Abstract
Substance addiction can be a chronic relapsing disorder. While different drugs of addiction have different primary molecular targets, it has been demonstrated that many share the common action of being able to increase dopamine within hard-wired reward circuitry. While this effect is widely conceived as a primary factor driving initial drug use, long-term adaptations within this hard-wired neural circuitry underlie the transition from drug use to drug dependence. Significantly, these neuroadaptations are responsible for triggering recurrent drug relapse in people recovering from addiction, even when following periods of long-term abstinence. While there is no animal model of addiction that can fully emulate the human condition, some animal models do permit the investigation of specific elements of drug addiction, particularly those involving the reward system and its role in drug-seeking behavior. Neuroimaging methods now also permit us to test hypotheses of addiction derived from such animal models, allowing the field of neuroscience to examine neural components of drug abuse and dependence in humans. These neuroimaging procedures permit neuroscientists to test hypotheses in humans at different stages of the addiction cycle, particularly with a view to developing better treatments.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Conditioned Stimulus
- Anterior Cingulate Cortex
- Ventral Tegmental Area
- Conditioned Place Preference
- Ventral Striatum
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Substance addiction is often defined as a chronic, relapsing disorder characterized by (1) compulsion to seek and take the substance, (2) loss of control in limiting substance intake, and (3) the emergence of a negative emotional state (e.g., dysphoria, anxiety, irritability) reflecting a motivational withdrawal syndrome when access to the substance is prevented (defined as Substance Dependence by the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) of the American Psychiatric Association). Individuals meeting such criteria are those commonly described in clinical and neuroscience literature; however, many people do not enter such a chronic relapsing pattern and recover without treatment. Although drugs of addiction (e.g., alcohol, amphetamines, cocaine, nicotine) have varying pharmacological profiles, their ability to activate the mesocorticolimbic system is known to mediate their acute reinforcing effects (Koob and Volkow 2010). With chronic drug use, however, it is proposed that long-term neuroadaptations within this same mesocorticolimbic circuitry underlie the transition from drug use to drug dependence and relapse to drug use during abstinence.
Animal models have been critical in developing theories regarding the evolution of addiction. The positive-reinforcing effects of drugs of abuse (i.e., their ability to induce a conscious feeling of pleasure) have been widely conceived as a primary factor behind continued drug use and eventual drug dependence (Koob and Volkow 2010). Both positive and negative reinforcement theories additionally provide some insight into both the initiation (i.e., pleasure) and maintenance (i.e., withdrawal avoidance) of compulsive drug use (Cami and Farre 2003; Koob and Volkow 2010). Such theories, however, are unable to account for the resumption of drug-seeking and drug-taking behaviors (i.e., relapse) following protracted periods of abstinence. Chronic drug use has been proposed to result in a pathological shift in the hedonic set point of the drug user (Koob and Le Moal 1997). This state of dysregulation within evolutionary hard-wired brain reward systems is proposed to ultimately lead to a loss of control over drug use; that is, drug abuse produces a disequilibrium within brain reward circuitry that cannot be biologically maintained without using the substance of addiction.
An alternative theory is that addiction involves the development of “incentive sensitization” (Berridge 2009; Berridge and Robinson 1998; Berridge et al. 2009; Robinson and Berridge 2000). The chronic use of drugs is theorized to produce alterations in neural systems involved in the motivation and reward for natural appetitive reinforcers (e.g., food, water). Drug abuse is thought to induce a hypersensitive state to the drug and drug-associated stimuli (e.g., people, places, and objects associated with the substance). This ultimately leads to a shift from drug “liking” to drug “wanting,” whereby there is an ensuing compulsion to seek out and use the substance at any cost.
Additional theories view the persistent nature of addictive behaviors as ingrained drug habits in the form of aberrant stimulus response learning (Everitt and Robbins 2005; Volkow et al. 2006a; Wise 2002) and alterations in prefrontal cortical activity that reduce behavioral control and decision-making skills (Bechara 2005; Goldstein and Volkow 2002). Although each of these theories contributes its own unique perspective to the evolution of addiction, it is likely that there is significant overlap in these concepts. Furthermore, in humans, initial drug use probably involves a complex interaction between various components (i.e., biological, sociological, economic, legal, and cultural) that, in some individuals, produce substance addiction.
Pharmacology of Major Drugs of Dependence
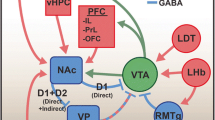
Understanding the pharmacology of drugs of abuse is vital to improving the prevention and treatment of addiction (Nutt and Lingford-Hughes 2008; Lingford-Hughes et al. 2010). Drugs of abuse are commonly classified into major categories (Feltenstein and See 2008) that include narcotics (e.g., opiates such as heroin), cannabinoids (e.g., marijuana), depressants (e.g., alcohol), and stimulants (e.g., nicotine, amphetamines, cocaine). While these substances all produce feelings of pleasure and relieve negative emotional states, they also possess highly diverse behavioral effects due to their varied neuropharmacological profiles in the brain. The mesocorticolimbic dopaminergic system is a key target for all substances of abuse. Modulation of the dopaminergic system may occur directly, as in the case of stimulants that block the dopamine transporter (DAT) in the nucleus accumbens (e.g., cocaine) or stimulate dopamine release (e.g., amphetamine). It may also be indirect, by increasing dopaminergic neuronal firing via disinhibition of inhibitory gamma amino butyric acid (GABA) interneurons in the ventral tegmental area (e.g., alcohol, opiates, nicotine) (see Everitt and Robbins 2005).
A key modulator of mesolimbic dopaminergic function is the endogenous endorphin system. It is the major target for the opioid drugs such as morphine and heroin, and it has long been implicated in processes such as interpersonal bonding (e.g., mother-child), love, and reward. Opiates reduce anxiety and induce euphoria and sedation (Heishman et al. 2000; Hill and Zacny 2000) by activating opioid receptors of which there are three subtypes: the mu opioid receptor (mOR), kappa opioid receptor (kOR), and delta opioid receptor (dOR). The activity of opiates at the mOR subtype underlies their abuse potential. The mOR is located in a variety of brain regions, including the cerebral cortex, thalamus, hippocampus, locus coeruleus, ventral tegmental area (VTA), nucleus accumbens (NAcc)/ventral striatum (VS), and the amygdala. Opiates mediate their reinforcing effects directly in the NAcc at the mOR and indirectly through mOR inhibition of GABA function on dopaminergic cells in the VTA, thereby increasing firing of dopamine VTA projections to the NAcc. Recent findings from human brain imaging studies suggest that addiction is associated with alterations within the endorphin system and that the craving, distress, and dysphoria found in early alcohol and drug abstinence are associated with alterations in mOR (see section “Neuroimaging Studies in Addiction” below).
Emerging research also suggests that the kOR subtype may play a role in addiction, particularly the experience of negative emotional states during drug withdrawal (Bruijnzeel 2009). Stimulation of kOR inhibits dopamine release in the striatum, with chronic administration of drugs of abuse shown to increase the release of dynorphin in this region. This suggests that the chronic abuse of substances (not just opiates) may have an enduring effect at the kOR subtype.
Cannabinoids induce feelings of euphoria, disinhibition, relaxation, and analgesia (Curran et al. 2002). Delta-9-tetrahydrocannabinol (or THC) is the principal psychoactive constituent of cannabis and exerts its central effects via the cannabinoid 1 receptor (CB1). CB1 receptors are highly expressed in the cerebral cortex, hippocampus, striatum, amygdala, and cerebellum (Herkenham et al. 1991; Tsou et al. 1998). CB1 receptors are also located in the VTA and NAcc, where they modulate dopaminergic firing (D’Souza et al. 2008; Huestis et al. 2007; Hunault et al. 2008). The presence of the CB1 receptor in the hippocampus is believed to underlie the effects of cannabinoids on memory.
Alcohol also induces euphoria, relaxation, and disinhibition, while reducing stress and anxiety (Koob 2004). The reinforcing effects of alcohol likely arise from its interaction with numerous neurotransmitter systems in the brain. Two key systems involved are opiate and GABA (the brain’s major inhibitory neurotransmitter). Alcohol increases endogenous endorphin release and modulates GABAA and GABAB receptors to increase dopamine levels (Koob 2004; Sullivan et al. 2011; Tang et al. 2003). Stimulation of GABA results in anxiolysis and sedation, which are major drivers for alcohol abuse in humans (Sieghart 2006). Tolerance to alcohol involves adaptations in the GABA system as well as excitatory glutamatergic N-methyl-d-aspartate (NMDA) receptors, making the GABA system less responsive and increasing NDMA receptor activity. In the absence of alcohol, these adaptions lead to a hyperexcitable brain, resulting in signs and symptoms of alcohol withdrawal that include tremor, fits, and delirium tremens.
