Abstract
This chapter deals with practical implications of the conceptual profile theory to the design and development of teaching sequences, using as an example a teaching project in thermal physics with 9th grade students in Brazil. We start with a discussion of a simplified version of the heat conceptual profile model built by Amaral and Mortimer, and then we show how the contents and activities of the teaching sequence fit with the learning demands related to this simplified model. Next, we examine the learning contexts and micro-contexts of the activities that have proved effective in promoting understanding of the potentialities and limitations of the prescientific zones of heat and introducing and strengthening the scientific way of thinking. Finally, we present classroom teaching episodes that indicate how the alternation between authoritative and dialogic discourse contributes to enhance students’ awareness of the heat conceptual profile. The work allowed us to indicate the heuristic potential of this theoretical framework for innovative teaching practices in science education.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
This chapter deals with successive actions in planning and developing teaching sequences to introduce the concepts of heat, temperature, and thermal balance in secondary education in Brazil. The conceptual profile theory was used in order to address concept formation in the study, which focused, thus, on the change of conceptual profiles, as discussed in other chapters of this book. Our objective in so doing is to show the heuristic potential of this approach when driving and inspiring effective actions in science teaching.
The first teaching experience reported here refers to the work carried out over two consecutive years with 9th grade classes of the Centro Pedagógico (university primary and secondary school) of the Federal University of Minas Gerais (UFMG). This school implements teaching innovations in the university and is a privileged forum for initial teaching training. The students come from different social classes and are selected in the first year through a public draw.
The second experience concerns the refining of this teaching planning for use in the following years by preservice physics teachers in training during their internships. Different from the first experience, we do not have a detailed record of interactions in the classroom, but only the written report of the experiences in the internship, from the trainee teachers’ point of view. The results of this approach became part of a chapter of a science textbook of which I am one of the authors (Aguiar Jr. et al. 2007).Footnote 1
Finally, from 2007 to 2009, I went back to this planning in training courses for science teachers of the Minas Gerais state public schools. Most of these teachers were initially trained as biology teachers and had limited knowledge of physics. Therefore, we believe that the teaching approach with these teachers should be compared to that which was used previously with students from secondary education.
The experiences in the university school (Centro Pedagógico) and in the teacher training courses were recorded on video, with the mapping of events and later selection of episodes for transcription and analysis. This enabled us to reflect about situations and, after some distancing from them, to correct some of the activities and pedagogical orientations for application in the classrooms, in a process of “developing the curriculum on a micro-scale” (Lijnse 1995). Here we present the results of these reflections, aligned with psychological, epistemological, and anthropological assumptions that support the view of science learning as changing and acquiring of awareness of conceptual profiles.
Therefore, the first item presented is a summary of the different zones that make up the conceptual profile of heat (see Chap. 1). Then follows a review of the teaching sequence, in terms of the relations established with these different ways of speaking and thinking about heat and thermal phenomena. At the same time, we will provide a detailed analysis of the conceptual aspects and learning demands (Leach and Scott 2002) that guided the preparation of the activities that make up the teaching sequence, highlighting the discussion around the status attributed by research in science education to the substantialist zone of the concept of heat. We believe this zone is not only an epistemological obstacle for the development of the scientific notion of heat, but can be considered, even if paradoxically, as a learning route for the development of an understanding of thermodynamics, because of its importance in differentiating between heat and temperature and in attributing invariants in physical transformations.
The next issue to be dealt with is the construction of learning contexts and their relation to conceptual profiles of heat to be developed. This seems to be a fundamental unfolding of the teaching approach inspired by the evolution of conceptual profiles, since there are contexts that evoke different elements of the profile. Also, some contexts can be more suitable to introduce new zones of the profile, according to a careful analysis of the learning demands. Therefore, the selection of adequate contextual situations is essential both in discussing the nonscientific zones of the concept as well as in introducing scientific zones and forging strategies for their appropriation by the students.
The third point treated in this chapter is the analysis of discursive interactions in the classroom, in which the processes of negotiation of meanings and consolidation of given meanings occur. For this we use the concept of communicative approach from Mortimer and Scott (2003) in order to analyze, throughout the teaching sequence, how the alternations between dialogic and authoritative discourses occur and how they can favor the appropriation by the students of the scientific ways of speaking and thinking about heat.
Finally, we highlight the importance of the activities that made it possible, throughout the teaching sequence, to raise awareness of the students’ own profiles, so as to guide the adequate use of scientific concepts in contexts and situations which demand them.
2 The Conceptual Profile of Heat
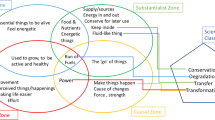
A profile for the concept of heat was developed by Amaral and Mortimer (2001) and is discussed in Chap. 1. This profile model includes five zones as follows: (1) heat and cold as opposite entities (sensorialist zone), (2) affinities for heat (animist zone), (3) heat as a substance (substantialist zone), (4) differentiation between heat and temperature (empiricist zone), and (5) heat as proportional to the difference between the temperatures of two systems (rationalist zone).
However, for the teaching actions reported here, a simplified version of this model was used. These five zones have been grouped into three, by merging the sensorialist and animist ways of thinking, making up the first zone of the simplified profile of heat, as well as merging the substantialist and empiricist ways of thinking, making up a second zone of the profile used for our teaching purposes.
This simplification of the profile model for teaching planning and action results from the different purposes of two spheres of human activity: on the one hand, the refinement and sophistication of research instruments and results, which can, in some circumstances, be excessive for teaching purposes, although necessary for a deeper understanding of the teaching and learning processes, and, on the other hand, teaching practice with its situated demands.
In the following sections, we will describe the zones taken as references for the planning and development of the teaching sequence on the topic “heat and temperature.”
2.1 Heat and Cold as Opposite Entities
In the first zone of the simplified profile of heat used in this study, cold and heat are treated as properties of objects. Mortimer et al. (2010) show that in the ontogenetic, sociohistorical, and microgenetic domains, there is strong evidence that the concept of heat is associated with hot things and often in an undifferentiated way. Field research in science education shows a strong tendency of students to consider the opposition between heat and cold, and sometimes they assume two types of heat: the hot heat and the cold heat (Cafagne 1996; Silva 1995; Erickson 1985). It is a logic which draws on attributes, qualities of materials and objects, as a way of providing explanations for the phenomena (Aguiar 2002). The experience of thermal sensations – cold and heat – seems to be at the root of this way of understanding thermal phenomena.
This view ignores the notion of thermal balance, since temperature is considered as a property of materials, which have a great affinity for cold or for heat. In order to explain the movements of heat, children tend to describe heat as something with an intrinsic driver. Silva (1995) presents ideas of students that attribute animistic properties to objects (wanting to give or receive) to explain the processes of heating or cooling.
