Abstract
The distribution of cadmium in the ocean is very similar to that of major nutrients suggesting that it may be taken up by marine phytoplankton at the surface and remineralized at depth. This interpretation is supported by recent data on Cd isotope distribution showing an increase in the 112Cd/110Cd ratio in Cd-depleted surface water. While at high concentrations, Cd is toxic to phytoplankton as it is to many organisms, at relatively low concentrations, Cd can enhance the growth of a number of phytoplankton species under zinc limitation. Kinetic studies suggest that Cd is taken up through either the Mn or the Zn transport system, depending on the ambient concentrations of these metals. In addition to inorganic Cd complexes (including the free Cd2+ ion), Cd complexes with relatively weak organic ligands may also be bioavailable. Cd is very effective to induce the production of phytochelatin and other thiols in phytoplankton, probably as a detoxification mechanism as well as a control of Cd homeostasis in cells. The only known biological function of Cd is to serve as a metal cofactor in Cd-carbonic anhydrase (CDCA) in diatoms. The expression of CDCA is regulated by Cd and Zn availabilities and by the pCO2/pH of the ambient seawater in cultured diatoms and natural assemblages. The conformation of CDCA active site is similar to that of β-CA and both Zn and Cd can be used as its metal cofactor and exchanged for each other. Understanding of the biological role of Cd in marine phytoplankton provides insights into the biogeochemical cycling of this element in the ocean.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Marine phytoplankton are a diverse group of unicellular photosynthetic microorganisms that live in the sunlit surface waters of the ocean. While their standing biomass is miniscule compared to that of land plants, they grow rapidly and account for nearly half of primary production on earth [1]. To grow, phytoplankton take up nutrients from surface seawater; part of the resulting biomass is continuously exported to deep water where the nutrients are remineralized and slowly returned to the surface by ocean mixing. The concentrations of major and trace nutrients in surface seawater thus reflect both the activity of the phytoplankton which export them to the abyss and the geochemical processes that cycles elements in and out of the oceans. The mutual influence of nutrient concentrations and phytoplankton physiology results in a coupling between the geochemistry of biologically essential elements and the evolution of phytoplankton over geological time [2].
Cadmium is a highly toxic element and there have been instances of Cd pollution with some well-documented cases of impact on human health (e.g., Itai-itai disease [3]). Elevated Cd concentrations occur in some polluted coastal regions and are cause for concern, particularly as a result of the ability of some marine bivalves to accumulate the metal, posing health risks to humans and other animals that consume them (e.g., [4,5]). However, the concentration of Cd in the open ocean is extremely low, and like that of Zn and other essential metals, its vertical profile is closely correlated to that of major nutrients such as phosphate. This “nutrient-like” concentration profile suggests that Cd maybe removed from surface seawater by the same mechanisms as algal nutrients and that it may itself serve as a nutrient to marine phytoplankton [6–8].
After many years of research, the first protein that uses Cd naturally has been discovered in marine diatoms: a carbonic anhydrase with Cd as its catalytic center (CDCA) [9,10]. It appears that CDCA plays a pivotal role in the acquisition of inorganic carbon in diatoms, and thus the use of Cd in CDCA provides a link between the biogeochemical cycles of carbon and Cd. The existence of CDCA is an example of the unique mechanisms phytoplankton have evolved over geological times as an adaptation to life in the metal-poor environment of surface seawater. But CDCA may not be the only biological use of Cd in seawater. While we are beginning to understand how and how much Cd is utilized by phytoplankton cells, there are still many challenging questions that need to be answered.
2 Cadmium Distribution in the Ocean
2.1 Vertical Profiles
As discussed in Chapter 2, the vertical distribution of Cd is very similar to that of major nutrients such as phosphate and nitrate; it is depleted in the surface water as a result of biological uptake by phytoplankton and regenerated in deep water (Figure 1). This nutrient-like profile has been observed across ocean basins and in both open ocean and coastal upwelling regions [6,7,11–14]. The strong correlation between dissolved Cd and P concentrations in seawater is also seen in suspended particles, further confirming that the distribution of Cd is governed by biological activity [11,12]. Vertical mixing of Cd-rich deep water to the surface is the main input of Cd to the surface waters of the open ocean while atmospheric deposition is insignificant [7,12].
The concentration of dissolved Cd in the open ocean is on the order a few pmol L–1 at the surface, around 1 nmol L–1 in the deep Pacific, and sub-nmol L–1 in the deep Atlantic [7,8,14–16] (Figure 1). In surface seawater, the bulk of dissolved Cd is complexed to organic ligands with high conditional stability constants (log\( K^{\prime}_{\rm CdL,Cd^{\prime}}\) ≈ 9.8–10.9) [14,16]. As a result, the inorganic Cd concentration, Cd′, which is dominated by chloride complexes (97.2%) [17] is maintained in the sub-pmol L–1 range, [14,16,18]. Microorganisms are presumably the source of the strong organic ligands that bind Cd in surface seawater; they likely also release weaker ligands that are not detectable by current electrochemical techniques. For example, in an estuary where the dissolved Cd concentration is elevated by anthropogenic activities, most of the Cd is complexed by organic ligands with a mean conditional stability constant of 8.9 (log K \( ^\prime_{\rm CdL,Cd^\prime} \)) [19]. As discussed below, the formation of weak Cd complexes may enhance the bioavailability of Cd to phytoplankton [20].
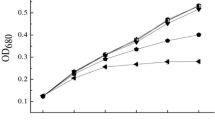
The demonstration that Cd might be utilized as a nutrient by phytoplankton – as implied by its nutrient-like profile in the ocean – came from culture studies in which the growth rate of Zn-limited phytoplankton species was markedly increased by addition of Cd to the medium (Figure 2) [21]. The concentration of bioavailable free Zn in the surface waters of the open ocean is indeed quite low [14,22–25] and in the range found to limit phytoplankton growth in cultures [24–26]. Some field studies have in fact found evidence of Zn-limitation of primary production in ocean water [27–29]. It is therefore plausible that phytoplankton may take up Cd to replace Zn for biological functions in the Zn-depleted conditions of the surface ocean.