While tobacco use is not associated with significant psychological and social impairment typical of other types of addiction, it is the leading cause of preventable death in developed countries (Benowitz 2008; Mathers and Loncar 2006; Peto et al. 1996). Nicotine is the main addictive component in cigarettes (Benowitz 2009; Gray et al. 1996; Mansvelder and McGehee 2002) and acts on nicotinic acetylcholine receptors (nAChRs) in the brain, including those in the VTA, that modulate dopaminergic cell firing (Grady et al. 2010; Klink et al. 2001; Wooltorton et al. 2003; Drenan et al. 2010; Mameli-Engvall et al. 2006). The high-affinity α4β2 subunit of the nAChR appears to be crucial to the positive-reinforcing and cognitive-enhancing effects of nicotine (Lippiello et al. 2006; Patterson et al. 2009).
Positron emission tomography (PET) studies in humans have demonstrated that smoking produces dopamine release in the VS (Brody et al. 2004). Furthermore, research in humans, investigating the effects of smoking on α4β2 nAChRs, has shown that smoking a full cigarette results in more than 88 % α4β2 subunit receptor occupancy, an effect which is accompanied by a significant reduction in cigarette craving (Brody et al. 2006). These PET research findings corroborate the value of medications that specifically target the α4β2 subunit of nAChRs (Gonzales et al. 2006; Jorenby et al. 2006) in reducing relapse in smokers attempting to quit.
Psychostimulants, such as cocaine and amphetamines, directly increase the concentration of dopamine in mesocorticolimbic brain regions (Kuhar et al. 1991; Wise 1996). Cocaine is a reuptake inhibitor that binds to the presynaptic dopamine transporter (DAT) (Amara and Kuhar 1993; Woolverton and Johnson 1992) that moves dopamine from the synapse back into presynaptic nerve terminals. By blocking the DAT, cocaine inhibits the reuptake of dopamine, increasing dopaminergic levels and amplifying its reinforcing effects. Amphetamines also block the DAT but directly trigger dopamine release as well (Rudnick and Clark 1993). The increase in dopamine produced by psychostimulants correlates with the resulting “high” that people experience (Volkow et al. 1999). While the pharmacological effects of psychostimulants also increase levels of serotonin and norepinephrine in the brain (Howell and Kimmel 2008; Rudnick and Clark 1993; Sora et al. 2009), it is primarily their effects on dopamine at the NAcc that underlie their abuse potential. Ecstasy or MDMA, another amphetamine-type drug, primarily targets the serotonergic system by blocking serotonin reuptake, producing a different set of euphoric experiences.
Animal Models of Addiction
There are a variety of increasingly sophisticated animal models that have provided invaluable insights into both the neurobiology of addiction and the pharmacological actions of drugs of abuse (Feltenstein and See 2008; Heidbreder 2011; Yahyavi-Firouz-Abadi and See 2009). Models that have particularly served to elucidate important neurobehavioral mechanisms in addiction include intracranial self-stimulation (ICSS), conditioned place preference (CPP), behavioral sensitization, and self-administration paradigms. Furthermore, these models, particularly those involving self-administration, have also proved beneficial in examining the neurobiology and neuropharmacology of drug relapse.
Knowledge about brain regions important for reward originally began with research in rodents by Olds and Milner, who found that rats would expend a great deal of effort to electrically stimulate areas of the brain that form part of a reward circuit (Olds and Milner 1954). Additional evidence suggested that the rewarding effects of ICSS activated a dopamine projection from the VTA to the NAcc, via a pathway known as the medial forebrain bundle (Heimer and Van Hoesen 2006; Wise 2005). Drugs of abuse have been observed to decrease ICSS thresholds; that is, the reinforcing properties of addictive drugs reduce the amount of brain stimulation required by the animal. Animal research has also revealed that the more addictive a substance is, the greater its ability to reduce the ICSS threshold. This model can be used to evaluate the abuse potential of different drugs. The ICSS model has also served as a unique experimental tool to assess alterations in the basal hedonic state of an animal following chronic drug exposure. In contrast, withdrawal from all major drugs of abuse produces an increase in ICSS thresholds; that is, the effects of drug withdrawal on reward circuitry increase the amount of brain stimulation required by the animal to overcome this state.
The CPP model utilizes the classical (Pavlovian) conditioning paradigm in which an animal learns associations between a conditioned stimulus (CS) and unconditioned stimulus (UCS). A CS (e.g., neutral object) is repeatedly paired with an UCS (e.g., drug), until the CS on its own comes to elicit the same response as the UCS. In the CPP model, an animal is exposed to an apparatus consisting of two neutral environments. These environments can differ in terms of a number of stimulus modalities, including color, texture, odor, and lighting (Bardo and Bevins 2000). One environment is paired with drug administration (CS+), while the other is paired with the administration of a control substance, usually saline (CS-). After a number of conditioning sessions, the animal (now in a drug-free state) is permitted free access to the environments of the apparatus, during which their preference for the two environments is measured (e.g., by frequency of entry into and the time spent in the environments). In accordance with the principles of classical conditioning, because the drug condition has reinforcing effects, the animal shows a significant preference for the drug-paired (CS+), over the saline-paired (CS−), environment.
Experimental studies show that various drugs of abuse (e.g., amphetamines, cocaine, heroin, nicotine) typically induce CPP for the drug-paired environment (Pastor et al. 2012; Sticht et al. 2010; Thorn et al. 2012), suggesting a role for classical conditioning in the acquisition of drug use behavior. The CPP model, however, possesses a number of limitations, including the method of drug administration (e.g., experimenter administered) that fails to model human drug use (i.e., self-administration), the potential confound of novelty on the day of testing, difficulties in generating dose–response curves, and the model being limited to use in rodents.
Behavioral sensitization involves a progressive increase in the motor stimulatory effects of a drug with repeated and intermittent administration. The development of behavioral sensitization has been hypothesized to represent the shift from drug “liking” to drug “wanting” that has been hypothesized to underlie compulsive drug use in humans (Berridge 2009; Berridge and Robinson 1998; Berridge et al. 2009; Robinson and Berridge 2000). The phenomenon of behavioral sensitization has been demonstrated for a variety of drugs of addiction, such as amphetamines (Degoulet et al. 2009), cocaine (Burger and Martin-Iverson 1994), and nicotine (Kosowski and Liljequist 2005). It may potentially model elements of drug craving and relapse in humans (Vanderschuren and Kalivas 2000). Although useful for studying several aspects of drug-induced neuroplasticity, the behavioral sensitization model, like CPP, is limited because animals never experience contingent drug self-administration, a hallmark of human addiction.
The most widely accepted animal model of drug abuse and addiction is the self-administration paradigm. During this operant conditioning procedure, the animal presses a lever (i.e., the operandum), which triggers the delivery of a reward (e.g., cocaine). Animals can be trained to perform a variety of different operant behaviors (e.g., nose pokes) in order to receive the drug after varying amounts of attempts. Like humans, animals will readily make operant responses in order to self-administer most drugs of abuse, including opiates, cannabinoids, alcohol, nicotine, amphetamines, and cocaine (Feltenstein and See 2008; Heidbreder 2011; Yahyavi-Firouz-Abadi and See 2009). Furthermore, studies almost universally demonstrate that animals will preferentially respond on a reinforced (i.e., active), rather than a non-reinforced (i.e., inactive) operandum. This suggests that like humans, animals are able to rapidly discriminate between responses that elicit the delivery of drug and nondrug rewards.
While a variety of species and routes of drug administration can be used, most animal studies of addiction involve the use of rodents or nonhuman primates. Drugs of abuse are typically self-administered intravenously, via a chronic indwelling catheter, or orally. The abuse potential of different compounds in humans is well predicted by animal intravenous self-administration models. This suggests that this paradigm mimics human abuse with greater ecological validity than repeated experimenter-delivered administration (e.g., intraperitoneal, subcutaneous). Therefore, the drug self-administration model appears to possess reasonable face, construct. and predictive validity for examining the neuropharmacological profiles of drugs that are readily abused by humans.
Craving and the recommencement of drug seeking and drug taking following drug abstinence are significant features of addiction (Sinha and Li 2007; Volkow et al. 2002a, 2006b). Factors believed to contribute to drug craving and relapse include exposure to conditioned drug cues, negative mood states, and stress. These triggers have been examined using animal models of relapse that employ an “extinction–reinstatement” approach. In the “extinction–reinstatement model,” animals are allowed to self-administer a drug (e.g., cocaine) for prolonged periods of time, mimicking chronic drug use in humans. The animals then undergo extinction training in which the previously reinforced behavior (e.g., pressing a lever) fails to elicit drug delivery. These animals are then exposed to small amounts of the previously administered drug (called drug priming) or environmental stressors (e.g., foot shock) to test the reinstatement of drug self-administration after extinction. Research has shown that conditioned cues, drug priming, and stress are all powerful triggers for the reinstatement of drug-seeking behavior, as indexed by an increase in a behavior previously paired with a drug (Shaham et al. 2003). The reinstatement of drug self-administration is believed to model relapse to drug use in humans. The application of the reinstatement model has also proved useful in examining the neural circuitry underlying drug relapse (Kruzich et al. 2001; Kruzich and See 2001; Weiss et al. 2000).
