Abstract
Osteoarthritis (OA) is the most common type of arthritis and the most common cause of disability in the elderly. It is a disease process that affects the total joint. OA may occur as the consequence of a number of different pathways that ultimately result in joint failure. It is increasingly recognized that inflammation is an important component in the pathophysiology of OA. The main clinical feature of OA is pain that tends to be transient early in the disease course but becomes persistent with disease progression. Radiographic features of OA may precede the development of pain. OA is the most common reason for joint replacement, and there have been striking increases in the rates of knee and hip replacement. Important OA risk factors include age, obesity, genetic factors and joint injury/trauma. With the aging of the population and the epidemic of obesity, there have been dramatic increases in the public health impact of OA, and the incidence, prevalence and public impact of OA are expected to rise.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aging
- Epidemiology
- Geriatrics
- Older adults
- Longevity
- Osteoarthritis
- Obesity
- Magnetic resonance imaging
- X-ray
- Joint failure
- Osteophytes
- Risk factors
- Obesity
- Joint injury
- Disability
- Joint replacement
1 Introduction
Osteoarthritis (OA), also known as degenerative joint disease, is the most common type of arthritis. OA is a disease of the synovial joints that encompasses the pathophysiologic changes that result from alterations in joint structure due to the failed repair of joint damage, as well as the individual’s illness experience, which is most characteristically manifested by pain.
OA may occur as the consequence of a number of different pathways that ultimately result in joint failure, a disease process that affects the total joint, including the subchondral bone, ligaments, joint capsule, synovial membrane, periarticular muscles, peripheral nerves, menisci (when present) and articular cartilage [1]. These pathways may consist of abnormal intra- and extra-articular processes that involve a combination of biomechanical, biochemical and genetic factors which results in matrix destruction. The matrix destruction is, in turn, defined as the failure of the repair response and mechanical failure that ultimately lead to joint destruction and the manifestations of joint pain and disability. Examples of these pathways include bone trauma and repetitive injury; malalignment; joint instability due to muscle weakness and ligamentous laxity; nerve injury, neuronal sensitization and/or hyper-excitability; low grade systemic inflammation due to subacute metabolic syndrome; or local inflammation due to synovitis. The destruction of the joint, including the wearing away of articular cartilage, is therefore best viewed as the final product of a variety of possible etiologic factors.
In this chapter, we will review the clinical features and pathophysiology of OA, as well as the diagnosis and natural history of the disease. We will then review the descriptive epidemiology of OA, including prevalence and incidence, describe the impact of OA on the public, and review the non-modifiable and potentially modifiable risk factors for OA. We will finish with a review of OA prevention and of the difficulties with and need for prevention clinical trials.
2 Clinical Features
The main clinical feature of OA is pain, though radiographic features of OA may be evident prior to the onset of the characteristic OA pain pattern. Pain is generally worse with activity and/or weight-bearing, and better with rest. Pain tends to be transient early in the course of the disease and more persistent with disease progression. In later stages, pain may also occur when at rest. Recent research has found that pain in OA may be reported as either a constant aching or as a more severe, intermittent pain [2]. OA pain tends to be localized to the specific joint involved, but may also be referred to a more distant site. A subset of individuals may experience neuropathic pain [3]. A number of patient-specific factors may modify pain reception and pain reporting. A patient’s affective status (e.g., depression, anxiety, anger) may impact the level of pain reported. Similarly, a patient’s cognitive status (e.g., pain beliefs, expectations, memories of past pain experiences, communication skills) may determine how pain is reported. Studies have shown that pain reporting may also be impacted by demographic factors such as age, sex, socio-economic status, race/ethnicity and cultural background [4].
The etiology of OA pain is unclear and is likely to be heterogeneous. OA pain may be the result of an interaction among the following factors: structural pathology; the motor, sensory and autonomic innervation of the joint; pain processing at both the spinal and cortical levels; and specific individual and environmental factors [4]. Cartilage is aneural, but the subchondral bone, periosteum, peri-articular ligaments, peri-articular muscle, synovium and joint capsule are all richly innervated and may be sources of nociceptive pain in OA. Sources of pain in subchondral bone include bone marrow lesions, perostitis with osteophyte formation, subchondral microfractures and bone ischemia due to decreased blood flow and/or elevated interosseous pressure [5], inflammation in the synovium and irritation of nerve endings by osteophytes. There may be peripheral sensitization as a result of hyperalgesia and central sensitization that leads to pain persistence. Allodynia may also be present. Pain in OA has been reported to be associated with the presence and size of bone marrow lesions seen on magnetic resonance imaging (MRI) [6]. A recent systematic review examined the associations of MRI findings (e.g., cartilage defects, bone marrow lesions [BML], osteophytes, meniscal lesion, effusion/synovitis, ligamentous abnormalities, subchondral cysts and bone attrition) with the presence of pain in patients with knee OA [7]. Only the presence of BMLs and effusion/synovitis were significantly associated with the presence of knee pain.
Stiffness in the affected joint may be present, particularly after prolonged inactivity, but it is not a major feature of OA and is of short duration, usually lasting for less than 30 minutes. In addition to loss of function, impaired quality of life, fatigue, sleep disturbance and mood disturbance may also be prominent features as the result of chronic pain [5]. Patients with knee OA may also complain of knee buckling.