Studies indicate that this zone of the profile is extremely powerful and present in everyday language when speaking about hot and cold things. Therefore, we refer to “warm clothing,” and we close the windows “for the cold not to get in.”
2.2 Heat as Substance and Differentiation Between Heat and Temperature
In the second zone of the profile used as a reference for teaching planning, heat is considered as a kind of subtle substance that can be stored or contained in objects and transferred from one place to another, disseminating itself in matter. The historical importance of this way of thinking is undeniable. It was originally formulated by Joseph Black and reflected in the works of Lavoisier and Laplace, at the end of the eighteenth century, and of Carnot, at the beginning of the nineteenth century. In fact, this assumption made it possible to highlight an invariant in thermal phenomena (the caloric, which cannot be created or destroyed) and a distinction between causes and effects, with the differentiation between heat and temperature. This differentiation made it possible to empirically investigate the phenomena of heat and the thermal properties of materials.
This notion of heat as substance has been superseded in scientific theory, but, even today, it is still present, especially in the ways of thinking and speaking of technicians and engineers operating thermal machines and refrigeration devices. The very language of thermodynamics is marked by substantialist expressions – flow or transfer of heat, specific heat, latent heat, as well as the use of diagrams that indicate the entrance or exit of heat with arrows. More than the historical reminiscences of a superseded ontology, the use of these expressions and representations shows the heuristic potential of the metaphor of heat contained and transferred from one place to another, which continues valid in situations in which work is nil (i.e., in which the energy transfers are carried out only by heat).
In science education, there prevails a view that substantialism represents a strong obstacle to the learning of scientific concepts. Besides heat, other physical measures, such as energy and electric current, are frequently treated as “material substances.” There is an undeniable difficulty in the transition from an ontology based on “things” to another based on “events” or “processes” (Chi 1992).
However, I believe that research in science education has given too little relevance to the heuristic potential or “pragmatic power” of substantialist notions which, among other things, are at the root of the attribution of invariants, that is, the consideration that something is conserved in transformation. Also, the substantialist view allows for the differentiation between heat and temperature. With this differentiation, heat becomes empirically distinct from the sensation of heat, a fundamental piece in the construction of a theory for thermal phenomena.
Therefore, according to Bachelard (1996), some ideas are, at the same time, powerful ways of understanding the world and obstacles to the later advance of knowledge. The evolution of the conceptual profile intends to examine this heuristic potential while not ignoring its limitations and, therefore, the need to go beyond it in treating particular problems.
2.3 Heat as Energy in Transit Proportional to the Differences of Temperature
The third zone of the conceptual profile of heat considers it as a way of transferring energy between systems at different temperatures. Heat is not considered permanent and exists only when there is a difference of temperature. In the language of thermodynamics, heat is not a state variable. This way of thinking involves, in the first place, the equivalence between the transfer of energy through heat and work, mechanical or electric. The phenomena of heat are dealt with using energy balance as the wider reference, while heat is only the exchange currency.
Second, this concept of heat involves the construction of the kinetic model of particles, with the attribution of intrinsic, incessant, and random movement to the particles, which explains the difficulties of conversion of heat into work. Therefore, the third zone of the profile model involves the differentiation between heat and internal energy and the understanding of the relations between heat and work. The problem of the irreversibility of processes and the concept of entropy are the unfolding of the idea of thermal movement and the probabilistic treatment that results from it.
3 Choices of Teaching Content
Having characterized the different zones of the conceptual profile of heat, we now indicate the approach to content and the focus of the activities to be carried out in the classroom. For an initial and introductory treatment of thermal physics for the 9th grade (14–15-year-olds), we have chosen as a focus the first two zones of the profile of heat and some aspects of the third zone. While still using aspects of the model of particles, already taught to these students, the emphasis would be on the appropriation by the students of the relations between the concepts of heat, temperature, and thermal balance.
Therefore, some decisions were made with regard to the treatment of thermal phenomena, which resulted in a teaching planning with the following characteristics:
-
1.
Analysis of the logic of hot/cold attributes in interpreting thermal phenomena
-
2.
Predominantly macroscopic treatment, through the concomitant construction of the concepts of heat, temperature, and thermal balance (Arnold and Millar 1994, 1996)
-
3.
General and qualitative approach, avoiding the presentation of equations and solution of numerical problems
-
4.
“Friendly coexistence” with the idea of heat “contained” in the bodies, understood as an intermediary stage in the construction of scientific concepts
-
5.
Considering heat as a form of energy, that is, that can be obtained through other sources of energy and transformed into other manifestations of energy
-
6.
Considering the energetic balance of organisms, showing the transfer of energy as heat dissipated into the environment and heat produced by cell respiration
The last point resulted from the contexts of approach to the theme justified below.
4 Defining the Contexts for Learning
Before advancing, it is necessary to briefly outline what is meant by context, referring the reader to Chap. 10 for deeper discussion. According to our approach, contexts are not given or static. On the contrary, they are created dynamically through interactions via negotiation and intersubjective contracts between participants in a given sphere of human activity.
Also, different contexts may coexist at the same place and be reviewed on different scales. A general context (macro-context) may include more than one specific context (micro-context). We may, for example, consider the macro-context of society, the meso-context of the school, and the micro-context of the classroom. The cross section to be used in this chapter considers the science classroom as the context, the teaching sequence (“temperature regulation in living beings”) as a meso-context, and the contexts created by the teaching activities throughout this sequence as micro-contexts.
Considering the science classroom context, we do not refer to an abstract being, but to a configuration that is forged in the relations and interactions between teacher, students, and objects of knowledge. These relations are also permeated by elements of social representation, such as the value attributed to the sciences in our society, by the materiality of the school, by teaching resources and methodologies, and by predispositions of what students are expected to learn. In the school we observed, natural sciences had been chosen by the students as one of their favorite subjects, only after physical education. We believe that this results from the work dynamics established by the teachers of the area and the incentive given to the students in bringing their experiences, curiosities, and interests in dialogue with science content. It must be remembered also that the context of science classrooms has an intrinsic complexity as it includes concepts, models, and languages of various subject fields with specific subcultures.