Neuroimaging Studies in Addiction
Studies Using Positron Emission Tomography (PET)
PET imaging directly assesses neurotransmitter systems in the brain by using a radioactive tracer that recognizes a particular target. There are a number of well characterized tracers for some neurotransmitter systems of interest in addiction (e.g., dopaminergic system), but not for others (e.g., glutamate). This limits the utility of PET investigations.
Dopamine
Cocaine and methamphetamine (or “crystal meth”) increase dopamine levels in ways that can be measured by an increase in the displacement of PET tracers that bind to dopamine (D2) receptors (e.g., [11C]raclopride). The increase in dopamine produced by stimulants in healthy volunteers is dose-related and reflects the “high” that people experience (Volkow et al. 1999). These findings, supported by earlier animal studies, showed that drugs of addiction increase dopamine in the NAcc. This led to the view that dopamine release was a necessary, perhaps even sufficient condition, for drugs to have addictive potential. Recent work, however, has cast doubt on this. Heroin and other opioids, nicotine, and cannabis do not appear to produce detectable increases in dopamine (Bossong et al. 2009; Brody et al. 2004; Daglish et al. 2008; Stokes et al. 2009).
In cocaine- and alcohol-dependent individuals, amphetamine- or methylphenidate-stimulated release of dopamine, particularly in the ventral striatum, is blunted compared with healthy volunteers (Martinez et al. 2005). This finding challenges the theory of sensitization, which would predict increased dopamine levels in addicted individuals. Cocaine-dependent individuals also reported a reduced “high” and blunted change in dopamine levels that predicted the choice for cocaine over money (Martinez et al. 2007; Volkow et al. 1997).
The dopamine receptor, however, may play a key role in addiction propensity. Low levels of dopamine receptors are associated with a greater rewarding effect of stimulants (Volkow et al. 1999), while high levels are possibly protective in alcoholism (Volkow et al. 2006a). The use of stimulants (e.g., cocaine and methamphetamine) has been shown to lower dopamine receptor numbers (Dagher et al. 2001; Heinz et al. 2004; Lee et al. 2009; Volkow et al. 2001). Similarly, in alcohol dependence, lower striatal D2/3 receptor availability has been reported (Volkow et al. 1996; Martinez et al. 2005). However, there is no evidence of reductions in D2/3 receptor availability in cannabis dependence (Sevy et al. 2008) or use (Stokes et al. 2012). A recent study has shown that DAT availability is significantly reduced in the striatum of long-term cannabis users and cigarette smokers (Leroy et al. 2011), suggesting that disturbances in dopamine functioning is associated with chronic use. Daglish et al. 2008 were also unable to detect lower striatal D2/3 receptor levels in methadone-maintained, opioid-addicted individuals. Martinez and colleagues did report a reduction in recently abstinent heroin-addicted individuals (Martinez et al. 2011), suggesting that the level of striatal D2/3 receptors may depend on whether opioid dependent individuals are free of or maintained on opioid drugs.
Opioid System
In cocaine addicted individuals, regional brain mOR levels remained elevated in the anterior frontal/cingulate cortex during 12 weeks of abstinence (Gorelick et al. 2005). These regions are strongly implicated in “top-down” cognitive regulation of impulses and behavior. Elevated mOR levels in the medial frontal and middle frontal gyri prior to psychosocial treatment were significantly associated with greater cocaine use during treatment (Ghitza et al. 2010). This study also found that elevations in mOR levels in the anterior cingulate cortex (ACC), medial frontal, and insular cortices correlated with a shorter duration of cocaine abstinence. Significantly, mOR binding was a more powerful predictor of treatment outcome than baseline drug and alcohol use.
The endogenous opioid system plays a significant role in alcohol dependence, as indicated by the efficacy of opiate antagonists (e.g., naltrexone) in pharmacotherapeutic trials (Lingford-Hughes et al. 2012a). Several studies have reported an increased availability of mOR in striatal regions in abstinent alcoholics (Heinz et al. 2005; Williams et al. 2009; Weerts et al. 2011). A similar increase in mOR availability has been found in abstinent opioid-dependent individuals (Williams et al. 2007). An increased availability of mOR has been demonstrated in addiction to a number of pharmacologically different substances of abuse and therefore may be involved in the vulnerability to and perpetuation of drug taking after abstinence.
GABA
Several studies have shown that GABA binding is lower in abstinent alcohol-dependent patients (Abi-Dargham et al. 1998; Lingford-Hughes et al. 1998) or its function reduced (Lingford-Hughes et al. 2005). The reduction in GABA binding may be the result of the downregulation of GABA in order to reduce the impact of sedative drugs on the GABA system. Individuals at risk of alcoholism, and addiction in general, may have preexisting reduced levels of GABA activity. The α5 subtype of the GABAA receptor is highly expressed in brain regions that regulate emotion and reward, such as the ventral striatum, and a reduction in these receptors is found in persons with alcoholism (Lingford-Hughes et al. 2012b).
Studies Using Functional MRI (fMRI)
fMRI exploits the fact that the magnetic properties of blood change as oxygen is removed. These changes can be detected using an MRI measure known as the Blood-Oxygen-Level-Dependent (BOLD) signal while a person performs a behavioral task (e.g., reward learning). The BOLD response represents the change in oxyhemoglobin to deoxyhemoglobin ratio in venous blood. The strength of this signal in a brain region (e.g., ventral striatum) indicates the relative level of oxygenated to deoxygenated blood at that location. Because neuronal activity requires oxygen, the BOLD signal is believed to indirectly reflect neuronal activity at that location during the psychological process being studied.
The use of behavioral assays that specifically tap into the neural circuitry on which drugs of abuse act allows fMRI neuroscientists to explore differences and similarities between the long-term effects of different drugs. The cognitive domains that have been under investigation in recent years, and which have provided some insight into the addicted brain, comprise memory, planning and impulse control, and more subjective experiences such as empathy (see below).
Drug-Related Stimuli
The production of strong emotional and cognitive responses to drug-related stimuli, referred to as cue reactivity, is a common and clinically important feature of drug addiction. Studies have investigated the neural correlates of cue reactivity and craving using cue-exposure techniques, which are ethically less challenging than giving drugs of abuse to addicted individuals. In particular the reactivity of neural reward circuits to drug-related cues has been widely studied to test whether there is an “overvaluation” of drug reinforcers, as has been hypothesized (Goldstein and Volkow 2002). Understanding how this system operates is important because drug-related cues may increase attentional bias and expectancy of drug delivery in both current and abstinent drug users. Such studies may potentially identify neural mechanisms and inform treatment development by providing potential cognitive or pharmacological targets (Muraven 2010; Schoenmakers et al. 2010; Shoptaw et al. 2008; Franklin et al. 2011; Goldstein et al. 2010).
fMRI studies have identified common brain regions (e.g., amygdala, OFC, and VS) that are involved in cue reactivity and craving elicited by drug-related cues in drug-using populations. We describe studies in nicotine addiction to illustrate how such imaging and reactivity has clinical relevance. Greater reactivity to smoking-related images has been reported in the insula and dorsal ACC (dACC) in those nicotine-dependent women who relapsed (Janes et al. 2010). The importance of the insula, which integrates interoceptive (i.e., bodily) states into conscious feelings and decision-making processes involving uncertain risk and reward, has recently emerged with evidence that damage to the insula disrupts nicotine addiction (Naqvi and Bechara 2008). Another study revealed that extinction-based smoking cessation treatment attenuated responses to smoking cues in the amygdala, and the same attenuation pattern in the thalamus predicted which smokers remained abstinent (McClernon et al. 2007). Concerning the impact of medication, varenicline has been shown to reduce responses in the VS and medial OFC to smoking-related cues as well as subjective craving (Franklin et al. 2011).
Imaging with alcohol-related cues has shown activation of similar brain areas. For instance, heavy drinkers show significantly greater activations in the dorsal striatum (DS) than social drinkers, and light drinkers show higher cue-induced activations in the VS and prefrontal areas than heavy social drinkers (Vollstadt-Klein et al. 2010). Detoxified alcoholics have less activation in the VS during the anticipation of nondrug rewards than healthy controls but increased VS activation in response to alcohol-associated cues (Wrase et al. 2007). This finding suggests that mesolimbic activation in alcoholics (and addiction as a whole) is biased toward the processing of alcohol, as opposed to conventional reward cues, supporting the hypothesis of a reward deficiency syndrome. Various medications such as naltrexone (mOR antagonist), ondansetron (serotonin 5HT3 receptor antagonist), and aripiprazole (D2/3 partial agonist) all reduce VS activation in response to alcohol-related cues in non-treatment-seeking alcoholics (Myrick et al. 2008, 2010).
