of the tiger to catch the prey, which is measured as the proportion of prey individuals that the tiger can capture in the prey population (d).
Studies of prey-predator interactions (Karanth et al. 2004; Muller 1998) have shown that a tiger removes 10% of the population of its prey each year. Therefore, we assumed for this study that d = 0.10.
Based on the above, the equation to calculate tiger carrying capacity is shown below:
where p is the diet of Amur tiger (animal units/per year); d is the depletion rate of the Amur tiger on its prey as the percentage of prey population. p (food consumption for tiger to survive) and d (depletion rate of prey) are constant coefficients for different forest structures. U is the variable changing with forest structures, measured as AUM/km2. U is divided by 12 because the animal unit would use a different area each month.
In our model, p = 33 Animal Units/year, and d = 0.1. The rationale for the value of p is that the tiger’s appetite is consistent among the five existing subspecies. Based on tiger research in India, a tiger needs to consume approximately 50 ungulates per year to maintain its regular activities (Karanth and Stith 1999, Karanth et al. 2004). Since an elk is equivalent to 0.67 animal unit (Ruyle and Ogden 1993), 50 ungulates are approximately equivalent to 33 animal units. Subsequent calculations assume that the Amur tiger eats 33 animal units per year (p = 33 AU/year).
The Amur tiger’s carrying capacity in different distributions of open, dense, and complex structures was estimated by Eq. (5.1):
5.3.1.5 Amur Tiger Minimum Home Range Estimation
The home range estimate based only on prey availability is the minimum home range because shelter and water availability would regulate the population at some level below the maximum number. When the animal is territorial, as the tiger is, and if its home range does not vary among individuals (or, say, we are interested in mean home range), the minimum home range (H) could be calculated as the area divided by carrying capacity (in our case, 1/T). Therefore, the equation to calculate the Amur tiger’s home range is shown below:
The Amur tiger’s home range with different distributions of open, dense, and complex structures was estimated by this Eq. (5.2), and the results were rounded to the closest “tens” integer:
These numbers could vary based on the assumptions stated above; however, given the magnitudes of the differences calculated, it is highly likely that the forest stand structure differences do strongly influence the size of the Amur tiger’s home range.
3.2 The Current Status of Forest Structures in Northeastern China
Although forest stand structures play an important role in determining biodiversity, the status of these structures in northeastern China has only been described very recently (Han et al. 2007). We evaluated the current status by three methods listed here and described below:
-
1.
Field observation and estimation from historical harvest patterns
-
2.
Official inventory statistical data analysis
-
3.
Satellite image interpretation
3.2.1 Field Observation and Estimation from Historical Harvest Patterns
We conducted a field survey in June-August, 2006 in the forests of northeastern China, sampling the natural forests and plantations in the Changbaishan Mountain area in eastern Jilin Province, and in the Xiaoxinganling Mountain, Wandashan Mountain, Zhangguangcailing Mountain, and Laoyeling Mountain areas in eastern Heilongjiang Province. We selected stands with different structures in each area. On random plots in these stands, we measured tree density, species, and diameter size, and sampled tree ages. Based on these measures and our observations, we visually assigned a structure to each stand. The current forest structure status in northeastern China was also qualitatively evaluated from field observation during a 2-month tour of the region.
Furthermore, we summarized historical harvest patterns based on the literature and forest records and estimated the possible resulting structures from harvest and regrowth.
3.2.2 Official Inventory Statistical Data Analysis
Detailed forest inventory plot data were available for only some extensive forest areas, but not for all the forests in the four regions within northeastern China where NFPP is being implemented. Therefore, we used the officially summarized output of this statistical data on forestland types (Chinese State Forestry Administration statistics for the years 1994–2003; Table 5.2) that covers all of the four northeastern China forest regions to develop the decision system for classifying the forest by stand structures shown in Fig. 5.6 .
Forestland type and forest age are both closely related to forest structure. According to the definitions of forestland types in China:
-
forested areas are those with canopy densities above 0.3 (or 0.2 after 1994), with 1.0 being 100% closed canopy;
-
afforestation areas are the newly afforested area (usually younger than 7 years old) without a canopy but with more than 41% survival rate of regeneration;
-
savanna areas are those with a canopy density of 0.1–0.3;
-
shrub areas are more than 40% shrub coverage; and
-
unforested areas are those forestlands to be forested but without trees at present, including openings from harvesting, fire, and old agriculture fields (State Forestry Administration of P. R. China 1982).
Forested areas most likely contain dense, understory, and complex forest structures; savanna and shrub areas can be considered as the savanna structure; and all others can be considered as open structures.
In addition, forested areas were further classified by the state forestry administration into five categories as “young,” “middle,” “nearly mature,” “mature,” and “over mature” based on their ages. The overall area and volume of forest in different age groups in northeastern China were reported as part of the official statistical data output. Forest stand structures are closely related to age classes in a given forest because, although the minor disturbances and silvicultural practices such as logging, thinning or human-assisted regeneration can cause variations of structures within an age class, forest stand structures generally change with forest development stages and correspond to forest ages (Oliver and Larson 1996). Consequently, within the forest area category of the forest inventory, we assigned the “middle” age class stands to the dense structure; the “near mature” age class to the understory structure; and “mature” and “over mature” age classes to the complex structure.