The second hierarchical level of the learning context refers us to the teaching sequence reported and analyzed here. This teaching sequence was drawn up around the theme “temperature regulation in living beings” and was made up of activities, concepts, and models of thermal physics and animal physiology. This sequence was presented to the students with a set of questions and issues involving thermal sensations and changes in our organism in response to environmental conditions. The issues raised in the opening activity referred to situations from everyday life but signaled a promise that these situations should be “explained” using concepts and models from physics and physiology. The context of the teaching sequence, therefore, promised a dialogue between questions from everyday life contexts (e.g., why do we transpire and feel hot when we practice sports? Why do we cool down when there is a wind?) and from scientific concepts (“after all, what do we understand by heat and temperature?”) to be used to re-signify these experiences. This context of issues related to thermal sensations extended and permeated all the activities carried out. One evidence that this context was shared by students and teachers was the dedication of students in answering questions placed by the teacher (“what happens to our body when we feel hot?”) and, especially, the fact that the students themselves formulated questions about the context presented (“why do we feel hot at 30 °C if my body is at 36 °C?”).Footnote 2
The third hierarchical level refers to the micro-context of the activities carried out in the classroom. Next we will present some of these situations guided by the temporal sequence of events. We can say that the context of the activities was closer to school physics, using experimental situations to build physical models of heat, temperature, and thermal balance.
These three hierarchical levels of contexts will be presented in dialogue with the zones of the conceptual profile of heat. According to our theoretical view, concepts are not stable mental entities, owned by individuals, but cultural references that are appropriated and rebuilt by each subject in the various contexts of social life. Therefore, it is essential to configure, in the planning, the contexts that are most adequate to enable the emergence of multiple views for the concept of heat in order to appropriate aspects of the scientific model of heat, temperature, and thermal balance.
The research on conceptual profiles indicates that it is the contexts that evoke the different zones of the profile. The context of the teaching sequence and of the problems evoked by it refers to the issues of the sensations of hot and cold and, beyond them, to temperature regulation of living beings. In fact, this issue, in the interface between biology and physics, makes it possible to highlight the interactions of the organism with the environment as an object of study and, thus, go beyond a simplistic view that sensations allow us to perceive the world “such as it is.” On the contrary, the relational view informs us that we only perceive the world while transforming its stimuli, that is, interacting with it. Another advantage of the context of study of temperature regulation in living beings, for the introduction of thermal physics, is that it makes it possible to deal with the energy balance of organisms, considering, on the one hand, the continuous transfer of heat from our organism to the environment and, on the other, the constant production of energy by the metabolism.
Before going forward, it must be said that we chose a curricular sequence in which the contexts evoke and raise certain concepts. This way of working the curriculum intends to reduce the distance between school content and contemporary life, its problems, and challenges. In the case of the study of temperature regulation in living beings, this theme requires, besides the physical concepts of heat, temperature, thermal balance, and energy balance, other concepts and models of animal physiology (the action of the hypophysis, thermal-regulatory mechanisms, and reactions of the organisms to hyper- and hypothermia).
5 Example of the Micro-context of Some Activities
The dynamics of the classroom involved experimental activities carried out in groups or presented by the teacher, always followed by raising questions and tasks to be carried out by the students. These activities ended with discussions involving the whole class, in which the teacher sought to build, together with the students, summaries of what had been said. Before the activities, there was a discussion to contextualize the specific activity and the general work plan of the teaching sequence. We would often combine the activities and the reading of short texts. This way of organizing the teaching had the objective of providing teaching and learning environments favoring student involvement and activity, through their engagement in tasks, mediated by language and interactions with their peers and the teacher. The intention was to configure the classroom in such a way that the students developed scientific ideas in the context of relevant tasks with the support and guidance of the teacher. Considering the crucial role we attribute to language in the conceptual development of students, we sought to identify and guide the ways of speaking of the students in the context of tasks and problems to be solved throughout the activities.
The first activities of the course had the objective of showing the previous concepts of the students, that is, the prescientific elements of the conceptual profile. This manifestation is important because it makes it possible to find cultural ways of thinking and speaking that are inevitably related to the scientific concepts to be developed. However, to recognize their legitimacy in these domains does not mean to dissolve them in an amalgam of scientific and everyday concepts without a clear distinction between them. On the contrary, we seek to highlight the need for new concepts for a scientific approach of the thermal phenomena. Therefore, we demanded that the students seek criteria of generality, coherence, and internal consistency, accepted values of the scientific way of getting to know the world.
Besides the pretest, the first two activities of the course had this aspect of eliciting and raising issues of prescientific concepts. In the first, we sought to show the inadequacy of touch to determine the thermal state of the materials. The groups handled three containers with water at different temperatures, moving their right hand from the hot water container to the room temperature container and their left hand from the cold water container to the room temperature container. They were encouraged to find explanations for the different sensations to touch brought about by the water. The second activity involved foreseeing and comparing the behavior of a block of ice and a hot potato rolled in flannel compared to other identical objects in contact with the environment.
When carried out in the classroom, these first activities evoked the sensorialist zone of the profile of heat. Then, we created strategies to question the validity of this idea as the general organizer of the thermal phenomena. One of the strategies used to discuss this idea involved proposing the possibility of a glass with melting ice to behave as a “source of heat.” The activity required preparing a system that was “colder” than the melting ice – chipped ice with salt – and observing what happens to a thermometer when moved from the glass with “ice + salt” to the glass with melting ice. This strategy was carried out as part of a discursive framework in which the students took part working in small groups and the teacher provoked and fed into the discussions. In his interventions, the teacher emphasized the relativeness of what could be considered cold or hot, an idea presented in contrast with the initial concept of cold and hot as opposite qualities of heat. At this moment, the teacher sought to highlight the simultaneity of the warming of the body that was initially at a lower temperature and the cooling of that which was at a higher temperature in order to explain these two simultaneous effects from a single and the same process of transfer of energy.
We sought to establish the concept of thermal balance progressively, beginning with the intuitive notions of the students who accept it in some circumstances. Although limited to certain typical “cases,” the final equal temperature between bodies in contact is not entirely strange to the students. The problem lies in the lack of generality of their proposition, whether counter to thermal sensations, or because they did not consider the special conditions in which the phenomenon occurred – isolated systems. Therefore, the formulation of the concepts of heat, temperature, and thermal balance was carried out in three activities with the progressive distancing from the initial notions.
In the first of these activities, students were asked to foresee, observe, and explain the temperature variations of two equal amounts of water – at 20 and 50 °C, respectively − placed in an aquarium with a metal plate separating the two environments (Fig. 9.1). This activity was carried out seeking not only to emphasize the final state of the system but also to describe in detail the process that leads to this state. The problem of frontiers – “what should be the temperature of the metal plate?” – and the consideration of the transfer of heat to the environment were aspects highlighted in interpreting the experiment. Also, the situation made it possible to show that the heated water, with red die, does not mix with the other, although something “passes” from one side to the other of the aquarium.