In abstinent heroin addicts and cocaine abusers, salient drug-related cues have been shown to result in activation in the ACC in all participants, but PFC activation was only seen in those that experienced craving (Wexler et al. 2001). The study by Daglish et al. (2001) also revealed that ACC activation increased, rather than decreased, with the duration of abstinence. This finding may support the long-held belief that addiction can be an enduring process involving long-term adaptations in various circuits. It has been suggested that increased activity in the OFC reflects a hypersensitivity to reward (Bolla et al. 2003), whereas reduced activity in ACC reflects hyposensitivity to punishment (Garavan and Stout 2005).
As with medications to treat alcoholism, medications used to treat opiate addiction, such as methadone and buprenorphine, have been shown to reduce responses in the insula and hippocampus to salient drug cues (Langleben et al. 2008; Mei et al. 2010). It appears however that activity in the OFC does not dissipate after medication, the impact and clinical implications of which requires further study.
The development of a conditioned, cue-induced neural attentional bias in response to drug-predictive stimuli is accompanied by craving in different drug-using populations. This bias may be implicit in maintaining addictive behaviors and provoking drug relapse among users attempting to remain abstinent. Significantly, functional brain imaging procedures have been shown to reliably measure the effect of neural responses to drug-related stimuli on drug relapse. This may be important when testing the effects of medications on these responses. Future research may improve treatment outcomes in addiction medicine by identifying neural signatures that predict relapse.
Reward Processing
Reward is a central driver of incentive-based learning that elicits appropriate responses to stimuli and shapes the development of goal-directed behaviors. Motivational theories of drug use make different predictions about how drug use may differentially recruit brain areas, such as the VS, in response to rewards (Bjork et al. 2008). The reward deficiency syndrome (RDS) and the allostatic hypotheses (AH), for example, both postulate that addiction is the result of a deficit in dopamine motivational circuitry for nondrug rewards and that only drugs of abuse are able to normalize dopamine at the VS (Blum et al. 2000; Koob et al. 2004). This may induce reflexive, conditioned responses to drug cues and diminish responses to cues that signal nondrug rewards. Alternatively, the “impulsivity hypothesis” of addiction suggests that persons who are vulnerable to, or suffering from, addiction have an excessive approach and reduced inhibitory control over their behavior (Bechara 2005; Bickel et al. 2007). This hypothesis is supported by longitudinal studies which have shown that both poor self-control and high novelty seeking in childhood are significant predictors of substance use in adolescence (Ding et al. 2004; Masse and Tremblay 1997; Myers et al. 1995) and addiction in later life (Fergusson et al. 2007).
Substance-dependent persons exhibit both impulsive and reward-centered choice behavior, and those with alcohol, cocaine, heroin, and nicotine dependence have an increased preference for small immediate over larger delayed rewards (Bechara et al. 2001; Bickel and Marsch 2001; Bjork et al. 2004; Heil et al. 2006; Reynolds and Fields 2011; Robles et al. 2011). This suggests that individuals who are both prone to, and engage in, chronic substance use have some combination of reward hypersensitivity and deficient inhibitory control (Bechara 2005; Bickel et al. 2007; Solomon and Corbit 1974). Assessing neural responses to nondrug rewards in substance abusers has particular value in evaluating these hypotheses and establishing patterns of reward functioning in addiction.
As described above, significantly lower numbers of D2 receptors or released dopamine have been found in the striatum of people addicted to alcohol, cocaine, and methamphetamine (Martinez et al. 2005, 2007, 2009, 2011; Volkow et al. 2004). While this evidence is consistent with the RDS and AH, there is less consistent evidence in heroin, nicotine, or opioid addiction. It is not easy to determine whether the impairment precedes or follows addiction. Those at risk of initiating substance abuse may be hyporesponsive to nondrug rewards due to deficient dopamine functioning in the striatum which is overcome by taking drugs of abuse that enhance dopamine levels.
Most fMRI studies in addiction have attempted to examine reward sensitivity using the Monetary Incentive Delay (MID) task (Knutson et al. 2001). The MID task allows researchers to measure brain activation while a person anticipates and receives monetary reward and punishment. The person first views a brief visual cue indicating the type of reward trial they will participate in. This is followed by a short delay after which the person responds to a target stimulus and does or does not receive a reward depending on their response to the target. Significantly, fMRI BOLD responses during the MID delay period correlate with dopamine release in the VS (Schott et al. 2008), appearing to substantiate its sensitivity to dopamine reward functioning.
In alcoholism, support for the RDS/AH is demonstrated by blunted VS responses during reward anticipation compared with nonalcoholics (Beck et al. 2009; Wrase et al. 2007). However, alcoholics did not differ from nonalcoholics during reward anticipation, but they did differ in their responses to reward outcomes (Bjork et al. 2008), a finding more consistent with the impulsivity hypothesis. In cannabis-using populations, there is support for both hypotheses. Greater activation in the VS has been shown in cannabis users than drug-naïve controls during reward anticipation, consistent with the impulsivity hypothesis (Nestor et al. 2010). By contrast, Van Hell and colleagues found that cannabis users had significantly less activation in the VS compared to non-cigarette smokers, but not cigarette smokers (van Hell et al. 2010), thereby supporting the RDS/AH.
Greater activation in the left and right VS, right caudate, and right insula has been shown in treatment-seeking cocaine-addicted individuals using the MID (Jia et al. 2011). Notably, some neural responses predicted treatment success with activation during reward anticipation in the bilateral thalamus and right caudate negatively associated with cocaine-negative urinalyses and activation in the left amygdala and parahippocampal gyrus correlated negatively with treatment retention. These findings suggest that in treatment-seeking cocaine-addicted persons, impulsive corticolimbic reward circuitry for nondrug rewards may be a neural biomarker that predicts treatment outcome (Jia et al. 2011). (Goldstein et al. 2007a) have also reported dysfunctional PFC activation during instrumental tasks in cocaine-addicted persons (Goldstein et al. 2007b). Finally, it has also been shown that in cigarette smokers, neural responses in the VS during reward anticipation are significantly lower than in control subjects (Peters et al. 2011). This has also been observed during the receipt of delayed rewards in this population (Luo et al. 2011).
The concepts of reward and impulsivity are both important in eliciting appropriate responses to stimuli and shaping the development of goal-directed behaviors. Since the empirical findings to date are consistent with both hypotheses, future research on the processing of nondrug rewards in addiction will need to address a potential disparity in neural responses in different types of addictions. In doing so, brain imaging research of reward processing in substance abusers may be able to delineate neural responses that are contingent upon the substance of abuse, the treatment-seeking status of the individual, and the duration of their abstinence.
Cognitive Control and Decision-Making
Flexible goal-directed behavior requires an adaptive cognitive control system for organizing and optimizing processing (Ridderinkhof et al. 2004a, b). Evidence from cognitive neuroscience is beginning to converge on the different contributions of the PFC in cognitive control. This convergence of evidence may identify potential biomarkers of compromised cognition that predict both the initiation and continuation of drug abuse. Since addiction is by definition continued drug use and recurrent drug relapse in the face of serious negative consequences, decrements in cognitive inhibitory control may be a core feature of the disease.
Laboratory tests of cognitive inhibitory control usually involve a person withholding a habitual motor response or ignoring the presentation of irrelevant stimuli while continually updating information and monitoring one’s performance. The processes of cognitive inhibitory control and monitoring have consistently been shown to involve the PFC and ACC (Carter et al. 1998; Garavan et al. 1999, 2002; Ullsperger and von Cramon 2001). If the ability to inhibit and monitor one’s behavior is important in the development and maintenance of addiction (Garavan and Stout 2005), then brain imaging assessments of cognitive inhibitory control may identify deficits in both behavior and brain functioning.
Dysfunctional activity in the PFC including ACC and OFC of different drug-using (i.e., alcohol, cannabis, cocaine, heroin, methamphetamine and nicotine) individuals has been shown in fMRI studies using tests of cognitive inhibitory control and monitoring when compared with demographically matched drug-naïve individuals (Volkow et al. 2007; Garavan et al. 2008; Goldstein et al. 2004, 2010; Hester and Garavan 2004; Kaufman et al. 2003). Importantly, it has been shown that severe global cognitive impairment makes cocaine-addicted individuals less amenable to behavioral treatments (Aharonovich et al. 2003, 2006). This underscores the need to uncover biomarkers of cognitive control in addiction to inform rehabilitation programs for individual substance abusers.
Error monitoring has also been shown to be impaired in substance using populations, for example. Reduced functioning in the ACC when cannabis users are required to indicate their awareness of errors (Hester et al. 2009) or in chronic heroin users during error monitoring (Forman et al. 2004). Notably, previous research in early cocaine and methamphetamine abstinence has shown cortical neural deficits during verbal and visuospatial (Kubler et al. 2005) working memory (Moeller et al. 2010; Tomasi et al. 2007), conflict resolution (Nestor et al. 2011a) and decision-making processes (Hoffman et al. 2008; Monterosso et al. 2007). These findings support the notion that disruptions in prefrontal circuits are important for general, flexible, goal-directed behavior. Interestingly one study has shown that ex-smokers who had been abstinent for a year or more had increased lateral PFC activation compared with both smokers and nicotine naïve participants (Nestor et al. 2011b). Increased lateral PFC activation may be an important characteristic of successful abstinence in former smokers.
