Abstract
-
1.
The term “non-indigenous species ” (NIS) represents a biogeographical category, which indicates human involvement in the introduction of a certain species to a particular ecosystem and has nothing to do with putting “good” or “bad” tags on these species.
-
2.
A biological invasion is the spread of a NIS or a cryptogenic species (of uncertain or unknown origin) to an area where it did not previously occur.
-
3.
About 130 NIS and cryptogenic species have been introduced to the Baltic Sea Area by anthropogenic activities.
-
4.
Most NIS have arrived to the Baltic Sea during recent decades due to intensification of global trade, human mobility and removal of custom barriers, although the first introductions are thought to have taken place already centuries ago.
-
5.
The NIS in the Baltic Sea mainly originate from the coastal waters of three source areas (the North American east coast , the Ponto-Caspian region and East Asia), which are connected to the Baltic Sea by a number of introduction pathways, such as shipping and human-made canals.
-
6.
In the Baltic Sea, NIS are represented by many taxonomic groups, from unicellular plankton organisms to crustaceans, molluscs , fish, waterbirds and mammals.
-
7.
Many of the NIS in the Baltic Sea have increased functional diversity, bringing new and unusual functions to the species-poor Baltic Sea ecosystem .
-
8.
Some NIS may spread, highly increase in abundance and cause an adverse impact on biological diversity, ecosystem functioning, socio-economic values and/or human health. These NIS are called “invasive alien species ”.
-
9.
As it cannot be predicted which NIS will become invasive and cause harm in a particular ecosystem, a precautionary approach, preventing the arrival of new NIS in general, is advisable.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Biodiversity
- Functional diversity
- Human-mediated introductions
- Invasive alien species
- Non-indigenous species
1 Who is “alien” and who is not?
1.1 Non-indigenous species and cryptogenic species
Generally, the term “non-indigenous species ” (NIS) is used for a species that through human interference has been moved from its native dispersal range to a new area (Box 5.1). Synonyms used for NIS are “alien”, “exotic”, “non-native ”, “allochthonous ” and “introduced” species. However, the true native area of a species may be uncertain or unknown, especially for unicellular organisms, and therefore they cannot be classified as either indigenous or non-indigenous. Such species are called “cryptogenic species ” (Carlton 1996).
Box 5.1: Key definitions in invasion biology
Non-indigenous species (NIS) is the preferred term used for species, subspecies or lower taxa (such as a variety or form), which are introduced outside of their past or present natural range and outside of their natural dispersal potential (Olenin et al. 2010). This includes any propagule of a NIS, such as a gamete , seed or resting stage , a gravid female, a pair of individuals of different sexes (in species with sexual reproduction ) or a vegetative reproductive organ or section of tissue (in species with asexual reproduction ), which might survive, reproduce and subsequently form a population. NIS also include hybrids between non-indigenous and native species , fertile polyploid organisms and artificially hybridised species, irrespective of their natural range or dispersal potential. The presence of a NIS in a given region is always due to intentional or unintentional introduction resulting from anthropogenic activities . Natural shifts in distributional ranges (e.g. due to climate change or dispersal by ocean currents ) do not qualify a species to be a NIS. However, secondary spread of NIS from the area(s) of their first arrival may occur without human involvement due to spread by natural means.
Cryptogenic species are species of uncertain or unknown origin, i.e. it cannot be reliably demonstrated if they are introduced or native (Carlton 1996). For example, the true origin of a species may remain obscure because of insufficient taxonomic knowledge or due to a lack of records from the time before they were possibly introduced to a certain area. Unicellular organisms with wide global distributions are especially often considered as cryptogenic.
Biological invasion is the spread of a NIS or a cryptogenic species to an area where it did not previously occur.
Invasive alien species (IAS) is a commonly accepted term to indicate a subset of established NIS and/or cryptogenic species, which have spread, are spreading or have demonstrated their potential to spread elsewhere, and have an adverse impact on biological diversity, ecosystem functioning, socio-economic values and/or human health in the invaded region (Olenin et al. 2010). Sometimes the term “invasive species” is used as a synonym to all NIS. This is not correct, because only a small part of NIS may actually reach high abundances and cause harm. Also, the term IAS should not be used to indicate native species , which can reach high abundances and thereby cause the same type of adverse effects as IAS. Such native species are included in the definitions of “pests ” and “outbreak-forming species”.
Biological pollution is the adverse effects of IAS on the quality of the environment by impacts at several levels of biological organisation: an individual organism (e.g. internal biological pollution by parasites or pathogens ), a population (e.g. hybridisation of native species with IAS or shifts in size/age structure due to predation by an IAS), a community (e.g. structural shifts such as replacement or total elimination of native species by IAS), a habitat (e.g. modification of physico-chemical conditions by IAS) or an ecosystem (e.g. changes in energy and organic matter flows caused by IAS). Commonly used synonyms of biological pollution are “biopollution ”, “biological invasion impact” and “bioinvasion impact”. Biological pollution may also cause economic losses and impacts on human health.
Pests are harmful native, cryptogenic or non-indigenous species living in places where they are unwanted and have an adverse impact on biological diversity, ecosystem functioning , socio-economic values and/or human health in the invaded regions.
Outbreak-forming species (OFS) are native, cryptogenic or non-indigenous species with pulse-like, short-term (days to a few months) exponential population growth , during which they have an adverse impact on biological diversity, ecosystem functioning, socio-economic values and/or human health in invaded regions.
Vagrant species, such as fish or planktonic organisms with a high dispersal capacity , may spread to areas outside their normal reproductive range by natural phenomena such as currents. This can even happen on a continuous basis and such species have often been mistakenly depicted as NIS. Moreover, climate change alters species distributions (Parmesan and Yohe 2003). However, fluctuations in distributional ranges due to gradual changes in temperature or ocean currents do not qualify a species to be a NIS either.
About 130 NIS and cryptogenic species have been introduced to the Baltic Sea Area by anthropogenic activities . The list of Baltic Sea NIS changes continuously. Mostly species are added when new introductions occur, but sometimes species are deleted from the list as well, e.g. when it appears that a species has been misidentified. The AquaNIS information system always provides the latest updated list of NIS in the Baltic Sea (AquaNIS 2015).
1.2 Biological invasions and invasive species
Biological invasions (bioinvasions ), in the broad sense, are the movements of organisms to areas where they did not previously occur. This includes natural slow gradual spread and natural rapid expansion due to unusual geological or climatic events. Such invasions took place well before anthropogenic activities began to play any notable role in changing the biogeography of the marine realm (cf. Sect. 4.6.2).
However, the strong increase of biological invasions in recent decades is almost entirely caused by the spread of NIS due to anthropogenic activities . This is why the term “biological invasions” is increasingly used to designate the spread of NIS and cryptogenic species to areas where they did not previously occur, and this is also how we use the term here (Box 5.1). The term “invasive alien species ” (IAS) is reserved for non-indigenous and cryptogenic species that have an adverse effect on biological diversity, ecosystem functioning , socio-economic values and/or human health .
Invasion biology is a complex interdisciplinary scientific research area, which involves both fundamental and applied aspects (Fig. 5.1). Biological invasions offer a unique opportunity to study fundamental processes in population, community, ecosystem and evolution across taxonomic groups . Many ecological disciplines perform bioinvasion studies. Population ecologists investigate the dynamics of NIS populations, their arrival, establishment, expansion and/or decrease. Community ecologists study the interactions between NIS and long-time residents, including the effects of the invaders on the diversity of communities and the responses of communities to the invaders. These interactions have implications for invasion resistance and ecosystem resilience. Invasions are put in a societal context in more applied disciplines like conservation biology, restoration ecology and pest management .
The Ponto-Caspian zebra mussel Dreissena polymorpha is a notorious ecosystem engineer, capable of forming dense aggregates and mussel beds . It only needs a small patch of stable substrate to form an attached aggregate. The new habitat created by Dreissena polymorpha serves as an “island” of high biodiversity on the otherwise rather monotonous soft bottoms in low-salinity areas of the southeastern Baltic Sea and facilitates the establishment of other species. Photo: © Sergej Olenin
The consecutive stages in a human-mediated primary introduction of a non-indigenous species (NIS), including direct dispersal from its native source area by crossing an environmental barrier with the help of a human-mediated vector , and its subsequent arrival and establishment in the recipient area, possibly followed by a secondary spread to other areas. In principle, a secondary spread follows the same stages as the primary introduction, but pathways may be human-mediated vectors and/or natural processes. (a) The dispersal of propagules from the source area to secondary spread. (b) The relative development of the population size during the different stages of (a). Population size usually declines during transportation, but during the expansion phase it is often (temporarily) higher in the recipient area than in the source area. During the adjustment phase three different scenarios are possible: 1 = the population size remains high, 2 = the population size declines to a lower level after which it remains more-or-less stable, 3 = the population becomes extinct. Figure modified from Olenin et al. (2011)
The bivalve mollusc Crassostrea gigas (Pacific oyster ) is a native species in estuarine and coastal marine waters in Japan and Southeast Asia . (a) Seven individuals, showing the sharp edges of the shells. (b) Open shells, showing the oyster’s soft body parts, ready to eat. Crassostrea gigas has been introduced throughout the world for use in aquaculture because it grows fast and tolerates a wide range of environmental variation. In the Baltic Sea Area, this oyster is only found in the westernmost Arkona Sea , the Belt Sea and the Kattegat. Photo: © Pauline Snoeijs-Leijonmalm
The oval shells of the North American common slipper shell Crepidula fornicata are up to 5 cm long and commonly build curved chains of up to 12 animals. The species is usually found attached to shells and stones on soft substrates in the upper littoral zone. Crepidula fornicata was first observed in Europe in 1872 on the west coast of Great Britain. In the Baltic Sea Area, it has only been found in the Belt Sea and the Kattegat, to where it probably arrived as a secondary spread from the North Sea. Photo: © Sergej Olenin
Introduction and secondary spread of non-indigenous species in the Baltic Sea: numbers indicate the year of the first record and shaded sea areas show the potential directions of spread with the darkest colour indicating the primary recipient area. (a) Secondary spread of the planktonic cladoceran Cercopagis pengoi from the Gulf of Finland and the Gulf of Riga from 1992 to 2004. (b) Gradual incursion of the planktonic dinoflagellate Prorocentrum cordatum from the North Sea into the inner parts of the Baltic Sea from 1979 to 1999. (c) Secondary spread of the round goby Neogobius melanostomus from the Gdańsk Bay between 1990 and 2005. (d) Intentional introduction of the Ponto-Caspian mysid Hemimysis anomala into the Curonian Lagoon and its secondary spread to other coastal regions from 1962 to 2002. Figure: © Anastasija Zaiko
Cumulative numbers of non-indigenous species (NIS) and cryptogenic species recorded in the North Sea and the Baltic Sea between 1900 and 2009. Figure modified from Olenin et al. (2014)
Interactions of various introduction vectors in estuarine and coastal areas: 1= Arrival of NIS with shipping. 2 = Range expansion through canal systems. 3 = Transfer of fouling organisms on small craft and to marina sites from sea and overland transport of boats. 4 = Stocking of organisms to provide leisure pursuits or for fisheries management , 5 = Releases from aquaria or from water ponds. 6 = Releases of organisms intended as live food, 7 = Releases by anglers or from their equipment. 8 = Aquaculture escapees. 9 = Discharges of wastes following processing, 10 = Movements associated with fishing gear or discards. Figure based on vector data in Minchin et al. (2006). Figure: © Vitalija Gasiunaite
Examples of the shipping pathway of non-indigenous species . (a) Ballast water release in a harbour. (b) Ballast water overflow on deck. (c) Ballast water release in a dock at night. (d) Sediment in a ballast water tank after release of the water. (e) Collection of organisms from a ship’s hull (bow propeller). Photo: © Stephan Gollasch
Map showing the positions of the six major human-made canals (1–6) that interconnect natural inland waterways and thereby link the Baltic Sea with other marine and estuarine regions: 1 = the Kiel Canal, 2 = the Dnepr -Bug Canal, 3 = the Dnepr-Nemunas Canal , 4 = the Volga-Baltic Waterway , 5 = the White Sea Canal , 6 = the Volga-Don Canal . Figure: © Anastasija Zaiko
Adults of the bay barnacle Amphibalanus improvisus usually grow up to ~10 mm in size. This species may have arrived to the Baltic Sea from North America as adults attached to ship hulls , or may have spread secondarily as larvae by sea currents or in ballast water from the neighbouring North Sea. This species was first observed in the Baltic Sea in the 1880s. Photo: (a) © Sergej Olenin, (b) © Pauline Snoeijs-Leijonmalm
Two non-indigenous species in the Baltic Sea that originate from North America, Orconectes limosus and Amphibalanus improvisus. The freshwater spiny-cheek crayfish Orconectes limosus is up to 12 cm long and was actively introduced to Germany for aquaculture in 1890. It has established in freshwaters in Europe, as well as in the Baltic Sea (Leppäkoski and Olenin 2000; Jaszczołt and Szaniawska 2011). In this photograph, Orconectes limosus is covered by the brackish-water bay barnacle Amphibalanus improvisus (cf. Fig. 5.12). Photo: © Sergej Olenin
Native areas of the non-indigenous species (NIS) established in the Baltic Sea Area by 2010. The proportion of Ponto-Caspian NIS is larger in the eastern Baltic Sea while NIS originating from the North Amerian East Coast and East Asia dominate in the western part of the Baltic Sea Area. The category “Other” includes species from other parts of the world, as well as cryptogenic species . Figure: © Anastasija Zaiko
The white-fingered mud crab Rhithropanopeus harrisii has a carapace up to 2 cm wide. The native area of this small brackish-water crab is the east coast of North America . It shows high fecundity , a long planktonic larval period, and a wide tolerance range for several environmental drivers , which has likely facilitated its invasion success. It was first observed in Europe in 1874 and in the Baltic Sea in 1951 (in Poland). Rhithropanopeus harrisii has been reported from coastal areas of the Baltic Sea in Germany, Poland, Lithuania , Estonia, Finland and Sweden (Hegele-Drywa and Normant 2009; Fowler et al. 2013). Photo: © Sergej Olenin
The Canada goose Branta canadensis is the only non-indigenous coastal bird species in the Baltic Sea region . Centuries ago it was intentionally introduced to Europe as an ornamental species and for hunting . Branta canadensis is a summer visitor, transit migrant and irregular winterer on Baltic Sea coasts , where it has been observed since the 1930s. Strictly speaking, this species is not a typical waterbird as it prefers open, grassy habitats where it feeds on grasses, herbs, and plant roots , but it can also feed on aquatic plants. The Canada goose can hybridise with native species and is considered a sanitary problem at e.g. bathing sites. Photo: © Sergej Olenin
Since invasion biology is a fast-developing discipline, its terminology also evolves rapidly and is influenced by a variety of sometimes contrasting academic, cultural and even political views (Elliott 2003; Carlton 2009; Olenin et al. 2010). The key definitions are centred around the opposing categories “native versus non-native”, “spreading versus non-spreading”, “impacting versus non-impacting” and “harmful versus harmless” (Box 5.1).
1.3 Continuous net immigration to the Baltic Sea
Like in other areas, the species pool of the Baltic Sea is a product of immigration and extinction . Being a young and environmentally unstable sea in a geological time perspective, the Baltic Sea has been exposed to several waves of species immigrations and extinctions during its respective geological stages (cf. Sect. 2.5). The current geological stage of the Baltic Sea is named the “Mya ” stage, after the sand gaper Mya arenaria (Box 5.2), which occurs abundantly in the Baltic Sea sandy habitats . After it was recognised that Mya arenaria is in fact a cryptogenic species , probably introduced to the Baltic Sea by humans a long time ago (Strasser 1999), the name of this geological stage seems even more appropriate; it not only illustrates a dominant species in the Baltic Sea ecosystem but also an era of human-induced changes in biodiversity.
Box 5.2: The sand gaper Mya arenaria
Invasion history
Mya arenaria (Box Fig. 5.1) is a typical cryptogenic species , which cannot reliably be ascribed as introduced or native. Originating in the Pacific Ocean ~12 million years ago (middle Miocene ), it was already present on the west and east coasts of the Atlantic Ocean in the Pliocene . However, in the eastern Atlantic Ocean it died out during the Pleistocene glaciations (Strasser 1999). From analyses of ancient kitchen middens and marine shell deposits from that period, it was concluded that Mya arenaria was not present in northwestern European coastal waters until ~500 years ago (Hessland 1946; Bernard 1979; Petersen et al. 1992, 2005). Later investigations have shown that the first shell deposits of Mya arenaria appeared on the Danish coast around the 13th century. Based on these observations, it is assumed that the species may have been transferred from the Atlantic coast of North America to Europe already before Columbus by the Vikings . Mya arenaria may have served as a food item during early ship travels crossing the Atlantic Ocean. Surprisingly, it is not exploited as a human food item in Europe, while it is highly valued as such in the USA and Canada. Nowadays, Mya arenaria inhabits the entire Baltic Sea, except for the Bothnian Bay and the eastern Gulf of Finland where salinity is too low for its survival.