The situation makes it possible to differentiate between heat (transfer of energy) and temperature (the variation of temperature is one of the effects of heat). It also allows to model the relations between the concepts of heat, temperature, and thermal balance: there is only heat while there is a difference of temperature between the systems, and the variations of temperature that the heat brings about result, in the end, in thermal balance. In the interpretation of the students, we observe that there prevails a substantialist notion of heat (“the red water has more heat and passes it to the other”). This interpretation was admitted as valid, although the teacher uttered it in terms of energy transfer.
In more recent teacher training activities, we added another approach in which the aquarium is divided into unequal volume sections of hot and cold water (see Fig. 9.2). In this case, the final balance temperature depends on the amounts of hot water. Even in an ideally isolated system, the reduction of the temperature of the hot water does not correspond, in this case, to the increase of the temperature of cold water. However, we admit that the amount of heat transferred by the hot water is equal to the amount of heat absorbed by the cold water, supposing the container was isolated and ideal. This situation, therefore, makes it possible to identify heat as the extensive variable and the temperature as the intensive variable, with various other situations raised and discussed (e.g., heating a coin until it glows and dropping it in a small glass of water or in a large tank full of water and comparing the results).
A second situation involving thermal balance was examining the generality of final equal temperatures when different materials are placed in the same environment. To do this, we used blocks of wood and aluminum with an orifice to enable the measurement of temperature (Fig. 9.3). This situation placed us before another obstacle which is epistemological, involving overcoming the idea that sensations correspond directly to the properties of the objects, not knowing the importance and the nature of the interaction between organism and environment that determine them. It was not enough to observe the empirical data supplied by the readings on the thermometer. It was also necessary to provide indications and inputs for a new synthesis that made it possible to explain the different sensations to touch by objects in thermal balance. The guideline provided for the activity was to monitor the process of warming inside each of the items used when we hold them in our hands, so that the students’ attention moved to the process of gradual heating of the materials when touched. In this way, we intended to observe the flows of energy that result from the interaction of the hand holding each of the blocks with the materials they are made up.
We identified the contexts of this second block of activities (aquarium with metal plate separating the two environments and cubes of wood and aluminum) in close relation to the empiricist zone of the profile of heat (2nd zone of the conceptual profile).
A third block of activities involved guiding the discussion for the notion of heat as a flow of energy between systems, that is, to the rational zone of the heat conceptual profile. In this case, we considered situations in which the physical system is not isolated, that is, it receives energy from a source, which means there is no thermal balance. We should note that isolated systems correspond, approximately, to some actual situations, but not all of them. In this case, the principle of thermal balance has its generality in the direction of the process – the system tends to thermal balance – and not its final state, indicated by equal temperatures. It is, therefore, a question of finding a common explanation to the occurrence and nonoccurrence of thermal balance from notions of system, surroundings, and flows of energy.
The conservation of energy was presented as a hypothesis in the context of the study of energy balance in organisms. The analogy with the soldering iron was the strategy used as a resource to assist in this construction. As in every analogical thinking used to explore new domains, we sought to highlight the common aspects and differences between the two systems – soldering iron and human body. In this case, the key question was: What are the physical conditions necessary for a body to maintain its temperature constant? Given the tendency of spontaneous thinking of concentrating on each aspect of a problem or entraining causes taken separately as a linear sequence of processes (Viennot 1997), we seek to assist students to consider, simultaneously, the processes linked to heat transfers and the production of energy by the system: while the soldering iron heats up, the energy supplied by the electricity system is higher than the energy transferred to the environment, and, thus, the temperature of the iron increases; this increase in the temperature of the system results in an increase of the flow of heat to the environment; since the supply of electricity is constant, after some time, the flows of energy will be equal, which explains, from that point, the maintenance of the temperature. In both cases, the system does not reach a thermal balance due to an additional source of energy. These transformations can be schematically represented in terms of flows of energy, as shown in Fig. 9.4.
On the other hand, contrary to what happens with the soldering iron, the supply of energy of a person’s organism does not happen at a constant rate, depending not only on the supply of oxygen and nutrients to the cells but also on a set of factors that can inhibit or favor metabolic rates, for example, the intensification of the muscle tonus and hormonal action. Therefore, as happens with metabolic rates, the rates of heat transfer to the environment are also regulated by the central nervous system. In this case, the variation of the amount of heat transferred depends not only on the difference between the temperature of the body and the environment but also on many other factors, such as: transpiration, relative humidity of the air, dilation or constriction of subcutaneous capillary vessels (which changes the thermal conductivity of the skin) and surface of the body exposed to the environment.
In conclusion, we sought to expand considerations about energy balance, considering the differences between homothermal and heterothermal animals in terms of intensity of energy exchanges with the environment and the structures covering the body through some “cases” of adaptation of organisms to the temperature conditions of the environment, which were otherwise unfavorable.
This brief description of the teaching activities makes it possible to highlight correspondences between the micro-contexts of the teaching activities, organized in time, and the conceptual profiles of heat, informed by the research. We should also note that the contexts evoked are almost always those of analogical construction of physical models in school situations. This didactic context was coordinated with the discussion of everyday situations, such as: What happens to my body when, on a very hot day, I go into a room with air conditioning? Why do we feel cold when we get out of the pool with our body wet?
6 Shifts Between Authoritative and Dialogic Discourse
When understanding science learning as an evolution and becoming aware of conceptual profiles, we should give special attention to the way teacher and students, in the classroom, develop discourse in the context of relevant tasks. After all, the conceptual profiles model different ways of thinking and speaking about the world which are used and appropriated by the subjects when participating in social practices in specific contexts.
For planning and analyzing teaching situations in the classroom, the concept of communicative approach proposed by Mortimer and Scott (2003) and discussed in Chap. 3 will be used.
Mortimer and Scott (2003) highlight the importance of transitions between dialogic and authoritative discourse to support the learning of scientific knowledge by the students. Therefore, when the teacher raises a theme with questions for debate, she explores the concepts of the students, raises questions, and, in this way, adopts a dialogic and interactive communicative approach. In other moments of the sequence, we see the same teacher guiding the work of the students in a more restricted way, with the intention of developing certain scientific ideas, seeking to introduce them into the students’ repertoire. She then adopts an authoritative discourse, with different degrees of participation of the students in speech turns. In this case, we see the teacher considering only those utterances from the students that contribute to the scientific point of view that is being developed and avoiding or correcting the utterances that indicate other, nonscientific points of view. At the end of the sequence, the teacher can introduce new situations and allow the students to explore them and go back to dialogic discourse but now at a different level. In this case the teacher gives the student greater freedom in solving the problems posed, so as to explore the relations between the concepts and between the concepts and the context of the problem to be solved.