To assess decision-making in individuals with substance dependence, studies have used the Iowa Gambling Task (IGT) (Bechara et al. 1994). On this task patients with damage to the ventromedial prefrontal cortex (VMPFC) appear to be oblivious to the future consequences of their actions (i.e., myopia for the future), appearing only to be guided by their immediate prospects. Subsequent neuroimaging studies in healthy participants using the IGT have shown increased activation in the VMPFC, ACC, parietal/insular cortices, amygdala, and striatum during the actual decision-making component of the task (Ernst et al. 2002; Matthews et al. 2004; Verney et al. 2003). All of these regions are known to be affected by drugs of abuse. This had led to the “somatic-marker” hypothesis in which decision-making depends on the neural substrates that regulate homeostasis, emotion, and feeling (Verdejo-Garcia and Bechara 2009). According to this model, there should be a link between alterations in processing emotions in substance abusers and their impairments in decision-making.
There is some support for this hypothesis. For example, after 3 weeks abstinence, cocaine abusers have greater activation during performance of the IGT in the right OFC and less activation in the right DLPFC and left medial PFC than a control group (Bolla et al. 2003). These results suggest that cocaine abusers show persistent functional abnormalities in prefrontal neural networks involved in decision-making that may undermine attempts to remain abstinent. In a similar study, after 4 weeks of abstinence cannabis users, particularly heavy users (53–84 joints/week), had greater activation in the left cerebellum and less activation in the right OFC and DLPFC than controls (Bolla et al. 2005). These preliminary findings suggest that prefrontal neural deficits in heavy cannabis users are manifested in decrements in decision-making.
Other studies have examined patterns of regional brain activation in abstinent drug users during decision-making. Imaging patterns in methamphetamine abusing individuals performing the two-choice prediction task have shown decreased activation of the OFC, DLPFC, insular, and inferior parietal cortices (Paulus et al. 2003). These patterns of brain activation were strong predictors of relapse. Here activation patterns in the right insular, posterior cingulate, and temporal cortex obtained in early recovery correctly predicted 90 % of subjects who did not relapse and 94 % of subjects who did (Paulus et al. 2005).
Summary
Our knowledge about how drugs affect brain functioning and neural circuits in abuse and dependence has substantially increased in the last few decades due to developments in neuroimaging. Animal studies have improved in their complexity to better reflect what happens in man. In human and animal studies, the mesolimbic dopaminergic system has continued to receive much attention, and evidence suggests that hypofunctioning in this system is involved in vulnerability to drug liking for naïve users and also to relapse in addicted individuals. The role of other neurotransmitter systems is receiving more attention, and the importance of opioid and GABAergic systems, for instance, is recognized and has led to improvements in clinical treatment. Psychological constructs of behaviors such as reward processing, decision-making, and impulsivity have been widely studied in substance use and abuse with impairments generally described. Challenges for the future include using our knowledge and neuroimaging to bring psychological and pharmacological theories closer together so that the interplay between the impact of the drugs and/or their psychological or pharmacological treatment on underlying psychological processes is clearer.
References
Abi-Dargham, A., Krystal, J. H., Anjilvel, S., Scanley, B. E., Zoghbi, S., Baldwin, R. M., et al. (1998). Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. The American Journal of Psychiatry, 155(11), 1550–1555.
Aharonovich, E., Nunes, E., & Hasin, D. (2003). Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug and Alcohol Dependence, 71(2), 207–211.
Aharonovich, E., Hasin, D. S., Brooks, A. C., Liu, X., Bisaga, A., & Nunes, E. V. (2006). Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence, 81(3), 313–322.
Amara, S. G., & Kuhar, M. J. (1993). Neurotransmitter transporters: Recent progress. Annual Review of Neuroscience, 16, 73–93.
Bardo, M. T., & Bevins, R. A. (2000). Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology, 153(1), 31–43.
Bechara, A. (2005). Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience, 8(11), 1458–1463.
Bechara, A., Damasio, A. R., Damasio, H., & Anderson, S. W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50(1–3), 7–15.
Bechara, A., Dolan, S., Denburg, N., Hindes, A., Anderson, S. W., & Nathan, P. E. (2001). Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia, 39(4), 376–389.
Beck, A., Schlagenhauf, F., Wustenberg, T., Hein, J., Kienast, T., Kahnt, T., et al. (2009). Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry, 66(8), 734–742.
Benowitz, N. L. (2008). Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology and Therapeutics, 83(4), 531–541.
Benowitz, N. L. (2009). Pharmacology of nicotine: Addiction, smoking-induced disease, and therapeutics. Annual Review of Pharmacology and Toxicology, 49, 57–71.
Berridge, K. C. (2009). Wanting and liking: Observations from the neuroscience and psychology laboratory. Inquiry (Oslo), 52(4), 378.
Berridge, K. C., & Robinson, T. E. (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews, 28(3), 309–369.
Berridge, K. C., Robinson, T. E., & Aldridge, J. W. (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73.
Bickel, W., & Marsch, L. (2001). Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction, 96(1), 73–86.
Bickel, W. K., Miller, M. L., Yi, R., Kowal, B. P., Lindquist, D. M., & Pitcock, J. A. (2007). Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence, 90(Suppl 1), S85–S91.
Bjork, J. M., Hommer, D. W., Grant, S. J., & Danube, C. (2004). Impulsivity in abstinent alcohol-dependent patients: Relation to control subjects and type 1-/type 2-like traits. Alcohol, 34(2–3), 133–150.
Bjork, J. M., Smith, A. R., & Hommer, D. W. (2008). Striatal sensitivity to reward deliveries and omissions in substance dependent patients. NeuroImage, 42(4), 1609–1621.
Blum, K., Braverman, E. R., Holder, J. M., Lubar, J. F., Monastra, V. J., Miller, D., et al. (2000). Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. Journal of Psychoactive Drugs, 32(Suppl i–iv), 1–112.
Bolla, K. I., Eldreth, D. A., London, E. D., Kiehl, K. A., Mouratidis, M., Contoreggi, C., et al. (2003). Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage, 19, 1085–1094.
Bolla, K. I., Eldreth, D. A., Matochik, J. A., & Cadet, J. L. (2005). Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage, 26(2), 480–492.
Bossong, M. G., van Berckel, B. N., Boellaard, R., Zuurman, L., Schuit, R. C., Windhorst, A. D., et al. (2009). Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology, 34(3), 759–766.
Brody, A. L., Olmstead, R. E., London, E. D., Farahi, J., Meyer, J. H., Grossman, P., et al. (2004). Smoking-induced ventral striatum dopamine release. The American Journal of Psychiatry, 161(7), 1211–1218.
Brody, A. L., Mandelkern, M. A., London, E. D., Olmstead, R. E., Farahi, J., Scheibal, D., et al. (2006). Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Archives of General Psychiatry, 63(8), 907–915.
Bruijnzeel, A. W. (2009). Kappa-Opioid receptor signaling and brain reward function. Brain Research Reviews, 62(1), 127–146.
Burger, L. Y., & Martin-Iverson, M. T. (1994). Increased occupation of D1 and D2 dopamine receptors accompanies cocaine-induced behavioral sensitization. Brain Research, 639(2), 228–232.
Cami, J., & Farre, M. (2003). Drug addiction. The New England Journal of Medicine, 349(10), 975–986.
Carter, C., Braver, T., Barch, D., Botvinick, M., Noll, D., & Cohen, J. D. (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280(5364), 747–749.
Curran, H. V., Brignell, C., Fletcher, S., Middleton, P., & Henry, J. (2002). Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology, 164(1), 61–70.
D’Souza, D. C., Braley, G., Blaise, R., Vendetti, M., Oliver, S., Pittman, B., et al. (2008). Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology, 198(4), 587–603.
Dagher, A., Bleicher, C., Aston, J. A., Gunn, R. N., Clarke, P. B., & Cumming, P. (2001). Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse, 42(1), 48–53.
Daglish, M. R., Weinstein, A., Malizia, A. L., Wilson, S., Melichar, J. K., Britten, S., et al. (2001). Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. The American Journal of Psychiatry, 158(10), 1680–1686.
Daglish, M. R. C., Williams, T., Wilson, S. J., Taylor, L. G., Brooks, D. J., Myles, J. S., Grasby, P. G., Lingford-Hughes, A. R., & Nutt, D. J. (2008). No measurable dopamine response to heroin in the brains of human addicts. The British Journal of Psychiatry, 193(1), 65–72.
Degoulet, M. F., Rostain, J. C., David, H. N., & Abraini, J. H. (2009). Repeated administration of amphetamine induces a shift of the prefrontal cortex and basolateral amygdala motor function. The International Journal of Neuropsychopharmacology, 12(7), 965–974.