Growth curves of Emiliania huxleyi (a) and Thalassiosira weissflogii (b) under various inorganic Zn (Zn′) and Cd (Cd′) concentrations. (a): low Zn = 0.7 pmol L–1 Zn′; low Zn +Cd = 0.7 pmol L–1 Zn′ and 20 pmol L–1 Cd′; high Zn = 15 pmol L–1 Zn′. Data from [37]. (b): low Zn = 4 pmol L–1 Zn′; low Zn + Cd = 4 pmol L–1 Zn′ and 30 pmol L–1 Cd′; high Zn = 20 pmol L–1 Zn′. Unpublished data from Y. Xu.
2.2 Isotope Composition
Recent measurements of the isotope composition of Cd in seawater and marine Fe-Mn deposits provide insight into the processes that affect the biogeochemical cycle of Cd [30–34]. An inverse correlation between dissolved Cd concentration and the 112Cd/110Cd and 114Cd/110Cd ratios in the upper water column suggests that the isotope composition of Cd is controlled by Rayleigh fractionation [30–33]. A systematic trend has also been observed in the isotope signal of Cd in marine Fe-Mn deposits [34]. This isotope fractionation is consistent with a preferential uptake of the light Cd isotope by phytoplankton, as also seen in the only culture study using a freshwater phytoplankton species [31]. In accord with this interpretation, the lack of measurable isotopic fractionation of Cd observed in the northwest Mediterranean Sea simply reflects the small depletion of Cd in these waters [31]. Different negative correlations between the 112Cd/110Cd ratio and Cd concentration in different regions of the Southern Ocean indicate that the physiological status and species composition of the phytoplankton may cause variations in the fractionation of Cd isotopes [30].
In contrast to the upper water column, deep water has rather uniform and small Cd isotopic fractionation despite the fact that Cd concentrations increase along the global deep-water pathway from the Atlantic to the Pacific Ocean [31–34]. The Cd in deep water that is remineralized from exported organic matter, must thus have a small Cd isotopic fractionation [32]. To the extent that the bulk of the surface Cd is taken up by phytoplankton, this result is not inconsistent with isotopic fractionation by phytoplankton: the isotopic composition of any nutrient in the biomass must converge to that of the source (upwelled) water when the fraction of nutrient taken up increases, as observed, for example, for nitrogen [35]. More studies on Cd isotopic fractionation by different phytoplankton taxa under various conditions are warranted to help understand the isotopic composition of Cd in the oceans.
3 Effects of Cadmium on Phytoplankton Growth
3.1 Beneficial Effect
As mentioned above, the first demonstration of the biological use of Cd in phytoplankton was obtained with Zn-limited cultures of the diatom Thalassiosira weissflogii [21]. Since then the positive effect of Cd on growth rate under conditions of Zn limitation has also been observed in several other phytoplankton species such as Thalassiosira pseudonana, Pleurophrysis carterae, Tetraselmis maculata, and Emiliania huxleyi (Figure 2) [36,37]. In E. huxleyi, different strains have similar response to Cd addition suggesting that the use of Cd is a general attribute of Zn-limited E. huxleyi [37].
Two lines of evidence indicate that Cd is used to substitute for Zn either as a metal center in Zn proteins or in Cd-specific proteins that replace Zn proteins: (i) the beneficial effect of adding Cd in phytoplankton cultures can only be observed when Zn is limiting and is more pronounced when Zn is more limiting [21,37,38]; (ii) the size distribution of Zn and Cd in soluble intracellular proteins of T. weissflogii is remarkably similar [21]. However, Zn in phytoplankton cells can only be replaced partially by Cd and there is a minimum requirement for Zn that cannot be replaced by Cd [37,38]. For example, in E. huxleyi cells, only up to 50% of cellular Zn can be replaced by Cd [37]. Moreover, the use of Cd by phytoplankton is less efficient than that of Zn such that the growth rates observed upon addition of Cd to Zn-limited cultures are never as high as those of Zn-sufficient cultures (Figure 2) [9,37].
In T. weissflogii, carbonic anhydrases (CA) account for a major fraction of soluble Zn proteins especially under low pCO2/high pH conditions. Addition of Cd to Zn-limited cultures of T. weissflogii restores CA activity [9,39]. This had led to the discovery of CDCA, the first and only known native Cd protein (see below). The utilization of Cd in CDCA explains, at least in part, the beneficial effect of Cd in Zn-limited cultures of other organisms that are known to also possess the cdca gene, including T. pseudonana [40]. But Cd must have other biochemical functions in phytoplankton than as a metal cofactor in CDCA since the beneficial effect of Cd is observed in organisms such as T. maculata and E. huxleyi that do not have the cdca gene. Cd also has a beneficial effect on cultures of T. weissflogii at high pCO2/low pH condition when CDCA expression is down regulated (Xu, unpublished data).
3.2 Toxic Effect
It is well known that elevated Cd concentrations are toxic to phytoplankton and that different species have different sensitivity to Cd toxicity [36,41–46]. For example, in a comparison of phytoplankton taxa, Brand and coworkers showed that cyanobacteria were the most sensitive to Cd toxicity, and diatoms the least sensitive with coccolithophores and dinoflagellates having intermediate sensitivity [41]. Another study found no systematic differences among taxa [44]. Differences in sensitivity can also be found within the same genus; for example, oceanic Thalassiosira species are more resistant to Cd toxicity than coastal ones [45]. The free Cd ion concentration that causes 50% reduction in growth rate ranges from a few pmol L–1 to several hundred nmol L–1 [41,44].