Some very young forests are in the stand initiation stage with an open structure, while others have grown into the dense structure. The forest inventory data did not provide enough information for us to distinguish these two structures within the age class of “young”; therefore, we categorized the “young” forest into the open and dense structures as two alternatives and then took the midpoint of these two alternatives as the final estimate of open and dense structures. The actual area of open and dense structures should be somewhere between these two alternatives and approximate to our final estimates.
We recognize that by simply classifying forest age classes into forest stand structures, we may overlook the variation of structures within age classes thus reduce the confidence of our estimates. To overcome this problem, we supplemented the present classification with satellite image interpretations to be described next.
3.2.3 Satellite Image Interpretation
Satellite image interpretation was used in two ways as an additional approach to estimating forest structures: to check the interpretation derived from official statistical data, and to portray the spatial distribution of all structures. Two sets of satellite image products were used to evaluate the forest stand structures in this study:
-
(i)
The forest canopy density map of Global Forest Resources Assessment (FRA 2000) with a resolution of 1 km from AVHRR was obtained from the USGS Earth Resources Observation and Science Center (FAO 2000; http://edc2.usgs.gov/glcc/fao/index.php);
-
(ii)
The 2001 MODIS/Terra land cover type L3 global grid data with a resolution of 1 km were obtained from the USGS-NASA Land Processes Distributed Active Archive Center (NASA 2001; http://LPDAAC.usgs.gov/dataproducts.asp).
Five scenes of MODIS/Terra data were mosaically fit together to cover the study area. Both data were re-projected to Lambert Azimuthal Equal Area projection. The MODIS/Terra land cover map with IGBP 16 classes was reclassified to 7 classes to distinguish open, savanna, and closed forest, non-forest and other land cover types. The reclassification terms were generalized as shown in Table 5.3. Closed forest was further classified into dense, understory, and complex structures according to pixel values of the forest canopy density map. Pixels with forest canopy densities of 81–100% were reclassified as dense structures; pixels with forest canopy densities of 61–80% were reclassified as understory structures; and pixels with forest canopy densities of 31–60% were reclassified as complex structures. Then the amount and percentage of each forest structure were calculated. The whole process is illustrated in Fig. 5.7.
4 Results
4.1 Model Estimates of Prey and Tiger Carrying Capacity and Home Range
The model described earlier estimated that the ungulate carrying capacity is 41 AUM/km2 in the open structure, 6 AUM/km2 in the dense structure, and 14 AUM/km2 in the complex structure. Consequently, the minimum home range of an Amur tiger where the prey is limited is estimated to be 100 km2 in the open structure, 660 km2 in the dense structure, and 280 km2 in the complex structure (Table 5.4). Home ranges for savanna structure areas would probably be intermediate between open and complex, and for understory areas would be intermediate between dense and complex.
A forest with a mixture of structures would have a minimum home range for N tigers:
N = A/100 + B/660 + C/280;
where A = the forest area in open structure; B = the forest area in dense structure; C = the forest area in complex structure.
4.2 Status of Stand Structures in Forests in Northeastern China
Although there are some variations among the numbers for the stand structure compositions resulting from the three approaches, they all demonstrate that the majority of the forests in northeastern China are in the dense structure and that northeastern China contains only a small amount of open and savanna structures.
In the first approach, field observations suggested that many forests are in very dense structures (Fig. 5.8). Because of the limited transportation and funding, the 2-month field survey did not follow well-designed sampling procedures. Nonetheless, our rough visual estimation is that more than three-quarters of the forests are fairly dense. They are either in the dense structure with little understory vegetation or in the early understory structure as planted or natural regeneration begins appearing in the selectively cut stands. Complex, savanna, and open structures are rare, together accounting for the remaining one quarter of the forest. This observation is reinforced by the timber harvest history in this area. Numerous, very heavy timber harvest operations from the 1950s to the 1980s (Lei 2005; Figs. 5.3 and 5.4) led to the secondary forest regrowing into the dense structure (stem exclusion stage) at present. The systematically sampled forest inventory in the Daxinganling Mountain area in 2000 showed that approximately half of the forest was between 10 and 50 years old (Han 2011), having resulted from clearcutting, heavy partial harvesting, or other major disturbances in the 1950s through 1980s.
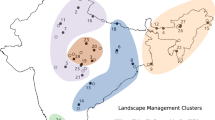
In the second approach, according to the official statistical data of forestland types, 15% of the forest is unforested, savanna and shrubs, or afforestation areas that contain open or savanna structures, while 85% of the forest area is forested and so contains dense, understory and complex structures (DAOW 2007; HFSDI 2001; Fig. 5.9; IMFSDI 2001; JFSDI 2002; Wang et al. 2005a).