Invasive traits
Mya arenaria possesses several traits that enable it to colonise new habitats, including high fecundity , pelagic larval development, rapid growth, and tolerance of a wide range of environmental conditions. An additional key feature of this species is its long life span ; individuals older than 27 years have been observed. Despite these features, Mya arenaria is unexpectedly characterised by a relatively low level of genetic polymorphism compared to other marine bivalves , both in its native and introduced range (Strasser and Barber 2009).
Biology
Mya arenaria is an infaunal species, i.e. it lives buried in sediments beneath the surface of the seafloor. After burying itself, an individual will stay in the same place for the rest of its life. During its first year the burial depth is only 5–10 cm, but >10 years old Mya arenaria can live down to ~40 cm deep in the sediment. This mode of life provides an excellent defence against predators and also against freezing during severe winters. Mya arenaria has a high filtration capacity ; one individual of 6–7 cm shell length can filtrate 1–10 L seawater h−1 (Jørgensen and Riisgård 1988; Riisgård and Seerup 2003). In the southern Baltic Sea, population filtration rates of Mya arenaria can be >8 m3 m−2 day−1 (Forster and Zettler 2004). Water passes in and out of the clam through two siphons that reach to the sediment surface (Box Fig. 5.1b). Populations of Mya arenaria often consist of adult specimens only, which suggests that long episodes without recruitment of new individuals are common. Sexual maturity usually sets in when the oval shells reach a length of 2–5 cm (Strasser 1999). Fertilisation is external and larvae are pelagic and planktotrophic for 10–35 days. Mortality is up to 90 % during the first year, and even higher after mild winters when predation pressure on newly settled clams is high. It is, however, lower after cold winters when predation pressure is lower (Beukema 1982).
Impacts in the Baltic Sea
Being an ancient invader, Mya arenaria has already passed through all invasion stages (cf. Fig. 5.3), and is so well established in the Baltic Sea ecosystem that it is difficult to identify its impacts. However, when Mya arenaria invades a new area it still shows its invasive properties. For example, a salinity increase from ~9 to ~12 in the Ringkøbing Fjord on the Danish west coast in the 1990s caused a shift in the dominating pathway of organic matter production from pelagic turnover to benthic-pelagic coupling through new recruitment and growth of Mya arenaria (Petersen et al. 2008).
Most species living at present in the Baltic Sea are post-glacial immigrants that have extended their native range from adjacent marine or freshwater regions (cf. Sect. 4.6.2). It is commonly accepted to consider these species as native to the Baltic Sea. However, there is a continuous net immigration of species into the Baltic Sea through both natural dispersal and human-mediated introduction of species, and that is why scientists sometimes call the Baltic Sea “a sea of invaders” (Leppäkoski et al. 2002a). The rate of new arrivals has greatly increased in recent decades due to the intensification of global trade, human mobility and removal of former custom barriers.
1.4 Are non-indigenous species “good” or “bad”?
Categorising species into “indigenous” and “non-indigenous” has nothing to do with putting “good” or “bad” tags on them. The term “non-indigenous” represents a biogeographical category, which indicates human involvement in the introduction of a certain species to a particular ecosystem. Most NIS do not cause harm to the biological diversity and ecosystem functioning of the Baltic Sea, nor to socio-economic values or human health associated with it, although for a large number of NIS in the Baltic Sea their impacts are still unknown (Ojaveer and Kotta 2015).
Adding a new NIS increases species richness , yet an invaded ecosystem loses its biogeographical peculiarities (Leppäkoski and Olenin 2001). From the beginning of the 19th century and up to the 1970s, intentional species introductions were in many European countries a popular measure to “improve nature” or compensate for destroyed stocks of native species (Leppäkoski et al. 2002b). Some of these acclimatisation experiments have had commercial success, e.g. by increased shellfish production, whereas others have caused devastating effects on local fauna and economic losses (Westman 2002). In recent decades our knowledge on ecosystem functioning has increased, and it is recognised that it is advisable to abstain from intentional introductions because of their often unpredictable consequences.
Since the brackish Baltic Sea is a species-poor system, at least at the level of multicellular organisms (cf. Fig. 4.10), its functional diversity is also low. Some of the NIS are known to bring new functions and increase the functional diversity of the Baltic Sea ecosystem , contributing to the circulation of nutrients and fostering the energy flow from the pelagic to the benthic system (Olenin and Leppäkoski 1999; Kotta et al. 2003; Norkko et al. 2012). However, faster turnover of nutrients does not a priori mean that an ecosystem becomes “better” and that a NIS that contributes with a new function is “good”. The same NIS can outcompete native species, release extra nutrients and/or chemical pollutants from bottom sediments into the water column, or cause economic losses (Leppäkoski 2002; Gren et al. 2009).
There are no “ultimately good or bad” species or functions. When making evaluations, we should always ask the question “good” for what or “bad” for what? In general, we should avoid judgment of NIS in a moralistic context, but rather objectively assess their impacts and role in the ecosystem on a case-by-case basis.
Finally, there are many documented examples worldwide showing that some NIS may become pests and pose serious threats to biodiversity, the economy and even human health. This is why bioinvasions remain high on the environmental conservation agenda and are the subject of intensive research. Since it cannot be predicted which NIS will become invasive and cause harm in a particular ecosystem, a precautionary approach , preventing the arrival of new NIS in general, is advisable.
1.5 Which non-indigenous species have the largest impacts?
The NIS with the largest identified impacts on the Baltic Sea ecosystem are the polychaete worms Marenzelleria spp., the zebra mussel Dreissena polymorpha , the cladoceran Cercopagis pengoi , the amphipods Gammarus tigrinus , Obesogammarus crassus and Pontogammarus robustoides and the fish Neogobius melanostomus (Zaiko et al. 2011; Ojaveer and Kotta 2015). These species were introduced to the Baltic Sea in 1975–1992, except for Dreissena polymorpha, which has been present in the Baltic Sea already for some centuries (Leppäkoski et al. 2002a).
1.6 Impacts of Marenzelleria spp. and Dreissena polymorpha
The benthic invertebrates Marenzelleria spp. (Box 5.3) and Dreissena polymorpha (Box 5.4) alter the physical habitat, nutrient cycling and trophic interactions . Marenzelleria spp. can reach densities of >30,000 individuals m−2 and a biomass of >400 g wet weight m−2 (Zettler 1996). These non-indigenous worms compete with native species , e.g. they reduce the abundance of the native polychaete Hediste diversicolor and the native amphipod Monoporeia affinis (Kotta et al. 2006). Bioturbation by dense Marenzelleria spp. populations may lead to an enhanced release of soluble nutrients and hazardous substances from the sediments to the water column (Hedman et al. 2011). On the other hand, they may also aid in the recovery of oxygen conditions on the seafloor (Norkko et al. 2012).
Box 5.3: The red-gilled mud worms Marenzelleria spp.
Invasion history
Two morphologically very similar spionid polychaetes , Marenzelleria neglecta and Marenzelleria viridis , have recently been introduced to the Baltic Sea from the east coast of North America (Box Fig. 5.2). A third species, Marenzelleria arctia , is of Arctic origin. After the first record of Marenzelleria in the southern Baltic Sea in 1985, it was thought that only one species had invaded the Baltic Sea as a secondary spread from the North Sea to where it had been introduced earlier. However, after more detailed studies, involving scanning electron microscopy and genetic analysis (Blank et al. 2008), it was revealed that two independent introduction events of two species had taken place: one to the North Sea and another one to the Baltic Sea, probably by transfer of ballast water (Bastrop et al. 1995). At the same time, Marenzelleria neglecta was identified as a new species to science in both its native and invaded range. The specific epithet “neglecta” indicates that the species has previously been misidentified and overlooked. Currently, all three Marenzelleria spp. are widely spread in the Baltic Sea, from coastal waters, estuaries and shallow bays to oxygen-deficient deep-water zones where most other macrozoobenthos species cannot survive. There are, however, differences in the distributions of the species based on differences in their salinity tolerance ranges and preferred substrate. Marenzelleria neglecta and Marenzelleria viridis may co-occur in the more saline southwestern part of the Baltic Sea, but Marenzelleria neglecta has a wider distribution at lower salinity inside the Baltic Sea. In the northern part of the Baltic Sea the distribution of Marenzelleria neglecta partly overlaps with that of the Marenzelleria arctia (Blank et al. 2008).
All three Marenzelleria species in the Baltic Sea have an elongated greenish body with rows of short chaeta along both sides, but without dorsal scales. (a) Marenzelleria neglecta. (b) Mucus-lined burrows of Marenzelleria spp. have a maximum diameter of 2 mm and can penetrate down to ~35 cm in the sediment. This activity brings oxygen to the sediments, as shown by the yellowish colour of oxygenated sediments around the middle burrow. Photo: (a) © Andrius Šiaulys, (b) © Sergej Olenin
Invasive traits
The invasion success of Marenzelleria spp. is favoured by their broad feeding strategy as they are both deposit and suspension feeders . Their dispersal potential is large since they have planktonic larvae and adult specimens are able to swim. Marenzelleria spp. also have a broad spectrum of habitat preferences and the ability to cope with low oxygen levels (Schiedek 1993; Fritzsche and von Oertzen 1995).
Biology
These infaunal polychaetes are up to 16 cm long and dwell in burrows in sediments beneath the surface of the seafloor (Box Fig. 5.2b). Typical faecal pellet strings are deposited near the openings of the burrows. Gametogenesis of Marenzelleria neglecta occurs in spring. After ~20 weeks the gametes reach maturity and the animals spawn in autumn. During spawning , the larval density near the coast can be as high as 21 million individuals m−3. The development of the pelagic larvae into juvenile benthic worms depends largely on water temperature and takes between 4 and 12 weeks. The larvae occur in the coastal water column mainly from September to November, but they can be found up to March (Bochert 1997; Sikorski and Bick 2004).
Impacts in the Baltic Sea
Marenzelleria spp. have a negative impact on the abundances of the native polychaete Hediste diversicolor and the native amphipod Monoporeia affinis (Kotta et al. 2001; Kotta and Ólafsson 2003). On the other hand, Marenzelleria spp. have positive impacts on Zostera marina , a species of high conservation value, as the worms bury the seeds of the vascular plant and thereby reduce seed predation and facilitate seed germination (Delefosse and Kristensen 2012). Since Marenzelleria spp. have high burrowing activity they improve oxygen circulation in the sediments, but burrowing may also lead to an enhanced release of nutrients and hazardous substances from the sediments into the water column (Kotta et al. 2001; Hedman et al. 2011; Norkko et al. 2012). Marenzelleria spp. have become a food source for demersal fish such as European plaice Pleuronectes platessa and European flounder Platichthys flesus (Winkler and Debus 1997).
Box 5.4: The zebra mussel Dreissena polymorpha
Invasion history
The native area of Dreissena polymorpha (Box Fig. 5.3) is the Ponto-Caspian region. It is likely that this species already occurred in the Baltic Sea Area during the last interglacial period, became extinct and re-established in the early 1800s (Buynevich et al. 2011). This re-establishment was probably related to the building of canals between rivers, which opened new inland waterways for ship traffic between eastern and central Europe in the beginning of the 19th century (cf.Fig. 5.11). Dreissena polymorpha may have been attached to timber rafts that reached the Curonian Lagoon via the Dnepr -Nemunas Canal system. The zebra mussel is today one of the most common species in the oligohaline southern and eastern coastal lagoons and inlets of the Baltic Sea (Kotta et al. 1998; Orlova et al. 2004; Daunys et al. 2006).
Invasive traits
The invasion success of Dreissena polymorpha is favoured by its high fecundity (up to 1.5 million eggs per female per year), rapid growth, a planktonic stage that is easily dispersed and wide environmental tolerances, e.g. a salinity range of 0 to ~4 and water temperature up to 29 °C. Dreissena polymorpha usually spawns in May-July and fertilised eggs give rise to veliger larvae of up to 100 μm in body size. Before the larvae form byssus and attach to a substrate, they live planktonically for 2–4 weeks and are then able to move by means of a velum (Orlova 2002).
Biology
Dreissena polymorpha attaches by its byssus to a variety of human-made and natural stable surfaces, such as rocky substrates, macrophytes, animals, garbage (Box Fig. 5.3b) and fishing gear . It can, however, also build mussel beds in soft bottom areas because it only needs a small patch of stable substrate to form an attached aggregate. The triangular-shaped shells of adult Dreissena polymorpha are ~3 cm long and have a characteristic prominent banding pattern to which the name “zebra mussel ” refers. Its specific epithet “polymorpha” is derived from the many variations in shell colour, pattern and shape depending on substrate, depth and density of aggregation.
Impacts in the Baltic Sea
Dreissena polymorpha is a notorious ecosystem engineer, capable of modifying the physical, morphological, biological and bio-geochemical properties of bottom habitats as it is able to form dense colonies and beds of living individuals and empty shells (Zaiko et al. 2009). Such modified habitats serve as “islands” of high biodiversity on the otherwise rather monotonous soft bottoms in low-salinity areas of the southeastern Baltic Sea and facilitate the establishment of other species, both native and non-indigenous. Dreissena polymorpha may overgrow native unionid bivalve s and seriously decrease the abundances of the native species in recently invaded areas. Large Dreissena polymorpha beds have a high filtration capacity. They can process huge amounts of particulate organic matter and release dissolved inorganic nutrients (Orlova et al. 2004). A positive effect of Dreissena polymorpha, owing to its efficient filtering of the water, is that it may help mitigate eutrophication, increase water transparency and ameliorate growth conditions for benthic macrophytes. Through biodeposition Dreissena polymorpha increases the density of benthic deposit feeders and the zebra mussel itself can be an important food item for some fish, crayfish and waterbirds . Economic losses caused by Dreissena polymorpha are fouling of intake pipes, ship hulls , navigational constructions and cages used in aquaculture , as well as injuries to bathers from the sharp edges of the shells (Minchin et al. 2002). As a powerful filter feeder, Dreissena polymorpha is known to bioaccumulate chemical pollutants and toxins.
Improved bottom-water oxygen conditions in the coastal areas of the northern Baltic Sea coincide with increased abundances of Marenzelleria spp., which bioturbate a relatively thick upper sediment layer. Using a reactive-transport model, Norkko et al. (2012) demonstrated that the long-term bioirrigation activities of high, but natural, abundances of Marenzelleria (>3,000 individuals m−2) lead to a substantial increase in the iron-bound phosphorus content of sediments while reducing the concentration of labile organic carbon. In contrast to short-term laboratory experiments, the model simulations, which covered a 10-year period, showed that Marenzelleria has the potential to enhance long-term phosphorus retention in muddy sediments. This may facilitate the switch from a seasonally hypoxic system (<2 mL O2 L−1) back to a normoxic system by reducing the potential for sediment-induced eutrophication in the upper water column.
Dreissena polymorpha beds (Fig. 5.2) occupy ~300 km2 in the part of the Curonian Lagoon that is directly exposed to the Nemunas river outflow, which is ~20 % of the lagoon’s total bottom area (Daunys et al. 2006). In this area, the 14–20 mm body size class of Dreissena polymorpha dominates, the density varies from only 40 up to 57,000 individuals m−2 and the biomass varies from 0.5 to 5,000 g shell-free dry weight m−2. Similarly dense Dreissena polymorpha beds occur in other places with a large freshwater input, e.g. the inner Neva Estuary (Orlova et al. 2004). The presence of this species, which contributes up to 95 % of total community biomass, has caused an essential redistribution of the native benthic communities in the Curonian Lagoon (Zaiko et al. 2011).
1.7 Impacts of Cercopagis pengoi
The Ponto-Caspian fish-hook water flea Cercopagis pengoi (Box 5.5) can fundamentally change community composition and food web structure in the pelagic zone . It adds an extra trophic level to the food web as a zooplankton predator on smaller zooplankton. Cercopagis pengoi has caused a decline in the native cladocerans Bosmina longispina maritima , Evadne nordmanni and Pleopis polyphemoides , probably by direct predation (Ojaveer et al. 2004; Kotta et al. 2006). Cercopagis pengoi itself is used as a food source by several fish species. In late summer and early autumn, it can constitute a large proportion of the diets of the major planktivorous fish species of the Baltic Sea, e.g. the sticklebacks Gasterosteus aculeatus and Pungitius pungitius , the Atlantic herring Clupea harengus and the European sprat Sprattus sprattus (Gorokhova et al. 2004; Ojaveer et al. 2004). As Cercopagis pengoi tends to attach to fishing gear and clog nets and trawls, it may cause substantial economic losses for fishermen (Leppäkoski and Olenin 2000).