Examination of an involved joint may reveal joint-line tenderness and bony enlargement of the joint. Joint effusion and/or soft-tissue swelling may be present, but tend to be intermittent. Crepitus with movement, limitation of joint motion, joint deformity and/or joint laxity may also be present. An involved joint does not generally show persistent inflammation with joint warmth, effusion and soft-tissue swelling.
Several subtypes of generalized OA have been identified. The nodal form of OA, involving primarily the distal interphalangeal joints, is most common in middle-aged women, typically those with a strong family history among first-degree relatives. Erosive, inflammatory OA is associated with prominent erosive and destructive changes, especially in the finger joints, and may suggest rheumatoid arthritis, though systemic inflammatory signs and other typical features of rheumatoid arthritis (e.g., nodules, proliferative synovitis, extra-articular features, rheumatoid factor) are absent.
The diagnosis of OA is based on history, physical examination and characteristic radiographic features. The physician must distinguish OA from other inflammatory joint diseases such as rheumatoid arthritis. Distinguishing OA from other inflammatory joint diseases involves identifying the characteristic pattern of joint involvement and the nature of the individual joint deformity. Joints commonly involved in OA include the distal interphalangeal joints, proximal interphalangeal joints, first carpometacarpal joints, first metatarsophalangeal joints, hips, knees and facet joints of the cervical and lumbar spine. Heberden’s and Bouchard’s nodes may be present in the hands. Involvement of the wrist, elbows, shoulders and ankles is uncommon except in the case of trauma, congenital disease, or endocrine or metabolic disease.
3 Pathophysiology
The causes of OA are complex and heterogeneous, but our understanding of its pathophysiology has increased over the years. The cardinal feature of OA is the progressive loss of articular cartilage with associated remodeling of subchondral bone. As noted previously, OA is ultimately joint failure due to a variety of pathways.
At the tissue level, normal cartilage exhibits a continuous extracellular matrix turnover with a balance of synthesis and degradation [8]. In OA, there is an imbalance of these two processes, with an excess of matrix degradation that exceeds the ongoing matrix synthesis. Excess degradation is the result of the overproduction of catabolic factors such as proinflammatory cytokines (e.g., interleukin [IL]-1, IL-6, IL-7, IL-8, and tumor necrosis factor alpha [TNFα]) and other catabolic factors such as transforming growth factor alpha (TGFα), nitric oxide and other reactive oxygen species (ROS), Oncostatin M, basic fibroblast growth factor (bFGF) and matrix fragments. These factors stimulate the chondrocyte to produce matrix metalloproteases (MMPs), aggrecanase and other proteases, which results in extracellular matrix degradation. At the same time, there is a decrease in the production of matrix and growth factors (e.g., bone morphogenic protein [BMP-7] and transforming growth factor beta [TGF- β]). An imbalance between tissue inhibitors of metalloproteases and the production of metalloproteases may be operative in OA.
Loeser [8] described a number of age-related changes that contribute to the susceptibility of OA. At the cellular level, decreased levels of growth factors and decreased growth factor responsiveness lead to reduced matrix synthesis and repair. There is an increased formation of advanced glycation end-products (AGEs), which leads to the increased cross-linking of collagen molecules, more brittle tissues and increased susceptibility to fatigue failure of cartilage. Decreased aggrecan size, increased cleavage of collagen and decreased cartilage hydration lead to the reduced tensile strength of cartilage. With aging, there is oxidative stress, an increase in ROS and an accumulation of oxidative damage that is manifested by reduced anabolic signaling and increased catabolic signaling, which ultimately result in decreased matrix synthesis and increased matrix degradation.
At the tissue level, an age-related decrease in the number of chondrocytes and an increase in chondrocyte senescence are manifested by telomere shortening. There is also the transformation of chondrocytes into the senescent secretory phenotype, which results in the increased production of inflammatory cytokines and MMPs that lead to matrix degradation. Aging chondrocytes are also less likely to respond to growth factors. Unlike in cartilage, within subchondral bone there is increased bone remolding with increased matrix calcification and the production of an abnormal bone matrix. This alters the mechanical properties of the bone, increasing its stiffness and making it less able to absorb loads, thereby transferring loadbearing to the cartilage. There are also age-related changes (e.g., sarcopenia) which result in decreased muscle strength and the resultant decreased ability of the muscles to act as internal shock absorbers to absorb the forces transmitted to the subchondral bone and cartilage. With aging, there is also loss of proprioception, and the degeneration and increased stiffness of ligaments and menisci.
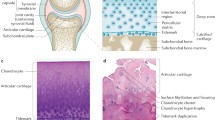
The pathology of OA is seen in Fig. 29.1. In OA, the entire joint is commonly involved. Cartilage degradation results in fibrillation, thinning and, ultimately, the loss of cartilage down to subchondral bone, leaving areas of denuded bone. There are changes in the subchondral bone with thickening; the development of BMLs which leads to subchondral bone cysts; the formation of marginal osteophytes; and bone remodeling, with bone attrition producing changes in bone curvature. There is often weakness of the bridging peri-articular muscles. If present, the menisci degenerate and may extrude beyond the bony margins. It is difficult to say which of these processes occurs first, but all of these features may be present in the later stages. Scanzello et al. [9] has described a number of changes in the synovium that occur in OA, including synovial hyperplasia, perivascular aggregates of small mononuclear cells, subintimal fibrosis, and increased vascularity.