It is also important to highlight the action of the teacher with comments about what is being done in order to generalize conclusions and systematically organize the content. Sometimes these reflections are done in a noninteractive and dialogic way, taking into account different points of view presented in relation to a given concept. These comments are teacher’s actions which intend to provide the students with awareness of the range of ways of speaking and thinking about the world and their suitability to the contexts and spheres of human activity.
Scott et al. (2006) highlight the existence of a tension between authoritative and dialogic discourse. According to them this tension is inevitable, since the social language of science is, essentially, a voice from authority, but its appropriation demands a responsive attitude from the student (Bakhtin 1986), confronting the school science view with other possible views, which generates dialogic discourse. When working with classroom content, the teacher must decide between encouraging the students to express their points of view and focusing on the ones that are closer to those accepted by science. It does not mean that dialogic discourse is superior to that from authority but rather to understand in which situations dialogic discourse is most appropriate and in which it is necessary to narrow the meanings so as to examine and deepen the scientific point of view in an authoritative discourse.
Now we will give examples and comments on this alternation between dialogic discourse and authoritative discourse, utilizing some classroom teaching episodes in the sequence reported here. These passages also make it possible to identify the manifestation by the students of elements that make up the zones of the profile of heat and review how the teacher deals with these situations.
7 Eliciting and Raising Questions on Students’ Conceptions: The First Zone of the Profile in Discussion
In one of the first lessons of the sequence, the teacher works with one of the groups and discusses, with the students, the meanings they attribute to “heat exchanges” between ice and the environment.Footnote 3
-
Teacher: In the case of the ice with the environment / the ice in the flannel. Is the ice losing heat to the environment when it melts?
-
Student 3: No / It’s not exchanging heat // it’s keeping it ((referring to the flannel, which keeps the ice cold for a while)).
-
Teacher: Exchanging heat // What is this heat exchange thing?
-
Student 3: There’s one thing that’s colder and another that’s warmer. Then they keep getting together like this until they’re equal. Then the heat kind of keeps passing heat from one to the other / from one to the other / until they are equal.
-
Student 2: Yeah. Until the heat is equal.
-
Student 1: It’s like this. There’s the ice. Put the ice here. Then put your hand over the ice ((He makes a gesture as if there were ice beneath his hand)). The air that’s colder here and the warmer air / so then they keep exchanging this air ((Student 3 interrupts him and completes his sentence)).
-
Student 3: with the warmer air until they get to the same temperature / then the flannel doesn’t let the air / I mean / the heat from outside / get to the ice and melt it.
-
Student 1; Next the ice melts and the water reaches the same temperature as the environment. The flannel stops this air / the warmth from outside / from getting to the ice and melting the ice.
-
Teacher: OK. Yes / The ice and the environment / it / Does it pass something to the environment when it melts? ((The teacher here retakes the group’s conclusion from the second experiment)).
-
Student 3: Uh huh. ((He agrees and gestures with his hands over the table as if there were an ice cube there)). It’s just that / it’s that if you get real close you see / you feel that something is passing / only that the air is much larger
-
Student 1: The space that the air takes up is larger than the space that the ice takes up.
-
Student 4: So then it exchanges more heat.
-
Student 3: Only that the air is much larger. If the ice was gigantic you could feel it better.
-
Student 4: If you put your hand there you’ll feel that the air is a lot colder.
-
Student 1: Like an iceberg. An iceberg for example //
-
Teacher: Is it transferring heat to the environment?
-
Student 4: Yeah. It is.
-
Student 1: It is. And the environment is transferring heat to the ice.
-
Student 3: Just a little compared to the environment / which is gigantic.
In this passage we can see the actions of the teacher, inviting the students to present and develop their points of view, even when they do not agree with the school science view. This action favors the rise of students’ utterances that reveal, in this context, a strong attribution of “heat exchanges,” cold and hot, between ice and the air around it. When asked to justify their positions, the students stated that they come from their perception of “cold heat” coming from the ice. Therefore, they refer to a hypothetical action of putting their hand close to the surface of the ice cube and also a large iceberg floating in the sea. The discourse shows a meaning of heat which belongs to the material itself and exchanges with the heat of the environment until they are equal. These utterances enable us to describe these hypotheses as belonging to the first zone of the conceptual profile of heat, as they consider heat and cold as two opposite kinds of “heat” and treat heat as a something that belongs to the materials.
In this same lesson, a few minutes later, we see the teacher working in a different way with the same group of students. After advancing the discussion with the students, he goes back to the meaning of heat as related to the movement of particles and then changes from dialogic to authoritative discourse.
-
Teacher: And what would be the difference between the cold part and the warm part? What’s the difference between them?
-
Student 3: Between the cold part and the warm part? The warm part has more heat / it’s moving more.
-
Student 1: It’s that it’s already received the heat that the ice has / and the other part hasn’t.
-
Teacher: OK. If we could see the particles / how would the cold part of the ice look and how would the warm part look?
-
Student 3: The cold part has less movement and the warm part has more heat.
-
Student 4: The warm part would have more movement and the cold part has less movement.
-
Student 1: It’s moving more.
-
Teacher: Then when it comes into contact ((with the colder part)) what will happen?
-
Student 1: It gets to a speed that’s close / their speeds are going to become equal / it will start moving the same amount / then the speeds will become equal.
-
Teacher: But it’s transferring energy. You were saying that heat is energy. So then / from where to where will it transfer energy? From the ice to the environment or from the environment to the ice?
-
Student 1: From the ice to the environment.
-
Student 4: From the ice to the liquid.
-
Teacher: From the ice to the environment? Is the energy being transferred from the ice to the environment?
-
Student 1: No, no // from the liquid to the ice because //
-
Student 4: It will gain more speed.
-
Student 1: Yeah //
-
Teacher: From the environment to the ice / there is an energy transfer from the environment to the ice ((He completes this statement with pauses between words and a professorial tone.))
-
Student 1: Because there’s more movement.
-
Teacher: That’s right.
-
Student 3: But why does the ice cool the environment too?
-
Student 4: Because there’s an exchange from the ice to the environment too.
-
Student 3: But then / it is not necessary to mix all of the parts. Then / if you take two solid materials like these / the temperatures don’t mix together and they exchange heat in the same way.
-
Teacher: They exchange heat in the same way / but the particles don’t mix together. There is an energy transfer.
-
((one student asks how the heat could move from one place to another and the teacher encourages them to imagine how this can be; this discussion takes 10 turns of speech))
-
Teacher: There’s one body that’s at a higher temperature / so it’s vibrating more, right? Then you put it next to another body that’s at a lower temperature. When it transfers energy to the other body / does something happen to this one that was vibrating more?