Forest composition in northeastern China. (a) separated by the four forest regions – Jinlin, Helongjiang, Inner Mongolia, and Daxinganling. Note that the area designated as savanna and shrubs in Daxinganling actually represents the sum of savanna and shrubs, afforested, and others. (b) Composition for the overall northeastern China forests. Data source: NFPP implementation scheme for Jinlin province (2002), Heilongjiang province (2001), Wang et al. (2005a), and Inner Mongolia (2001); Data from 5th National Forest Resource Inventory, 1994–1998
The reported official summarization of statistical output data by forest age classes shows that 67% of the forested area and 50% of the forest volume are in young or middle ages, therefore in the dense structure; 15% of the forested area and 21% of the forest volume are in nearly middle age, therefore in the understory structure; and 18% of the forested area and 29% of the forest volume is in mature or over mature age, therefore in the complex structure (Fig. 5.10).
Forest area and volume by age classes in Northeast China and Inner Mongolia. Data source: 6th forest resource inventory conducted in 1999–2003 (Lei 2005)
Based on the forest land types and age classes (Fig. 5.6), we estimated the area and percentage of all forest structures (Table 5.5). The results agree with the field observations and the interpretation of growth from the harvesting history. The majority of the forests (47%) is in the dense structure; open structure accounts for 23% of the forest area; savanna accounts for 2%; understory structure accounts for 13%; and complex accounts for 15%.
In the third approach, the satellite imagery shows that in the subregions where NFPP is implemented, there are 50 million ha of forest area, or 79% of the total area. The dense structure forest accounts for the largest proportion of both the total area (37%) and the forest area (48%); the open structure accounts for 15%, the savanna accounts for 9%, the understory structure accounts for 7%, and the complex structure accounts for 6%. Besides these areas assigned definitely to structures, 5% of the forest area is in transition – either spatial transition as a mosaic of grassland, cropland, and closed forest; or temporal transition undergoing an apparent conversion between two different structures (Table 5.6, Fig. 5.11).
5 Discussion
Although the general trend of the estimated percentages of each structure based on the official statistical data (second approach) is consistent with the estimates from satellite imagery interpretation (third approach), especially for the dense structure (47% vs. 48%), quantitatively there are discrepancies between the estimates with the two approaches (Tables 5.5 and 5.6). The discrepancies may have been caused by the following: (i) the estimation of official data was based on the approximation of forest structure from forest age, which inevitably omits the variation of structures within age classes; (ii) the satellite imagery interpretation was based on the approximation of forest structures from the land cover types and forest canopy densities. Forest canopy density was further derived from NDVI (USGS 2005) in which estimated vegetation density can be affected by the tree species. In addition, the canopy density value may become saturated above a certain level. These factors may also result in some uncertainty in our estimation; (iii) the time difference between the taking of the field inventory and the satellite data could result in the discrepancies. The Fifth National Forest Resource Inventory conducted from 1994 to 1998 in the different regions was used. The FAO forest canopy density map was drawn from the AVHRR satellite data captured in 1995, and the land cover map was based on the MODIS satellite data captured in 2001. The land cover change between 1994 and 2001 may have affected the accuracy of satellite imagery interpretation. For example, the slight decrease of open structure suggested by satellite images (20%) compared to official data (23%) may be caused by some forests growing from the open structure into the dense structure between 1994 and 2001; the increase of savanna structure suggested by satellite images (14%) compared to official data (2%) may be caused by selective harvesting operations in the late 1990s; (iv) the scale differences may also be influential. The satellite images used in this study are all at 1 km2 resolution. However, the forest structure variation within 1 km2 can be detected and reported by the official survey, but cannot be detected with the applied satellite images. The combinations of stand structures across a 1 km2 pixel would result in an averaged result of pixel values, leading to a probable increase in estimation of forests in the intermediate canopy closure categories, such as savanna and transition structures.
This study demonstrates a way of making use of publicly available generalized data to estimate roughly the quantity and distribution of forest stand structures in the northeastern China region. To avoid the drawbacks elaborated on in the last paragraph and to get a more reliable and accurate estimation of the quantity and distribution of forest structures, we are working on (Han 2011) interpreting forest stand structures directly using finer resolution radar data and examining the use of forest relative density for estimating the vegetation in the understory.
5.1 Northeastern China’s Shortage of Open and Savanna Structures
Open and savanna structures can assure sufficient prey density for the Amur tiger, but our study suggests that northeastern China is experiencing an imbalance of forest structures. The majority of the forests are in the dense structure based on observation, official statistical data, and satellite imagery interpretation. Massive forest areas were clear cut or severely selectively cut between the 1950s and 1980s, leading to much of the forest regrowing to the dense structure at present. Furthermore, fire protection and control after the 1990s further maintained the existing dense structures and reduced the possibility of natural disturbances creating open and savanna structures.