The biochemical mechanisms of Cd toxicity in phytoplankton are in some respects similar to those in higher plants (see Chapter 13). One of the well-known effects is that Cd can compete with essential metals for uptake sites on the cell surface. High concentration of Cd inhibits the uptake of Mn and thus causes Mn deficiency in cells at low Mn concentrations [47–49]. Similarly, Cd also inhibits Fe uptake and assimilation and thus causes Fe deficiency, as evidenced by decreases in cytochrome f to chlorophyll a ratio and nitrate reductase activity [50,51]. Interestingly, even at a concentration where it is beneficial to growth, Cd can become toxic if the Zn concentration becomes severely limiting [37,38]. This presumably reflects a loss of activity caused by Cd substitution for Zn in some essential Zn enzymes ([36,37] and references therein). Other mechanisms of Cd toxicity include oxidative stress, as reviewed in [52] and inhibition of photosynthesis via interference with the xanthophyll cycle in the diatom Phaeodactylum tricornutum [53].
The differences in sensitivity to Cd toxicity between phytoplankton species may be related to differences in their ability to detoxify the metal [43,44]. Cd-induced phytochelatin production is the most common detoxification mechanism in phytoplankton (see Section 5.1), but other thiol-containing peptides or proteins may also be involved. For example, a Cd-tolerant phytoplankton species, Isochrysis galbana, produces a metal-binding protein rich in cysteine [54]; the cyanobacterium Synechococcus sp. produces a metallothionein-like protein to complex metals [55]. In addition, some species may induce efflux systems to remove intracellular Cd [47,56,57], or sequester Cd into the vacuole to reduce the cytosolic Cd concentration [58].
4 Cadmium Uptake by Phytoplankton
4.1 Transport System and Effect of Manganese and Zinc
The molecular mechanism of Cd uptake by phytoplankton is not known but kinetic studies in the laboratory using cultured model species show that Cd may be taken up through two separate metal transport systems depending on the concentrations of competing metals [47]. Several studies showed that the rate of Cd uptake depends not only on the concentration of Cd, but also on the concentrations of Zn and Mn (Figure 3) [37,38,47,59,60]. The data can be interpreted quantitatively by considering that Cd is taken up competitively by the high affinity Zn uptake system under Zn-limited conditions and that Cd, Zn, and Mn share the same uptake system under Zn-sufficient conditions. Accordingly, Cd uptake kinetics follow a competitive saturation equation [59]:
(a) Short-term Cd uptake rate by T. weissflogii preconditioned at different Zn concentrations in the presence of Cd. Low Zn + Cd: 1.6 pmol L–1 Zn′ and 460 pmol L–1 Cd′; High Zn + Cd: 16 pmol L–1 Zn′ and 460 pmol L–1 Cd′. Data are from [38]. (b) Short-term Cd uptake by T. pseudonana at different Mn concentrations. Data are from [48].
K values are the affinity constants for binding of the subscripted metals to the uptake ligands; V max1 and V max2 are the maximum uptake rates for the two systems; the free metal ion concentrations of Cd, Zn and Mn are those at the cell surface. The first term gives Cd uptake by the system induced at low cellular Zn and the second term uptake by Mn transport system. Interestingly, V max2 was found to equal V maxMn in the coastal diatom T. pseudonana, indicating that both metals have similar internalization rate constants (k in ). This suggests that V maxCd is controlled by a rate-limiting internalization step, e.g., physical movement of Cd across the membrane by transport molecules [47]. Similar effects of Mn and Zn on Cd uptake have been observed in field studies as well: the short-term Cd uptake rate and the cellular Cd:P ratio in natural assemblages were lower with addition of Zn or Mn [61,62] (Xu unpublished data).
Unlike Zn and Co, whose uptake rates can reach 60 to 90% of the maximum diffusion rates of unchelated Zn and Co to the cell surface, Cd uptake rate only reaches up to 20% of the maximum diffusion rate of unchelated Cd in E. huxleyi [37]. This may be because the dissociation of Cd2+ from the strong chloride complexes in seawater slows down the binding to uptake molecules [37].
4.2 Effect of Other Metals and Macronutrients
Both laboratory culture studies and shipboard incubation experiments have shown that cellular Cd concentrations are elevated in Fe-limited phytoplankton cells [60,63]. For example, the average estimated particulate Cd:P ratio in samples from Fe-limited waters was 2.3-fold higher than that from other samples [64]. The effect of Fe-limitation can be partly explained by biodilution since cells often grow slower under Fe limitation while the Cd uptake rate remains unchanged [60]. Another possible mechanism is uptake of Cd via a putative divalent metal transporter that is up-regulated under Fe-limitation [65].
Colloid-bound Cd is taken up by phytoplankton either through dissolution of Cd2+ or, possibly, through direct internalization of lipophilic colloid-bound Cd [66]. As is the case for other metals, Cd complexes with low molecular weight lipophilic organic ligands enter phytoplankton by passive diffusion across the plasma membrane [67].
4.3 Effect of pH/pCO2 on and Role of Weak Ligands in Cadmium Uptake
Since Cd is used in CDCA in diatoms and CDCA expression is regulated by the pH/pCO2 of seawater (see Section 6.1), we might expect that Cd uptake should be affected by pH/pCO2 as well. Indeed, field studies showed that the Cd:P ratio in natural assemblages dominated by diatoms increased as seawater pCO2 decreased/pH increased and short-term Cd uptake was inversely related to seawater pCO2 [20,61,62]. However, at low ambient Zn concentrations, the Cd uptake rate is only controlled by the bioavailable concentration, Cd′, and independent of seawater pH/pCO2 [20]. In field samples, the Cd uptake rate decreased at lower pH [20]. This effect is likely explained by the role of weak organic complexes that make Cd bioavailable through a ligand exchange reaction with uptake ligands [68]. The decrease in Cd uptake at lower pH is caused by a lower concentration of bioavailable weak complexes, while Cd′ is maintained constant by complexation to a strong ligand. This effect has been demonstrated in the laboratory using simultaneously EDTA and cysteine to complex Cd [20,68]. Therefore, both strong and weak complexing agents in surface seawater may play an important role in controlling the bioavailability of Cd (and other essential metals) to phytoplankton.