-
Student 3: I think it loses energy in order to make the other body move more.
-
Student 1: Because it receives the //
-
Teacher: And what happens to its temperature?
-
Student 3: It will drop.
-
Student 1: It will drop and become equal.
-
Student 4: It will try to become equal.
-
Teacher: Great. ((The teacher moves away to help other groups. The students make notes and answer the questions presented by the experiment.))
Comparing this passage with the previous one, we observe a change in the questions asked by the teacher: instead of encouraging the students to formulate their hypotheses or opinions about the themes, the questions are now guided theoretically and provide support and direction to the students’ thinking. The teacher asks about the difference in terms of the particle model of the portion of hot water when compared to the cold water and then asks what happens when they are in contact. By formulating these questions, the teacher seeks answers in agreement with the scientific view, and, when the students answer differently, he evaluates them soliciting and giving support to a new formulation by the students. The discourse is, therefore, predominantly interactive and authoritative. The teacher introduces the idea of heat as energy, which had not yet been evoked by the students and keeps the expression “transfer of energy” in all its interventions. When doing so, he introduces new zones of the profile, molding ways of speaking and ways of thinking about the phenomena under discussion. In his discourse, the teacher brings aspects of the third zone of the conceptual profile of heat, the rationalist one. However, at some stages, it can be seen that the ways of speaking of the students evoke aspects of the substantialist zone, such as the lack of distinction between heat and the particles that make up matter.
As we see it, the transition from dialogic discourse, shown previously, to the authoritative discourse is necessary when introducing and strengthening new zones of the conceptual profile, making them available for the students to be able to gradually appropriate them, under the supervision of the teacher.
On the other hand, it was the dialogic discourse at the beginning which made it possible for the teacher to recognize why the students had a difficulty in abandoning the idea of the ice transmitting its heat (cold) to the environment. The question asked by student S3 (“why then does the ice cool the environment?”) shows a way of thinking frequently used in everyday life, in which each effect has a single cause. To answer the student’s question, the teacher again uses guided questions so that S3 and his colleagues can perceive that the transfer of heat from the environment to the ice can explain both processes simultaneously: on the one hand, the warming and, later, the melting of the ice and, on the other hand, the cooling of the air in the nearby environment.
In the next lesson, the teacher, after discussing with the whole class the activities carried out with the groups in the previous lesson, establishes the difference between everyday ways of speaking about heat and cold and those of scienceFootnote 4:
-
Teacher: Now let’s return to our question. Last week some groups were talking about there being two kinds of heat // hot and cold heat. In fact / this is not a new idea. In the history of science it’s been around for a long time.
-
Also / we often think about heat in terms of our sense of touch and we have distinct senses of hot and of cold. So / we naturally tend to accept that there are two opposite and separate things / hot heat / which warm objects have and cold heat / which cool objects have.
-
But we have to examine these ideas to see whether they can help us understand the notion of heat or not. So / there are two things. The first relates to what we call ‘cold’ or ‘the cold’. There is nothing which is absolutely cold is there? For example / melting ice // we think it is really cold / but is it compared to ice plus salt? Is it cold?
-
Student?: No.
-
Teacher: No / it’s warm. It’s a source of heat. If you put both in contact / pure melting ice will pass heat to the ice with salt. What is cold? I can say that it is less hot and the opposite is also true / hot is less cold. Cold and hot are relative ideas / aren’t they? It’s a matter of comparing things. So / does it help to think about two kinds of heat, one associated with hot objects and the other with cold? There is a second point, an important one //
Here we see the teacher going back to the initial idea of the students that there are two types of heat, hot or cold, recognizing that this idea has historical roots in the development of scientific thinking. Next he uses a group activity done by the students (with melting ice and ice with salt) to discuss the idea of two types of heat and support the scientific view that “heat and cold are relative concepts.”
Thus, this episode constitutes one turning point in the flow of discourse of this lesson sequence, as there is a clear transition from dialogic to authoritative discourse. The teacher brings together everyday and scientific views, through a dialogic discourse, in the first paragraph, and then makes an authoritative case for the scientific view that there are not two kinds of heat. The teacher has developed the case by engaging the students in an activity (“Can cold be hot?”) which offers a vivid example of a “cold object” (melting ice) actually being “warm” in relation to another object (ice plus salt), and the noninteractive/authoritative argument that the teacher develops is based on the shared outcomes of this activity. At this point the teacher is doing all of the talking, and it would certainly be wrong to assume that all of the students in the class have taken on the scientific view. Nevertheless, in subsequent small group and whole class discussions, there are many opportunities for students to articulate their developing ideas about heat, and the two kinds of heat idea is not raised again, by teacher or students.
The function of this turning point in the discourse is to enhance the awareness, by the students, of the conceptual profile of heat. Thus, the students have the opportunity to position the authoritative discourse of the disciplinary knowledge in relation to their everyday views, and in this way, we believe that they are better placed to appropriate this discourse and to make it their own. In simple terms, the students are better placed to see how the different ideas fit together.
Finally, we bring a fourth episode to show how the teacher seeks to consolidate through interactive and authoritative discourse the empiricist zone of heat. This discussion comes after an experimental activity with the class, shown in Fig. 9.1 (aquarium with metal plate dividing two systems with water at different temperatures). The data of the experiment were collected by two students and written on the board, and then the teacher turns back to the issues related to the activity.
-
Teacher: Well, then // what is the condition for two objects that are in thermal contact / initially with different temperatures / to reach thermal balance characterized by the final equal temperature? Hum? Two objects in thermal contact, at different temperatures / how are they going to reach the balance?
-
Student 1: It is when the temperature is the same / of course.
-
Teacher: But what happened for //
-
Student 1: One passes heat to the other.
-
Teacher: Hum
-
Student 1: The one that has more heat / the higher temperature / will pass it to the one with the lower temperature
-
Prof.: I can’t hear what student 1 is saying. Would you please pay attention and stop parallel conversation. Go on student 1.
-
Student 1: That the higher temperature // the one with the higher temperature will pass it to the one with the lower temperature.
-
Teacher: Passed what?
-
Student 1: Passed the heat.
-
Teacher: What do you mean? Passed heat / what is heat? What do you mean? How do you understand heat there?
-
Student 1: Ah / the difference / the difference from one to the other.
-
Teacher: Ham?
-
Student 1: Of energy / of energy from one to the other.
-
Prof.: From the one that had?