5.2 Implications for the Amur Tiger’s Home Range, Population Size, and Behavior
The Amur tiger’s home range is probably largely related to the prey density that the habitat can provide, as described earlier. Our estimations of prey carrying capacity and thus the Amur tiger’s home range quantitatively demonstrate that Amur tigers would benefit from open and savanna forest stand structures if only prey availability is considered. The home range required by the tiger in the open structure is the smallest; the dense structure range is the largest; and the complex structure range is in between, because the open structure provides the best forage quality for its prey, cervids and wild boars (Fig. 5.12). The average home range documented in the Russian Far East forest is 400 km2 (Carroll and Miquelle 2006), which is close to, but slightly larger than, our estimation of the minimum home range in the complex structure of 280 km2. This suggests that the model constructed in this study is reasonable and perhaps that: (i) the majority of forests in the Russian Far East are in the complex structure, with a mixture of dense, open, and other structures; (ii) water and shelter may sometimes be restricted, especially in the winter time. Shelter could be limiting for either the tiger or its prey.
Besides prey, suitable habitat also requires shelter and water for the Amur tiger. The dense and old-growth forest structures might have been important for providing shelter for Amur tigers to hide themselves from hunters. Amur tigers were known for avoiding encounters with people; however, since hunting has been reduced in northeastern China, the Amur tigers have shown signs of changing their behavior. In recent years, Amur tigers have been documented as coming into villages and attacking livestock and people in the Hunchun, Dongning, and Mulin areas in northeastern China (Ma 2000; Sun 2004; Wang 2007a, b; Wang et al. 2005b). They apparently roam for food on the forest edges and in farmlands, and rely on the peripheral dense forest structures for hiding. The attacks enhance the Amur tiger’s conflict with human beings and frustrate conservation efforts by the local community. This unfortunate situation appears to be driven by both a shortage of food inside the forest and the Amur tiger’s behavior change.
The Amur tiger is not the only large cat which is learning not to fear people and changing its habits. Cougars (Puma concolor) in North America have increasingly come to the suburbs and attacked people since the 1990s, although they were historically believed to be “shy creatures with no history of attacking humans” (Canby 2005). There are frequent recent reports of Bengal tigers (Panthera tigris tigris) attacking humans in India, Nepal and Bhutan as well (Dema 2010; Gurung et al. 2008; Quammen 2003). It is suggested that increasing attacks from big cats are probably caused by two factors (Canby 2005; Shaw 2009):
-
(i)
The increasing density of forests, so that much of it is in the dense structure and contains little suitable prey as food, so the big cats move to openings near human populated areas; and
-
(ii)
The reduction in hunting of big cats, so the big cats are learning not to fear people.
Proactively creating openings and savanna stands within the forest could help alleviate the unpleasant situation described above. It must be appreciated that more openings and savanna stands within the forest may make the tiger and its prey more visible to hunters. If these structures lead to more hunting and killing of the tiger and its prey, the forest management will be counterproductive. Consequently, the proactive management of stand structures must be accompanied by an appreciation of the greater hunting opportunities and a concomitant increase in efforts to control it.
5.3 Considerations for Expanding the Population and Managing the Amur Tiger
According to the intermediate disturbance hypothesis (Connell 1978; Wilkinson 1999), an ecosystem will obtain the highest biodiversity when the disturbances are neither too rare nor too frequent. One of the major objectives of the NFPP in northeastern China is to conserve biodiversity. Disturbances have been reduced by restricting fires, prohibiting hunting, and severely reducing timber harvesting.
The current conservation effort of prohibiting hunting and setting aside reserve areas has promoted the tiger’s ability to survive in northeastern China, especially compared to 30 years ago when the importance of wildlife and biodiversity was not appreciated by the policy-makers or the public. However, our study shows that these conservation efforts directed solely at top-down regulation may no longer be sufficient. To expand the population and manage the Amur tiger, a necessary next step would be to address the imbalance of forest structures and to use active management to create open and savanna structures inside the dense forest. By doing so, the Amur tiger would benefit from the increased prey density; meanwhile, people would benefit by avoiding tiger attacks and by getting some economic return from wood harvesting. A well-planned forest management approach could be implemented that will provide both economic and ecological benefits, especially if it is combined with strong control of human hunting of tigers and their prey. A demonstration of this kind of forest management is studied in more detail in Han (2011). The dynamic nature of forests and the current understanding of wildlife ecology provide the theoretic bases for this approach.
6 Conclusions and Management Implications
Hunting and poaching of tigers seem now largely to be under control due to conservation efforts. The population of Amur tigers in northeastern China seem, however, not to recover and remains at a very low and unsatisfactory level. This may be caused in part by a shortage of forest stand structures that are not suitable for its prey. Consequently, an additional step to protecting this tiger and other species may be to ensure that all types of stand structures are maintained in the forest to elevate the forest landscape carrying capacity of the tiger’s prey. If the forest is found to be dense, it is important to manage the forests actively across the landscape to ensure an appropriate diversity of forest stand structures, especially ensuring open and savanna structures. Forest management in this area could design silvicultural pathways that include well-planned harvesting, thinning, or controlled fires that would create the desirable stand structures or reduce the dense structures to complement other efforts to enhance the Amur tiger’s population as part of the next step in implementing NFPP and subsequent forest restoration programs.