Box 5.5: The fish-hook water flea Cercopagis pengoi
Invasion history
The first records of the carnivorous cladoceran Cercopagis pengoi (Box Fig. 5.4) in the Baltic Sea are from the Gulf of Finland and the Gulf of Riga in 1992 (Kotta et al. 2006). Most probably it arrived to the Baltic Sea with ships from its native area , the Ponto-Caspian region . By 2004, Cercopagis pengoi had expanded to the whole Baltic Sea proper, the Pomeranian Bay and the northern parts of the Gulf of Bothnia. The introduction of Cercopagis pengoi to the Laurentian Great Lakes in North America , where it was first recorded in 1999, was most likely a secondary spread from the Baltic Sea through shipping (Cristescu et al. 2001).
The carnivorous cladoceran Cercopagis pengoi. (a) One individual, showing the head with one large compound eye and the caudal process with a distinctive loop-like curvature at the end. (b) Aggregates of Cercopagis pengoi form cotton-like masses that can clog fishing gear. Photo: (a) © Soili Saesmaa, (b) © Teemu Lehtiniemi
Invasive traits
Cercopagis pengoi tolerates a wide salinity range (0–17) and temperature (3–38 °C). It is a generalist predator, capable of feeding on a variety of prey species of different sizes, such as small cladocerans , larvae of the bay barnacle Amphibalanus improvisus and adults of the copepods Eurytemora affinis and Acartia spp. (Laxson et al. 2003; Plichlová-Ptácniková and Vanderploeg 2009).
Biology
The most conspicuous body parts of Cercopagis pengoi (Box Fig. 5.4a) are the head with one large compound eye, a well-developed second pair of antennae, four pairs of thoracic legs with the first pair larger than the others, an abdomen, a brood pouch in females, and a caudal process (posterior extension of the body) that in the summer has a distinctive loop-like curvature at the end (Grigorovich et al. 2000; Kotta et al. 2006). The body length, without the caudal process , is larger in females (~1.2–2.0 mm) than in males (~1.1–1.4 mm). The caudal process is usually 5–7 times longer (up to ~10 mm long) than the main body, but this seems to vary regionally (Grigorovich et al. 2000). Cercopagis pengoi is a cyclic parthenogen, which mainly reproduces asexually in summer when the parthenogenetic young develop in a brood pouch that ruptures to release them (Mordukhai-Boltovskoi and Rivier 1971, 1987). In early autumn, parthenogenetic females produce eggs that develop into males and gametogenic females, which copulate. Sexual reproduction intensifies in late autumn when water temperature declines. This results in resting eggs that are released when the brood pouch ruptures and overwinter in the sediment. These eggs hatch in spring-summer when water temperature increases after winter. Resting eggs guarantee survival during unfavourable environmental conditions such as low winter temperatures (Katajisto et al. 2013). Resting eggs may also act as an effective means of dispersal for Cercopagis pengoi as they can withstand extreme conditions during transport in ballast water tanks.
Impacts in the Baltic Sea
Cercopagis pengoi is one of the most impactive invasive species in the Baltic Sea. The enormous expansion of the distribution area of Cercopagis pengoi in the Baltic Sea has caused changes in the pelagic food web and increased competition for food. In some areas of the Baltic Sea, e.g. in the Gulf of Riga , the population of the native water flea Bosmina longispina maritima has drastically decreased as a result of the Cercopagis pengoi invasion, probably by direct predation (Ojaveer et al. 2004). In spring and summer, Cercopagis pengoi competes for food with small planktivorous fish, which enhances eutrophication because of heavy predation on phytoplankton -grazing zooplankton. Aggregates of Cercopagis pengoi form cotton-like masses (Box Fig. 5.4b). Biofouling of fishing equipment by Cercopagis pengoi is a problem and the clogging of nets and trawls by the species causes substantial economic losses for fishermen (Leppäkoski and Olenin 2000).
1.8 Impacts of non-indigenous amphipods
The North American amphipod Gammarus tigrinus and the Ponto-Caspian amphipod Pontogammarus robustoides are able to fundamentally change community composition in the phytobenthic zone. Especially in the lagoons of the southeastern Baltic Sea proper and the eastern Gulf of Finland , they outcompete their native relatives Gammarus duebeni and Gammarus zaddachi (cf. Sect. 11.4.3), probably because the introduced amphipods are more versatile feeders (Orlova et al. 2006). In many places, Gammarus tigrinus completely dominates the nektobenthos, e.g. in some sections of the Wisła Lagoon , where it has replaced not only the native species , but also the previously introduced amphipod Pontogammarus robustoides (Grabowski et al. 2006).
1.9 Impacts of Neogobius melanostomus
In the Gdańsk Bay, a trophic cascade has occurred as a result of the introduction of the Ponto-Caspian round goby Neogobius melanostomus (Box 5.6). The great cormorant Phalacrocorax carbo sinensis, a top predator, has shifted its diet from the European eel Anguilla anguilla and European sprat Sprattus sprattus to Neogobius melanostomus, which caused population increases in eel and sprat. In turn, sprat feeding has reduced zooplankton biomass and the subsequent reduced zooplankton grazing on the phytoplankton has caused an increase in the phytoplankton biomass (Corkum et al. 2004). The expanding populations of Neogobius melanostomus in the coastal areas of the southeastern Baltic Sea may have also reduced the blue mussel Mytilus trossulus population, since the non-indigenous fish preys upon these mussels (Karlson et al. 2007).
Box 5.6: The round goby Neogobius melanostomus
Invasion history
The native area of Neogobius melanostomus (Box Fig. 5.5) is the Ponto-Caspian region . The first Baltic Sea individuals were caught near the tip of the Hel Peninsula (Poland) in 1990, but judging from the age of these specimens, it is likely that they have been inhabiting the Gdańsk Bay since at least 1987. Neogobius melanostomus rapidly dispersed over large areas in the Gdańsk Bay, e.g. in the surroundings of piers >350 individuals larger than 8 cm per 100 m−2 have been observed (Sapota and Skóra 2005). It was most probably brought to the Baltic Sea in ballast water as fertilised eggs or larvae. The population in the Gdańsk Bay was probably the base for the introduction of Neogobius melanostomus into other regions of the Baltic Sea. This is assumed because this fish’s range of migration is small, comprising a distance of some hundred m. The longest migrations (up to some km) take place in late autumn and early spring when parts of the population move to and from deeper waters. Currently, Neogobius melanostomus is present in all countries surrounding the Baltic Sea. Its comparatively rapid secondary spread within the Baltic Sea may have been aided by shipping. For example, in Lithuania it was first found in the harbour of Klaipėda before it spread inside the Curonian Lagoon and further along the open coast. At the Swedish coast , Neogobius melanostomus was first reported in Karlskrona in 2008, and by 2014 it spread to Öland , Gotland and the Stockholm archipelago (data: Swedish Agricultural University). Further records from the Archipelago Sea , Gulf of Finland and the coast of southern Denmark (Kornis et al. 2012) confirm that this species is becoming well-established throughout the Baltic Sea. The introduction of Neogobius melanostomus to the Laurentian Great Lakes in North America took place at about the same time as that to the Baltic Sea (Corkum et al. 2004; Sapota and Skóra 2005).
Invasive traits
The invasion success of Neogobius melanostomus is favoured by its long spawning period. It is a multiple spawner , i.e. the females release their eggs in portions throughout the reproductive season (April to September). Adults aggressively defend their spawning sites and they can thus prevent the native fish from occupying prime spawning areas . Neogobius melanostomus has a broad diet of benthic animals, ranging from chironomids , amphipods and isopods to bivalves such as Dreissena polymorpha , Macoma balthica and Mytilus trossulus (Corkum et al. 2004; Sapota 2004; Rakauskas et al. 2013).
Biology
Neogobius melanostomus can live in limnic and brackish-water environments . They are typically found near sandy, stony bottoms, marine structures, sunken objects and mussel beds . The eggs are deposited in nests guarded by males, and several females can use the same nest. The nests are built on solid substrate such as stones, rocks, wood, roots of vascular plants or human-made constructions such as piers (Tomczak and Sapota 2006). Neogobius melanostomus has a relatively large head and its pelvic fins are fused to form a suctorial disk that is used for anchoring to substrates, especially in running waters. The fish usually stays in one place with only limited repositioning with its pectoral fins (Box Fig. 5.5a). Neogobius melanostomus can be distinguished from the black goby Gobius niger , which is native to the Baltic Sea, by a distinct black spot on the first dorsal fin of the invader. Sexual dimorphism is marked in Neogobius melanostomus, with males having a larger body size with age, enlarged cheeks and darker brownish-grey colour (Kornis et al. 2012). Breeding males are black with white-edged caudal fins. The maximum length of Neogobius melanostomus in the Gdańsk Bay is ~24 cm in males and ~18 cm in females (Sokołowska and Fey 2011).
Impacts in the Baltic Sea
In the southern Baltic Sea, Neogobius melanostomus seems to have a longer life span (up to 6 years) and a larger body size with age than its Ponto-Caspian and North American conspecifics. This suggests that this invasive species has found favourable conditions and a vacant niche in the Baltic Sea, including food resources, suitable habitats and spawning grounds. Where Neogobius melanostomus is numerous it can seriously affect populations of benthic invertebrates and may outcompete native benthivorous fish such as Eurasian ruffe Gymnocephalus cernuus and European flounder Platichthys flesus (Karlson et al. 2007; Rakauskas et al. 2013). Neogobius melanostomus may also feed on eggs and fry of native fish species. Neogobius melanostomus has been shown to significantly contribute (by 7–18 %) to the diet of piscivorous fish such as zander Sander lucioperca and the European perch Perca fluviatilis and waterbirds such as the great cormorant Phalacrocorax carbo sinensis and the grey heron Ardea cinerea (Rakauskas et al. 2013). In the Ponto-Caspian region Neogobius melanostomus is commonly caught for human consumption , and even in the Baltic Sea it has begun to be commercially exploited (Box Fig. 5.5b).
2 The invasion process
2.1 Dispersal from the source area
A human -mediated biological invasion process of a NIS includes several consecutive stages (Fig. 5.3). The process starts in a source (donor) area when a species interacts with an introduction pathway (e.g. shipping). The source area of a NIS in the Baltic Sea may be the native region of the species, e.g. the Caspian Sea for Cercopagis pengoi , but it can also be an area to which it has already been introduced. Examples of the latter case are the Pacific oyster Crassostrea gigas (Fig. 5.4) and the common slipper shell Crepidula fornicata (Fig. 5.5). Both these species were first introduced from their native areas to the North Sea (primary introduction ), and from there they have later spread to the Baltic Sea (secondary introduction ).
The number of species involved in a pathway is always higher than the number of species that manage to survive transportation over an environmental barrier (Fig. 5.3). With respect to primary introductions of NIS to the Baltic Sea, the environmental barriers between the Baltic Sea and the source areas (brackish or limnic systems elsewhere) are either land masses or vast open ocean spaces. Secondary introductions of NIS to the Baltic Sea may occur through human-mediated vectors but also by natural processes such as tidal movements, alongshore drift, flooding events, turbidity currents, and transfer by wind and animals. These natural processes may also greatly facilitate the dispersal of NIS within a recipient ecosystem.
In general, there are comparatively few primary introductions recorded in the Baltic Sea. Some examples include mainly Ponto-Caspian NIS such as Cercopagis pengoi, Dreissena polymorpha and Neogobius melanostomus (Fig. 5.6). Secondary introductions from both adjacent inland waters and the North Sea have historically been, and still are, more common than primary introductions.
2.2 Propagule pressure
The potential of a species to establish a stable population in an area where it previously did not occur is called “propagule pressure ”. Propagule pressure differs from settlement or recruitment because it represents the potential for an introduction, not the realised introduction (Johnston et al. 2009). For species with sexual reproduction, a propagule may be a gamete , a seed or a resting stage , a gravid female or a pair of individuals of different sex. For species with asexual reproduction, this may be a vegetative reproductive organ or a tissue section. NIS propagules also include hybrids between non-indigenous and indigenous species, fertile polyploid organisms and artificially hybridised species (Box 5.1).
The propagule pressure of a NIS can be calculated as the number of its propagules released into a region that they are not able to reach naturally (i.e. without transport by humans), multiplied by the number of discrete release events. When the number of propagules or the number of releases increases, the propagule pressure also increases. Thus, species that are constantly being introduced in large quantities are more likely to survive in the recipient area, provided they tolerate the environmental conditions in this area, and can utilise the habitats and energy resources present, whereas species introduced in small numbers with only one or a few release events are more likely not to establish.
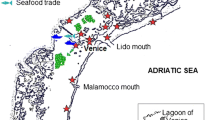
However, one introduction on one occasion may be enough for a NIS to establish in the recipient area. There is a well-documented case of such a single introduction event from the Mediterranean Sea , where the green alga Caulerpa taxifolia began to spread rapidly after release from the Monaco aquarium (Jousson et al. 1998). For the Baltic Sea, such evident cases are not known with certainty, but several invasions may have started from single-event introductions. For example, the wedge clam Rangia cuneata (Box 5.7) was most likely transferred from the Belgian or Dutch waters of the North Sea by Dutch ladder-dredge boats, which were dredging the waterway of the harbour of Kaliningrad in 2008, although transfer by ship ballast water cannot be ruled out completely (Rudinskaya and Gusev 2012).
Box 5.7: The North American wedge clam Rangia cuneata
Invasion history
One of the most recent newcomers to the eastern Baltic Sea coast , Rangia cuneata (Box Fig. 5.6), is considered to be native to the Gulf of Mexico (North America ), where it mainly occurs in brackish-water estuaries (Wakida-Kusunoke and MacKenzie 2004; Verween et al. 2006). In the Baltic Sea, Rangia cuneata was first recorded in the Russian part of the Wisła Lagoon in 2010 (Rudinskaya and Gusev 2012). Its pathway to the Wisła Lagoon was attributed to ladder-dredge boats, which operated in the sea channel of the harbour of Kaliningrad in 2008, from areas where the species had been introduced earlier (e.g. the harbour of Antwerpen, Belgium). There are indications that Rangia cuneata has begun to spread to adjacent Polish and Lithuanian coastal waters (Warzocha and Drgas 2013).
Shell of Rangia cuneata found in the Wisła Lagoon where recorded shell length was up to 40 mm (Rudinskaya and Gusev 2012). Photo: © Andrey Gusev
Invasive traits
Rangia cuneata is highly tolerant to varying environmental conditions. It has high fecundity and a planktonic larval stage, which provides the clam with a good dispersal ability. Conditions that are unfavourable for many native species in the Baltic Sea, like sudden salinity fluctuations , may trigger an outburst of Rangia cuneata.
Impacts in the Baltic Sea
Although there is not enough information on ecosystem-wide impacts of Rangia cuneata, the rapid spread of this species within the Wisła Lagoon , and its ability to reach high abundances fast, suggest that it may induce adverse transformations in the local benthic communities. By modifying soft-bottom habitats and restructuring the benthic communities, Rangia cuneata might affect food webs of the coastal areas and therefore the resource availability for economically important fish species. However, in its native area Rangia cuneata is harvested for human consumption and is considered a valuable economic resource. This aspect should be taken into account when planning management and mitigation measures for Rangia cuneata in the Baltic Sea.
2.3 Arrival, establishment and expansion
The successful invasion of a NIS into a recipient area always begins with one or more incidences of arrival, followed by the establishment of a small group of reproducing individuals, which may proceed into an expansive phase (Fig. 5.3; Reise et al. 2006). During an expansion peak the impacts of a NIS on the recipient ecosystem are the strongest. Generally, the expansion of planktonic species (e.g. Cercopagis pengoi and Prorocentrum cordatum ) is more rapid and covers larger areas than the expansion of nektobenthic crustaceans (e.g. Hemimysis anomala ) or demersal fish (e.g. Neogobius melanostomus ) (Fig. 5.6).
Some species enter the expansion phase almost immediately after arrival. An example of this is Cercopagis pengoi, which was first found in 1992 in the Gulfs of Finland and Riga. It spread rapidly to the Baltic Sea proper, and by 2002 this cladoceran reached the Gulf of Bothnia, including the Bothnian Bay in the north and by 2004 also the German coast in the south (Fig. 5.6a).
Other NIS may be “sleepers” for decades and then expand when conditions become favourable. For example, the Chinese mitten crab Eriocheir sinensis (Box 5.8), which was first found in Germany ~100 years ago, was also recorded in the less saline northern Gulf of Finland in the 1930s. Altogether, 25 individuals were found in an area extending from the Archipelago Sea in the west to Vyborg Bay in the east. From the 1930s until the early 2000s, an average of 1–2 individuals was reported annually from this area. However, in 2002 Eriocheir sinensis suddenly expanded and at least 103 individuals were documented during 2002–2004; several individuals were often caught together (Ojaveer et al. 2007). The reason for the apparent increasing occurrence and abundance of Eriocheir sinensis may be related to increased surface-water temperature and relatively mild winters, which may reduce the environmental stress experienced by Eriocheir sinensis in the low-saline parts of the Baltic Sea.