Pathology of osteoarthritis. The osteoarthritic joint is characterized by degradation and loss of the articular cartilage, thickening of the subchondral bone accompanied by formation of bone marrow lesions and cysts, osteophytes at the joint margins, variable degrees of synovitis with synovial hypertrophy, meniscal degeneration (knee), and thickening of the joint capsule (Reprinted with permission from Loeser [8])
In OA, the earliest finding is fibrillation of the most superficial layer of the articular cartilage. With time, the disruption of the articular surface becomes deeper, with extension of the fibrillations to subchondral bone, fragmentation of cartilage with release into the joint, matrix degradation, and eventually, the complete loss of cartilage, leaving only exposed bone. Early in this process, the cartilage matrix undergoes significant change, with increased water content and decreased proteoglycan content. This progression is in contrast to the dehydration of cartilage that occurs with aging. The tidemark zone, which separates the calcified cartilage from the radial zone, becomes invaded with capillaries. Chondrocytes are initially metabolically active and release a variety of cytokines and metalloproteases that contribute to the matrix degradation, which, in later stages, results in the penetration of fissures to the subchondral bone and the release of fibrillated cartilage into the joint space. Subchondral bone increases in density, and cyst-like bone cavities occur which contain myxoid, fibrous or cartilaginous tissue. Osteophytes (bony proliferations at the margin of joints at the site of bone-cartilage interface) may also form at capsule insertions. Osteophytes contribute to joint-motion restriction and are thought to be the result of new bone formed in response to the degeneration of articular cartilage; however the precise mechanism for their production remains unknown.
The diversity of risk factors that predispose an individual to OA suggests that a wide variety of insults to the joints (e.g., biomechanical trauma, chronic articular inflammation, and genetic and metabolic factors) can contribute to or trigger the cascade of events that results in the characteristic pathologic features of OA. At some point, the cartilage degradative process becomes irreversible, perhaps the result of an imbalance of regulatory molecules such as tissue inhibitors of metalloproteases. With progressive changes in articular cartilage, joint mechanics become altered which, in turn, perpetuates the degradative process.
4 Measurement of Clinical and Subclinical Disease: Diagnosis and Natural History
The diagnosis of OA is based on symptoms of pain, stiffness and/or poor sleep; the presence of characteristic radiographic features; or the presence of bony and intermittent soft tissue swelling in a joint commonly affected by OA such as distal interphalangeal joints, proximal interphalangeal joints, first carpometacarpal joints, first metatarsophalangeal joints, hips, knees, or facet joints of the cervical and lumbar spine.
The natural history of OA is depicted schematically in Fig. 29.2. Initially, one or more triggering event(s) initiates the disease process in a susceptible individual. Susceptibility may be increased by aging-related cellular and tissue changes that may occur either before or after the triggering events. Currently, clinically detectable OA is defined by the presence of abnormalities on plain radiographs. Unfortunately this stage occurs late in the disease course and is indicative of “joint failure.” Depending on the joint and other circumstances, symptoms may precede or follow evidence of clinically detectable radiographic OA. Since there are no currently approved disease-modifying OA drugs (DMOADs) that slow disease progression, patients may progress to end-stage disease (i.e., joint death) where the only effective treatment is joint replacement. The challenge is to identify pre-clinical OA through morphologic changes in joint structures that are detectable by MRI, ultrasound or—at an even earlier stage—by molecular changes in joint structures that are detectable by MRI or other biomarkers.
The characteristic radiographic features of OA are the result of pathologic changes. Joint space narrowing is felt to be a consequence of cartilage loss. Osteophytes may be a consequence of marginal lipping and outgrowths of bone. Subchondral bone cysts and sclerosis may be the result of osteonecrosis and the healing of microfractures. Altered bone contours may be due to bone attrition and the remodeling of bone surfaces.
The current definition of OA is based on the presence of features that are seen on conventional radiography. that is, osteophytes and joint space narrowing as a surrogate for the loss of cartilage. In most epidemiologic studies, the definition of OA is based on a combination of pain and the radiographic disease as assessed by the Kellgren-Lawrence grading system, which is scored using an ordinal scale from 0 to 4. On the scale, 0 indicates normal (i.e., no features of OA); 1 indicates “doubtful” and is characterized by the presence of a minute osteophyte of doubtful significance; 2 indicates the level most used to categorize “definite OA” and is characterized by the presence of a definite osteophyte without impairment of joint space; 3 indicates moderate OA and is characterized by the presence of multiple osteophytes and moderate diminution of joint space as a surrogate for cartilage loss; and 4 indicates severe OA and is characterized by the marked impairment of joint space, often bone on bone and with the presence of a sclerosis of subchondral bone [10]. These categories may often be difficult to assess and are based on the presumption of a progression of radiographic features with increasing severity, which may not always be the case. This has led to the development of an alternative scale developed by the Osteoarthritis Research Society International (OARSI), which provides an accompanying radiographic atlas that enables the separate scoring of features such as osteophytes, joint space narrowing and sclerosis [11]. Unfortunately, radiographs are limited in that they only provide images of bony structure and are two-dimensional projections of the three-dimensional joint(s) involved in OA.