Ding, Y. S., Gatley, S. J., Thanos, P. K., Shea, C., Garza, V., Xu, Y., et al. (2004). Brain kinetics of methylphenidate (Ritalin) enantiomers after oral administration. Synapse, 53(3), 168–175.
Drenan, R. M., Grady, S. R., Steele, A. D., McKinney, S., Patzlaff, N. E., McIntosh, J. M., et al. (2010). Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors. Journal of Neuroscience, 30(29), 9877–9889.
Ernst, M., Bolla, K., Mouratidis, M., Contoreggi, C., Matochik, J. A., Kurian, V., et al. (2002). Decision-making in a risk-taking task: A PET study. Neuropsychopharmacology, 26(5), 682–691.
Everitt, B. J., & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–1489.
Feltenstein, M. W., & See, R. E. (2008). The neurocircuitry of addiction: An overview. British Journal of Pharmacology, 154(2), 261–274.
Fergusson, D. M., Horwood, L. J., & Ridder, E. M. (2007). Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence: Results of a 25-year longitudinal study. Drug and Alcohol Dependence, 88(Suppl 1), S14–S26.
Forman, S. D., Dougherty, G. G., Casey, B. J., Siegle, G. J., Braver, T. S., Barch, D. M., et al. (2004). Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biological Psychiatry, 55(5), 531–537.
Franklin, T., Wang, Z., Suh, J. J., Hazan, R., Cruz, J., Li, Y., et al. (2011). Effects of varenicline on smoking cue-triggered neural and craving responses. Archives of General Psychiatry, 68(5), 516–526.
Garavan, H., & Stout, J. C. (2005). Neurocognitive insights into substance abuse. Trends in Cognitive Science, 9(4), 195–201.
Garavan, H., Ross, T. J., & Stein, E. A. (1999). Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America, 96(14), 8301–8306.
Garavan, H., Ross, T. J., Murphy, K., Roche, R. A., & Stein, E. A. (2002). Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. NeuroImage, 17(4), 1820–1829.
Garavan, H., Kaufman, J. N., & Hester, R. (2008). Acute effects of cocaine on the neurobiology of cognitive control. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1507), 3267–3276.
Ghitza, U. E., Preston, K. L., Epstein, D. H., Kuwabara, H., Endres, C. J., Bencherif, B., et al. (2010). Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biological Psychiatry, 68(8), 697–703.
Goldstein, R. Z., & Volkow, N. D. (2002). Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry, 159, 1642–1652.
Goldstein, R. Z., Leskovjan, A. C., Hoff, A. L., Hitzemann, R., Bashan, F., Khalsa, S. S., et al. (2004). Severity of neuropsychological impairment in cocaine and alcohol addiction: Association with metabolism in the prefrontal cortex. Neuropsychologia, 42(11), 1447–1458.
Goldstein, R. Z., Tomasi, D., Alia-Klein, N., Cottone, L. A., Zhang, L., Telang, F., et al. (2007a). Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and Alcohol Dependence, 87(2–3), 233–240.
Goldstein, R. Z., Tomasi, D., Rajaram, S., Cottone, L. A., Zhang, L., Maloney, T., et al. (2007b). Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience, 144(4), 1153–1159.
Goldstein, R. Z., Woicik, P. A., Maloney, T., Tomasi, D., Alia-Klein, N., Shan, J., et al. (2010). Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proceedings of the National Academy of Sciences of the United States of America, 107(38), 16667–16672.
Gonzales, D., Rennard, S. I., Nides, M., Oncken, C., Azoulay, S., Billing, C. B., et al. (2006). Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA: The Journal of the American Medical Association, 296(1), 47–55.
Gorelick, D. A., Kim, Y. K., Bencherif, B., Boyd, S. J., Nelson, R., Copersino, M., et al. (2005). Imaging brain mu-opioid receptors in abstinent cocaine users: Time course and relation to cocaine craving. Biological Psychiatry, 57(12), 1573–1582.
Grady, S. R., Salminen, O., McIntosh, J. M., Marks, M. J., & Collins, A. C. (2010). Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. Journal of Molecular Neuroscience, 40(1–2), 91–95.
Gray, R., Rajan, A. S., Radcliffe, K. A., Yakehiro, M., & Dani, J. A. (1996). Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature, 383(6602), 713–716.
Heidbreder, C. (2011). Advances in animal models of drug addiction. Current Topics in Behavioral Neurosciences, 7, 213–250.
Heil, S. H., Johnson, M. W., Higgins, S. T., & Bickel, W. K. (2006). Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors, 31(7), 1290–1294.
Heimer, L., & Van Hoesen, G. W. (2006). The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neuroscience and Biobehavioral Reviews, 30(2), 126–147.
Heinz, A., Siessmeier, T., Wrase, J., Hermann, D., Klein, S., Grusser, S. M., et al. (2004). Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. The American Journal of Psychiatry, 161(10), 1783–1789.
Heinz, A., Reimold, M., Wrase, J., Hermann, D., Croissant, B., Mundle, G., et al. (2005). Correlation of stable elevations in striatal mu-opioid receptor availability in detoxified alcoholic patients with alcohol craving: A positron emission tomography study using carbon 11-labeled carfentanil. Archives of General Psychiatry, 62(1), 57–64.
Heishman, S. J., Schuh, K. J., Schuster, C. R., Henningfield, J. E., & Goldberg, S. R. (2000). Reinforcing and subjective effects of morphine in human opioid abusers: Effect of dose and alternative reinforcer. Psychopharmacology, 148(3), 272–280.
Herkenham, M., Lynn, A. B., Johnson, M. R., Melvin, L. S., de Costa, B. R., & Rice, K. C. (1991). Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. Journal of Neuroscience, 11(2), 563–583.
Hester, R., & Garavan, H. (2004). Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience, 24(49), 11017–11022.
Hester, R., Nestor, L., & Garavan, H. (2009). Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology, 34(11), 2450–2458.
Hill, J. L., & Zacny, J. P. (2000). Comparing the subjective, psychomotor, and physiological effects of intravenous hydromorphone and morphine in healthy volunteers. Psychopharmacology, 152(1), 31–39.
Hoffman, W. F., Schwartz, D. L., Huckans, M. S., McFarland, B. H., Meiri, G., Stevens, A. A., et al. (2008). Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology, 201(2), 183–193.
Howell, L. L., & Kimmel, H. L. (2008). Monoamine transporters and psychostimulant addiction. Biochemical Pharmacology, 75(1), 196–217.
Huestis, M. A., Boyd, S. J., Heishman, S. J., Preston, K. L., Bonnet, D. L., Fur, G., et al. (2007). Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology, 194(4), 505–515.
Hunault, C. C., Mensinga, T. T., de Vries, I., Kelholt-Dijkman, H. H., Hoek, J., Kruidenier, M., et al. (2008). Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology, 201(2), 171–181.
Janes, A. C., Pizzagalli, D. A., Richardt, S., deB Frederick, B., Chuzi, S., Pachas, G., et al. (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry, 67(8), 722–729.
Jia, Z., Worhunsky, P. D., Carroll, K. M., Rounsaville, B. J., Stevens, M. C., Pearlson, G. D., et al. (2011). An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biological Psychiatry, 70(6), 553–560.
Jorenby, D. E., Hays, J. T., Rigotti, N. A., Azoulay, S., Watsky, E. J., Williams, K. E., et al. (2006). Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA: The Journal of the American Medical Association, 296(1), 56–63.
Kaufman, J. N., Ross, T. J., Stein, E. A., & Garavan, H. (2003). Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience, 23(21), 7839–7843.
Klink, R., de Kerchove d’Exaerde, A., Zoli, M., & Changeux, J. P. (2001). Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience, 21(5), 1452–1463.
Knutson, B., Adams, C. M., Fong, G. W., & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159.
Koob, G. F. (2004). A role for GABA mechanisms in the motivational effects of alcohol. Biochemical Pharmacology, 68(8), 1515–1525.
Koob, G. F., & Le Moal, M. (1997). Drug abuse: Hedonic homeostatic dysregulation. Science, 278(5335), 52–58.
Koob, G. F., & Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238.
Koob, G. F., Ahmed, S. H., Boutrel, B., Chen, S. A., Kenny, P. J., Markou, A., et al. (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neuroscience and Biobehavioral Reviews, 27(8), 739–749.
Kosowski, A. R., & Liljequist, S. (2005). Behavioural sensitization to nicotine precedes the onset of nicotine-conditioned locomotor stimulation. Behavioural Brain Research, 156(1), 11–17.
Kruzich, P. J., & See, R. E. (2001). Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. Journal of Neuroscience, 21(14), RC155.
Kruzich, P. J., Congleton, K. M., & See, R. E. (2001). Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behavioral Neuroscience, 115(5), 1086–1092.
Kubler, A., Murphy, K., & Garavan, H. (2005). Cocaine dependence and attention switching within and between verbal and visuospatial working memory. European Journal of Neuroscience, 21(7), 1984–1992.