4.4 Phytoplankton Species
A survey study that measured the elemental composition of marine eukaryotic phytoplankton species in cultures showed that all 15 species take up Cd under Zn replete condition, and in general, coccolithophores have the highest Cd quota followed by diatoms and then green algae [69]. Another culture study showed that oceanic diatoms have higher Cd quota than prymnesiophytes [64]. It seems that higher Cd quota in oceanic phytoplankton is an intrinsic trait reflecting their potential to substitute Cd for Zn in a Zn-poor environment. Consistently, Cd quotas in oceanic particles are much larger than the reported coastal values ([69] and references therein).
5 Cadmium and Thiol Production
5.1 Phytochelatins
Phytochelatins are small metal-binding polypeptides with the general formula (γ-Glu-Cys)n-Gly (n = 2–11) produced by organisms in response to metal exposure. Cd is the most effective inducer of phytochelatins in marine phytoplankton, resulting in cellular phytochelatin content more than 10 times that induced by other metals such as Cu or Pb at similar concentrations [70–74]. Although phytoplankton produce phytochelatins even without metal exposure, the production of intracellular phytochelatins is elevated significantly with Cd addition even at picomolar concentrations [71,75] (Figure 4). In general, the production of intracellular phytochelatins is well correlated with the free or inorganic Cd concentrations in phytoplankton culture medium [71,75–79] (Figure 4). The ubiquity of phytochelatin synthesis in response to Cd exposure suggests that phytochelatin production is the primary detoxification mechanism in phytoplankton.
Although the oligomer chain lengths of phytochelatins may vary in different species, the shorter oligomers (n = 2 and 3) are the predominant forms in most species and the stoichiometric ratio of phytochelatin to intracellular Cd is maintained at 2 to 4 γ-Glu-Cys per Cd at high Cd concentrations [75,80,81]. However, other studies also found that the ratio of intracellular Cd to phytochelatin increased with increasing [Cd2+] [82,83]. It seems that the production of phytochelatins is tightly regulated in cells to detoxify Cd and to maintain Cd homeostasis. A kinetic study showed that phytochelatin was rapidly accumulated in T. weissflogii and P. tricornutum cells shortly after Cd exposure and then the concentration rapidly decreased once Cd stress was removed [70,84]. At high Cd concentrations (Cd′ >1 nM), T. weissflogii and T. pseudonana cells export Cd as well as phytochelatin, suggesting that cells export the Cd-phytochelatin complexes to maintain low internal Cd concentrations as part of the detoxification mechanism [57,85]. Although the dissolved phytochelatin may be removed quickly by microbial degradation, phytochelatins released by phytoplankton may nonetheless be a source of organic metal-complexing agents in seawater [85].
The production of phytochelatins in response to Cd exposure is also modulated by the presence of other metals. For example, the production of phytochelatins induced by Cd exposure decreased with addition of Zn or Co, probably because these metals compete with each other for transporters and cellular binding sites and addition of Zn and Co decreases Cd uptake and thus the cellular Cd concentrations [74,86,87]. A similar antagonistic effect has been observed between Cd and Mn as well [87]. However, synergistic effects were also observed between Cd and Cu with higher production of phytochelatins in the presence of both Cd and Cu than that in the presence of individual metals [87]. Therefore, the interaction of metals may partly explain the lack of correlation between phytochelatin concentrations measured in natural seawater and metal concentrations [74,85].
Major nutrients may also affect the production of phytochelatins in response to Cd exposure but the effect may vary between species. For example, at stationary phase caused by major nutrient limitation, E. huxleyi did not increase the production of phytochelatins with time of Cd exposure but T. pseudonana did [76].
Besides detoxification, phytochelatins likely play a role as a buffer of intracelllar Cd and perhaps, more generally, in trace metal homeostasis. Such a role is suggested by the fact that phytochelatin synthesis is induced even at metal concentrations much below those that affect the growth of phytoplankton [57,72,75]. This is consistent with the remarkably high affinity of the phytochelatin synthase of T. pseudonana for one of its substrates, Cd-GS2 [88]. Also, contrary to the general trend of decreasing phytochelatins with decreasing metal concentrations, phytochelatin concentrations increase at very low Zn concentration when Cd becomes beneficial to phytoplankton [74]. This is likely the explanation for the relatively high concentrations of phytochelatin observed in the metal-poor surface water of the Equatorial Pacific (Figure 4). In vitro experiments show that Cd complexed to phytochelatin can be incorporated in CDCA [89]. However, there is yet no evidence that phytochelatin delivers Cd to a functional protein in vivo.
5.2 Other Thiols
Besides phytochelatins, other thiols are also produced in marine phytoplankton in response to Cd exposure. The cellular glutathione [GSH = (γ-Glu-Cys)-Gly, the precursor of phytochelatin] concentration remains relatively constant upon Cd exposure in various phytoplankton species even as phytochelatin concentration increases many folds [73,76,87,90]. Apparently, cells tightly regulate their GSH concentration, presumably because of its essential role in cellular functions, particularly the detoxification of reactive oxygen species [91]. The production of other low molecular weight thiols varies among species. For example, γ-Glu-Cys increased in response to Cd exposure in E. huxleyi, T. weissflogii, and P. tricornutum but remained constant in T. pseudonana and was below detection in Dunaliella sp. [76,87]. E. huxleyi also produces Cys, Arg-Cys, and Gln-Cys and the concentrations of the former two thiols increased at higher [Cd2+]. The presence of other metals like Zn and Cu at high concentrations suppresses thiol production in E. huxleyi [90].
The coccolithophore E. huxleyi releases a variety of thiols into the external medium upon exposure to elevated Cd concentrations [90]. Cys and GSH were the primary thiols released by cells exposed to Cd only, whereas γ-Glu-Cys was the primary thiol when Cu and Zn were also present at high concentrations. Like phytochelatins, these low molecular weight thiols released by phytoplankton may also serve as organic metal-complexing agents in surface seawater.