The advent of MRI has greatly advanced our knowledge of OA and has enabled the visualization of pre-radiographic OA. MRI has demonstrated that additional morphologic abnormalities (e.g., BMLs, synovitis) may also be important features of OA [12]. MRI also has the advantage of greater resolution and the ability to visualize all of the joint tissues to assess their involvement. Other features of joint morphology that may be important in OA and can be assessed by MRI include subchondral cyst-like lesions; subchondral bone attrition; joint effusion; meniscal degeneration and/or subluxation; periarticular cysts and bursae; marginal and central osteophytes; and the integrity of the anterior cruciate, posterior cruciate, medial collateral and lateral collateral ligaments [13]. These features may be assessed using recently-developed semi-quantitative scoring systems [14, 15]. High resolution images have also enabled the quantitative assessment of joint structures through the manual segmentation of joint morphology, including cartilage volume (VC), total area of subchondral bone (tAB), the area of the cartilage surface (AC), the cartilage thickness over the total bone area (ThCtAB), the area of cartilage-covered subchondral bone (cAB) and the area of denuded subchondral bone that is not covered by cartilage (dAB) [16]. These parameters may be calculated for specific regions or subregions within a joint.
Advances in MRI have also enabled the development of non-contrast and contrast-enhanced imaging methods for assessing morphometric and compositional parameters that occur with degradation of the extracellular matrix as potential imaging biomarkers of preclinical OA. Examples include T2 mapping, T1 rho mapping, Ultrashort TE imaging, sodium imaging, diffusion-weighted imaging and delayed gadolinium-enhanced MR imaging of cartilage (dGEMRIC) [17].
A recent review has recommended a panel of 12 OA-related biomarkers that have been validated for a variety of OA outcomes [18]. All 12 are commercially available and include the following: Urinary carboxy-telopeptide of type II collagen (CTX-II), Serum CTX-II, Serum hyaluronan (HA), Serum and urine collagenase-generated neoepitope of types I and II collagens (C1, 2C), Serum and urine collagenase-generated neoepitope of type II collagen (C2C), Serum and urine Coll2-1 and Coll2-1NO2, Serum type II collagen propeptide (CPII or PIICP), type IIA procollagen amino propeptide (PIIANP), Urine/serum N-telopeptide of type I collagen (NTX-1), Urine/serum carboxy-telopeptide of type I collagen (CTX-1), Serum aggrecan chondroitin sulfate 846 epitope (CS846), and Serum metalloproteinase of stormelysin (MMP-3). The identification of these biomarkers and of changes in bone and cartilage composition is a step toward the classification of preclinical OA prior to morphologic changes in joint structure which may be apparent on conventional radiographs and MRI.
5 Descriptive Epidemiology
5.1 Prevalence
Over 26.9 million Americans >25 years of age have some form of OA, and the prevalence of OA increases with age. The prevalence of radiographic OA varies by the joint involved, with 27.2% of all adults and over 80% of those >65 years of age having evidence of hand OA (Table 29.1). With regard to knee OA, 37.4% of those ≥60 years of age have radiographic evidence of disease. The prevalence of symptomatic OA is lower, with 6.8% of all adults having evidence of symptomatic hand OA and 16.7% of those ≥45 years of age having evidence of symptomatic knee involvement. Hand and knee OA is more common among women, especially after age 50, and also more common among African-Americans. Nodal OA, involving the distal and proximal interphalangeal joints, is significantly more common in women and also more common among female first-degree relatives of those who have nodal OA.
In the Framingham study, radiographic evidence of knee OA increased from 27.4% in participants <70 years of age to 43.7% in those ≥80 years of age [20]. There was a slightly higher prevalence of radiographic changes of OA in women than in men (34 versus 31%); however, there was a significantly higher proportion of women with symptomatic disease (11% of all women versus 7% of all men; p = 0.003).
A meta-analysis of population-based studies of OA estimated that, compared to women, men have a decreased risk of prevalent hand and knee OA, but not hip OA [26]. The worldwide age-standardized prevalence rates per 100,000 individuals of hip and knee OA are 426 and 1,170 in men, respectively, and 371 and 2,693 for women, respectively [24]. For hip OA in men and women, these estimated age-standardized prevalence rates per 100,000 individuals range from a low of 273 and 145, respectively, to a high of 700 and 601, respectively, across World Health Organization (WHO) epidemiologic subregions. For knee OA in men and women, these estimated age-standardized prevalence rates per 100,000 individuals range from a low of 1,163 and 1,773, respectively, to a high of 3,089 and 3,942, respectively.
5.2 Incidence
The age- and sex-standardized incidence rates of symptomatic OA are 100 per 100,000 person-years for hand OA, 240 per 100,000 person-years for knee OA, and 88 per 100,000 person-years for hip OA [27]. The rates in both sexes rise with increasing age, especially after age 50. The rate of incident symptomatic knee OA is estimated to be 1% per year, and the rate of incident radiographic knee OA is estimated to be 2% per year [28]. Men <55 years of age have a greater risk of incident cervical spine OA than do women of the same age group, whereas women have a greater risk of incident knee and hip OA than do men [26]. The lifetime risk of developing symptomatic knee OA is estimated to be about 40% in men and 47% in women [29]. The worldwide age-standardized incidence rates of hip and knee OA per 100,000 individuals are 35.0 and 119.7 in men, respectively, and 30.8 and 178.6 for women, respectively [24]. For hip OA in men and women, these estimated age-standardized incidence rates per 100,000 individuals range from a low of 22.2 and 12.8, respectively, to a high of 40.5 and 55.4, respectively, across WHO epidemiologic subregions. For knee OA in men and women, these estimated age-standardized incidence rates per 100,000 individuals range from a low of 67.7 and 136.8, respectively, to a high of 194.9 and 253.1, respectively.