Box 5.8: The Chinese mitten crab Eriocheir sinensis
Invasion history
The native area of Eriocheir sinensis (Box Fig. 5.7) is East Asia in the South and East China Seas (Gollasch 2009). In Europe, the crab was first found in a tributary of the Weser river (Germany), approximately a century ago. It was probably introduced by ballast water . Eriocheir sinensis is known to actively migrate over long distances (hundreds of km), and about a decade after the first record it was found in the German part of the Baltic Sea where it possibly arrived via active migration through the Kiel Canal . Currently, Eriocheir sinensis has spread all over the Baltic Sea, including the inner parts of the Gulf of Bothnia and the Gulf of Finland. It is also found in rivers flowing into the Baltic Sea (Ojaveer et al. 2007).
Biology
Eriocheir sinensis reproduces in marine water and juveniles migrate inland by travelling upstream in rivers, or along the Baltic Sea coast east- and northwards to lower salinity and freshwater habitats . Adult crabs migrate back to the sea for reproduction. Mass developments of crabs occur every 15–30 years. Such population oscillations do occur in the Baltic Sea, but they are more pronounced in North Sea estuaries. As the reproduction is limited to more saline waters (salinity >10), it is believed that specimens of Eriocheir sinensis found north and east of the Baltic Sea proper have migrated here from distant places.
Impacts in the Baltic Sea
Eriocheir sinensis is an active predator that feeds on benthic organisms, but also on fish caught in traps and on nets, which damages fishing gear . The crabs can clog industrial constructions such as water intake filters. Since Eriocheir sinensis burrows in sediments, it can destabilise sediment structure and increase the recirculation of nutrients. The burrowing activity also increases the erosion of dikes, as well as river and lake embankments. Eriocheir sinensis is the second intermediate host for the human lung fluke parasite in Asia, but this parasite has not been recorded in European crabs yet. In Asia, Eriocheir sinensis is served in restaurants as a delicacy.
An expansion phase is usually followed by a phase of stasis or decline, which may be termed an adjustment or accommodation phase (Zaiko et al. 2014). There are numerous examples for such “boom and bust” phenomena of NIS in the Baltic Sea, but often the actual causes of the declines cannot be identified. Possible causes include a lower availability of the resources that initially allowed for rapid population expansions or that more predators and/or pathogens become focused on the invading species.
Besides more intensive human-mediated transport, the increase in the numbers of NIS introductions to the Baltic Sea during the past three decades (Fig. 5.7) may also reflect a higher awareness and larger research efforts. Nevertheless, the number of known Baltic Sea NIS is still only about one-sixth of that recorded in the Mediterranean Sea and almost one-third of that recorded on the European Atlantic coast. This difference is not only due to the smaller size of the Baltic Sea, but also to the hostility of its brackish waters for both marine and freshwater species, a comparatively lower trans-oceanic shipping activity and fewer species used in aquaculture .
3 Pathways and vectors
3.1 How do non-indigenous species cross environmental barriers?
A pathway is the route a NIS takes to invade a non-native ecosystem, and by definition the pathway for a primary introduction of a NIS is always human-mediated. The variety of pathways known worldwide may be classified into nine principal categories (Table 5.1). The main pathways to the Baltic Sea are shipping, human-made canals and fisheries while aquaculture , so far, is less important compared to other European seas. The remaining five pathways are of lower significance or not (yet) known to be involved in NIS transfer to the Baltic Sea Area.
Each pathway contains several vectors (Table. 5.1 and Fig. 5.8). A vector is the actual transfer mechanism, the direct physical means, by which a NIS is transported from one geographical region to another. Several vectors within a pathway may be involved in the transfer of one species, e.g. transfer by the pathway “shipping” may include several vectors such as a ship’s ballast water , its hull and its anchoring equipment.
3.2 Shipping and canals
Ships from more than 50 countries arrive directly to ports in the Baltic Sea. It has been estimated that ~2,000 large ships (excluding pleasure boats) travel across the Baltic Sea each day (HELCOM 2010). Most of these ships transport cargo and passengers between ports within the Baltic Sea or to and from ports in western Europe. Other shipping routes connect to areas further away, such as the Mediterranean Sea , the Ponto-Caspian region , Northwest Africa, the North American east coast and Asia. Even assuming low numbers of propagules in the total volume of the ship ballast tanks, billions of NIS propagules are released into the Baltic Sea ecosystem each year (Fig. 5.9).
The Baltic Sea is connected to other sea regions by human-made canals that interconnect natural inland waterways . For example, the Kiel Canal (Fig. 5.10) connects the mouth of the Elbe river in the North Sea to the Kiel Bay in the southwestern Belt Sea. This allows the spread of NIS from the North Sea to the Baltic Sea, either by natural means or by shipping. A second connection with the North Sea is via the Limfjorden system, a natural waterway that cuts across the northern part of the Jylland peninsula (Denmark) to the Kattegat. Many NIS that were primarily introduced to the North Sea have arrived in the Baltic Sea by secondary spread through these two inland waterways or by travelling around the northern tip of Denmark.
In the eastern part of the Gulf of Finland, NIS can penetrate into the Baltic Sea through a ramified network of inland waterways and human-made canals (Fig. 5.11). The Volga-Baltic Waterway is a series of canals and rivers in Russia, which links the Baltic Sea to the Volga river basin . Ultimately, this connects the Neva Bay in the vicinity of Sankt-Petersburg (Russia) to the large Ponto-Caspian region , which comprises the Black Sea , the Sea of Azov and the Caspian Sea. Some organisms have gradually spread to the Baltic Sea via the Volga river due to the removal of previous environmental barriers and the emergence of new suitable habitats, e.g. cold hypolimnions in water reservoirs or the opposite, thermal discharges from power plants. The White Sea Canal connects the White Sea with Lake Onega (Russia), which is further connected to the Baltic Sea via the Volga-Baltic Waterway. This connection is also a potential pathway for NIS spread to the Baltic Sea.
Two additional entrance points for Ponto-Caspian species to the Baltic Sea are situated in the southeastern part of the Baltic Sea proper and were also opened by linking rivers with canals (Fig. 5.11). These canals are the Dnepr-Bug Canal to the Gdańsk Bay and the Dnepr-Nemunas Canal to the Curonian Lagoon. While the former is still being used for shipping, the latter has been closed for navigation since World War II (Karatayev et al. 2008). The role of the Dnepr-Nemunas Canal was especially important for the transfer of NIS in the 19th century when several species, including Dreissena polymorpha , penetrated into the coastal lagoons of the southeastern Baltic Sea.
3.3 Fisheries and aquaculture
The import of stocking material for fisheries has been an important introduction vector for non-indigenous fish in the Baltic Sea. Particularly in the 1950s–1970s, a number of fish species (including nine salmonid and four sturgeon species) were introduced intentionally, but none of them managed to establish self-reproducing populations (AquaNIS 2015). Living food supplements for commercial fish were intentionally introduced during the 1950s–1970s as well. For example, the Ponto-Caspian mysids ( Hemimysis anomala , Limnomysis benedeni and Paramysis lacustris ) and amphipods ( Chaetogammarus ischnus , Chaetogammarus warpachowskyi , Chelicorophium curvispinum , Obesogammarus crassus and Pontogammarus robustoides ) were transferred from Dnepr water reservoirs into the inland waters of Lithuania and the Curonian Lagoon in 1960 (Arbaciauskas 2002). All these crustaceans have successfully acclimatised and most of them have later spread to other coastal areas in the Baltic Sea.
Compared to many other European marine and inland water bodies, aquaculture is less developed in the Baltic Sea and is thus of minor importance as a potential vector for introductions of NIS. Besides native species such as the Atlantic salmon Salmo salar and the European eel Anguilla anguilla , fish aquaculture in the Baltic Sea Area commonly relies on the North American rainbow trout Oncorhynchus mykiss , which is not able to reproduce in the Baltic Sea, but may occur in the wild through continuous escapes from fish farms . Several cultivation experiments with the Pacific oyster Crassostrea gigas (Fig. 5.4) were conducted in the Kiel Bay (southwestern Belt Sea), although without success (Meixner and Gerdener 1976). The first free-living Pacific oysters in the Baltic Sea were found in 2009, and they are thought to have been dispersed from the Kattegat by natural means (Wrange et al. 2010).
3.4 Certainty of pathways and vectors
In order to take appropriate management decisions, it is necessary to identify the active vector(s) for a specific NIS. Such knowledge can help to prevent other NIS using the same transfer mechanism in the future. The highest certainty level (“direct evidence”) is provided when the transport of a NIS to a particular locality is clearly associated with a specific vector , such as the intentional stock movements of Ponto-Caspian mysids and amphipods to the Curonian Lagoon (Arbaciauskas 2002). The “very likely” level of certainty is applied if a NIS has appeared for the first time in a locality where a single pathway /vector is known to operate and the conclusion is deduced from the analysis of the introduction event. For example, since the zooplankton crustacean Cercopagis pengoi was first found in the harbour areas of Tallinn and Pärnu it can be assumed that this NIS was most likely introduced with ballast water (Leppäkoski and Olenin 2000).
In many cases, the introduction of a NIS cannot be convincingly ascribed to a single pathway/vector, because more than one pathway could be involved and/or different life stages of the same species may be transported by different vectors of the same pathway. In such cases, the “possible” level of certainty is applied. For example, the bay barnacle Amphibalanus improvisus (Figs. 5.12 and 5.13) may have arrived to the Baltic Sea from North America as adults attached to ship hulls or spread as larvae by sea currents or in ballast water from the neighbouring North Sea . The overlap between pathways and vectors can be even more complicated (Fig. 5.8; Minchin et al. 2006). For example, a species that initially arrived via canals could be further transported within the recipient area via the shipping pathway or fishing gears , or have naturally spread to adjacent waters with currents.
The assumed pathway by which a species arrives is often based on known anthropogenic activities in the area. The role of different pathways and vectors may shift due to climate change (e.g. warm-water species may more easily survive in northern areas when these areas become warmer), changes in environmental quality (e.g. new ecological niches may emerge due to construction of artificial habitats or degradation of native biota), political and social-economic events (removal of custom control, closure of acclimatisation programmes, changes in aquacultural practices), management policy (e.g. ballast water management), and the emergence of new trading routes .
4 Origin and distribution
4.1 Source areas
NIS that have been introduced to the Baltic Sea originate from coastal marine (brackish) and freshwater bodies in many regions of the world. The most important source areas are the Ponto-Caspian region , the North American east coast and East Asia (Fig. 5.14).
The Ponto-Caspian species have evolved in the watersheds and estuarine areas of brackish water bodies : the Black Sea, the Sea of Azov and the Caspian Sea (cf. Table. 2.1), and are therefore well adapted to the brackish-to-limnic conditions of the estuarine systems of the Baltic Sea (cf. Sect. 13.2). Most of these species have settled in the Gulf of Finland, particularly in the Neva Bay off Sankt-Petersburg , and in the large coastal lagoons of the southeastern Baltic Sea proper (the Curonian Lagoon) and southern Baltic Sea proper (the Wisła Lagoon and the Szczecin Lagoon ).
The proportion of Ponto-Caspian NIS diminishes westward and also northward in the Baltic Sea (Fig. 5.14). Only three NIS belonging to this group ( Dreissena polymorpha , Neogobius melanostomus and the hydrozoan Cordylophora caspia ) are found in the Kattegat and the Belt Sea, where they occur in limnic and/or oligohaline coastal conditions. For most of the Ponto-Caspian NIS, the Baltic Sea is the area of primary introduction outside their native range, and some of them have continued to spread from the Baltic Sea to other regions of the world. For example, Cercopagis pengoi , Cordylophora caspia, Dreissena polymorpha and Neogobius melanostomus have become established in the Laurentian Great Lakes in North America (Mills et al. 1993; Cristescu et al. 2001).
In contrast to the introductions from the Ponto-Caspian region, the occurrences of most NIS originating from North America, East Asia as well as from other parts of the world (South America, Africa and the Indo-Pacific region) are the results of secondary spread from the North Sea or other western European sea areas as primary recipients. Most of these NIS occur in the western, more saline and warmer parts of the Baltic Sea Area, e.g. the common slipper shell Crepidula fornicata (Fig. 5.5) and the white-fingered mud crab Rhithropanopeus harrisii (Fig. 5.15), and practically all of them are results of unintentional introductions .
Only a few NIS of North American origin have been introduced intentionally to the Baltic Sea region , e.g. three freshwater crayfish species ( Orconectes limosus (Fig. 5.13), Orconectes virilis and Pacifastacus leniusculus ), the Canada goose Branta canadensis (Fig. 5.16) and the mammals: American mink Neovison vison and muskrat Ondatra zibethicus (Westman 2002; Jaszczołt and Szaniawska 2011). Ten species have been intentionally imported into the Baltic Sea and adjacent water bodies from Siberian and Russian Far East inland waters, but only two of those (the Baikalian amphipod Gmelinoides fasciatus and fish Perccottus glenii ) have established self-reproducing populations within the Baltic Sea.
4.2 The Baltic Sea bioinvasion gradient
The distinct environmental gradients of the Baltic Sea (cf. Sect. 2.4) determine the boundaries of spread and colonisation potential for both native and non-native species. The primary factor shaping the large-scale geographical distributions of NIS is salinity (Paavola et al. 2005). Temperature and oxygen concentrations are additional significant factors for the spread of NIS, but their roles are less known than that of salinity. On a local scale , the distributions of NIS are, like those of native organisms, modified by factors such as food supply , competition , predators, and availability of suitable substrates.
The lowest number of established NIS is found in the northernmost part of the Baltic Sea, the Bothnian Bay (19 NIS), where salinity is low and temperature conditions are subarctic . The highest number (37 NIS) occurs in the transition zone to the North Sea (Belt Sea and Kattegat), mainly because of the proximity to the North Sea and intensive ship traffic in combination with higher salinity and milder winters. In this area a larger proportion of NIS originate from North America and the Pacific Ocean (Fig. 5.14). In contrast, in the Baltic coastal lagoons with pronounced local salinity gradients and ice cover in winter, the Ponto-Caspian NIS prevail.
The lowest species richness of macroscopic organisms in brackish waters occurs in salinity of 5–7 (Remane 1934; cf. Sect. 4.5.6), which is the salinity in most of the Baltic Sea (cf. Fig. 2.15). Thus, the human-mediated species introductions of NIS from brackish source areas to the Baltic Sea flatten the “Remane diagram ” (cf. Fig. 4.21) by filling in the trough between fully limnic and fully marine waters. For example, Paavola et al. (2005) analysed the distributions of 84 NIS belonging to 15 phyla that are established in different salinity zones of the three large European brackish water bodies : the Baltic Sea, the Black Sea (including the Sea of Azov ) and the Caspian Sea . They found that the majority of these 84 NIS (72–83 %) tolerate more than one salinity zone, and nearly half of them occur in at least three salinity zones in all three water bodies. It turned out that most NIS are well adapted to the salinities holding the lowest species richness already in their native area , and that a NIS richness maximum in brackish water bodies occurs in the salinity intervals of the native species richness minimum. This predictable pattern in the salinity range of NIS provides a tool for the initial risk assessment of future invasions in brackish water bodies , especially when mapping potential source and recipient areas.
Since Elton’s (1958) seminal work, there has been a general belief that diverse native communities use resources to a larger extent and thus leave fewer opportunities for potential invaders. Seemingly, this concept may hold true for the Baltic Sea since NIS tend to proliferate in areas of this naturally species-poor brackish ecosystem. However, many studies have also shown the opposite: the degree of invasion seems to be more often positively correlated with the species richness of natives, particularly in systems where the biodiversity distribution is largely determined by environmental drivers (Zaiko et al. 2007). Interestingly, along the Baltic Sea bathymetric gradient, from coastal areas to deeper basins, the species richness of both native and non-native species declines rapidly. So far, species-poor and oxygen-deficient subhalocline areas have been invaded only by three spionid polychaete species, all belonging to the genus Marenzelleria (Norkko et al. 2012).
4.3 Invasion “hotspots”
The invasion success of a NIS, and its further distribution in the Baltic Sea, is determined by the similarity in environmental conditions between the source and recipient areas, the proximity to shipping routes and/or inland waterways , and the level of anthropogenic or/and invasive disturbance. Therefore, many NIS are abundant or even dominant in coastal areas, especially in lagoons and inlets with intensive anthropogenic activities such as shipping routes, harbour areas, marinas and hydrotechnical constructions.
The facilitative effect of environmental modifications that promote new NIS invasions may be asserted through physical or biological mechanisms. An example of a physical mechanism is the provision of hard substrate in an area with natural sandy beaches by e.g. harbour constructions or wind farms. In these cases, a sessile NIS that needs a hard substrate for attachment is likely to experience only low competition for space and resources from local organisms. Biological mechanisms include altered habitat conditions caused by an already established NIS so that the invasion of a new NIS is favoured. This may create a feedback system that accelerates the accumulation of NIS and forms an invasion “hotspot ” (Simberloff and Von Holle 1999). For example, dense aggregates of the zebra mussel Dreissena polymorpha provide an attractive habitat for numerous native and non-native benthic fauna in coastal lagoons with large freshwater influences in the southeastern Baltic Sea (Zaiko et al. 2007).