6 Cadmium Carbonic Anhydrase
Cadmium carbonic anhydrase (CDCA) is the first member of a new class of carbonic anhydrases, the ζ class. CDCA1, which uses Cd as its metal cofactor when Zn is limiting, was isolated from the marine diatom T. weissflogii. The amino acid sequence of CDCA1 contains a triple repeat with ~85% identity between repeats [10]. CDCA1 is a key enzyme in the carbon concentrating mechanism (CCM) through which T. weissflogii increases the concentration of CO2 at the site of fixation by RuBisCO [92].
6.1 Cadmium Carbonic Anhydrase Expression
The regulation of CDCA expression, has been studied in detail in the diatom T. weissflogii. The expression of CDCA1 at transcript and protein levels is modulated by both pCO2 and metal concentrations in the growth medium [61,93]. The cdca1 transcript level at steady state increases with decreasing pCO2 [93]. At low pCO2, the CDCA protein abundance increases with the concentration of available Zn in the absence of Cd and increases with the concentration of available Cd when Zn is limiting (Figures 5a and b). Interestingly, the cellular concentration of another CA, TWCA1 also increases upon Cd addition, although this enzyme can only use Zn or Co as its metal center. The effect of Cd on the concentration of TWCA1 is interpreted as the re-allocation of Zn from CDCA1 upon incorporation of Cd (Figure 5b). Upon addition of Cd in Zn-limited cultures, the cdca1 transcript level increases by a factor of 2 in 4 hours and then decreases slightly at 24 hours, while CDCA1 protein abundance increases gradually over time [93].
(a) and (b): CA expression in T. weissflogii under various Zn′ and Cd′ (unpublished data from Y. Xu). 3Zn = 3 pmol L–1 Zn′; 3Zn5Cd = 3 pmol L–1 Zn′ and 5 pmol L–1 Cd′; 3Zn10Cd = 3 pmol L–1 Zn′ and 10 pmol L–1 Cd′; 15Zn = 15 pmol L–1 Zn′. (a): black bars are growth rate and white bars are relative CA activity; (b): black bars are relative CDCA1 abundance and white bars are relative TWCA1 abundance. The pH of the cultures was around 8.8 at the time of measurements for CA activity and CA abundance. (c) and (d): CA expression in natural assemblages collected from the Great Bay, New Jersey (data are from [93,94]). pH values at the time of measurements for CDCA abundance and total CA activity are indicated on the x-axis.
A CDCA homolog, TpCDCA, containing only a single sequence (25.5 kDa) has been identified in the genome of the diatom T. pseudonana, [10]. The expression of TpCDCA at both transcript and protein levels is also regulated by metal availability and pCO2 level, with an increase in expression with decreasing pCO2 and increasing Zn availability [94]. Similar results have been observed in the field. In water samples from Great Bay, New Jersey, cdca transcript level and protein abundance increased as the pCO2 of the medium decreased, coincident with an increase in CA activity (Figure 5 c and d) [93,94]. In samples collected off the Peruvian coast, synthesis of a CDCA-like protein was induced by incubation at low pCO2 (pH = 8.6) or with addition of Cd [93]. Interestingly, only a 26-kDa protein was revealed by CDCA antiserum in samples from the Great Bay whereas a 70-kDa protein in samples from the Peruvian coast, suggesting that a single sequence of CDCA was the dominant form expressed in the Great Bay and a three-repeat form off the Peruvian coast [93,94].
6.2 Diversity of cdca
CDCA, the only known Cd protein, was first identified in the model diatom T. weissflogii. Homolog genes have so far been found exclusively in diatom species [40,93]. They have also been found in all environmental samples that have been tested, suggesting that the use of Cd in CDCA likely accounts, at least partially, for the nutrient-like behavior of Cd in the oceans.
cdca-like genes have been amplified from about two thirds of the diatom species that have been tested but not from any other phytoplankton taxa [40]. The translated amino acid sequences from these genes are very similar to each other and over 64% identical to TpCDCA. Phylogenetic analysis showed that these sequences can be clustered into three groups: the Tw group, the Np group, and the Tp group (Figure 6). Interestingly, unlike the 18 S rRNA gene, the CDCA sequences show no clear difference between pennate and centric diatoms.
Phylogenetic tree of translated amino acid sequences of CDCA-like genes from cultured diatoms and environmental samples [40].
cdca-like sequences have also ben found in four geographically distinct environments: the New Jersey coast, Lake Carnegie in New Jersey, the Arabian Sea, and the Peruvian coast [40,93]. These environmental sequences are over 80% identical to TpCDCA at the amino acid level. The variation in these sequences is substantial between environments but very small within the same environment and even smaller within the same water sample. Most of the environmental sequences fall within the Np group and can be clustered into four subgroups. The rest of the sequences fall within the Tw group and are clustered into one subgroup that is similar to Skeletonema costatum but separated from other centric diatoms (Figure 6). Interestingly, two sequences closely related to CDCA1 were identified in a freshwater sample (Lake Carnegie); it should be noted that Thalassiosira species are widely distributed in both freshwater and marine environments.
6.3 Structure and Properties of Cadmium Carbonic Anhydrase
The 3D structures of all three repeats of CDCA1 have been determined by X-ray [89,95]. The overall structure is a novel protein fold without similarity to any other proteins reported in the Protein Data Bank (Figure 7a). In the active site, Cd is bound to three conserved residues (Cys, His, and Cys) and a water molecule to form a complete tetrahedral coordination (Figure 7b). Interestingly, although CDCA has no sequence homology to any other CAs, it contains five highly conserved residues found in β-CAs [93]. The active site conformation of CDCA1 also closely resembles that of the β-CA, indicating a common catalytic mechanism between these two types of CA. Strikingly, a monomer of a CDCA single repeat is a structural and functional mimic of a β-CA dimer. The fundamental differences between the structures of ζ-CA and β-CA, together with the similarity of their functional units offer a remarkable example of convergent evolution.