6 Public Health Impact
OA is associated with major morbidity and is one of the top five causes of long-term disability in the United States (US) [30]. Lower extremity OA is the most common cause of difficulty with walking or climbing stairs, preventing an estimated 100,000 older US adults from independently walking from bed to bathroom. Overall loss of joint function as a result of OA is a major cause of work disability and reduced quality of life [31]. About 80% of patients with OA have some degree of movement limitation. About 40% of adults with knee OA report their health as “poor” or “fair.” In 1997, a total of 4.9 million women and 2.2 million men had ambulatory medical care visits for OA, accounting for 19.5% of all arthritis-related ambulatory medical care visits [32].
In 1999, adults with knee OA reported more than 13 days of lost work due to health problems. In the year 2000, the years lived with disability (YLDs) [33] for men and women with OA were 5,549 and 8,667, respectively. In the year 2000, the disability-adjusted life years (DALYs) for men and women with OA were 5,554 and 8,675, respectively. These YLDs and DALYs rank high among chronic diseases, and both have increased since 1990.
The Centers for Disease Control (CDC) estimates that osteoarthritis and related arthritic conditions cost the US economy nearly $81 billion per year in direct medical care, with indirect expenses (including lost wages and lost production) of about $47 billion. CDC figures further estimate the total annual direct cost per person of OA and related conditions is approximately $1,752 [31]. A large proportion of these costs are associated with total joint replacement, with costs for total joint replacement in the US estimated to be $79 billion in 1997 [34]. The job-related costs of OA are estimated to be $3.4–$13.2 billion per year [35].
OA wields a large economic impact as the result of both direct medical costs (e.g., physician visits, laboratory tests, medications, surgical procedures) and indirect costs (e.g., lost wages, home care, lost wage-earning opportunities). With the aging of the US population, the burden of OA is expected to increase throughout the coming years.
7 Risk Factors
OA is a complex disorder with identifiable risk factors that include biomechanical, metabolic or inflammatory processes; congenital or developmental deformities of the joint; and genetic factors. As noted above, age, sex and race are prominent risk factors for OA. Biomechanical contributors include repetitive or isolated joint trauma related to certain occupations or physical activities that involve repeated joint stress. These can predispose an individual to early OA. Obesity may contribute from a biomechanical perspective, or from a systemic perspective related to a subacute metabolic syndrome. Certain metabolic disorders (e.g., hemochromatosis, ochronosis) are also associated with OA. High bone mineral density (BMD) has been shown to be associated with hip or knee OA. Estrogen deficiency may also be a risk factor for hip or knee OA. Inflammatory joint diseases, such as rheumatoid arthritis, may result in cartilage degradation and biomechanical factors that lead to secondary OA. Candidate gene studies and genome-wide scans have identified a number of potential genetic markers of OA.
7.1 Non-Modifiable Risk Factors
7.1.1 Demographic Risk Factors
Prior studies have reported an increase in the risk of radiographic knee OA (RKOA) with advancing age, as well as an increased risk of RKOA in women compared to men. Data from the NHANES III and the Johnston County OA Study have reported an increased risk of RKOA among African-Americans compared to whites, particularly among African-American women [21, 22]. NHANES III did not find either education level or income to be associated with risk of RKOA [22].
7.1.2 OA in Other Joints
Studies have suggested that risk of knee OA might be related to the presence of hand OA [36–38]. In the Bristol cohort [36] the association was with Herberden’s nodes. Data from the Rotterdam study [38] reported an increased risk of RKOA associated with radiographic hand OA of the metacarpophalangeal and carpometacarpal joints, with a higher risk among individuals who were overweight. In the Croatian study [37], the association was greater in women compared to men, and was greater for OA in the distal interphalangeal joints compared to the proximal interphalangeal joints. That study also found an increased risk of RKOA with carpometacarpal involvement in men. Radiographic hand OA has been reported to be associated with increased risk of RKOA in the both the index knee after menisectomy and in the contralateral knee [39].
7.2 Potentially Modifiable Risk Factors
7.2.1 Body Composition
In prior studies, obesity and increased body mass index (BMI) have been reported to increase the risk of RKOA. Data on more detailed measures of body composition are now available. Multiple studies have suggested that increased waist circumference and increased waist-to-hip ratio may be associated with increased risk of RKOA, but this increased risk was no longer significant after adjusting for BMI [40, 41]. In contrast, analysis of the NHANES III data suggested that waist circumference was still an important risk factor when analyzed by different strata of BMI, particularly in the medium and highest BMI tertiles [42]. Data from the Johnston County OA Study suggested that increasing fat mass and increasing lean mass are both associated with increased risk of radiographic knee OA, but neither were significant after adjusting for BMI [40]. Data from a study in Sweden suggest that the presence of metabolic syndrome may also be associated with increased risk of radiographic knee OA, but this association was also no longer significant after adjusting for BMI [43]. Obesity also increases with increasing age, at least until reaching very old age when weight begins to decrease.
Data from a case–control study from southern Sweden suggest that changes in BMI from younger ages to older ages may be an important consideration [44]. Increasing BMI after age 30 was associated with increased risk of RKOA in both men and women. Decreasing BMI after age 30 helped to decrease the risk of RKOA. The highest risk of RKOA was seen in men and women who were already obese at a young age.
7.2.2 Bone Mineral Density
A number of prior studies, including some using data from the Framingham Study, have reported an association between increased BMD and increased risk of OA in a separate joint [45]. Data from the Johnston County OA Study reported that increased bone mass and lower percent bone mass were both associated with increased risk of RKOA, but this association was no longer significant after controlling for either BMI or weight [40]. The mechanisms behind this association are still unclear and require further investigation.