Kuhar, M. J., Ritz, M. C., & Boja, J. W. (1991). The dopamine hypothesis of the reinforcing properties of cocaine. Trends in Neurosciences, 14(7), 299–302.
Langleben, D. D., Ruparel, K., Elman, I., Busch-Winokur, S., Pratiwadi, R., Loughead, J., et al. (2008). Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. The American Journal of Psychiatry, 165(3), 390–394.
Lee, B., London, E. D., Poldrack, R. A., Farahi, J., Nacca, A., Monterosso, J. R., et al. (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience, 29(47), 14734–14740.
Leroy, C., Karila, L., Martinot, J. L., Lukasiewicz, M., Duchesnay, E., Comtat, C., et al. (2011). Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: A high-resolution PET study. Addiction Biology, 17(6):981–90.
Lingford-Hughes, A. R., Acton, P. D., Gacinovic, S., Suckling, J., Busatto, G. F., Boddington, S. J. A., Bullmore, E., Woodruff, P. W., Costa, D. C., Pilowsky, L. S., Ell, P. J., Marshall, E. J., & Kerwin, R. W. (1998). Reduced levels of the GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. The British Journal of Psychiatry, 173, 116–122.
Lingford-Hughes, A. R., Wilson, S. J., Cunningham, V. J., Feeney, A., Stevenson, B., Brooks, D. J., & Nutt, D. J. (2005). GABA-benzodiazepine receptor function in alcohol dependence: A combined 11C-flumazenil PET and pharmacodynamic study. Psychopharmacology, 180, 595–606.
Lingford-Hughes, A. R., Watson, B., Kalk, N., & Reid, A. (2010). Neuropharmacology of addiction and how it informs treatment. British Medical Bulletin, 96, 93–110.
Lingford-Hughes, A., Welch, S., Peters, L., Nutt, D. on behalf of expert group. (2012a). Evidence-based guidelines for the pharmacological management of substance misuse, addiction and comorbidity: Recommendations from BAP. Journal of Psychopharmacology, 26(7), 899–952.
Lingford-Hughes, A. R., Reid, A. G., Myers, J., Feeney, A., Hammers, A., Taylor, L. G., Rosso, L., Turkheimer, F., Brooks, D. J., Grasby, P., & Nutt, D. J. (2012b). A [11C]Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced a5 benzodiazepine receptors in limbic regions. Journal of Psychopharmacology, 26(2), 273–281.
Lippiello, P., Letchworth, S. R., Gatto, G. J., Traina, V. M., & Bencherif, M. (2006). Ispronicline: A novel alpha4beta2 nicotinic acetylcholine receptor-selective agonist with cognition-enhancing and neuroprotective properties. Journal of Molecular Neuroscience, 30(1–2), 19–20.
Luo, S., Ainslie, G., Giragosian, L., & Monterosso, J. R. (2011). Striatal hyposensitivity to delayed rewards among cigarette smokers. Drug and Alcohol Dependence, 116(1–3), 18–23.
Mameli-Engvall, M., Evrard, A., Pons, S., Maskos, U., Svensson, T. H., Changeux, J. P., et al. (2006). Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron, 50(6), 911–921.
Mansvelder, H. D., & McGehee, D. S. (2002). Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology, 53(4), 606–617.
Martinez, D., Gil, R., Slifstein, M., Hwang, D. R., Huang, Y., Perez, A., et al. (2005). Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological Psychiatry, 58(10), 779–786.
Martinez, D., Narendran, R., Foltin, R. W., Slifstein, M., Hwang, D. R., Broft, A., et al. (2007). Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. The American Journal of Psychiatry, 164(4), 622–629.
Martinez, D., Greene, K., Broft, A., Kumar, D., Liu, F., Narendran, R., et al. (2009). Lower level of endogenous dopamine in patients with cocaine dependence: Findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. The American Journal of Psychiatry, 166(10), 1170–1177.
Martinez, D., Saccone, P. A., Liu, F., Slifstein, M., Orlowska, D., Grassetti, A., et al. (2011). Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: Commonalities and differences with other types of addiction. Biological Psychiatry, 71(3):192–8.
Masse, L. C., & Tremblay, R. E. (1997). Behavior of boys in kindergarten and the onset of substance use during adolescence. Archives of General Psychiatry, 54(1), 62–68.
Mathers, C. D., & Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine, 3(11), e442.
Matthews, S. C., Simmons, A. N., Lane, S. D., & Paulus, M. P. (2004). Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport, 15(13), 2123–2127.
McClernon, F. J., Hiott, F. B., Liu, J., Salley, A. N., Behm, F. M., & Rose, J. E. (2007). Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: Results of a preliminary functional magnetic resonance imaging study. Addiction Biology, 12(3–4), 503–512.
Mei, W., Zhang, J. X., & Xiao, Z. (2010). Acute effects of sublingual buprenorphine on brain responses to heroin-related cues in early-abstinent heroin addicts: An uncontrolled trial. Neuroscience, 170(3), 808–815.
Moeller, F. G., Steinberg, J. L., Schmitz, J. M., Ma, L., Liu, S., Kjome, K. L., et al. (2010). Working memory fMRI activation in cocaine-dependent subjects: Association with treatment response. Psychiatry Research, 181(3), 174–182.
Monterosso, J. R., Ainslie, G., Xu, J., Cordova, X., Domier, C. P., & London, E. D. (2007). Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping, 28(5), 383–393.
Muraven, M. (2010). Practicing self-control lowers the risk of smoking lapse. Psychology of Addictive Behaviors, 24(3), 446–452.
Myers, M. G., Brown, S. A., & Mott, M. A. (1995). Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcoholism, Clinical and Experimental Research, 19(6), 1528–1536.
Myrick, H., Anton, R. F., Li, X., Henderson, S., Randall, P. K., & Voronin, K. (2008). Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Archives of General Psychiatry, 65(4), 466–475.
Myrick, H., Li, X., Randall, P. K., Henderson, S., Voronin, K., & Anton, R. F. (2010). The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. Journal of Clinical Psychopharmacology, 30(4), 365–372.
Naqvi, N. H., & Bechara, A. (2008). The hidden island of addiction: The insula. Trends in Neurosciences, 32(1):56–67.
Nestor, L., Hester, R., & Garavan, H. (2010). Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage, 49(1), 1133–1143.
Nestor, L., McCabe, E., Jones, J., Clancy, L., & Garavan, H. (2011a). Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: Evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. NeuroImage, 56(4), 2258–2275.
Nestor, L. J., Ghahremani, D. G., Monterosso, J., & London, E. D. (2011b). Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Research, 194(3), 287–295.
Nutt, D. J., & Lingford-Hughes, A. R. (2008). Addiction: The clinical interface. British Journal of Pharmacology, 154(2), 397–405.
Olds, J., & Milner, P. (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of Comparative and Physiological Psychology, 47(6), 419–427.
Pastor, V., Andres, M. E., & Bernabeu, R. O. (2012). The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology (Berlin), 226(3):551–60.
Patterson, F., Jepson, C., Strasser, A. A., Loughead, J., Perkins, K. A., Gur, R. C., et al. (2009). Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry, 65(2), 144–149.
Paulus, M. P., Hozack, N., Frank, L., Brown, G. G., & Schuckit, M. A. (2003). Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry, 53(1), 65–74.
Paulus, M. P., Tapert, S. F., & Schuckit, M. A. (2005). Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry, 62(7), 761–768.
Peters, J., Bromberg, U., Schneider, S., Brassen, S., Menz, M., Banaschewski, T., et al. (2011). Lower ventral striatal activation during reward anticipation in adolescent smokers. The American Journal of Psychiatry, 168(5), 540–549.
Peto, R., Lopez, A. D., Boreham, J., Thun, M., Heath, C., Jr., & Doll, R. (1996). Mortality from smoking worldwide. British Medical Bulletin, 52(1), 12–21.
Reynolds, B., & Fields, S. (2011). Delay discounting by adolescents experimenting with cigarette smoking. Addiction, 107(2):417–24.
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., & Nieuwenhuis, S. (2004a). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443–447.
Ridderinkhof, K. R., van den Wildenberg, W. P., Segalowitz, S. J., & Carter, C. S. (2004b). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56(2), 129–140.
Robinson, T. E., & Berridge, K. C. (2000). The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction, 95(Suppl 2), S91–S117.
Robles, E., Huang, B. E., Simpson, P. M., & McMillan, D. E. (2011). Delay discounting, impulsiveness, and addiction severity in opioid-dependent patients. Journal of Substance Abuse Treatment, 41(4), 354–362.
Rudnick, G., & Clark, J. (1993). From synapse to vesicle: The reuptake and storage of biogenic amine neurotransmitters. Biochimica et Biophysica Acta, 1144(3), 249–263.
Schoenmakers, T. M., de Bruin, M., Lux, I. F., Goertz, A. G., Van Kerkhof, D. H., & Wiers, R. W. (2010). Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug and Alcohol Dependence, 109(1–3), 30–36.