(a) Overall structure of CDCA repeat 2 with Cd (purple ball) at the active site. (b) The active site of CDCA repeat 2. The substrate analog, acetate, a water molecule (blue ball), and Cd (purple ball) are shown in the active site. (c) Comparison of the active site conformation between metal-free CDCA repeat 1 (cyan) and the Cd-bound CDCA repeat 1 (grey). (d) Metal exchange in CDCA1 repeat 2. Bars represent the amount of exchanged metal at the active site after 24 hr incubation with Zn- or Cd-phytochelatin complexes. Zn/Cd: Zn in CDCA-R2 was exchanged by Cd; Cd/Zn: Cd in CDCA-R2 was exchanged by Zn; G316A and G324A: Cd in mutants was exchanged by Zn (see text). Data are from [89].
Studies showed that CDCA1 repeats are sensitive to sulfonamide and sulfamate derivatives and other anion inhibitors and the inhibition constants are comparable to that observed in β-CAs [95–97]. Intriguingly, in vitro experiments have demonstrated that Cd in the active site of CDCA1 can be readily substituted by Zn, and vice versa (Figure 7d). The facile metal exchange is explained by a stable opening of the active site in the absence of metal (Figure 7c). A 9-amino acid linker sequence with a Gly residue on each end provides flexibility to open and close the active site. Loss of the metal exchange capability in the mutants in which either of the two Gly residues was mutated to Ala has been observed (Figure 7d). CDCA1 is a very fast enzyme with a catalytic efficiency near the diffusion limit. Although the Zn form of the enzyme has higher catalytic activity than the Cd form, both are sufficiently fast to satisfy the needs of fast growing diatoms, a significant competitive advantage in the low metal environment of the oceans [89].
7 Concluding Remarks and Future Directions
It is now clear that, like many other trace metals, Cd can be either beneficial or detrimental to phytoplankton depending on conditions. The bioavailability of Cd is mainly determined by the presence of complexing agents. Although Cd complexed by strong organic ligands is generally not bioavailable, new data show that Cd complexed by relatively weaker organic ligands can be taken up by phytoplankton. Elucidating the chemistry, origins, and function of the strong and weak ligands in seawater remains a great challenge.
Phytochelatins and other thiol-containing compounds produced and released by phytoplankton presumably contribute to the pool of metal binding ligands, but it is unclear how stable these compounds are in seawater and whether they play a significant role in the complexation of metals such as Cd.
According to kinetic studies, Cd is actively taken up by phytoplankton via either the Mn or Zn transport systems depending on ambient concentrations. Other factors such as Fe limitation and ambient pH may also contribute to the variation in Cd uptake rates observed in cultures or natural assemblages. Cd has toxic effects on growth at elevated concentrations and various species have different sensitivities and detoxification mechanisms. Cd also enhances the growth of a number of phytoplankton species under Zn-limitation. Fundamental studies are required to characterize molecules involved in transporting Cd in and out of cells, shuttling Cd into different cellular compartments for storage/detoxification or biochemical use and maintaining Cd homeostasis. At present, the only known biochemical use of Cd is to serve as a metal cofactor in CDCA in marine diatoms. However, Cd is beneficial to the growth of Zn-limited phytoplankton even in situations where CDCA is clearly not involved. There are likely other biochemical functions of Cd in marine phytoplankton that await to be discovered.
Abbreviations
- CA:
-
carbonic anhydrase
- Cd′:
-
inorganic Cd, including Cd complexes with the major inorganic ligands of seawater and Cd2+
- CDCA:
-
cadmium carbonic anhydrase
- Cd-GS2 :
-
(gluathionato)-Cd complex
- EDTA:
-
ethylenediamine-N,N,N’,N’-tetraacetic acid
- GSH:
-
glutathione
- RuBisCO:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase
- Zn′:
-
inorganic Zn, including Zn complexes with the major inorganic ligands of seawater and Zn2+