7.2.3 Malalignment
There is limited data on the association of the presence of malalignment with the risk of RKOA. A number of studies have demonstrated the importance of malalignment in the progression of RKOA, but few have looked at malalignment as a risk factor for the development of RKOA. Data from the Framingham Study was unable to demonstrate a relationship between various measures of alignment and increased risk of RKOA [46], whereas data from the Rotterdam Study indicated that varus malalignment was associated with an increased risk of RKOA, and that varus and valgus malalignment were both important in the development of RKOA in obese individuals [47]. It is important to note that both of these studies were based on the assessment of alignment using standing, fully-extended anteroposterior knee radiographs. These findings should be replicated using the measurement of alignment from full limb films, which may be more accurate in assessing the mechanical angle.
7.2.4 Physical Characteristics
A number of physical features have been associated with the risk of developing RKOA. Data from the Johnston County OA Study indicated that leg length inequality increased the risk of RKOA [48]. Data from the Beijing OA Study suggested that higher knee height was associated with increasing prevalence of both radiographic and symptomatic OA [49]. In addition, data from the Matsudai Knee Survey suggested that the presence of a round back also increased the risk of developing RKOA [50].
Data from Nottingham, UK suggested that the pattern of the second digit being shorter than the fourth digit is also associated with increased risk of knee OA [51]. The relationship between differences in joint shape (often related to congenital abnormalities of the hip) and the increased risk of hip OA has been clearly demonstrated. More work is needed to determine whether differences in joint shape and limb development may increase the risk of RKOA.
7.2.5 Knee Injury
Studies have shown a relationship and increased risk of RKOA with previous knee injury. The risk is increased in both men and women, and in both white women and African-American women [52]. With regard to knee surgery, the risk seems to be increased with subtotal or total menisectomy, as well as with degenerative tears of the meniscus [53]. Data from the Beijing OA Study indicated a trend toward increased quadriceps strength having a protective effect against the development of both tibiofemoral and patellofemoral RKOA, with the results becoming significant when both outcomes were considered together [54].
7.2.6 Recreational Activity
A number of studies have looked at the association of recreational activities and the risk of RKOA. It does not appear that the risk of RKOA is increased by walking for exercise, recreational walking or other levels of recreational activity such as working up a sweat or having a higher level of activity compared to peers [55, 56]. Regular sports participation may increase the risk of RKOA, particularly specific types of activities such as soccer, ice hockey or tennis in men. However, the increased risk was more likely due to the occurrence of injury rather than to participation in these activities [36, 57]. The association with these activities was no longer significant after controlling for prior knee injury.
7.2.7 Occupational Activity
NHANES III demonstrated an increase in risk of RKOA in men with manual labor occupations [22]. Data from southern Sweden suggest that working in building construction increases the risk of RKOA in overweight men, and farm work increases the risk of RKOA in both men and women if they are overweight. Certain specific occupational activities (e.g., climbing more than 15 flights of stairs per day, lifting more than 10 kg ten times/week, squatting) seem to increase the risk of RKOA.
7.2.8 Hormone Therapy
Few studies have examined the relationship between the use of hormone therapy in women and the risk of radiographic OA. Previous data had been conflicting, depending on the joint of interest. Recent data from the Rotterdam study and the Melbourne Women’s Life Study have not demonstrated an association of radiographic OA with the use of hormone therapy [56, 58]. Although the prevalence of radiographic OA was higher in the Rotterdam study among women who had previously used hormone therapy, this did not reach statistical significance (27 vs. 21%, p = 0.26). Data from the Melbourne Women’s Life Study suggested that women who had never used hormone therapy had an increased risk of 0.29, but this did not reach statistical significance (95% CI: 0.8–11.6, p = 0.12).
8 Prevention, Including Prevention Clinical Trials
Possible targets of primary prevention, alone or in combination, include weight gain/obesity, joint injury related to recreational and/or occupational activities, or structural issues such as joint biomechanics. Secondary prevention in individuals with early disease could also be directed toward these targets, or directed toward other joints in individuals who already have OA in a joint.
It is difficult to design prevention trials in OA due to current definitions of the disease being based on radiographic changes, which are insensitive to detecting preclinical disease or changes early in the disease course [59]. With advances in biomarker technology (both imaging and biochemical markers), it will be possible to identify high-risk individuals with preclinical disease and to characterize changes early in the disease course. Such trials would likely involve large study samples with follow-up over several years, and would therefore be costly and complex to conduct. However, prevention studies are ultimately needed to decrease the large burden of disease due to OA.
9 Summary
OA is a disease of the whole joint, with alterations in joint structure due to the failed repair of joint damage. OA is a common disease and a leading cause of disability, particularly in older populations. Pain is the presenting symptom of the individual’s illness experience, and the treatment for end-stage OA is joint replacement. With the aging of the population and the epidemic of obesity, the prevalence and public health impact of OA are expected to increase dramatically. Obesity and joint trauma have been identified as important modifiable risk factors and are potential targets for prevention studies.