Also, natural or anthropogenic disturbances may create new ecological niches that favour NIS invasions. For example, the outcompeting of native gammarid populations by the North American Gammarus tigrinus in many places along the coasts of the Wisła Lagoon may be partly due to eutrophication and chemical contamination by hazardous substances , which may strengthen the competitive capacity of the invader (Grabowski et al. 2006). Another example of disturbed environments being favourable for species invasions are the discharge areas of cooling water from nuclear power plants into the Baltic Sea (Box 5.9). These discharges create habitats with continuously elevated water temperatures on a scale of a few km2. Some notorious NIS, such as the New Zealand mud snail Potamopyrgus antipodarum and Conrad’s false mussel Mytilopsis leucophaeata , thrive in these environments exceptionally well and/or were first introduced to these environments and spread from there, e.g. the eel parasite Anguillicoloides crassus .
A generalised model of an “invader-friendly” habitat where invasion “hotspots” may be found can be defined by the following features:
-
1.
The habitat has favourable physical conditions for maintaining diverse communities in general. In this case native species richness can be considered as an indicator of a habitat’s invasibility .
-
2.
The habitat has an increased amount of usable resources, e.g. through anthropogenic nutrient inputs of nitrogen and/or phosphorus. Both spatial and temporal variation in the availability of resources facilitates NIS invasion by providing resource pools to new colonists.
-
3.
The habitat is severely disturbed by natural or anthropogenic stressors , e.g. a heavy storm or bottom dredging. Every additional disturbance event may promote a new surge of NIS invasions.
-
4.
The habitat properties are altered due to previous NIS introductions. A successfully established habitat-engineering species should be considered as a powerful facilitative factor for further invasions.
Box 5.9: Thermal discharges and non-indigenous species
Pauline Snoeijs-Leijonmalm
Thermal discharges stimulate the spread of non-indigenous species
Nuclear power plants discharge large volumes of cooling water into the coastal environment at several places in the Baltic Sea. This creates locally disturbed habitats that are notorious for high abundances of some non-indigenous species such as the diatom Pleurosira inusitata (syn. Pleurosira laevis fo. polymorpha ), the Conrad’s false mussel Mytilopsis leucophaeata and the mud snail Potamopyrgus antipodarum . The vicinities of large cooling-water outlets are not only hotspots where non-indigenous species can build up high-density populations, but they can also be “first bridgeheads” for non-indigenous species that later on invade other areas of the Baltic Sea. The year-round increased water temperature in the cooling-water discharge areas may promote the adaptation and spread of the newcomers to a new habitat. For example, the polychaete Marenzelleria sp. and the amphipod Gammarus tigrinus were first observed in heated water at Loviisa (Ilus 2009) before they spread to other Finnish coastal areas in the eastern Gulf of Finland . Similarly, high numbers of the eel parasite Anguillicoloides crassus were first discovered in heated water at Oskarshamn (Höglund and Andersson 1993) before it invaded the rest of the Baltic Sea.
Large cooling-water discharges in the Baltic Sea Area
Five large Swedish and Finnish nuclear power plants with 14 reactor units use brackish seawater to dispose of waste heat in the Baltic Sea Area (Box Fig. 5.8). Other coastal nuclear power plants (e.g. in Sankt-Petersburg , Russia) use cooling towers. About one-third of the energy produced in a nuclear power plant , by either a boiling-water reactor or a pressurised-water reactor, is transferred to electricity. The other two-thirds of the energy produced is excess heat, and thus the reactors need to be cooled down. At full operation, the 14 reactor units together produce 12,226 MW of electricity and 23,107 MW of waste heat. For ~1,000 MW (MJ s−1) of electricity production ~45 m3 s−1 of cooling water is needed. The water is heated by ~10 °C when it is returned to the sea, which creates a coastal area of a few km2 with significantly increased water temperature . For example, at Forsmark the warm water keeps 2–3 km2 of the Bothnian Sea free of ice in winter. On the coasts of the Baltic Sea many other industries use brackish cooling water as well, e.g. paper mills and even a large data centre in Finland (Hamina, Gulf of Finland), but these thermal discharges are more limited than those of the nuclear power plants.
The diatom Pleurosira inusitata
The large chain-forming diatom Pleurosira inusitata (Box Fig. 5.9) was probably introduced to the Forsmark area (Sweden) during an experimental release of eels that had been raised in aquaria in southern Europe. This diatom was never observed in Forsmark before 1989, but since 1990 it forms up to 0.5-meter high colonies in water heated by ~10 °C each year in September-November. The colonies are attached to stones and macroalgal vegetation and they hang like fishing nets in the water, especially at sites with slow-flowing water (Snoeijs and Weckström 2010).
Conrad’s false mussel Mytilopsis leucophaeata
In the cooling water discharge at Loviisa (Finland) a strong recruitment of young dreissenid bivalves of the species Mytilopsis leucophaeata (Box Fig. 5.10) was observed in 2003. Already one year later, a dense population with up to 28,000 adult individuals m−2 (9.8 kg wet weight m−2) completely covered boulders and stones (Laine et al. 2006). In 2011 the species also arrived at Forsmark and spread fast in the heated water (Florin et al. 2013). Mytilopsis leucophaeata has most probably been transported to the Baltic Sea by ballast water . Its body size, shape and habitat are very similar to those of the blue mussel Mytilus trossulus, which is native to the Baltic Sea, but in contrast to Mytilopsis leucophaeata, Mytilus trossulus avoids the heated water in the cooling-water discharges .
The New Zealand mud snail Potamopyrgus antipodarum
The native habitat of the deposit-feeding prosobranch snail Potamopyrgus antipodarum (syn. Paludestrina jenkinsi , Box Fig. 5.11) is freshwater in New Zealand (Ponder 1988). Molecular studies have identified two mitochondrial haplotypes from the North Island of New Zealand that are identical to those found in Europe (Städler et al. 2005). The original introduction to Europe was probably a secondary spread from Australia , and transport may have been in drinking water barrels on board ships. The first European finds of Potamopyrgus antipodarum around 1890 were from estuaries and the brackish coasts of the Baltic Sea, from where it spread further to European freshwaters. In the cooling-water discharges at Forsmark, Oskarshamn , Olkiluoto and Loviisa , Potamopyrgus antipodarum is a common to dominant species (Snoeijs 1989; Ilus 2009). In most places it lives in soft bottoms , but it colonises rocky shores as well. In sediments at Forsmark it has been observed at densities of almost 30,000 individuals m−2 (Sandström 1990) and with densities of 8,000–10,000 individuals m−2 on rocky substrates at ~10 °C in winter, as well as at ~28 °C in summer (Snoeijs and Mo 1987). These numbers are from macrofaunal samples (body size >1 mm), but the true densities of the species were much higher since many specimens of Potamopyrgus antipodarum are <1 mm in size. The species is viviparous, reproduces year-round and has wide temperature and salinity tolerances, which are traits that explain its invasion success (Snoeijs 1989).
5 Diversity and ecology of non-indigenous species
5.1 Correct taxonomic identification is crucial
The presence of a NIS often remains unnoticed until it becomes abundant and/or creates trouble because of incomplete taxonomic knowledge at the time of its arrival in the recipient ecosystem. In most cases it is also difficult to check a species’ identity afterwards because reference specimens of first introductions have seldom been kept.
For example, there has been a great deal of confusion around the introduced Marenzelleria spp. (Box 5.3) in the Baltic Sea Area. These polychaetes can be up to 16 cm long, but still they can only be reliably identified to the species level by genetic analysis (Blank et al. 2008). Three species with different geographical distributions live in the Baltic Sea. Marenzelleria viridis occurs from the Skagerrak to the Öresund and has also been observed in the southwestern and southeastern Baltic Sea proper as well as in the Gulf of Riga . Marenzelleria neglecta occurs in the whole Baltic Sea proper up to the Åland Sea and Marenzelleria arctia occurs in the northern Baltic Sea proper and the Bothnian Sea. Both Marenzelleria viridis and Marenzelleria neglecta have been introduced from the North American east coast while Marenzelleria arctia is an Arctic species .
5.2 Taxonomic confusion about Mnemiopsis leidyi
Another good example of taxonomic confusion is the case of the American comb jelly Mnemiopsis leidyi (Box 5.10) in the Baltic Sea. This species is an actively hunting ctenophore , a hermaphrodite with a translucent body and a length of up to 14 cm, which is native to the American Atlantic coast from Narragansett Bay (USA) in the north to the Valdés Peninsula (Argentina) in the south. Mnemiopsis leidyi invaded the Black Sea in the early 1980s (Purcell et al. 2001) and the Caspian Sea in mid 1990s, to which it was likely transported through the Volga-Don Canal in ballast water (Kideys 2002). In 2005, the species appeared in coastal areas of the North Sea (Oliveira 2007; Tendal et al. 2007) and in 2006 on the Swedish west coast (Hansson 2006), with up to 92 individuals per m3 in the Kiel Bay in the southwestern Belt Sea (Javidpour et al. 2006). In 2007 it was reported that Mnemiopsis leidyi had spread to the Bothnian Sea and the Gulf of Finland as well, but molecular evidence proved that the comb jelly observed in these northern areas was in fact the Arctic comb jelly Mertensia ovum (Gorokhova et al. 2009). Mertensia ovum has a broad Arctic and circumboreal distribution , but had never been reported from the Baltic Sea before.
Box 5.10: The American comb jelly Mnemiopsis leidyi
Invasion history
The native area of the American comb jelly Mnemiopsis leidyi (Box Fig. 5.12) is the east coast of North and South America. Outside its native area it was first discovered in the Black Sea in 1982, after which it rapidly invaded the Sea of Azov , the Aegean Sea, and the Marmara Sea. In 1999 it was also found in the Caspian Sea . It reached Northern Europe in 2005 (North Sea ) and the Belt Sea in 2006. At present, Mnemiopsis leidyi occurs in the transition zone and the southern Baltic Sea with its northernmost established population in the Bornholm Sea . Genetic studies have revealed multiple introductions: the populations in northern Europe originate from the northeastern coast of America while the southern European populations, including the Mediterranean and Ponto-Caspian regions , originate from the Gulf of Mexico.
Invasive traits
Mnemiopsis leidyi has wide salinity and temperature ranges and tolerates low oxygen levels . However, it seems unlikely that Mnemiopsis leidyi would establish permanently in the northern or eastern parts of the Baltic Sea as its spread and population growth is limited by low salinity and low temperature (Lehtiniemi et al. 2012).
Impacts in the Baltic Sea
After its introductions to the Black Sea and the Caspian Sea, Mnemiopsis leidyi reproduced rapidly and formed very large populations. In these seas it found optimum conditions: plenty of food due to eutrophication, high temperature and overfished populations of potential competitors. Being a highly efficient predator of zooplankton , fish eggs and small larvae, and due to its voracious appetite, Mnemiopsis leidyi has caused drastic shifts in the Black Sea and Caspian Sea ecosystems. In the Baltic Sea its impact is less pronounced due to low population density and small body size . While the local fisheries collapsed in the Black Sea and the Caspian Sea during the periods of Mnemiopsis leidyi peak abundances, there is no evidence so far of it threatening the Baltic herring, sprat or cod stocks (Jaspers et al. 2011).
Thus, the search for a northward expansion of Mnemiopsis leidyi in the Baltic Sea had instead yielded increased knowledge of native biodiversity. Later it was shown that while the invader Mnemiopsis leidyi would perhaps be able to survive for a short time in vast areas of the northern Baltic Sea, if it would be transported there, its reproduction is prevented by salinity <10 and temperature <12 °C. Thus, due to the combined effect of low salinity and low temperature , it is not probable that Mnemiopsis leidyi will establish permanent populations in the central or northern Baltic Sea (Lehtiniemi et al. 2012).
Zooplankton and fish species that live in deep water can be transported from the Kattegat into the Baltic Sea with saltwater inflows . Their dispersal is thus mainly controlled by the baroclinic flow field and bottom topography (cf. Box 2.1). Hydrodynamic drift modelling has shown that the potential dispersion of e.g. comb jellies follows the deep-water currents from the Bornholm Sea towards the north and the east of the Baltic Sea and is limited by topographic features and low advection velocities (Lehtiniemi et al. 2012). However, if such species are new invaders in the area, and the conditions for growth and reproduction are favourable in the Baltic Sea, they will be able to form stable populations despite the fact that most individuals are hampered by the hydrodynamics of the deep water.
5.3 Phytoplankton
At least 13 non-native phytoplankton species, six diatoms, five dinoflagellates and two silicoflagellates have been recorded in the Baltic Sea Area (Olenina et al. 2010; Kownacka et al. 2013). None of these phytoplankton NIS originate from the Ponto-Caspian region and most of them are certain secondary introductions . They account for less than 1 % of the more than 2,000 phytoplankton species that are known from the Baltic Sea (Hällfors 2004). However, since phytoplankton organisms are easily distributed by ships’ ballast water , the number of non-indigenous/cryptogenic phytoplankton species in the Baltic Sea is probably underestimated.
There is usually a high level of uncertainty when assigning a unicellular plankton organism to NIS (Gómez 2008). In fact, all 13 non-indigenous phytoplankton species recorded in the Baltic Sea are cryptogenic, i.e. their native area is uncertain or unknown because they have already spread to many places on Earth. However, for all 13 species it was possible to show that they were new to the Baltic Sea because the phytoplankton community composition in the area has been studied for more than 100 years (Wasmund et al. 2008). If a species can be identified by light microscopy , and is abundant today despite not having been recorded earlier, it has most probably invaded the Baltic Sea. Remnants of some phytoplankton organisms accumulate in the sediments, e.g. diatoms and chrysophytes , and in these cases it is even possible to prove the absence of a species from the Baltic Sea for much longer than a time period of 100 years.
A number of notorious IAS-classified phytoplankton species increase in abundance worldwide with negative impacts on biological diversity, ecosystem functioning and socio-economic values (Anderson 2009). The only recognised phytoplankton IAS in the Baltic Sea is the dinoflagellate Prorocentrum cordatum . This cryptogenic dinoflagellate spread from the western part of the Baltic Sea up to the Gulf of Finland between 1979 and 1993 (Fig. 5.6b). The summer-autumn blooms of Prorocentrum cordatum can have a massive bioinvasion impact on ecosystem functioning; when the species’ abundance exceeds 1 million cells L−1 it can completely dominate the phytoplankton community (by up to 98 % of the total biomass) and change physical (water transparency ) and chemical (nutrient concentrations) properties of seawater (Olenina et al. 2010).
Prorocentrum cordatum also has the potential of forming toxic blooms that can kill crustaceans, fish and other marine organisms, but in the Baltic Sea the species has not been observed to be toxic. Other potentially toxic cryptogenic phytoplankton species in the Baltic Sea are the dinoflagellates Alexandrium minutum , Alexandrium ostenfeldii , Gymnodinium catenatum and Karenia mikimotoi , and the silicoflagellates Heterosigma akashiwo and Pseudochattonella verruculosa . All six species are known to cause “red tides” (large toxic blooms) elsewhere, but this phenomenon has not been recorded in the Baltic Sea so far, although it has been shown that Alexandrium ostenfeldii is able to produce paralytic shellfish poisoning toxins on the southwestern coast of Finland (Hakanen et al. 2012).
Another type of damage to the ecosystem can be caused by phytoplankton species that form dense blooms accompanied by copious amounts of mucilage , such as the diatom Coscinodiscus wailesii . Because of its comparatively large cell size with a 175–500 μm diameter, Coscinodiscus wailesii is inedible to most grazing zooplankton, and when its blooms decay the cells aggregate, sink and may cause anoxia at the seafloor. A direct socio-economic impact of mucilage is the clogging of equipment such as nets and cages used in fisheries and aquaculture .
5.4 Macrophytes
Thirteen non-native benthic macrophytes, including 11 algae and two vascular plants , have been recorded in the Baltic Sea Area (AquaNIS 2015). None of them originates from the Ponto-Caspian region and most of them are secondary introductions . There is no macrophyte NIS that has become an IAS in the Baltic Sea like the green alga Caulerpa taxifolia in the Mediterranean Sea (Meinesz et al. 2001).
Seven of the Baltic macrophyte NIS, the red algae Bonnemaisonia hamifera , Dasya baillouviana and Gracilaria vermiculophylla , the brown algae Colpomenia peregrina , Fucus evanescens and Sargassum muticum , and the reed Spartina anglica , occur inside the Baltic Sea, but only in the more saline areas in the Arkona Sea , the Belt Sea and/or the Öresund (Nyberg 2007). Four other macroalgal NIS, the green alga Codium fragile and three red algae ( Aglaothamnion halliae , Dasysiphonia japonica and Neosiphonia harveyi ) have not entered the Baltic Sea, but occur in the Kattegat (Nyberg 2007). The charophyte Chara connivens and the American pondweed Elodea canadensis are the only two macrophyte NIS that are restricted to the most limnic parts of the Baltic Sea.