-
Student (?): Higher temperature
-
Teacher: From the higher temperature to //
-
Student 1: Lower
-
Teacher: Ok / and then it stops there. Let’s say there was an object measuring 50º and the other at 20o / ok? When they reach a balance / do they remain there forever at that limit?
-
Students (?): No.
-
Teacher: What can happen?
-
Student 1: It can drop.
-
Teacher: Hum?
-
Student (?): It can drop to the room temperature
-
Teacher: They can / if they are in an open system / in contact with the air. What can happen to it?
-
Student (?): Stay at room temperature
-
Teacher: They exchange with another / another system / right? In other words the one around it / if it is the air / for example. So are they going to remain at balance at a constant temperature?
-
Student (?): No.
-
Teacher: The tendency is to reach a balance with other bodies they are in contact with / right? They go for example / from 50 to 20° / they will reach a balance. What will this balance be? This one at 20° or this at 50? 50 will remain 50 and the other will increase? What will happen?
-
Student 3: The temperature will be at the average.
-
Student 2: When there is air in our shirt / how can it be hotter than your body?
-
Teacher: You feel hot because you are producing heat at a rate faster than the heat is being transferred to the environment / so there is an even higher temperature. Then you begin to have many reactions / you begin to sweat / your start the dilation of your skin capillary vessels //
-
Student 2: So this happens because heat is not transferred.
-
Teacher: It transfers less heat than the heat being supplied / right? And it automatically adjusts a number of processes for this to continue to happen so you feel hot / you feel bad.
-
Student 2: In fact / we get hotter.
-
Teacher: What?
-
Student 2: We get hotter?
-
Teacher: The internal temperature?
-
Student 2: Yes
-
Teacher: No. You soon have several reactions that increase the transfer of heat so as to maintain the body temperature. Right?
In the first part of the episode, the teacher intends to remember, with the students, the meanings attributed to the terms heat, temperature, and thermal balance in interpreting the data from the experiment. For this he uses questions that are answered by the students and then evaluated – a typical pattern of authoritative discourse. The teacher also used follow-up strategies in order to encourage the students to produce more complete and justified utterances. Initially, we see student 1 and another colleague talking of the one-way transfer of heat, although they find it difficult to say what heat is. In spite of the ambiguity of the utterances, they appear to refer to the third zone, the rationalist, of the conceptual profile of heat. We can see progress, by some students, in the appropriation of important scientific ideas: besides uttering a solely direction for the propagation of heat within the context of the activity, these girls speak of heat as a difference of energy, and they also consider the heat transfers involving the environment. The utterances are guided to the appropriation of elements of the empiricist profile of heat, with the difference between heat and temperature and the coordination of these concepts among themselves and with the notion of thermal balance.
At the end of the episode, student 2 proposes a radical change of the context of the situations dealt with by the classroom discourse. Instead of the experiment of the transfer of heat between two sections of water at different temperatures, the student asks the teacher to consider a much more complex system: the human body covered by clothing. Why do we feel hot in this case? Here we see how the modeling activity contexts intercalate with the contexts of everyday situations which, as we saw, permeate the construction of the teaching sequence. The teacher answers the student seeking to maintain the generality of the contexts dealt with but considering the problems of the sensation of heat from the physiological point of view. The solution involves the idea that with the reduction of heat transfer, body temperature tends to rise, which triggers heat regulation mechanisms (sweat and dilation of capillaries) which, in turn, increase the heat transfer to the environment and, therefore, make it possible to keep a constant body temperature. The idea of dynamic balance is later taken up by the teacher with the use of analogies and diagrams, such as those discussed previously.
When dealing with the question by this student, the teacher opens the discourse to contexts and situations that had not been planned for that moment. In spite of this, the discourse produced in answering this question is also predominantly authoritative, since the teacher considers the sensation of heat only from the point of view of the scientific model that is being presented and developed. At that moment, the teacher did not want to go back to the prescientific zone of heat as a sensation, associated to “hot things.”
In the same way, the discourse of the first part of the episode can be described as predominantly interactive and authoritative, which occurs by the compliance of the students to the school scientific model in analyzing the situations. In this case, it can be said that there is a sharing of contexts between the teacher and the students, that is, the students seem to perceive context markers, and they adapt their discourse according to the context.
It should also be noted that the substantialist zone of heat was evoked by the students throughout the sequence, especially in experimental situations. In these cases, the students speak of “the heat contained in the water,” of the clothing that “preserves body heat,” and of the heat that is “passed from hot things to cooler things.”
8 Final Remarks
The situations presented here show the potential of the conceptual profile theory as a framework to organize teaching and learning situations in sciences.
We believe that the following questions can assist teachers in preparing their teaching plans based on this theoretical framework:
-
What are the common sense views and how do they intervene in the zone of the conceptual profile to be taught?
-
How can the common sense and scientific views of the topic be taught differentlyFootnote 5?
-
What are the main obstacles to the appropriation of the scientific ideas?
-
What strategies should be used to introduce scientific ideas in the classroom?
-
What problems should be proposed to help students to recognize the differences between intuitive ideas and scientific concepts?
-
How to encourage students to work with scientific concepts and models?
Another conclusion from this experience is that we learn to speak and think scientifically in the coming and going between concepts and contexts. The contexts of the teaching activities often refer to the use of activities building physical models, belonging to the sphere of school scientific knowledge. However, as we saw, these contexts dialogue with the context of everyday situations related to thermal sensations. We also seek to indicate that the micro-contexts of the activities were forged and temporarily developed in correspondence with zones of the conceptual profile evoked, introduced, and consolidated throughout the teaching sequence.
Every time we use a concept in the face of a specific contextual situation, in one way or another we relearn the concept in the situation, and it gains new contours and a new potential meaning in the end. The stabilization of senses of a given concept occurs in these spheres of use and generalization in contexts of signification.
Another conclusion refers to the discursive interactions that are inherent to the process of forming and developing concepts. In fact, concept is irreducible for both the subject and the world. It emerges from interaction, but the forms of interaction between teacher and students around these situations and tasks to be solved allow wide variation. When the teacher intends to reaffirm and discuss the prescientific elements of the concept, he uses dialogic discourse, accepting the students’ statements and analyzing them. On the contrary, when introducing new zones of the profile or even consolidating or stabilizing some meanings that circulate about the concept, the teacher uses authoritative discourse. At other moments, when introducing new problems as challenges to the understanding of the students, the teacher may go back to dialogic discourse, with transitions to authoritative discourse (after all, the students should have access to the scientific solution of the proposed problem).