A shortage of forest stand structures suitable for the tiger’s prey may significantly/severely keep the small and threatened population of the Amur tiger from recovery in northeastern China:
-
Maintaining an appropriate diversity of forest stand structures can help enhance the populations of the Amur tiger and its prey species by providing forage areas for prey species as well as cover for both the tiger and its prey;
-
If the forest is shown to be overly dense, it is important to manage the forests actively across the landscape to ensure an appropriate diversity of forest stand structures, especially ensuring open and savanna structures that will further support efforts to enhance the Amur tiger’s population;
-
Forest management in this area should consider, through well-considered silvicultural pathways, enhancing the Amur tiger’s habitat by creating open and savanna stand structures and reducing the dense structures in the deep forest, while protecting local livelihoods by building dense structure buffers near the villages to prevent tigers from preying on people or livestock;
-
An adaptive management approach is recommended to face the novel management and silvicultural challenges when conserving a highly profiled Red List species such as the tiger. The long-term management outcomes can be improved by developing silvicultural systems in a close cooperation between research and management, and provided such factors as hunting the Amur tiger are under control.
Abbreviations
- AUM:
-
Animal Unit Month
- FRA:
-
Forest Resources Assessment
- GIS:
-
Geographic Information System
- IUCN:
-
the International Union for Conservation of Nature
- km:
-
kilometer
- MODIS:
-
Moderate Resolution Imaging Spectroradiometer
- NFPP:
-
the Natural Forest Protection Program
- UN FAO:
-
Food and Agriculture Organization of the United Nations
References
Angermeier PL (2000) The natural imperative for biological conservation. Conserv Biol 14:373–381
Begon M, Harper JL, Townsend CR (1996) Ecology: individuals, populations, and communities, 3rd edn. Blackwell Science, Boston
Canby P (2005) The cat came back: alpha predators and the new wilderness. Harper’s Mag 310:95–102
Carroll C, Miquelle DG (2006) Spatial viability analysis of Amur tiger Panthera tigris altaica in the Russian Far East: the role of protected areas and landscape matrix in population persistence. J Appl Ecol 43:1056–1068
Chen P (2004) Natural forest protection: an overview abroad and a review at home. J Beijing For Univ 3:50–54
Connell JH (1978) Diversity in tropical rain forest and coral reefs. Science 199:1302–1310
Conniff R (2009) Can China save the Amur tiger? J Sch Yale For Environ Stud 2009(5–11):37
Cook JG, Irwin LL, Bryant LD et al (1998) Relations of forest cover and condition of elk: a test of the thermal cover hypothesis in summer and winter. Wildl Monogr 141:5–61
DAOW (Daxinganling Administrative Official Website) (2007) General information of Daxinganling Forestry Company. http://dxal.gov.cn/zyj/E_ReadNews.asp?NewsID=131. Accessed 3 Mar 2008
Dema T (2010) Human-wildlife conflict under the shadow of a big cat: residents live uneasily with a tigress in their midst. In: Kuensel (Saturday August 21, p15), vol. Saturday August 21, 2010, Thimphu
Deng W, Zhang P, Zhang B (2004) Development report on northeast region. Science Press, Beijing
Duncan S (2000) Why do elk seek shelter? the case against the need for thermal cover. PNW Sci Find 22:1–6
FAO (the Food and Agricultural Organization of the United Nations) (2000) Global Forest Resources Assessment (FRA 2000). Global Land Cover Characterization USGS http://edc2.usgs.gov/glcc/fao/index.php
Feldhamer GA (2007) Mammalogy: adaptation, diversity, ecology, 3rd edn. Johns Hopkins University Press, Baltimore
Fisher JT, Wilkinson L (2005) The response of mammals to forest fire and timber harvest in the North American boreal forest. Mamm Rev 35:51–81
Fuller TK, Sievert PR (2001) Carnivore demography and the consequences of changes in prey availability. In: Gittleman JL, Funk SM, Macdonald D et al (eds) Carnivore conservation. Cambridge University Press, Cambridge, pp 163–178
Gittleman JL, Harvey PH (1982) Carnivore home-range size, metabolic needs and ecology. Behav Ecol Sociobiol 10:57–63
Gross L (2005) Why not the best? How science failed the Florida Panther. PloS Biol 3:1525–1531
Gurung B, Smith JLD, McDougal C et al (2008) Factors associated with human-killing tigers in Chitwan National Park, Nepal. Biol Conserv 141:3069–3078
Gutierrez RJ, Carey AB (1985) Ecology and management of the spotted owl in the Pacific Northwest. Series
Han X (2011) The forest stand structures in northeastern China; their potential effects on the suitability of forests for animals, plants, and other values; and their possible relationships to Amur tiger (Panthera tigris altaica) conservation. Doctoral Dissertation. PhD. School of Forestry and Environmental Studies, Yale University, New Haven
Han X, Oliver CD, Ge J et al (2007) Could forest management that ensures a balance of stand structures enhance biodiversity conservation in Northeast China’s Natural Forest Protection Program need? In: Stanturf JA (ed) IUFRO conference on forest landscape restoration, Korea Forest Research Institute, Seoul, Korea, pp 40–43
Hao Z, Wang Q, Dai L (1999) The importance of the national programme for natural forests conservation on biodiversity conservation in northeast state owned forest areas of China. In: Xu Z (ed) China’s biodiversity conservation toward the 21st century. China Forestry Press, Beijing, pp 21–26
Heady HF, Child RD (1994) Rangeland ecology & management. Westview Press, Boulder
Herfindal I, Linnell JDC, Odden J et al (2005) Prey density, environmental productivity and home-range size in the Eurasian lynx (Lynx lynx). J Zool 265:63–71
HFSDI (Heilongjiang Forestry Survey and Design Institute) (2001) NFPP implementation scheme for Heilongjiang province. Issued by the Forestry Bureau of Heilongjiang Province. No. G00001.