5.5 Zooplankton
At least eight zooplankton NIS are established in the Baltic Sea (AquaNIS 2015). Six crustaceans : the cladocerans Cercopagis pengoi , Cornigerius maeoticus , Evadne anonyx and Penilia avirostris , and the copepods Acartia tonsa and Ameira divagans , comprise ~10 % of the total crustacean zooplankton species richness in the Baltic Sea, although this percentage varies somewhat between the different subregions of the Baltic Sea.
Due to a low number of (known) native gelatinous zooplankton species, two non-indigenous gelatinous species (the jellyfish Maeotias marginata and the comb jelly Mnemiopsis leidyi ) represent more than 30 % of the species richness of the jellyfish (Cnidaria ) in the northern Baltic Sea and more than 15 % of the comb jellies ( Ctenophora ) in the southern Baltic Sea.
The principal ecological difference between the two groups of zooplankton NIS (crustaceans and gelatinous) is the way they are utilised as a food source by higher trophic levels . Cladocerans and copepods are often valuable additions to the diet of a range of predators, while gelatinous zooplankton organisms are mainly preyed upon by carnivorous gelatinous top predators that utilise secondary production otherwise consumed by fish (Mills 1995; Boero et al. 2008). Therefore, gelatinous zooplankton organisms are often regarded as “dead ends” in marine food webs (Verity and Smetacek 1996).
The distribution of zooplankton NIS in the Baltic Sea is mainly governed by salinity. Ameira divagans and Penilia avirostris occur only in the more saline conditions of the Arkona Sea , Belt Sea and Kattegat. Cornigerius maeoticus and Maeotias marginata have been observed only in the Gulf of Finland, but it is not certain that these two species are absent from the Baltic Sea proper. The other four zooplankton NIS are more widely distributed: Acartia tonsa in the entire Baltic Sea, Cercopagis pengoi and Evadne anonyx in the northern and eastern parts, and Mnemiopsis leidyi in the western and southern parts.
Some benthic invertebrate NIS, e.g. the mussels Dreissena polymorpha and Mytilopsis leucophaeata and the barnacle Amphibalanus improvisus , have a planktonic larval stage. Also juveniles of the spionid polychaetes Marenzelleria spp. may occur in the water column above the sediments. The possession of such free-living life stages is a useful trait for a NIS as it provides an advantage over obligate sessile species when spreading to new areas.
Some zooplankton NIS that have established permanent populations are now part of the pelagic and benthic food webs in the Baltic Sea. In some cases, they have changed the energy flow in the food webs by adding an extra trophic level to the system. For example, Cercopagis pengoi and Evadne anonyx , predators of smaller zooplankton, and Acartia tonsa and Penilia avirostris , which graze on phytoplankton , have extended the native food webs (Saiz and Kiørboe 1995; Lehtiniemi and Gorokhova 2008). The zooplankton NIS are also preyed upon by planktivorous fish as well as by carnivorous invertebrates such as mysids and gelatinous zooplankton . Cercopagis pengoi is the only NIS in the Baltic Sea that seems to have a strong impact on ecosystem functioning in the pelagic zone . However, the distribution of the other introduced carnivorous cladoceran Evadne anonyx is increasing in the Baltic Sea (Põllupüü et al. 2008; Bielecka et al. 2014), and this species may also be a relevant food source for planktivorous fish in late summer when its population peaks.
5.6 Benthic and nektobenthic invertebrates
The largest group of NIS recorded in the Baltic Sea (~60 species) are benthic and nektobenthic invertebrates, mainly crustaceans, molluscs and polychaetes . Of these, ~45 species are currently established in the Baltic Sea (cf. Fig. 4.18c). The same three taxonomic groups also dominate the native benthic invertebrate fauna of the Baltic Sea, and NIS constitute ~8 % of the crustaceans, ~4 % of the molluscs and ~3 % of the polychaetes.
No shallow hard- or soft-bottom habitat in the Baltic Sea is entirely free from human-mediated benthic invaders anymore. Non-indigenous species can even be abundant or dominant in these habitats, e.g. Dreissena polymorpha on hard bottoms and Marenzelleria spp. on soft bottoms in the low-salinity lagoons of the southeastern Baltic Sea proper (Leppäkoski et al. 2002a).
Today, the native freshwater amphipods have disappeared from the central freshwater part as well as from the more brackish northern part of the Curonian Lagoon while the Ponto-Caspian amphipods Obesogammarus crassus and Pontogammarus robustoides proliferate here now, together with the North-American amphipod Gammarus tigrinus (Grabowski et al. 2006). High densities of Pontogammarus robustoides are associated with a reduced biomass of the green habitat-forming filamentous alga Cladophora glomerata (Arbaciauskas and Gumuliauskaite 2005), suggesting a grazing effect.
5.7 Fish
About 30 non-indigenous fish species have been introduced to the Baltic Sea and adjacent waters (AquaNIS 2015). Most of them were introduced intentionally between the 1950s and the 1970s. They have added a considerable number of species to the ~120 native marine, freshwater and migratory fish species known from the Baltic Sea. However, the majority of the intentionally introduced fish species have not been able to form self-reproducing populations in the Baltic Sea and their rare encounters in the wild concern specimens that have escaped from fish cultures. Examples of such NIS are the Siberian sturgeon Acipenser baerii , the Russian sturgeon Acipenser gueldenstaedtii , the sterlet Acipenser ruthenus , the spotted silver carp Aristichthys nobilis , the longnose sucker Catostomus catostomus , the beluga sturgeon Huso huso , the silver carp Hypophthalmichthys molitrix , the pink salmon Oncorhynchus gorbuscha , the chum salmon Oncorhynchus keta , and the North American rainbow trout Oncorhynchus mykiss .
Three of the intentionally introduced NIS that are able to reproduce in the Baltic Sea are the Chinese sleeper Perccottus glenii (introduced in 1916), which occurs in the most diluted low-salinity eastern parts of the Gulf of Finland (Orlova et al. 2006), the Prussian carp Carassius gibelio (introduced in the 17th century), which now is common in the Wisła Lagoon (Witkowski and Grabowska 2012) and along the Estonian coast (Vetemaa 2006) and Cyprinus carpio (introduced in the 14th century), which is common in the Curonian Lagoon (Virbickas 2000).
The most notable unintentional fish introduction is that of the Ponto-Caspian round goby Neogobius melanostomus . After being first recorded in the Gdańsk Bay in 1990 its incursion was reported from several other areas in the Baltic Sea. It is believed that the secondary spread of this species has been facilitated by shipping because in new localities it was first found mainly in or near habours.
In general, the estuarine and inshore waters of the Baltic Sea are more amenable to invasions of non-indigenous fish species than the open sea areas because most of the NIS originate from limnic or brackish-water source areas. Marine non-indigenous fish species are unable to form self-reproducing populations in the Baltic Sea for any longer time due to the, for them, unfavourable low salinity.
5.8 Mammals
Three mammal IAS, two native to North America and introduced to Europe in the 1920s–1930s and one native to East Asia , have spread along the Baltic Sea coasts (Nummi 2002). The American mink Neovison vison , the racoon dog Nyctereutes procyonoides and the muskrat Ondatra zibethicus were originally introduced for fur farming and large populations of these three mammals have built up in the Baltic Sea region from escaped and released individuals.
The mink and the racoon dog prey, for example, on eggs in bird nests and on incubating waterbirds in the archipelagos of the Baltic Sea. The mink may also cause losses for fish farms . The muskrat disturbs the structure of the littoral vegetation as it mainly feeds in reed belts and digs for plant roots . This may create floods and mud flats and has a negative impact on macrofauna, fish and bird nests due to habitat destruction . The muskrat also bears a large number of parasites , including the dwarf tapeworm Echinococcus multilocularis , which may infect humans (Nummi 2002).
5.9 Non-indigenous species associates
An aspect that has only rarely been studied is that one NIS can in fact be more than one. For example, the zebra mussel Dreissena polymorpha was shown to carry at least 14 types of parasites and other symbionts within the mantle cavity and/or associated with internal tissues, including ciliates ( Ancistrumina limnica , Conchophthirus acuminatus and Ophryoglena sp.), trematodes (Echinostomatidae , Aspidogaster sp., Bucephalus polymorphus and Phyllodistomum sp.), nematodes, oligochaetes , mites, chironomids and leeches (Karatayev et al. 2000). It is complicated to study such associated species because it is difficult to prove where the host became infected: in the source area, on the pathway or in the recipient area.
Transport of the host can be the vector for the introduction of a parasitic NIS that can also infest native species . This has happened e.g. with the nematode Anguillicoloides crassus , which was probably introduced to Europe with eels imported from Japan (Lefebvre et al. 2012). This parasite feeds on host tissues and reproduces in the swimbladder lumen of eels. In less than three decades, driven by intercontinental eel trade, it has spread over four continents, infecting six of the 20 eel species and subspecies described worldwide, including the European eel Anguilla anguilla . In the Baltic Sea, Anguillicoloides crassus is distributed from the Kattegat to the Archipelago Sea .
6 Environmental quality and invasive species
6.1 Biological pollution
NIS can change the biological, chemical and/or physical properties of an aquatic ecosystem and cause a decline in ecological quality. Such changes include, but are not limited to, local elimination of sensitive and/or rare species, alteration of native communities, harmful blooms , modification of the substrate, changes in oxygen and nutrient concentrations , pH, water transparency , and accumulation of hazardous substances . The outcomes of biological invasions that decrease ecological quality are called “biological pollution ” or “biopollution ” and the species involved are IAS (Box 5.1).
An IAS can affect one or more levels of biological organisation, e.g. internal biological pollution by parasites or pathogens , genetic changes (e.g. hybridisation ) or shifts in the age structure of a prey population at the population level, structural shifts at the community level, modification of physical-chemical conditions at the habitat level and/or alteration of energy and organic material flow at the ecosystem level.
There is a fundamental difference between various forms of pollution. IAS do not respond to remedial efforts in the same way as eutrophication or chemical pollution , which can be diminished if appropriate measures are taken. The risk of biological pollution can be most effectively reduced by a precautionary approach (e.g. vector and pathway management) while eradication or control of existing IAS are more challenging. IAS usually expand their distribution and increase their abundance from a local source via processes that are not controllable through management. The spatial extent, rate of spread, and impacts on the environment will depend on the biological traits of a NIS and the environmental conditions within an invaded ecosystem.
6.2 Environmental status of the Baltic Sea
The environmental status of marine waters is traditionally evaluated by taking into account the effects of eutrophication, chemical pollution , habitat destruction and overexploitation of fish stocks . However, biological pollution, which may even surpass the impacts of the “traditional” stressors, can also have pronounced effects on the environment, and should be included in environmental assessment s. One of the “good environmental status ” (GES, cf. Sect. 17.8.1) descriptors in the EU Marine Strategy Framework Directive (MSFD, cf. Sect. 17.8) specifically addresses the bioinvasion problem: “Non-indigenous species introduced by anthropogenic activities are at levels that do not adversely alter the ecosystem”. Thus, the absence or minimal level of biological pollution is one of the goals of achieving a GES of the Baltic Sea.
NIS cause adverse environmental impact and economic losses only after attaining a critical level of abundance and only when occupying a sufficiently large area. To classify the level of bioinvasion impacts, an integrative method called the “biopollution level index ” (BPL) was proposed for aquatic ecosystems (Olenin et al. 2007). This index is based on a classification of the abundance and distributional range of NIS and the magnitude of their impacts on native communities, habitats and ecosystem functioning . It includes five BPL classes: 0 = no impact, 1 = weak impact, 2 = moderate impact, 3 = strong impact and 4 = massive impact. An overall bioinvasion impact assessment based on the BPL of the entire Baltic Sea revealed that strong biopollution (BPL 3) often occurs in coastal lagoons , inlets and gulfs, and moderate biopollution (BPL 2) in the open sea areas (Zaiko et al. 2011). However, despite continuously accumulating information, documented ecological impacts are known so far for only one-third of the ~130 NIS in the Baltic Sea. Our understanding of both the direction and magnitude of impacts at the ecosystem level of even the most widespread NIS is still poor (Ojaveer and Kotta 2015).
Bioinvasion impacts may compromise the value of some indicators used for the ecological status assessment of coastal waters. For example, the ability of Dreissena polymorpha to modify bottom habitats and to form local patches of elevated biological diversity may bias the results of species richness -based environmental quality assessments by showing a false improvement of ecological status (Zaiko and Daunys 2015). Thus, the assessment may reflect the IAS impact rather than that of anthropogenic pressure .
7 Risk assessment and management
7.1 Risk assessment of impacts by non-indigenous species
Risk assessment of impacts by NIS includes the prediction of whether a species is capable of spreading from its native or introduced area, as well as the identification of possible impacts it might have in a new area if it were introduced (Gollasch and Leppäkoski 2007; Olenin et al. 2014). Such an assessment contains a high degree of uncertainty due to the lack of information on the probability of a species to be transported and established under certain environmental conditions. Additional uncertainty results from the scarcity of data on effects such species may provoke (David et al. 2013a). Risk analyses are aided by predictive habitat and niche modelling which helps to identify areas susceptible to new introductions. This in turn helps to design and target monitoring efforts and to plan control measures.
However, effective risk assessment requires detailed knowledge on the traits and ecology of the introduced species as well as on their ecological interactions with native species , which are most often poorly known. Moreover, while impacts on the invaded habitat and community structure may be tractable, information about IAS impacts on ecosystem functioning is mostly lacking. Extrapolating information on non-indigenous species impacts from one area to another is often problematic and should be performed with caution.
It is essential to compare the traits of successful and unsuccessful invaders with those of related native species to better understand why some species become pests in some areas or under certain environmental conditions. The traits of NIS vary and their effects may therefore be unpredictable and opposite to impacts of other NIS in the area.
7.2 Information support
While biological invasions attract increasing attention from scientists, policy makers and various management authorities, the knowledge base on NIS is continuously expanding. With the implementation of the EU MSFD and similar legislation addressing the problem of biological invasions, the availability of advanced, scientifically validated and up-to-date information support on NIS is essential for aquatic ecosystem assessment and management.
The Baltic Marine Biologists (BMB) initiated the first regional open information source worldwide, the Baltic Sea Alien Species Database (BSASD, online since 1997), which contains detailed information on NIS origin, introduction history, pathways and vectors for the Baltic Sea Area. Now this BSASD is part of a larger, new generation information system (AquaNIS ), dealing with aquatic NIS introduced to the marine, brackish and coastal freshwater environments of Europe and adjacent regions. This system is designed to assemble, store and disseminate comprehensive data on NIS and to assist in the evaluation of the progress made towards achieving management goals (AquaNIS 2015).
7.3 Early detection and molecular techniques
In order to enhance the opportunities and efficacy of management measures, it is important to detect a NIS at an initial stage of incursion, i.e. when a population is still confined to a small area and has low density (Fig. 5.3). Therefore, NIS monitoring and surveys should be prioritised in bridgehead sites and dispersal hubs which are often the first recipient areas for new introductions (Lehtiniemi et al. 2015).
Early detection requires proper species identification , which in many cases depends on explicit taxonomic expertise . Traditional taxonomic approaches are laborious, and often fail to identify cryptic species (two or more species hidden under one species name) or larval stages. Access to the appropriate taxonomic expertise, intercalibration exercises and searchable digital databases with image recognition functions may aid identification and enhance the quality of taxonomic assignment. Increasingly, genetic methods allow tracking of the source population and identifying pathways of the introductions. Population genetics can reveal the relatedness of two populations (e.g. native and introduced) and make it possible to roughly estimate the timing of the introduction in order to assess if the introduced species has one or more source regions and its possible pathways.
Rapidly advancing new molecular techniques provide promising tools for species identification from environmental samples. Novel molecular approaches such as metabarcoding have huge potential to provide more accurate and standardised, high-resolution taxonomic data on all organisms present in a sample, including hosts with all their parasites . Metabarcoding allows taxonomical assignment of a specimen based on sequencing of a short standardised DNA fragment (molecular marker or barcode), across entire biological communities (cf. Box 4.2). The recent development of high-throughput sequencing offering massive sequencing capacities allows multiple samples to be processed faster and cheaper than can be achieved by traditional morphological approaches (Pochon et al. 2013; Kelly et al. 2014). This new technique is expected to revolutionise NIS surveillance in the near future.
7.4 Precaution and mitigation
Thus far, the Baltic Sea has not been exposed to devastating biological pollution to the extent experienced by some other aquatic ecosystems of the world, e.g. the Mediterranean Sea , the Black Sea , the Caspian Sea and the Laurentian Great Lakes of North America . Still, this does not mean that large bioinvasion impacts cannot occur in the Baltic Sea in the future. Among the vast spectrum of potential NIS, it is practically impossible to predict which species may become invasive. Therefore, precaution is recommended as species introductions are irreversible and accumulate over time (David et al. 2013a).