The transitions between dialogic and authoritative discourses seem to be extremely important. These transitions should be clearly indicated to the students so as to permit both the recognition of the legitimacy of the nonscientific spheres of the concept and the specificity of its scientific treatment. The students should recognize that although legitimate, everyday concepts are not of the same kind as scientific concepts, because they were created and should be used for different purposes. It is the nature of the problem to be solved that enables us to choose the appropriate cultural tool for each situation.
Therefore, the teaching strategies highlighted here should make possible:
-
1.
The evoking and discussion of the prescientific zones of the concept and understanding of the aspects that justify and strengthen them, on the one hand, and their limitations and gaps, on the other
-
2.
The introduction and strengthening of new zones of the conceptual profile in the contexts of relevant tasks and problems to be solved
-
3.
The awareness of different zones of the conceptual profile in order to prevent their meanings being used without distinction or in a mixed way
Notes
- 1.
The first edition of the collection “Construindo Consciências” was published in 2003, and the second revised edition, in 2007. The chapter we refer to is “The Control of Body Temperature by Living Beings”, which is part of the content of the 8th year of secondary education.
- 2.
This and other questions from the students, as well as the answers given by them in the dynamics of the activities in the science classrooms, were examined in the article by Aguiar et al. (2010), published by JRST.
- 3.
This episode was analyzed in detail by Aguiar and Mortimer (2005).
- 4.
This is one of the episodes analyzed in Scott et al. (2006).
- 5.
For some teaching content and levels, it is also a matter of examining the passage from one scientific zone to another, with the domains and fields of validation that belong to it. This does not happen here, where heat is being taught at an elementary level.
References
Aguiar, O. G., Jr. (2002). Planejar o ensino considerando a perspectiva da aprendizagem: uma análise de abordagens didáticas na introdução da física térmica [Planning teaching considering the learning perspective: An analysis of pedagogical approaches in the introduction of thermal physics]. Caderno Catarinense de Ensino de Física, 19, 219–241.
Aguiar, O. G., Jr., & Mortimer, E. F. (2005). Tomada de consciência de conflitos: análise da atividade discursiva de uma aula de ciências [Becoming conscious aware of conflicts: Analysis of a classroom discursive activity]. Investigações em Ensino de Ciências, 10, 179–207.
Aguiar, O. G., Jr., De Caro, C., Paula, H. F., Loureiro, M., Lima, M. E., Castro, R. S., Braga, S. M., & Silva, N. S. (2007). Construindo Consciências [Constructing consciousness] (Vol. 3, 2nd ed.). São Paulo: Scipione.
Aguiar, O. G., Jr., Mortimer, E. F., & Scott, P. (2010). Learning from and responding to students’ questions: The authoritative and dialogic tension. Journal of Research in Science Teaching, 47, 174–193. doi:10.1002/tea.20315.
Amaral, E. M. R., & Mortimer, E. F. (2001). Uma proposta de perfil conceitual para o conceito de calor [A proposal of a conceptual profile of heat]. Revista Brasileira de Pesquisa em Educação em Ciências, 1, 5–18.
Arnold, M., & Millar, R. (1994). Children’s and lay adults’ views about thermal equilibrium. International Journal of Science Education, 16, 405–419. doi:10.1080/0950069940160403.
Arnold, M., & Millar, R. (1996). Learning the scientific ‘story’: A case study in the teaching and learning of elementary thermodynamics. Science Education, 80, 249–281. doi:10.1002/(SICI)1098-237X(199606)80:3<249::AID-SCE1>3.0.CO;2-E.
Bachelard, G. (1996). The formation of the scientific mind. London: Clianmen Press.
Bakhtin, M. M. (1986). Speech genres & other late essays (C. Emerson & M. Holquist, Eds., V. W. McGee, Trans.). Austin, TX: University of Texas Press.
Cafagne, A. (1996). Concepções em termodinâmica: o senso comum e o conhecimento científico [Conceptions in thermodynamics: The common sense and the scientific knowledge]. Doctoral dissertation, Faculdade de Educação, USP, São Paulo.
Chi, M. T. H. (1992). Conceptual change within and across ontological categories: Examples from learning and discovery in science. In R. N. Giere (Ed.), Cognitive models of science (Minnesota studies in the philosophy of science, Vol. XV). Minneapolis, MN: University of Minnesota Press.
Erickson, G. (1985). Heat and temperature, Part A. In R. Driver, E. Guesnes, & A. Tiberghien (Eds.), Children’s ideas in science (pp. 52–84). Milton Keynes: Open University Press.
Leach, J. T., & Scott, P. H. (2002). Designing and evaluating science teaching sequences: An approach drawing upon the concept of learning demand and a social constructivist perspective on learning. Studies in Science Education, 38, 115–142. doi:10.1080/03057260208560189.
Lijnse, P. (1995). ‘Developmental research’ as a way to an empirically based ‘didactical structure’ of science. Science Education, 79, 189–199. doi:10.1002/sce.3730790205.
Mortimer, E. F., & Scott, P. (2003). Meaning making in secondary science classrooms. Maidenhead: Open University Press.
Mortimer, E. F., Scott, P., Amaral, E. M. R., & El-Hani, C. N. (2010). Modeling modes of thinking and speaking with conceptual profiles. In S. D. J. Pena (Ed.), Themes in transdisciplinary research (pp. 105–137). Belo Horizonte: Editora UFMG.
Scott, P., Mortimer, E. F., & Aguiar, O. G. (2006). The tension between authoritative and dialogic discourse: A fundamental characteristic of meaning making interactions in high school science lessons. Science Education, 90, 605–631. doi:10.1002/sce.20131.
Silva, D. (1995). Estudo das trajetórias cognitivas de alunos na diferenciação dos conceitos de calor e temperatura [A study of students’ cognitive paths in the differentiation of heat and temperature]. Doctoral dissertation. São Paulo: Faculdade de Educação da USP.
Viennot, L. (1997). Experimental facts and ways of reasoning in thermodynamics: Learners’ common approach. In A. Tiberghien, E. L. Jossem, & J. Barojas (Eds.), Connecting research in physics education with teacher education. London: The International Commission on Physics Education (ICPE). http://www.physics.ohio-state.edu/jossem/ICPE/BOOKS.html
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Aguiar, O.G. (2014). The Implications of the Conceptual Profile in Science Teaching: An Example from a Teaching Sequence in Thermal Physics. In: Mortimer, E., El-Hani, C. (eds) Conceptual Profiles. Contemporary Trends and Issues in Science Education, vol 42. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9246-5_9
Download citation
DOI: https://doi.org/10.1007/978-90-481-9246-5_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9245-8
Online ISBN: 978-90-481-9246-5
eBook Packages: Humanities, Social Sciences and LawEducation (R0)