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants – a comparative view of their digestive-system. Oecologia 78:443–457
Hornocker MG, Quigley HB, Miquelle DG et al (1998) Final report to the SAVE THE TIGER FUND at the national fish and wildlife foundation: ecology and conservation of the Siberian tiger. Series Hornocker Wildlife Institute Moscow, Idaho
Hull RB, Robertson DP, Richer D et al (2002) Assumptions about ecological scale and nature knowing best hiding in environmental decisions. Conserv Ecol 6:2
IMFSDI (Inner Mongolia Forestry Survey and Design Institute) (2001) NFPP implementation scheme for Inner Mongolia. Issued by the Inner Mongolia People’s Government. No. 0501141
IUCN (2007) IUCN Red List of threatened species. http://www.iucnredlist.org. Accessed 10 Sept 2012
Jedrzejewska B, Jedrzejewski W (2005) Large carnivores and ungulates in European temperate forest ecosystems: bottom-up and top-down control. In: Ray JC, Redford KH, Steneck RS et al (eds) Large carnivores and the conservation of biodiversity. Island Press, Washington, DC, pp 230–246
JFSDI (Jilin Forestry Survey and Design Institute) (2002) NFPP implementation scheme for Jilin province. Issued by the Forestry Bureau of Jilin Province. No. 2001016
Johnson DH, O’Neil TA (2001) Wildlife-habitat relationships in Oregon and Washington. Oregon State University Press, Corvallis
Kaplanov LG (1948a) Tigers in Sikhote-Alin. In: Tiger, red deer and moose. O-vo Ispyt. Prir., Moscow
Kaplanov LG (1948b) Tigr, izyubr’, los’ (Tiger (Felix tigris), Manchurian Wapiti (Cervus elaphus xanthopygus), and Moose (Alces alces)). MOIP, Moskva, Izd
Karanth KU, Stith BM (1999) Prey depletion as a critical determinant of tiger population viability. In: Seidensticker J, Christie S, Jackson P (eds) Riding the tiger: tiger conservation in human-dominated landscapes. Cambridge University Press, Cambridge, pp 100–113
Karanth KU, Nichols JD, Kumar NS et al (2004) Tigers and their prey: predicting carnivore densities from prey abundance. Proc Natl Acad Sci USA 101:4854–4858
Kerley L (2003) A final report to SAVE THE TIGER FUND: scent dog monitoring of Amur tiger-II. (2002-0301-016) Lazovsky State Nature Zapovednik. Lazo, Primorski Region, Russia. http://www.nfwf.org/AM/Template.cfm?Section=Final_Reports3&TEMPLATE=/CM/ContentDisplay.cfm&CONTENTID=8629. Accessed 10 Sept 2012
Kostyack J, Hill K, Fuller MK (2006) Science behind Panther decisions discredited. News &Views. http://www.nwf.org/news/story.cfm?pageId=3DDF4881-65BF-09FE-B779B9AA9C8D66CF. Accessed 21 Sept 2006
Lei J (2005) Forest resources of China. China Forestry Press, Beijing
Li L (1999) Shaping and expanding of the agricultural area is Heilongjiang Basin during the Qing Dynasty. Collections of essays on Chinese historical geography 3:201–252
Li W (2003) Observation of roe deers’ ecological habits and analysis of their food. J Liaoning For Sci Technol 5:16–18
Li Z (2008) The land use pattern and dynamics of Amur tiger’s distribution area. In: College of life sciences. Beijing Normal University, Beijing
Li T, Jiang J, Wu Z (2001) Survey on Amur tigers in Jilin province (in Chinese). Acta Theriol Sin 21:1–6
Ma Y (1998) Panthera tigris. In: Wang S (ed) China red data book of endangered animals, vol Mamalia. Science Press, Beijing, pp 119–125
Ma Y (2000) Amur tiger is filled, cattle is lost. Electronic Xinhua Daily, p 005
Marquis DA (1975) The Allegheny hardwood forests of Pennsylvania. USDA Forest Service General Technical Report NE-15. 32 pp.