No control of IAS without affecting other components of the ecosystem is feasible once an invasion process is underway. Given the severity of the problems that can be caused by IAS, it is mandatory for policy and management to focus on the vectors of introduction to prevent introductions of species in general. Regarding vector management two prime instruments are applied: (1) the Ballast Water Management Convention (BWMC) of the UN International Maritime Organization (IMO) , and (2) the Code of Practices of the European Union (EU) and the International Council for Exploration of the Sea (ICES, cf. Box 18.1) for planned species introductions in aquaculture .
7.5 The Ballast Water Management Convention
The aim of the BWMC is to prevent, minimise and ultimately eliminate the risks associated with species transfers in ballast water (IMO 2004). Ballast water may be managed by either exchanging the water at high sea or by ballast water management systems. Several of the countries around the Baltic Sea have ratified the BWMC, but its entry-into-force requirements have not yet been met. HELCOM (cf. Sect. 17.8.4) and OSPAR (cf. Box 14.1) countries have voluntary ballast water management measures in place, which are based on ballast water exchange (BWE) (David and Gollasch 2008).
When BWE is applied, coastal ballast water is taken up in a harbour and later exchanged by seawater from a sea area with a water depth of at least 200 m and at least 200 nautical miles away from land. If this is impossible the ballast water is exchanged in a sea area with a water depth of at least 200 m and at least 50 nautical miles away from land. If this also is impossible, the arrival harbour State may, in accordance with IMO Guidelines, designate a ballast water exchange area that may be closer to land and in less deep waters. However, this procedure is of limited efficiency and cannot be applied in shallow seas like the Baltic Sea. This highlights that BWE needs to be phased-out over time and replaced by a more stringent ballast water performance standard.
A ballast water performance standard sets maximum permitted numbers of living organisms in ballast water discharged from ships. This may be achieved by ballast water management systems installed on board. Methods include mechanical separation of objects in the ballast water (e.g. filtration) followed by ultraviolet radiation or the use of so-called “active substances” (e.g. chemical reagents) (Gollasch et al. 2007; Gollasch and David 2012; David et al. 2013b; David and Gollasch 2015).
7.6 Code of Practices in aquaculture
In the EU, the import of living organisms for use in aquaculture is regulated by the EU Council Regulation No. 708/2007 regarding the use of non-indigenous and locally absent species in aquaculture (EU Council Regulation 2007). This instrument applies to both open and closed aquaculture facilities. It contains provisions on which species can be imported and concerns measures intended to combat possible risks of NIS movements. These measures include the requirement to obtain a permit for species movements, risk assessments , quarantine and monitoring. The instrument does not apply to movements of organisms within a EU Member State (except if there is a risk to the environment), pet-shops, garden centres or aquaria where there is no contact with EU waters, and selected species listed in Annex IV to the EU Council Regulation No. 708/2007.
A similar document was developed by ICES as a voluntary instrument (ICES 2005; Gollasch 2007). It indicates that a desk evaluation should be conducted well in advance of the introduction to include the following: (1) any previous known introduction(s) of the species elsewhere, (2) a review of all known diseases , parasites and other pests associated with the species, (3) a review of its physical tolerances and ecological interactions, (4) a determination of whether there are any possible genetic interactions in the new environment, and (5) a determination of the possible consequences of such an introduction and a hazard assessment. The document also prescribes quarantine and monitoring.
8 Review questions
-
1.
What is the difference between the range expansion of species and biological invasions?
-
2.
What are the main stages of a biological invasion?
-
3.
What are the major pathways of species introductions into the Baltic Sea?
-
4.
Which habitats are most susceptible to biological invasions in the Baltic Sea?
-
5.
What is biological pollution?
9 Discussion questions
-
1.
What do environmental managers need to know about bioinvasions?
-
2.
Why is it not correct to put “good” or “bad” tags on non-indigenous species?
-
3.
How would you rank prospective areas of bioinvasion research according to their importance for (a) the development of basic science and (b) direct societal applications?
-
4.
What are the differences between biological pollution and other forms of aquatic pollution?
-
5.
What is the most effective management option for (a) Prorocentrum cordatum , (b) Dreissena polymorpha and (c) Neogobius melanostomus, and why?
References
Anderson DM (2009) Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean and Coastal Management 52:342–347
AquaNIS (2015) Information system on aquatic non-indigenous and cryptogenic species. World Wide Web electronic publication. [http://www.corpi.ku.lt/databases/aquanis; Version 2.36+, viewed 2015-02-16]
Arbaciauskas K (2002) Ponto-Caspian amphipods and mysids in the inland waters of Lithuania: history of introduction, current distribution and relations with native malacostracans. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe – distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 104–115
Arbaciauskas K, Gumuliauskaite S (2005) Invasion of the Baltic Sea basin by the Ponto-Caspian amphipod Pontogammarus robustoides and its ecological impact. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Springer, Berlin, pp 463–477
Bastrop R, Röhner M, Jürss K (1995) Are there two species of the polychaete genus Marenzelleria in Europe? Marine Biology 121:509–516
Bernard FR (1979) Identification of the living Mya (Bivalvia: Myoida). Venus 38:185–204
Beukema JJ (1982) Annual variation in reproductive success and biomass of the major macrozoobenthic species living in a tidal flat area of the Wadden Sea. Netherlands Journal of Sea Research 16:37–45
Bielecka L, Mudrak-Cegiołka S, Kalarus M (2014) Evadne anonyx G.O. Sars, 1897 – the first record of this Ponto-Caspian cladoceran in the Gulf of Gdańsk (Baltic Sea). Oceanologia 56:141–150
Blank M, Laine AO, Jürss K, Bastrop R (2008) Molecular identification key based on PCR/RFLP for three polychaete sibling species of the genus Marenzelleria, and the species’ current distribution in the Baltic Sea. Helgoland Marine Research 62:129–141
Bochert R (1997) Marenzelleria viridis (Polychaeta: Spionidae): a review of its reproduction. Aquatic Ecology 31:163–175
Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S (2008) Gelatinous plankton: irregularities rule the world (sometimes). Marine Ecology Progress Series 356:299–310
Buynevich IV, Damušyte A, Bitinas A, Olenin S, Mažeika J, Petrošius R (2011) Pontic-Baltic pathways for invasive aquatic species: geoarchaeological implications. GSA Special Papers 473:189–196
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77:1653–1655
Carlton JT (2009) Deep invasion ecology and the assembly of communities in historical time. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems – ecological, management, and geographic perspectives. Springer, Berlin, Ecological Studies 204:13–56
Corkum LD, Sapota M, Skóra KE (2004) The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biological Invasions 6:173–181
Cristescu MEA, Hebert PDN, Witt JDS, MacIsaac HJ, Grigorovich IA (2001) An invasion history for Cercopagis pengoi based on mitochondrial gene sequences. Limnology and Oceanography 46:224–229
Daunys D, Zemlys P, Olenin S, Zaiko A, Ferrarin C (2006) Impact of the zebra mussel Dreissena polymorpha invasion on the budget of suspended material in a shallow lagoon ecosystem. Helgoland Marine Research 60:113–120
David M, Gollasch S (2008) EU shipping in the dawn of managing the ballast water issue. Marine Pollution Bulletin 56:1966–1972
David M, Gollasch S (eds) (2015) Global maritime transport and ballast water management – issues and solutions. Springer, Dordrecht, Invading Nature – Springer Series in Invasion Ecology 8:1–306
David M, Gollasch S, Leppäkoski E (2013a) Risk assessment for exemptions from ballast water management – the Baltic Sea case study. Marine Pollution Bulletin 75:205–217
David M, Gollasch S, Pavliha M (2013b) Global ballast water management and the “same location” concept: a clear term or a clear issue? Ecological Applications 23:331–338
Delefosse M, Kristensen E (2012) Burial of Zostera marina seeds in sediment inhabited by three polychaetes: laboratory and field studies. Journal of Sea Research 71:41–49
Elliott M (2003) Biological pollutants and biological pollution – an increasing cause for concern. Marine Pollution Bulletin 46:275–280
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London 181 pp
EU Council Regulation (2007) (No.708/2007 of 11 June 2007) concerning use of alien and locally absent species in aquaculture
Florin AB, Mo K, Svensson F, Schagerström E, Kautsky L, Bergström L (2013) First records of Conrad’s false mussel, Mytilopsis leucophaeata (Conrad, 1831) in the southern Bothnian Sea, Sweden, near a nuclear power plant. Bioinvasions Records 2:303–309
Forster S, Zettler ML (2004) The capacity of the filter-feeding bivalve Mya arenaria L. to affect water transport in sandy beds. Marine Biology 144:1183–1189
Fowler AE, Forsström T, von Numers M, Vesakoski O (2013) The North American mud crab Rhithropanopeus harrisii (Gould, 1841) in newly colonized Northern Baltic Sea: distribution and ecology. Aquatic Invasions 8:89–96
Fritzsche D, von Oertzen JA (1995) Metabolic responses to changing environmental conditions in the brackish water – polychaetes Marenzelleria viridis and Hediste diversicolor. Marine Biology 121:693–699
Gollasch S (2007) International collaboration on marine bioinvasions – the ICES response. Marine Pollution Bulletin 55:353–359
Gollasch S (2009) Eriocheir sinensis Milne Edwards, Chinese mitten crab (Varunidae, Crustacea). In: Drake JA (ed) Handbook of alien species in Europe. Springer, Dordrecht, Invading Nature – Springer Series in Invasion Ecology 3:312
Gollasch S, David M (2012) A unique aspect of ballast water management requirements – the same location concept. Marine Pollution Bulletin 64:1774–1775
Gollasch S, David M, Voigt M, Dragsund E, Hewitt C, Fukuyo Y (2007) Critical review of the IMO international convention on the management of ships’ ballast water and sediments. Harmful Algae 6:585–600
Gollasch S, Leppäkoski E (2007) Risk assessment and management scenarios for ballast water mediated species introductions into the Baltic Sea. Aquatic Invasions 2:313–340
Gómez F (2008) Phytoplankton invasions: comments on the validity of categorizing the non-indigenous dinoflagellates and diatoms in European Seas. Marine Pollution Bulletin 56:620–628
Gorokhova E, Fagerberg T, Hansson S (2004) Predation by herring (Clupea harengus) and sprat (Sprattus sprattus) on Cercopagis pengoi in a western Baltic Sea bay. ICES Journal of Marine Science 61:959–965
Gorokhova E, Lehtiniemi M, Viitasalo-Frösen S, Haddock SHD (2009) Molecular evidence for the occurrence of ctenophore Mertensia ovum in the northern Baltic Sea and implications for the status of the Mnemiopsis leidyi invasion. Limnology and Oceanography 54:2025–2033
Grabowski M, Konopacka A, Jażdżewski K, Janowska E (2006) Invasions of alien gammarid species and retreat of natives in the Vistula Lagoon (Baltic Sea, Poland). Helgoland Marine Research 60:90–97
Gren IM, Isacs L, Carlsson M (2009) Costs of alien invasive species in Sweden. AMBIO 38:135–140
Grigorovich IA, MacIsaac HJ, Rivier IK, Aladin NV, Panov VE (2000) Comparative biology of the predatory cladoceran Cercopagis pengoi from Lake Ontario, Baltic Sea and Caspian Sea. Archiv für Hydrobiologie 149:23–50
Hakanen P, Suikkanen S, Franzén J, Franzén H, Kankaapää H, Kremp A (2012) Bloom and toxin dynamics of Alexandrium ostenfeldii in a shallow embayment at the SW coast of Finland, northern Baltic Sea. Harmful Algae 15:91–99
Hällfors G (2004) Checklist of Baltic Sea phytoplankton species (including some heterotrophic protistan groups). Baltic Sea Environment Proceedings 95:1–208
Hansson HG (2006) Ctenophores of the Baltic and adjacent Seas – the invader Mnemiopsis is here! Aquatic Invasions 1:295–298
Hedman JE, Gunnarsson JS, Samuelsson G, Gilbert F (2011) Particle reworking and solute transport by the sediment-living polychaetes Marenzelleria neglecta and Hediste diversicolor. Journal of Experimental Marine Biology and Ecology 407:294–301
Hegele-Drywa J, Normant M (2009) Feeding ecology of the American crab Rhithropanopeus harrisii (Crustacea, Decapoda) in the coastal waters of the Baltic Sea. Oceanologia 51:361–375
HELCOM (2010) Maritime activities in the Baltic Sea – an integrated thematic assessment on maritime activities and response to pollution at sea in the Baltic Sea region. Baltic Sea Environment Proceedings 123:1–64
Hessland I (1946) On the Quaternary Mya period in Europe. Arkiv för Zoologi 37A(8):1–51
Höglund J, Andersson (1993) Prevalence and abundance of Anguillicola crassus in the European eel (Anguilla anguilla) at a thermal discharge site on the Swedish coast. Journal of Applied Ichthyology 9:115–122
ICES (2005) International Council for the Exploration of the Sea code of practice on the introduction and transfers of marine organisms. Denmark, Copenhagen 14 pp
Ilus E (2009) Environmental effects of thermal and radioactive discharges from nuclear power plants in the boreal brackish-water conditions of the northern Baltic Sea. STUK – Radiation and Nuclear Safety Authority, Helsinki, Finland, 380 pp
IMO (2004) International convention on the control and management of ship’s ballast water and sediments. International Maritime Organization, London, UK, 36 pp
Jaspers C, Titelman J, Hansson LJ, Haraldsson M, Ditlefsen CR (2011) The invasive ctenophore Mnemiopsis leidyi poses no direct threat to Baltic cod eggs and larvae. Limnology and Oceanography 56:431–439
Jaszczołt J, Szaniawska A (2011) The spiny-cheek crayfish Orconectes limosus (Rafinesque, 1817) as an inhabitant of the Baltic Sea – experimental evidences for its invasion of brackish waters. Oceanological and Hydrobiological Studies 40:52–60
Javidpour J, Sommer U, Shiganova T (2006) First record of Mnemiopsis leidyi A. Agassiz 1865 in the Baltic Sea. Aquatic Invasions 1:299–302
Johnston EL, Piola RF, Clark GF (2009) The role of propagule pressure in invasion success. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems – ecological, management, and geographic perspectives. Springer, Berlin, Ecological Studies 204:133–151
Jørgensen CB, Riisgård HU (1988) Gill pump characteristics of the soft clam Mya arenaria. Marine Biology 99:107–109
Jousson O, Pawlowski J, Zaninetti L, Meinesz A, Boudouresque CF (1998) Molecular evidence for the aquarium origin of the green alga Caulerpa taxifolia introduced to the Mediterranean Sea. Marine Ecology Progress Series 172:275–280
Karatayev AY, Burlakova LE, Molloy DP, Volkova LK (2000) Endosymbionts of Dreissena polymorpha (Pallas) in Belarus. International Review of Hydrobiology 85:543–559
Karatayev AY, Mastitsky SE, Burlakova LE, Olenin S (2008) Past, current, and future of the central European corridor for aquatic invasions in Belarus. Biological Invasions 10:215–232
Karlson AML, Almqvist G, Skóra KE, Appelberg M (2007) Indications of competition between non-indigenous round goby and native flounder in the Baltic Sea. ICES Journal of Marine Science 64:479–486
Katajisto T, Karjala L, Lehtiniemi M (2013) Fifteen years after invasion: egg bank of the predatory cladoceran Cercopagis pengoi in the Baltic Sea. Marine Ecology Progress Series 482:81–92
Kelly RP, Port JA, Yamahara KM, Martone RG, Lowell N et al (2014) Harnessing DNA to improve environmental management. Science 344:1455–1456
Kideys AE (2002) Fall and rise of the Black Sea ecosystem. Science 297:1482–1483
Kornis MS, Mercado-Silva N, Van der Zanden MJ (2012) Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80:235–285
Kotta J, Kotta I, Simm M, Lankov A, Lauringson V et al (2006) Ecological consequences of biological invasions: three invertebrate case studies in the northeastern Baltic Sea. Helgoland Marine Research 60:106–112
Kotta J, Ólafsson E (2003) Competition for food between the introduced exotic polychaete Marenzelleria viridis (Verrill) and the native amphipod Monoporeia affinis Lindström in the Baltic Sea. Journal of Sea Research 50:27–35