References
C. B. Field, M. J. Behrenfeld, J. T. Randerson, P. Falkowski, Science 1998, 281, 237–240.
F. M. M. Morel, Geobiology 2008, 6, 318–324.
M. Kasuya, Water Sci. Technol. 2000, 42, 147–154.
T. Y. T. Ng, W. X. Wang, Environ. Toxicol. Chem. 2005, 24, 2299–2305.
K. Chong, W. X. Wang, Environ. Toxicol. Chem. 2000, 19, 1660–1667.
E. A. Boyle, F. Sclater, J. M. Edmond, Nature 1976, 263, 42–44.
K. W. Bruland, Earth Planet. Sci. Lett. 1980, 47, 176–198.
J. H. Martin, S. E. Fitzwater, R. M. Gordon, C. N. Hunter, S. J. Tanner, Deep-Sea Res. Part II 1993, 40, 115–134.
T. W. Lane, F. M. M. Morel, Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631.
T. W. Lane, M. A. Saito, G. N. George, I. J. Pickering, R. C. Prince, F. M. M. Morel, Nature 2005, 435, 42–42.
K. W. Bruland, G. A. Knauer, J. H. Martin, Limnol. Oceanogr. 1978, 23, 618–625.
K. W. Bruland, K. J. Orians, J. P. Cowen, Geochim. Cosmochim. Acta 1994, 58, 3171–3182.
L. G. Danielsson, Mar. Chem. 1980, 8, 199–215.
M. J. Ellwood, Mar. Chem. 2004, 87, 37–58.
J. H. Martin, R. M. Gordon, S. Fitzwater, W. W. Broenkow, Deep-Sea Res., Part A 1989, 36, 649.
K. W. Bruland, Limnol. Oceanogr. 1992, 37, 1008–1017.
R. H. Byrne, L. R. Kump, K. J. Cantrell, Mar. Chem. 1988, 25, 163–181.
C. M. Sakamoto-Arnold, A. K. Hanson, D. L. Huizenga, D. R. Kester, J. Mar. Res. 1987, 45, 201–230.
P. B. Kozelka, K. W. Bruland, Mar. Chem. 1998, 60, 267–282.
Y. Xu, D. Shi, L. Aristilde, F. M. M. Morel, Limnol. Oceanogr. 2012, 57, 293–304.
N. M. Price, F. M. M. Morel, Nature 1990, 344, 658–660.
K. W. Bruland, Limnol. Oceanogr. 1989, 34, 269–285.
M. C. Lohan, P. J. Statham, D. W. Crawford, Deep-Sea Res. Part II 2002, 49, 5793–5808.
L. E. Brand, W. G. Sunda, R. R. L. Guillard, Limnol. Oceanogr. 1983, 28, 1182–1198.
W. G. Sunda, S. A. Huntsman, Limnol. Oceanogr. 1992, 37, 25–40.
M. A. Anderson, F. M. M. Morel, R. R. L. Guillard, Nature 1978, 276, 70–71.
K. H. Coale, Limnol. Oceanogr. 1991, 36, 1851–1864.
D. W. Crawford, M. S. Lipsen, D. A. Purdie, M. C. Lohan, P. J. Statham, F. A. Whitney, J. N. Putland, W. K. Johnson, N. Sutherland, T. D. Peterson, P. J. Harrison, C. S. Wong, Limnol. Oceanogr. 2003, 48, 1583–1600.
V. M. Franck, K. W. Bruland, D. A. Hutchins, M. A. Brzezinski, Mar. Ecol.: Prog. Ser. 2003, 252, 15–33.
W. Abouchami, S. J. G. Galer, H. J. W. de Baar, A. C. Alderkamp, R. Middag, P. Laan, H. Feldmann, M. O. Andreae, Earth Planet. Sci. Lett. 2011, 305, 83–91.
F. Lacan, R. Francois, Y. C. Ji, R. M. Sherrell, Geochim. Cosmochim. Acta 2006, 70, 5104–5118.
S. Ripperger, M. Rehkamper, D. Porcelli, A. N. Halliday, Earth Planet. Sci. Lett. 2007, 261, 670–684.
Z. C. Xue, M. Rehkamper, M. Schonbachler, P. J. Statham, B. J. Coles, Anal. Bioanal. Chem. 2012, 402, 883–893.
A. D. Schmitt, S. J. G. Galer, W. Abouchami, Earth Planet. Sci. Lett. 2009, 277, 262–272.
D. M. Sigman, M. A. Altabet, D. C. McCorkle, R. Francois, G. Fischer, J. Geophys. Res., [Oceans] 2000, 105, 19599–19614.
J. G. Lee, F. M. M. Morel, Mar. Ecol. Prog. Ser. 1995, 127, 305–309.
Y. Xu, D. Tang, Y. Shaked, F. M. M. Morel, Limnol. Oceanogr. 2007, 52, 2294–2305.
J. G. Lee, S. B. Roberts, F. M. M. Morel, Limnol. Oceanogr. 1995, 40, 1056–1063.
F. M. M. Morel, J. R. Reinfelder, S. B. Roberts, C. P. Chamberlain, J. G. Lee, D. Yee, Nature 1994, 369, 740–742.
H. Park, B. Song, F. M. M. Morel, Environ. Microbiol. 2007, 9, 403–413.
L. E. Brand, W. G. Sunda, R. R. L. Guillard, J. Exp. Mar. Biol. Ecol. 1986, 96, 225–250.
G. S. Braek, D. Malnes, A. Jensen, J. Exp. Mar. Biol. Ecol. 1980, 42, 39–54.
M.-J. Wang, W.-X. Wang, Aquat. Toxicol. 2009, 95, 99–107.
C. D. Payne, N. M. Price, J. Phycol. 1999, 35, 293–302.
P. D. Tortell, N. M. Price, Mar. Ecol. Prog. Ser. 1996, 138, 245–254.
A. J. Miao, W. X. Wang, P. Juneau, Environ. Toxicol. Chem. 2005, 24, 2603–2611.
W. G. Sunda, S. A. Huntsman, Sci. Total Environ. 1998, 219, 165–181.
W. G. Sunda, S. A. Huntsman, Limnol. Oceanogr. 1996, 41, 373–387.
J. R. Reinfelder, R. E. Jablonka, M. Cheney, Environ. Toxicol. Chem. 2000, 19, 448–453.
G. I. Harrison, F. M. M. Morel, J. Phycol. 1983, 19, 495–507.
P. L. Foster, F. M. M. Morel, Limnol. Oceanogr. 1982, 27, 745–752.
E. Pinto, T. C. S. Sigaud-Kutner, M. A. S. Leitao, O. K. Okamoto, D. Morse, P. Colepicolo, J. Phycol. 2003, 39, 1008–1018.