Matyushkin EN, Pikunov DG, Dunishenko YM et al (1996) Numbers, distribution, and habitat status of the Amur tiger in the Russian Far East: ‘Express-report’. Final report to the USAID Russian Far East Environmental Policy and Technology Project, USAID
McNab BK (1963) Bioenergetics and determination of home range size. Am Nat 97:133–140
McNaughton SJ, Oesterheld M, Frank DA et al (1989) Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 341:101–105
Meijaard E, Sheil D, Nasi R et al (2005) Life after logging: reconciling wildlife conservation and production forestry in Indonesian Borneo. Center for International Forestry Research, Jakarta
Miquelle DG, Smirnov EN, Merrill TW et al (1999) Hierarchical spatial analysis of Amur tiger relationships to habitat and prey. In: Seidensticker J, Christie S, Jackson P (eds) Riding the tiger: tiger conservation in human-dominated landscapes. Cambridge University Press, Cambridge, pp 71–99
Molles MC (2002) Ecology: concepts and applications, 2nd edn. McGraw-Hill, Boston
Morgantini LE, Woodard PM, Eslinger D (1994) Logging and elk in the Bighorn Creek Valley in Western Alberta (1988–1991). Final Report: Department of Renewable Resources in cooperation with Alberta Environmental Protection, Fish and Wildlife Service. Edmonton, 57 pp
Muller P (1998) Hunting by humans perverse, too efficient – nature has solutions. The C.A.S.H. Courier Fall, special articles http://www.all-creatures.org/cash/cc98fa-perverse.html
NASA (The National Aeronautics and Space Administration) (2001) MODIS/Terra land cover type L3 global grid data Land Processes Distributed Active Archive Center USGS http://LPDAAC.usgs.gov/dataproducts.asp
NFPP SFA (NFPP Management Office and Center of State Forestry Administration) (2006) Natural forest resource protection program manual. China Forestry Press, Beijing
Nowell K, Jackson P, Group ISCS (1996) Wild cats: status survey and conservation action plan. IUCN, Gland
Odum EP (1969) Strategy of ecosystem development. Science 164:262
O’Hara KL, Latham PA, Hessburg P et al (1996) A structural classification for inland northwest forest vegetation. West J Appl For 11:97–102
Oliver CD (1998) Passive versus active forest management. In: Calhoun JM (ed) Forest policy: ready for renaissance. Institute of Forest Resources, College of Forest Resources, University of Washington, Seattle, pp 237–257
Oliver CD, Larson BC (1996) Forest stand dynamics, Updated edn. Wiley, New York
Oliver CD, O’Hara KL (2004) Effects of restoration at the stand level. In: Stanturf JA, Marsden P (eds) Restoration of boreal and temperate forests. CRC Press, New York, pp 31–59
Oliver CD, Osawa A, Camp A (1998) Forest dynamics and resulting animal and plant population changes at the stand and landscape levels. J Sustain For 6:281–312
Quammen D (2003) Monster of God: the man-eating predator in the jungles of history and the mind. W.W. Norton, New York
Ruyle G, Ogden P (1993) What is an A.U.M.? http://cals.arizona.edu/arec/pubs/rmg/1%20rangelandmanagement/1%20aum93.pdf. Accessed 8 Apr 2007
Shaw JH (2009) Personal communication. November 15, 2009. Shaw JH is a professor in the School of Natural Resource Ecology and Management, Oklahoma State University
Shaw JH (2010) Personal communication. May 10, 2010. Shaw JH is a professor in the School of Natural Resource Ecology and Management, Oklahoma State University
Shi D (2005) Investigation and thoughts on managing guns in national forest. For Public Secur 2:14–15
Shi P, Xu J (2006) Deforestation in China. Discussion of the Agriculture Policy Research Center. http://www.ccap.org.cn/cn/research/discussc.htm. Accessed 15 Sept 2006
Smith JLD, McDougal CW, Sunquist ME (1987) Female land-tenure system in tigers. In: Tilson RL, Seal US (eds) Tigers of the world: the biology, biopolitics, management, and conservation of an endangered species. Noyes Publications, Park Ridge, pp 97–109
Smith JLD, Ahearn SC, McDougal C (1998) Landscape analysis of tiger distribution and habitat quality in Nepal. Conserv Biol 12:1338–1346
Song J, Li W, Zhang H et al (2005) Observation of wild boars’ ecological habits and analysis of their food in Baishilazi area. J Liaoning For Sci Techonol 2:25–26
State Forestry Administration of P. R. China (1982) Technical guidelines for the forest resource inventory. Issued by the Ministry of Forestry on 25 Dec, 1982. No. 10
Stevens WK (1990) New eye on nature: the real constant is eternal turmoil. The New York Times
Stroganov SU (1969) Carnivorous mammals of Siberia. Israel Program for Scientific Translations, Jerusalem