Abbreviations
- 2C:
-
Type II collagen
- AC:
-
Cartilage Surface
- AGEs:
-
Advanced Glycation End-products
- bFGF:
-
Basic Fibroblast Growth Factor
- BMD:
-
Bone Mineral Density
- BML:
-
Bone Marrow Lesions
- BMI:
-
Body Mass Index
- C1:
-
Type I collagen
- C2C:
-
Collagenase-generated neoepitope of type II collagen
- cAB:
-
Cartilage-covered subchondral bone
- CDC:
-
Centers for Disease Control
- CPII:
-
Type II collagen propeptide
- CS846:
-
Aggrecan chondroitin sulfate 846 epitope
- CTX-I:
-
Carboxy-telopeptide of type I collagen
- CTX-II:
-
Carboxy-telopeptide of type II collagen
- dAB:
-
Subchondral bone that is not covered by cartilage
- DALYs:
-
Disability-Adjusted Life Years
- dGEMRIC:
-
Delayed gadolinium-enhanced MR imaging of cartilage
- DMOADs:
-
Disease-Modifying OA Drugs
- HA:
-
Hyaluronan
- IL:
-
Interleukin
- MMPs:
-
Matrix Metalloproteases
- MMP-3:
-
Metalloproteinase of stormelysin
- MRI:
-
Magnetic Resonance Imaging
- NTX-1:
-
N-telopeptide of type I collagen
- OA:
-
Osteoarthritis
- OARSI:
-
Osteoarthritis Research Society International
- PIIANP:
-
Type IIA procollagen amino propeptide
- PIICP:
-
Type II collagen propeptide
- RKOA:
-
Radiographic Knee Osteoarthritis
- ROS:
-
Reactive Oxygen Species
- tAB:
-
Total area of subchondral bone
- TGFα:
-
Transforming Growth Factor Alpha
- TGFβ:
-
Transforming Growth Factor Beta
- ThCtAB:
-
Cartilage thickness over the total bone area
- TNFα:
-
Tumor Necrosis Factor Alpha
- US:
-
United States
- VC:
-
Cartilage Volume
- WHO:
-
World Health Organization
- YLDs:
-
Years Lived with Disability
References
Lane NE, Brandt K, Hawker G et al (2011) OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 19(5):478–482
Hawker GA, Davis AM, French MR et al (2008) Development and preliminary psychometric testing of a new OA pain measure–an OARSI/OMERACT initiative. Osteoarthritis Cartilage 16(4):409–414
Hochman JR, Gagliese L, Davis AM et al (2011) Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage 19(6):647–654
Marchand S (2008) The physiology of pain mechanisms: from the periphery to the brain. Rheum Dis Clin North Am 34(2):285–309
Fitzcharles MA, Shir Y (2008) New concepts in rheumatic pain. Rheum Dis Clin North Am 34(2):267–283
Felson DT, Chaisson CE, Hill CL et al (2001) The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 134(7):541–549
Yusuf E, Kortekaas MC, Watt I et al (2011) Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 70(1):60–67
Loeser RF (2010) Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med 26(3):371–386, PMCID: 2920876
Scanzello CR, Plaas A, Crow MK (2008) Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol 20(5):565–572
Kellgren J, Lawrence J (1963) Atlas of standard radiographs. The epidemiology of chronic rheumatism. Blackwell Scientific Publications, Oxford
Altman RD, Gold GE (2007) Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 15(Suppl A):A1–A56
Roemer FW, Eckstein F, Guermazi A (2009) Magnetic resonance imaging-based semiquantitative and quantitative assessment in osteoarthritis. Rheum Dis Clin North Am 35(3):521–555
Crema MD, Roemer FW, Marra MD et al (2009) Magnetic resonance imaging assessment of subchondral bone and soft tissues in knee osteoarthritis. Rheum Dis Clin North Am 35(3):557–577
Hunter DJ, Lo GH, Gale D et al (2008) The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score). Ann Rheum Dis 67(2):206–211
Peterfy CG, Guermazi A, Zaim S et al (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12(3):177–190
Wirth W, Eckstein F (2008) A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging 27(6):737–744
Crema MD, Roemer FW, Marra MD et al (2011) Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 31(1):37–61
Kraus VB, Burnett B, Coindreau J et al (2011) Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage 19(5):515–542
Zhang Y, Niu J, Kelly-Hayes M et al (2002) Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: the Framingham Study. Am J Epidemiol 156(11):1021–1027
Felson DT, Naimark A, Anderson J et al (1987) The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 30(8):914–918
Jordan JM, Helmick CG, Renner JB et al (2007) Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 34(1):172–180
Dillon CF, Rasch EK, Gu Q et al (2006) Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol 33(11):2271–2279
Helmick CG, Renner JB, Luta G et al (2003) Prevalence of hip pain, radiographic hip osteoarthritis (OA), severe radiographic hip OA, and symptomatic hip OA: the Johnson County Osteoarthritis Project [abstract]. Arthritis Rheum 48(Suppl 9):S212
Symmons D, Mathers C, Pfleger B (2006) Global burden of osteoarthritis in the year 2000. World Health Organization web site. http://www.who.int/healthinfo/statistics/bod_osteoarthritis.pdf. Accessed 12 Apr 2012
Lawrence RC, Felson DT, Helmick CG et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 58(1):26–35
Srikanth VK, Fryer JL, Zhai G et al (2005) A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 13(9):769–781
Oliveria SA, Felson DT, Reed JI et al (1995) Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum 38(8):1134–1141
Felson DT, Zhang Y, Hannan MT et al (1995) The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 38(10):1500–1505
Murphy L, Schwartz TA, Helmick CG et al (2008) Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 59(9):1207–1213