Schott, B. H., Minuzzi, L., Krebs, R. M., Elmenhorst, D., Lang, M., Winz, O. H., et al. (2008). Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. Journal of Neuroscience, 28(52), 14311–14319.
Sevy, S., Smith, G. S., Ma, Y., Dhawan, V., Chaly, T., Kingsley, P. B., et al. (2008). Cerebral glucose metabolism and D(2)/D (3) receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology, 197(4), 549–556.
Shaham, Y., Shalev, U., Lu, L., De Wit, H., & Stewart, J. (2003). The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology, 168(1–2), 3–20.
Shoptaw, S., Heinzerling, K. G., Rotheram-Fuller, E., Kao, U. H., Wang, P. C., Bholat, M. A., et al. (2008). Bupropion hydrochloride versus placebo, in combination with cognitive behavioral therapy, for the treatment of cocaine abuse/dependence. Journal of Addictive Diseases, 27(1), 13–23.
Sieghart, W. (2006). Structure, pharmacology, and function of GABAA receptor subtypes. Advances in Pharmacology, 54, 231–263.
Sinha, R., & Li, C. S. (2007). Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug and Alcohol Review, 26(1), 25–31.
Solomon, R. L., & Corbit, J. D. (1974). An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review, 81(2), 119–145.
Sora, I., Li, B., Fumushima, S., Fukui, A., Arime, Y., Kasahara, Y., et al. (2009). Monoamine transporter as a target molecule for psychostimulants. International Review of Neurobiology, 85, 29–33.
Sticht, M., Mitsubata, J., Tucci, M., & Leri, F. (2010). Reacquisition of heroin and cocaine place preference involves a memory consolidation process sensitive to systemic and intra-ventral tegmental area naloxone. Neurobiology of Learning and Memory, 93(2), 248–260.
Stokes, P. R., Mehta, M. A., Curran, H. V., Breen, G., & Grasby, P. M. (2009). Can recreational doses of THC produce significant dopamine release in the human striatum? NeuroImage, 48(1), 186–190.
Stokes, P. R. A., Egerton, A., Watson, B., Reid, A., Lappin, J., Nutt, D., & Lingford-Hughes, A. (2012). History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. Journal of Psychopharmacology, 26(1), 144–149.
Sullivan, J. M., Risacher, S. L., Normandin, M. D., Yoder, K. K., Froehlich, J. C., & Morris, E. D. (2011). Imaging of alcohol-induced dopamine release in rats: Preliminary findings with [(11) C]raclopride PET. Synapse, 65(9), 929–937.
Tang, A., George, M. A., Randall, J. A., & Gonzales, R. A. (2003). Ethanol increases extracellular dopamine concentration in the ventral striatum in C57BL/6 mice. Alcoholism, Clinical and Experimental Research, 27(7), 1083–1089.
Thorn, D. A., Winter, J. C., & Li, J. X. (2012). Agmatine attenuates methamphetamine-induced conditioned place preference in rats. European Journal of Pharmacology, 680(1–3), 69–72.
Tomasi, D., Goldstein, R. Z., Telang, F., Maloney, T., Alia-Klein, N., Caparelli, E. C., et al. (2007). Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Research, 1171, 83–92.
Tsou, K., Brown, S., Sanudo-Pena, M. C., Mackie, K., & Walker, J. M. (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience, 83(2), 393–411.
Ullsperger, M., & von Cramon, D. Y. (2001). Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage, 14(6), 1387–1401.
van Hell, H. H., Vink, M., Ossewaarde, L., Jager, G., Kahn, R. S., & Ramsey, N. F. (2010). Chronic effects of cannabis use on the human reward system: An fMRI study. European Neuropsychopharmacology, 20(3), 153–163.
Vanderschuren, L. J., & Kalivas, P. W. (2000). Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology, 151(2–3), 99–120.
Verdejo-Garcia, A., & Bechara, A. (2009). A somatic marker theory of addiction. Neuropharmacology, 56(Suppl 1), 48–62.
Verney, S. P., Brown, G. G., Frank, L., & Paulus, M. P. (2003). Error-rate-related caudate and parietal cortex activation during decision making. Neuroreport, 14(7), 923–928.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Hitzemann, R., Ding, Y. S., et al. (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, Clinical and Experimental Research, 20(9), 1594–1598.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Hitzemann, R., et al. (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature, 386(6627), 830–833.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Wong, C., et al. (1999). Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. Journal of Pharmacology and Experimental Therapeutics, 291(1), 409–415.
Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Ding, Y. S., Sedler, M., et al. (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. The American Journal of Psychiatry, 158(12), 2015–2021.
Volkow, N., Fowler, J., Wang, G., & Goldstein, R. (2002a). Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiology of Learning and Memory, 78(3), 610–624.
Volkow, N. D., Wang, G. J., Maynard, L., Fowler, J. S., Jayne, B., Telang, F., et al. (2002b). Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: A preliminary study. Psychiatry Research, 116(3), 163–172.
Volkow, N. D., Fowler, J. S., Wang, G. J., & Swanson, J. M. (2004). Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Molecular Psychiatry, 9(6), 557–569.
Volkow, N. D., Wang, G. J., Begleiter, H., Porjesz, B., Fowler, J. S., Telang, F., et al. (2006a). High levels of dopamine D2 receptors in unaffected members of alcoholic families: Possible protective factors. Archives of General Psychiatry, 63(9), 999–1008.
Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., Childress, A. R., et al. (2006b). Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. Journal of Neuroscience, 26(24), 6583–6588.
Volkow, N. D., Wang, G. J., Telang, F., Fowler, J. S., Logan, J., Jayne, M., et al. (2007). Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. Journal of Neuroscience, 27(46), 12700–12706.
Vollstadt-Klein, S., Wichert, S., Rabinstein, J., Buhler, M., Klein, O., Ende, G., et al. (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105(10), 1741–1749.
Weerts, E. M., Wand, G. S., Kuwabara, H., Munro, C. A., Dannals, R. F., Hilton, J., et al. (2011). Positron emission tomography imaging of mu- and delta-opioid receptor binding in alcohol-dependent and healthy control subjects. Alcoholism, Clinical and Experimental Research, 35(12), 2162–2173.
Weiss, F., Maldonado-Vlaar, C. S., Parsons, L. H., Kerr, T. M., Smith, D. L., & Ben-Shahar, O. (2000). Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America, 97(8), 4321–4326.
Wexler, B. E., Gottschalk, C. H., Fulbright, R. K., Prohovnik, I., Lacadie, C. M., Rounsaville, B. J., et al. (2001). Functional magnetic resonance imaging of cocaine craving. The American Journal of Psychiatry, 158(1), 86–95.
Williams, T. M., Daglish, M. R. C., Lingford-Hughes, A. R., Taylor, L. G., Hammers, A., Brooks, D. J., Grasby, P. G., Myles, J. S., & Nutt, D. J. (2007). Increased availability of opioid receptors in early abstinence from opioid dependence: A [11C]diprenorphine PET study. The British Journal of Psychiatry, 191(1), 63–69.
Williams, T. M., Davies, S. J., Taylor, L. G., Daglish, M. R., Hammers, A., Brooks, D. J., Nutt, D. J., & Lingford-Hughes, A. (2009). Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: An [(11)C]diprenorphine PET study. European Neuropsychopharmacology, 19(10), 740–748.
Wise, R. A. (1996). Neurobiology of addiction. Current Opinion in Neurobiology, 6(2), 243–251.
Wise, R. A. (2002). Brain reward circuitry: Insights from unsensed incentives. Neuron, 36(2), 229–240.
Wise, R. A. (2005). Forebrain substrates of reward and motivation. The Journal of Comparative Neurology, 493(1), 115–121.
Wooltorton, J. R., Pidoplichko, V. I., Broide, R. S., & Dani, J. A. (2003). Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. Journal of Neuroscience, 23(8), 3176–3185.
Woolverton, W. L., & Johnson, K. M. (1992). Neurobiology of cocaine abuse. Trends in Pharmacological Sciences, 13(5), 193–200.
Wrase, J., Schlagenhauf, F., Kienast, T., Wustenberg, T., Bermpohl, F., Kahnt, T., et al. (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage, 35(2), 787–794.
Yahyavi-Firouz-Abadi, N., & See, R. E. (2009). Anti-relapse medications: Preclinical models for drug addiction treatment. Pharmacology & Therapeutics, 124(2), 235–247.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Lingford-Hughes, A., Nestor, L. (2015). Neuroscience Perspectives on Addiction: Overview. In: Clausen, J., Levy, N. (eds) Handbook of Neuroethics. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4707-4_68
Download citation
DOI: https://doi.org/10.1007/978-94-007-4707-4_68
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4706-7
Online ISBN: 978-94-007-4707-4
eBook Packages: Humanities, Social Sciences and LawReference Module Humanities and Social SciencesReference Module Humanities