Suding KN, Gross KL, Houseman GR (2004) Alternative states and positive feedbacks in restoration ecology. Trends Ecol Evol 19:46–53
Sun Y (2004) Heilongjiang forests: wild Amur tiger ate cattle again. Electronic Xinhua Daily, p 8
Sunquist ME (1981) The social organization of tigers (Panthera tigris) in Royal Chitawan National Park, Nepal. Smithson Contrib Zool 336:1–98
Tian Y, Wu J, Kou X et al (2009) Spatiotemporal pattern and major causes of the Amur tiger population dynamics. Biodivers Sci 17:211–225
U.S. Fish and Wildlife Service (1985) Red-cockaded woodpecker recovery plan. U.S. Fish and Wildlife Service, Atlanta
USGS (2005) Global Forest Resources Assessment (FRA 2000). Earth Resources Observation and Science Center. http://edcsns17.cr.usgs.gov/glcc/fao/methodology.html. Accessed 15 Mar 2008
Wallington TJ, Hobb RJ, Moore SA (2005) Implications of current ecological thinking for biodiversity conservation: a review of the salient issues. Ecol Soc 10:15
Wang Y (1995) Korean pine forest. Northeast Forest University Press, Harbin
Wang S (2003) The system analysis of Heilongjiang modern forest management since 1949s. In: Forest management. Beijing Forestry University, Beijing
Wang H (2007a) Wild Amur tiger appeared in Hunchun again. Science and Technology Daily, p 010
Wang J (2007b) Frequent occurrence of Amur tiger in Mulin forest, Heilongjiang. China Green Times, p A01
Wang L (2007c) Modern forestal industrialization in Northeast China and its influence. In Chinese
Wang S, Guo Q, Song R (2005a) Land resources optimum and forest-type structure regulation for Daxing’anling forest region. J Northeast For Univ 33:23–24
Wang Z, Wang Z, Wang L (2005b) Wild Amur tiger comes to Dongning. Heilongjiang Daily News, p 009
Wilkinson DM (1999) The disturbing history of intermediate disturbance. Oikos 84:145–147
Wu Q (2007) Exploring the change of Heilongjiang forests and its cause in the modern age. Stud Explor Chin 170:220–224
Wu Q, Huang Q (2007) Clarify and explain the changes of forest area and volume in Heilongjiang in modern times. For Econ 3:60–62
WWF (2008) Tigers: tiger fact. WWF International http://www.panda.org/species
Yan K, Meng X, Yang Q (2006) Identification manual for mammals in China. China Forestry Press, Beijing
Yao C, Man X (2004) Historical evolvement and dynamic swing of forest resources in Heilongjiang. J Northeast For Univ 32:8–11
Yi B, Ye Y (2004) The development of forest reserves in Northeast China since the end of Qing Dynasty and its environmental cost. Agricultural History of China 4:115–123
Young MR (1992) Conserving insect communities in mixed woodlands. In: Cannell MGR, Malcolm DC, Robertson PA (eds) The ecology of mixed-species stands of trees. Blackwood Scientific Publications, London, pp 277–296
Yu X, Sun B, Sun H (2000) Amur tigers distribution and population amount in Heilongjiang Province. Wild Anim 2:14–16
Zhang E, Miquelle DG, Wang T et al (2005) Recovery of the wild Amur tiger population in China: progress and prospect. China Forestry Publishing House, Beijing
Acknowledgments
This study is supported by Yale School of Graduate Studies Fan Fellowship, Yale School of Forestry and Environmental Studies, Yale Institute for Biospheric Studies Center for Field Ecology Pilot Grant, the Jubitz Family Endowment for Research from United States, and the National Key Project for Cooperative Research on key issues concerning the environment and resources in China and Russia (Grant No. 2005CB724800), the National Natural Science Foundation of China (31270567, 31210103911) from China. This study has received valuable advice and encouragement from Dr. Maung Moe Myint, Professor Timothy G. Gregoire of Yale University, Professor Herman H. Shugart of the University of Virginia, Professor C. Dana Tomlin of University of Pennsylvania and Professor Jim Shaw of Oklahoma State University. We appreciate the help of Mr. Xuejun Chen, Vice Director of China’s NFPP Office, for his advice and for providing materials. We also thank the Hunchun National Reserve in Jilin Province for helping with this Amur tiger study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Han, X., Oliver, C.D., Ge, J., Guo, Q., Kou, X. (2012). Managing Forest Stand Structures to Enhance Conservation of the Amur Tiger (Panthera tigris altaica). In: Stanturf, J., Madsen, P., Lamb, D. (eds) A Goal-Oriented Approach to Forest Landscape Restoration. World Forests, vol 16. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5338-9_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-5338-9_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5337-2
Online ISBN: 978-94-007-5338-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)