Kotta J, Orav H, Kotta I (1998) Distribution and filtration activity of zebra mussel, Dreissena polymorpha (Pallas) in the Gulf of Riga and the Gulf of Finland. Proceedings of the Estonian Academy of Sciences, Biology and Ecology 47:32–41
Kotta J, Orav H, Sandberg-Kilpi E (2001) Ecological consequence of the introduction of the polychaete Marenzelleria viridis into a shallow water biotope of the northern Baltic Sea. Journal of Sea Research 46:273–280
Kotta J, Orav-Kotta H, Paalme T, Kotta I, Kukk H (2003) Benthos studies in the Estonian coastal sea during 1998-2001. Proceedings of the Estonian Academy of Sciences, Biology and Ecology 52:85–90
Kownacka J, Edler L, Gromisz S, Łotocka M, Olenina I et al (2013) Non-indigenous species Chaetoceros cf. lorenzianus Frunow 1863 – a new, predominant components of autumn phytoplankton in the southern Baltic Sea. Estuarine, Coastal and Shelf Science 119:101–111
Laine AO, Mattilla J, Lehikoinen A (2006) First record of the brackish water dreissenid bivalve Mytilopsis leucophaeata in the northern Baltic Sea. Aquatic Invasions 1:38–41
Laxson CL, McPhedran KN, Makarewicz JC, Telesh I, MacIsaac HJ (2003) Effects of the non-indigenous cladoceran Cercopagis pengoi on the lower food web of Lake Ontario. Freshwater Biology 48:2094–2106
Lefebvre F, Wielgoss S, Nagasawa K, Moravec F (2012) On the origin of Anguillicoloides crassus, the invasive nematode of anguillid eels. Aquatic Invasions 7:443–453
Lehtiniemi M, Gorokhova E (2008) Predation of the introduced cladoceran Cercopagis pengoi on the calanoid copepod Eurytemora affinis in the Gulf of Finland, Baltic Sea. Marine Ecology Progress Series 362:193–200
Lehtiniemi M, Lehmann A, Javidpour J, Myrberg K (2012) Spreading and physico-biological reproduction limitations of the invasive American comb jelly Mnemiopsis leidyi in the Baltic Sea. Biological Invasions 14:341–354
Lehtiniemi M, Ojaveer H, David M, Galil B, Gollasch S et al (2015) Dose of truth – monitoring marine non-indigenous species to serve legislative requirements. Marine Policy 54:26–35
Leppäkoski E (2002) Harmful non-native species in the Baltic Sea – an ignored problem. In: Schernewski E, Schiewer U (eds) Baltic coastal ecosystems – structure, function and coastal management. Springer, Berlin, pp 253–275
Leppäkoski E, Gollasch S, Gruszka P, Ojaveer H, Olenin S, Panov V (2002a) The Baltic – a sea of invaders. Canadian Journal of Fisheries and Aquatic Sciences 59:1175–1188
Leppäkoski E, Gollasch S, Olenin S (2002b) Alien species in European waters. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe – distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 1–6
Leppäkoski E, Olenin S (2000) Non-native species and rates of spread: lessons from the brackish Baltic Sea. Biological Invasions 2:151–163
Leppäkoski E, Olenin S (2001) The meltdown of biogeographical peculiarities of the Baltic Sea: the interaction of natural and manmade processes. AMBIO 30:202–209
Meinesz A, Belsher T, Thibaut T, Antolic B, Mustapha KB et al (2001) The introduced green alga Caulerpa taxifolia continues to spread in the Mediterranean. Biological Invasions 3:201–210
Meixner R, Gerdener C (1976) Untersuchungen über die Möglichkeiten einer Fortpflanzung der eingeführten Pazifischen Auster Crassotrea gigas in deutschen Küstengewässern. Archiv für Fischereiwissenschaft 26:155–160 [in German]
Mills CE (1995) Medusae, siphonophores, and ctenophores as planktivorous predators in changing global ecosystems. ICES Journal of marine Science 51:575–581
Mills EL, Leach JH, Carlton JT, Secor CL (1993) Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. Journal of Great Lakes Research 19:1–54
Minchin D, Galil BS, David M, Gollasch S, Olenin S (2006) Overall introduction. In: Gollasch S, Galil BS, Cohen AN (eds) Bridging divides – maritime canals as invasion corridors. Springer, Dordrecht, Monographiae Biologicae 83:1–4
Minchin D, Gollasch S, Cohen AN, Hewitt CL, Olenin S (2009) Characterizing vectors of marine invasion. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems – ecological, management, and geographic perspectives. Springer, Berlin, Ecological Studies 204:109–116
Minchin D, Lucy F, Sullivan M (2002) Zebra mussel: impacts and spread. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe – distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 135–146
Mordukhai-Boltovskoi FD, Rivier IK (1971) A brief survey of the ecology and biology of the Caspian Polyphemoidea. Marine Biology 8:160–169
Mordukhai-Boltovskoi FD, Rivier IK (1987) Predatory cladocerans (Podonidae, Polyphemidae, Cercopagidae and Leptodoridae) in the world’s fauna. Nauka, Leningrad 1–182 [in Russian]
Norkko J, Reed DC, Timmermann K, Norkko A, Gustafsson BG et al (2012) A welcome can of worms? – hypoxia mitigation by an invasive species. Global Change Biology 18:422–434
Nummi P (2002) Introduced semiaquatic birds and mammals in Europe. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe – distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 162–172
Nyberg (2007) Introduced marine macroalgae and habitat modifiers – their ecological role and significant attributes. Göteborg University, 61 pp [PhD Thesis]
Ojaveer H, Gollasch S, Jaanus A, Kotta J, Laine AO et al (2007) Chinese mitten crab Eriocheir sinensis in the Baltic Sea – a supply-side invader? Biological Invasions 9:409–418
Ojaveer H, Kotta J (2015) Ecosystem impacts of the widespread non-indigenous species in the Baltic Sea: literature survey evidences major limitations in knowledge. Hydrobiologia 750:171–185
Ojaveer H, Simm M, Lankov A (2004) Population dynamics and ecological impact of the non-indigenous Cercopagis pengoi in the Gulf of Riga (Baltic Sea). Hydrobiologia 522:261–269
Olenin S, Alemany F, Cardoso AC, Gollasch S, Goulletquer P et al (2010) Marine Strategy Framework Directive – Task Group 2 Report. Non-indigenous species. EUR 24342 EN. Office for Official Publications of the European Communities, Luxembourg, p 44
Olenin S, Elliott M, Bysveen I, Culverhouse PF, Daunys D et al (2011) Recommendations on methods for the detection and control of biological pollution in marine coastal waters. Marine Pollution Bulletin 62:2598–2604
Olenin S, Leppäkoski E (1999) Non-native animals in the Baltic Sea: alteration of benthic habitats in coastal inlets and lagoons. Hydrobiologia 393:233–243
Olenin S, Minchin D, Daunys D (2007) Assessment of biopollution in aquatic ecosystems. Marine Pollution Bulletin 55:379–394
Olenin S, Narščius A, Minchin D, David M, Galil B et al (2014) Making non-indigenous species information systems practical for management and useful for research: an aquatic perspective. Biological Conservation 173:98–107
Olenina I, Wasmund N, Hajdu S, Jurgensone I, Gromisz S et al (2010) Assessing impacts of invasive phytoplankton: the Baltic Sea case. Marine Pollution Bulletin 60:1691–1700
Oliveira OMP (2007) The presence of the ctenophore Mnemiopsis leidyi in the Oslofjorden and considerations on the initial invasion pathways to the North and Baltic Seas. Aquatic Invasions 2:185–189
Orlova M, Golubkov S, Kalinina L, Ignatieva N (2004) Dreissena polymorpha (Bivalvia: Dreissenidae) in the Neva Estuary (eastern Gulf of Finland, Baltic Sea): is it a biofilter or source for pollution? Marine Pollution Bulletin 49:196–205
Orlova MI (2002) Dreissena polymorpha (Pallas, 1771): evolutionary origin and biological peculiarities as prerequisites of invasion success. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe – distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 127–134
Orlova MI, Telesh I, Berezina NA, Antsulevich AE, Maximov AA, Litvinchuk LF (2006) Effects of non-indigenous species on diversity and community functioning in the eastern Gulf of Finland (Baltic Sea). Helgoland Marine Research 60:98–105
Paavola M, Olenin S, Leppäkoski E (2005) Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuarine, Coastal and Shelf Science 64:738–750
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Petersen JK, Hansen JW, Laursen MB, Clausen P, Carstensen J, Conley DJ (2008) Regime shift in a coastal marine ecosystem. Ecological Applications 18:497–510
Petersen KS, Rasmussen KL, Heinemeier J, Rud N (1992) Clams before Columbus? Nature 359:679
Petersen KS, Rasmussen KL, Rasmussen P, von Platen-Hallermund F (2005) Main environmental changes since the Weichselian glaciation in the Danish waters between the North Sea and the Baltic Sea as reflected in the molluscan fauna. Quaternary International 133–134:33–46
Plichlová-Ptácniková R, Vanderploeg HA (2009) The invasive cladoceran Cercopagis pengoi is a generalist predator capable of feeding on a variety of prey species of different sizes and escape abilities. Fundamental and Applied Limnology 173:267–268
Pochon X, Bott NJ, Smith KF, Wood SA (2013) Evaluating detection limits of Next-Generation Sequencing for the surveillance and monitoring of international marine pests. PLoS ONE 8(9):e73935
Põllupüü M, Simm M, Põllumäe A, Ojaveer H (2008) Successful establishment of the Ponto-Caspian alien cladoceran Evadne anonyx G.O. Sars 1897 in low-salinity environment in the Baltic Sea. Journal of Plankton Research 30:777–782
Ponder WF (1988) Potamopyrgus antipodarum: a molluscan coloniser of Europe and Australia. Journal of Molluscan Studies 54:271–285
Purcell JE, Shiganova TA, Decker MB, Houde ED (2001) The ctenophore Mnemiopsis in native and exotic habitats: U.S. estuaries versus the Black Sea basin. Hydrobiologia 451:145–176
Rakauskas V, Putys Z, Dainys J, Lesutiene J, Lozys L, Arbaciauskas K (2013) Increasing population of the invader round goby Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae) and its trophic role in the Curonian Lagoon, SE Baltic Sea. Acta Ichthyologica et Piscatoria 43:95–108
Reise K, Olenin S, Thieltges DW (2006) Are aliens threatening European aquatic coastal ecosystems? Helgoland Marine Research 60:106–112
Remane A (1934) Die Brackwasserfauna. Verhandlungen der Deutschen Zoologischen Gesellschaft 36:34–74 [in German]
Riisgård HU, Seerup DF (2003) Filtration rates in the soft clam Mya arenaria: effects of temperature and body size. Sarsia 88:415–428
Rudinskaya LV, Gusev AA (2012) Invasion of the North American wedge clam Rangia cuneata (G.B. Sowerby I, 1831) (Bivalvia: Mactridae) in the Vistula Lagoon of the Baltic Sea. Russian Journal of Biological Invasions 3:220–229
Saiz E, Kiørboe T (1995) Predatory and suspension feeding of the copepod Acartia tonsa in turbulent environments. Marine Ecology Progress Series 122:147–158
Sandström (1990) Environmental monitoring at the Forsmark nuclear power plant. Swedish Environmental Protection Board, Report 3860:1–37
Sapota MR (2004) The round goby (Neogobius melanostomus) in the Gulf of Gdańsk – a species introduction into the Baltic Sea. Hydrobiologia 514:219–224
Sapota MR, Skóra KE (2005) Spreading of alien (non-indigenous) fish species Neogobius melanostomus in the Gulf of Gdańsk (South Baltic). Biological Invasions 7:157–164
Schiedek D (1993) Marenzelleria viridis (Verrill) (Polychaeta), a new benthic species within European coastal waters: some metabolic features. Journal of Experimental Marine Biology and Ecology 211:85–101
Sikorski AV, Bick A (2004) Revision of Marenzelleria Mesnil, 1896 (Spionidae, Polychaeta). Sarsia 89:253–275
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1:21–32
Snoeijs P (1989) Effects of increasing water temperatures and flow rates on epilithic fauna in a cooling-water discharge basin. Journal of Applied Ecology 26:935–956
Snoeijs P, Mo K (1987) Macrofauna on rocky substrates in the Forsmark Biotest basin, March 1984-March 1985. Swedish Environmental Protection Board, Report 3397:1–55
Snoeijs P, Weckström K (2010) Diatoms and environmental change in large brackish-water ecosystems. In: Smol JP, Stoermer EF (eds) The diatoms: applications for the environmental and Earth sciences, 2nd edn. Cambridge University Press, Cambridge, pp 287–308
Sokołowska E, Fey DP (2011) Age and growth of the round goby Neogobius melanostomus in the Gulf of Gdánsk several years after invasion. Is the Baltic Sea a new promised land? Journal of Fish Biology 78:1993–2009
Städler T, Frye M, Neiman M, Lively CM (2005) Mitochondrial haplotypes and the New Zealand origin of clonal European Potamopyrgus, an invasive aquatic snail. Molecular Ecology 14:2465–2473
Strasser CA, Barber PH (2009) Limited genetic variation and structure in soft-shell clams (Mya arenaria) across their native and introduced range. Conservation Genetics 10:803–814
Strasser M (1999) Mya arenaria – an ancient invader of the North Sea coast. Helgoländer Meeresuntersuchungen 52:309–324
Tendal OS, Jensen KR, Riisgård HU (2007) Invasive ctenophore Mnemiopsis leidyi widely distributed in Danish waters. Aquatic Invasions 2:455–460
Tomczak MT, Sapota MR (2006) The fecundity and gonad development cycle of the round goby (Neogobius melanostomus Pallas 1811) from the Gulf of Gdańsk. International Journal of Oceanography and Hydrobiology, Oceanological and Hydrobiological Studies 35:353–367
Verity PG, Smetacek V (1996) Organism life cycles, predation and the structure of marine pelagic ecosystems. Marine Ecology Progress Series 130:277–293
Verween A, Kerckhof F, Vincx M, Degraer S (2006) First European record of the invasive brackish water clam Rangia cuneata (G.B. Sowerby I, 1831) (Mollusca: Bivalvia). Aquatic Invasions 1:198–203
Vetemaa M (2006) Invasion history and population structure of the alien gibel carp Carassius gibelio in Estonian marine waters. In: Ojaveer H, Kotta J (eds) Alien invasive species in the northeastern Baltic Sea: population dynamics and ecological impacts. Estonian Marine Institute Report Series 14:30–34
Virbickas J. (2000) Lietuvos žuvys (“Fish of Lithuania”). Vilnius: Trys žvaigždutės, 192 p. [in Lithuanian]
Wakida-Kusunoke AT, MacKenzie CL (2004) Rangia and marsh clams, Rangia cuneata, R. flexuosa and Polymesoda caroliniana, in eastern Mexico: distribution, biology and ecology, and historical fisheries. Marine Fisheries Review 66:13–20
Warzocha J, Drgas A (2013) The alien gulf wedge clam (Rangia cuneata G.B. Sowerby I, 1831) (Mollusca: Bivalvia; Mactridae) in the Polish part of the Vistula Lagoon (SE Baltic). Folia Malacologica 21:291–297
Wasmund N, Göbel J, von Bodungen B (2008) 100-years-changes in the phytoplankton community of Kiel Bight (Baltic Sea). Journal of Marine Systems 73:300–322
Westman (2002) Alien crayfish in Europe: negative and positive impacts and interactions with native crayfish. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe – distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, pp 76–95
Winkler HM, Debus L (1997) Is the polychaete Marenzelleria viridis an important food item for fish? In: Andrusaitis A (ed) Proceedings of the 13th Symposium of the Baltic Marine Biologists, Riga, pp 147–151
Witkowski A, Grabowska J (2012) The non-indigenous freshwater fishes of Poland: threats to the native ichthyofauna and consequences for the fishery: a review. Acta Ichthyologica et Piscatoria 42:77–87
Wrange AL, Valero J, Harkestad LS, Strand Ø, Lindegarth S et al (2010) Massive settlements of the Pacific oyster, Crassostrea gigas, in Scandinavia. Biological Invasions 12:1145–1152
Zaiko A, Daunys D (2015) Invasive ecosystem engineers and biotic indices: giving a wrong impression of water quality improvement? Ecological Indicators 52:292–299
Zaiko A, Daunys D, Olenin S (2009) Habitat engineering by the invasive zebra mussel Dreissena polymorpha (Pallas) in a boreal coastal lagoon: impact on biodiversity. Helgoland Marine Research 63:85–94
Zaiko A, Lehtiniemi M, Narščius A, Olenin S (2011) Assessment of bioinvasion impacts on a regional scale: a comparative approach. Biological Invasions 13:1739–1765
Zaiko A, Minchin D, Olenin S (2014) “The day after tomorrow”: anatomy of an “r” strategist aquatic invasion. Aquatic Invasions 9:145–155
Zaiko A, Olenin S, Daunys D, Nalepa T (2007) Vulnerability of benthic habitats to the aquatic invasive species. Biological Invasions 9:703–714
Zettler ML (1996) Successful establishment of the spionid polychaete, Marenzelleria viridis (Verrill, 1873), in the Darß-Zingst estuary (southern Baltic) and its influence on the indigenous macrozoobenthos. Archive of Fishery and Marine Research 43:273–284
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Olenin, S., Gollasch, S., Lehtiniemi, M., Sapota, M., Zaiko, A. (2017). Biological invasions. In: Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T. (eds) Biological Oceanography of the Baltic Sea. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0668-2_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-0668-2_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0667-5
Online ISBN: 978-94-007-0668-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)