Guccione AA, Felson DT, Anderson JJ et al (1994) The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health 84(3):351–358, PMCID: 1614827
Samson D, Grant M, Ratko T et al (2007) Treatment of primary and secondary osteoarthritis of the knee. Evidence Report/Technology assessment No. 157 (Prepared by Blue Cross and Blue Shield Association Technology Evaluation Center Evidenced-based Practice Center under contract No. 290-02-0026) AHRQ Publication No. 07-E012. Rockville, MD
Hootman JM, Helmick CG, Schappert SM (2002) Magnitude and characteristics of arthritis and other rheumatic conditions on ambulatory medical care visits, United States, 1997. Arthritis Rheum 47(6):571–581
Michaud CM, McKenna MT, Begg S et al (2006) The burden of disease and injury in the United States 1996. Popul Health Metr 4:11, PMCID: 1635736
Lethbridge-Cejku M, Helmick CG, Popovic JR (2003) Hospitalizations for arthritis and other rheumatic conditions: data from the 1997 National Hospital Discharge Survey. Med Care 41(12):1367–1373
Buckwalter JA, Saltzman C, Brown T (2004) The impact of osteoarthritis: implications for research. Clin Orthop Relat Res (427 Suppl):S6–S15
Cooper C, Snow S, McAlindon TE et al (2000) Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum 43(5):995–1000
Cvijetic S, Campbell L, Cooper C et al (2000) Radiographic osteoarthritis in the elderly population of Zagreb: distribution, correlates, and the pattern of joint involvement. Croat Med J 41(1):58–63
Dahaghin S, Bierma-Zeinstra SM, Reijman M et al (2005) Does hand osteoarthritis predict future hip or knee osteoarthritis? Arthritis Rheum 52(11):3520–3527
Englund M, Paradowski PT, Lohmander LS (2004) Association of radiographic hand osteoarthritis with radiographic knee osteoarthritis after meniscectomy. Arthritis Rheum 50(2):469–475
Abbate LM, Stevens J, Schwartz TA et al (2006) Anthropometric measures, body composition, body fat distribution, and knee osteoarthritis in women. Obesity (Silver Spring) 14(7):1274–1281
Lohmander LS, Gerhardsson de Verdier M, Rollof J et al (2009) Incidence of severe knee and hip osteoarthritis in relation to different measures of body mass: a population-based prospective cohort study. Ann Rheum Dis 68(4):490–496
Janssen I, Mark AE (2006) Separate and combined influence of body mass index and waist circumference on arthritis and knee osteoarthritis. Int J Obes (Lond) 30(8):1223–1228
Engstrom G, Gerhardsson de Verdier M, Rollof J et al (2009) C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage 17(2):168–173
Holmberg S, Thelin A, Thelin N (2005) Knee osteoarthritis and body mass index: a population-based case–control study. Scand J Rheumatol 34(1):59–64
Zhang Y, Hannan MT, Chaisson CE et al (2000) Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol 27(4):1032–1037
Hunter DJ, Niu J, Felson DT et al (2007) Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum 56(4):1212–1218
Brouwer GM, van Tol AW, Bergink AP et al (2007) Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum 56(4):1204–1211
Golightly YM, Allen KD, Renner JB et al (2007) Relationship of limb length inequality with radiographic knee and hip osteoarthritis. Osteoarthritis Cartilage 15(7):824–829, PMCID: 2836720
Hunter DJ, Niu J, Zhang Y et al (2005) Knee height, knee pain, and knee osteoarthritis: the Beijing Osteoarthritis Study. Arthritis Rheum 52(5):1418–1423
Aoda H, Nakamura K, Omori G et al (2006) Independent predictors of knee osteoarthritis in an elderly Japanese population: a multivariate analysis. Acta Med Biol 54(2):33–41
Zhang W, Robertson J, Doherty S et al (2008) Index to ring finger length ratio and the risk of osteoarthritis. Arthritis Rheum 58(1):137–144
Lachance L, Sowers M, Jamadar D et al (2001) The experience of pain and emergent osteoarthritis of the knee. Osteoarthritis Cartilage 9(6):527–532
Englund M, Lohmander LS (2004) Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum 50(9):2811–2819
Baker KR, Xu L, Zhang Y et al (2004) Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese: the Beijing osteoarthritis study. Arthritis Rheum 50(6):1815–1821
Felson DT, Niu J, Clancy M et al (2007) Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis Rheum 57(1):6–12
Szoeke CE, Cicuttini FM, Guthrie JR et al (2006) Factors affecting the prevalence of osteoarthritis in healthy middle-aged women: data from the longitudinal Melbourne Women’s Midlife Health Project. Bone 39(5):1149–1155
Thelin N, Holmberg S, Thelin A (2006) Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports 16(5):329–333
Bergink AP, Uitterlinden AG, Van Leeuwen JPTM et al (2005) Bone mineral density and vertebral fracture history are associated with incident and progressive radiographic knee osteoarthritis in elderly men and women: the Rotterdam Study. Bone 37(4):446–456
Jordan JM, Sowers MF, Messier SP et al (2011) Methodologic issues in clinical trials for prevention or risk reduction in osteoarthritis. Osteoarthritis Cartilage 19(5):500–508
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kwoh, C.K. (2012). Epidemiology of Osteoarthritis. In: Newman, A., Cauley, J. (eds) The Epidemiology of Aging. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5061-6_29
Download citation
DOI: https://doi.org/10.1007/978-94-007-5061-6_29
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5060-9
Online ISBN: 978-94-007-5061-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)