M. Bertrand, B. Schoefs, P. Siffel, K. Rohacek, I. Molnar, FEBS Letters 2001, 508, 153–156.
G. H. Wikfors, A. Neeman, P. J. Jackson, Mar. Ecol. Prog. Ser. 1991, 79, 163–170.
R. W. Olafson, W. D. McCubbin, C. M. Kay, Biochem. J. 1988, 251, 691–699.
T. Brembu, M. Jorstad, P. Winge, K. C. Valle, A. M. Bones, Environ. Sci. Technol. 2011, 45, 7640–7647.
J. G. Lee, B. A. Ahner, F. M. M. Morel, Environ. Sci. Technol. 1996, 30, 1814–1821.
Y. Nassiri, J. L. Mansot, J. Wery, T. Ginsburger-Vogel, J. C. Amiard, Arch. Environ. Contam. Toxicol. 1997, 33, 147–155.
W. G. Sunda, S. A. Huntsman, Environ. Sci. Technol. 1998, 32, 2961–2968.
W. G. Sunda, S. A. Huntsman, Limnol. Oceanogr. 2000, 45, 1501–1516.
J. T. Cullen, T. W. Lane, F. M. M. Morel, R. M. Sherrell, Nature 1999, 402, 165–167.
J. T. Cullen, R. M. Sherrell, Limnol. Oceanogr. 2005, 50, 1193–1204.
J. T. Cullen, Z. Chase, K. H. Coale, S. E. Fitzwater, R. M. Sherrell, Limnol. Oceanogr. 2003, 48, 1079–1087.
E. S. Lane, D. M. Semeniuk, R. F. Strzepek, J. T. Cullen, M. T. Maldonado, Mar. Chem. 2009, 115, 155–162.
E. S. Lane, K. Jang, J. T. Cullen, M. T. Maldonado, Limnol. Oceanogr. 2008, 53, 1784–1789.
W. X. Wang, L. D. Guo, Mar. Ecol. Prog. Ser. 2000, 202, 41–49.
J. T. Phinney, K. W. Bruland, Environ. Sci. Technol. 1994, 28, 1781–1790.
L. Aristilde, Y. Xu, F. M. M. Morel, Environ. Sci. Technol. 2012, 46, 5438–5445.
T. Y. Ho, A. Quigg, Z. V. Finkel, A. J. Milligan, K. Wyman, P. G. Falkowski, F. M. M. Morel, J. Phycol. 2003, 39, 1145–1159.
B. A. Ahner, F. M. M. Morel, Limnol. Oceanogr. 1995, 40, 658–665.
B. A. Ahner, N. M. Price, F. M. M. Morel, Proc. Natl. Acad. Sci. USA 1994, 91, 8433–8436.
B. A. Ahner, F. M. M. Morel, in Prog. Phycol. Res., Eds F. E. Round, D. J. Chapman, Biopress Ltd., Bristol, UK, 1999, Vol. 13, pp. 1–31.
S. K. Kawakami, M. Gledhill, E. P. Achterberg, J. Phycol. 2006, 42, 975–989.
B. A. Ahner, J. G. Lee, N. M. Price, F. M. M. Morel, Deep-Sea Res. Part II 1998, 45, 1779–1796.
B. A. Ahner, S. Kong, F. M. M. Morel, Limnol. Oceanogr. 1995, 40, 649–657.
B. A. Ahner, L. P. Wei, J. R. Oleson, N. Ogura, Mar. Ecol. Progr. Ser. 2002, 232, 93–103.
M. J. Wang, W. X. Wang, Environ. Sci. Technol. 2008, 42, 8603–8608.
E. Morelli, G. Scarano, Chem. Speciat. Bioavail. 1995, 7, 43–47.
E. Morelli, L. Fantozzi, Bull. Environ. Contam. Toxicol. 2008, 81, 236–241.
E. Torres, A. Cid, P. Fidalgo, C. Herrero, J. Abalde, Aquat. Toxicol. 1997, 39, 231–246.
J. W. Rijstenbil, J. A. Wijnholds, Mar. Bio. 1996, 127, 45–54.
M. J. Wang, W. X. Wang, Aquat. Toxicol. 2009, 95, 99–107.
M. J. Wang, W. X. Wang, Aquat. Toxicol. 2011, 101, 377–386.
E. Morelli, G. Scarano, Mar. Environ. Res. 2001, 52, 383–395.
L. P. Wei, B. A. Ahner, Limnol. Oceanogr. 2005, 50, 13–22.
S. Kawakami, M. Gledhill, E. Achterberg, Biometals 2006, 19, 51–60.
L. P. Wei, J. R. Donat, G. Fones, B. A. Ahner, Environ. Sci. Technol. 2003, 37, 3609–3618.
T. Gupton-Campolongo, L. M. Damasceno, A. G. Hay, B. A. Ahner, J. Phycol., in press.
Y. Xu, L. Feng, P. D. Jeffrey, Y. G. Shi, F. M. M. Morel, Nature 2008, 452, 56–U53.
C. L. Dupont, B. A. Ahner, Limnol. Oceanogr. 2005, 50, 508–515.
C. L. Dupont, T. J. Goepfert, P. Lo, L. P. Wei, B. A. Ahnerz, Limnol. Oceanogr. 2004, 49, 991–996.
F. M. M. Morel, E. H. Cox, A. M. L. Kraepiel, T. W. Lane, A. J. Milligan, I. Schaperdoth, J. R. Reinfelder, P. D. Tortell, Funct. Plant Biol. 2002, 29, 301–308.
H. Park, P. J. McGinn, F. M. M. Morel, Aquat. Microb. Ecol. 2008, 51, 183–193.
P. J. McGinn, F. M. M. Morel, Physiol. Plant. 2008, 133, 78–91.
V. Alterio, E. Langella, F. Viparelli, D. Vullo, G. Ascione, N. A. Dathan, F. M. M. Morel, C. T. Supuran, G. De Simone, S. Maria Monti, Biochimie, in press.
F. Viparelli, S. M. Monti, G. De Simone, A. Innocenti, A. Scozzafava, Y. Xu, F. M. M. Morel, C. T. Supuran, Bioorg. Med. Chem. Lett. 2010, 20, 4745–4748.
Y. Xu, C. T. Supuran, F. M. M. Morel, in Handbook of Metalloproteins, Ed A. Messerschmidt, John Wiley & Sons Ltd., Chichester, UK, 2010, Vol. 4 & 5, pp. 717–721.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Xu, Y., Morel, F.M.M. (2013). Cadmium in Marine Phytoplankton. In: Sigel, A., Sigel, H., Sigel, R. (eds) Cadmium: From Toxicity to Essentiality. Metal Ions in Life Sciences, vol 11. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5179-8_16
Download citation
DOI: https://doi.org/10.1007/978-94-007-5179-8_16
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5178-1
Online ISBN: 978-94-007-5179-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)