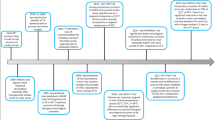
Abstract
Therapeutic hypothermia (TH) in unconscious victim resuscitated after cardiac arrest (CA) is currently the only intervention that has shown in recent years to positively impact on survival and neurological outcome. Therefore, the European and American 2010 guidelines fully acknowledge the role of TH as cornerstone in the treatment of the post-CA syndrome, suggesting its extension to “non-shockable rhythms”, in-hospital CA and paediatric population. However, TH is demanding and its implementation requires protocols providing the staff with clear strategies and targets. Furthermore, there is still uncertainty on few aspect of target temperature management: when to start cooling? How long for? Which length of the rewarming phase? Which is the ideal target temperature? In view of these areas of concern and of the pitfalls of the landmark trials, the “Target Temperature Management Trial” has been designed to compare two different subfebrile temperatures (33 °C vs. 36 °C) and its results should be available soon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neurological outcome
- Therapeutic hypothermia
- Target temperature management
- Post-cardiac arrest syndrome
- Hypoxic brain injury
- Post-resuscitation care
1 Effectiveness of Therapeutic Hypothermia
Over a decade has passed since the publication on the New England Journal of Medicine of the landmark studies that led to widespread clinical application of therapeutic hypothermia (TH) [1, 2]. After decades of clinical research, finally we found evidence in two randomized clinical trials (RCTs) of an intervention improving neurological outcome at hospital discharge. Indeed the largest RCT, the European study “Hypothermia After Cardiac Arrest” (HACA-Trial) unprecedentedly detected a survival benefit at 6 months after hospital discharge [1]. Both RCTs enrolled unconscious victims of cardiac arrest (CA) presenting with a shockable rhythm [1, 2].
Moreover in the same years a single center feasibility study looked at cooling patients in asystole or pulseless electrical activity (PEA) [3]. A subsequent meta-analysis was published that pooled the two RCTs and a feasibility study on cooling with a refrigerated head cap [4]. The results provided an astonishingly low number needed to treat only six patients to observe a benefit of hypothermia on both short- and long-term effects—i.e., improved neurological outcome and survival at hospital discharge and 6 months later.
As mentioned, these results are not trivial when considering that there are only three treatments supported by sound evidence in the field of cardiopulmonary resuscitation (CPR): (1) chest compressions and ventilation, (2) defibrillation for ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT), and now (3) mild TH (range 32–34 °C) for 24 h in unconscious victims resuscitated from CA. This is unprecedented news since much effort devoted to other advanced life support (ALS) interventions over the past 50 years was unable to show any impact on CA and CPR outcome at hospital discharge. This includes widely accepted interventions such as airway management by endotracheal intubation and the use of drugs like epinephrine [5–7].
The majority of patients initially resuscitated from CA (approximately 70 %) die in hospital [8]. Hypothermia introduced a paradigm shift in the in-hospital treatment of post-CA patients. Indeed, because 75 % of in-hospital deaths are attributed to irreversible anoxic neurological injury, such a high mortality is likely to offer room for improvement, and TH challenged the previous widespread nihilistic approach that clinicians had toward resuscitated patients [9].
2 Post-Resuscitation Care
The success of hypothermia gave momentum to an in-hospital standardized approach, similar to the one employed in the links of the Chain of Survival in the out-of-hospital setting. The increased appreciation of the numerous opportunities of care held by the in-hospital phase ultimately led to what is currently named as post-resuscitation care (PRC). The first group to systematically apply a standardized 72 h treatment protocol, including hypothermia, was the Norwegian group in Oslo led by Sunde et al. [10]. A whole set of therapeutic options to limit ongoing injury, to sustain organ function and to normalize physiological parameters were considered. Clear procedures identified objectives, goals, and strategies to achieve them. Realizing that there is much to do in little time while inducing, maintaining, and gradually restoring normothermia, and while maintaining optimal intensive care standards at all time, the authors acknowledged the need for a clear understanding of clinical priorities. To achieve such objectives, at least at the beginning, a protocol of standardized care was necessary. Much effort was also posed to aggressive hemodynamic support (both pharmacological and mechanical) and urgent coronary angiography with percutaneous or surgical revascularization if necessary. Survival with good neurological recovery at 1 year from hospital discharge significantly improved from 26 % in historical controls to 56 % after the intervention period [10].
Association of TH concurrently with an aggressive PRC policy led to very consistent outcome improvements over different countries, suggesting a clear margin of benefit. Interestingly, numerous centers now report very similar rates of survival with good neurological outcome, all ranging between 54 and 59 % [11–14].
The optimism imposed by the results of TH led the International Liaison Committee on Resuscitation (ILCOR) to officially acknowledge the existence of a post-CA syndrome [15]. Negovsky was the first to understand the complexity of this state, named in 1972 as post-resuscitation disease [16]. The ILCOR consensus statement published in 2008 defines the epidemiology, pathophysiology, treatment, and even elements useful for prognostication of this syndrome [15]. With a bold step forward ILCOR provides physicians and nurses with clear objectives that can be summarized in the treatment of the four key components of the Post-CA syndrome: (1) post-CA brain injury, (2) post-CA myocardial dysfunction, (3) systemic response to ischemia and reperfusion, and (4) persistent precipitating pathology.
The 2005 European resuscitation council (ERC) guidelines did not devote much attention to TH [17]. On the contrary, the following guidelines issued in 2010 strongly emphasized, among the changes introduced, the need for implementing TH and comprehensive PRC [18]. For instance, the name of the fourth link of the Chain of Survival was changed from ALS to PRC; furthermore for the first time the brain was depicted in the logo, quite peculiar when considering that this is the ultimate goal of resuscitation but was never portrayed before [18]. Finally, almost half of the summary of changes in the ALS chapter was dedicated to TH and PRC.
On the other side of the Atlantic, the American Heart Association (AHA) 2010 guidelines introduced a dedicated fifth link to the Chain of Survival and a whole new chapter within the guidelines entitled “post-cardiac arrest care” [19].
3 New Evidence Supporting Therapeutic Hypothermia
Presently, there are over 4,400 references on PubMed when searching for TH and CA, but very little is new in terms of scientific evidence, since the only RCTs remain the landmark studies on which we still rely.
New information might be sought in registries, yet these are only hypothesis generating. The largest database—the Hypothermia Network Registry—including over 1,000 patients suggests that TH is beneficial not only for patients in VF as demonstrated by the landmark trials, but also for those presenting with the so-called agonal rhythms [20]. An astonishingly high survival rate was reported by the registry when compared to that of historical controls: 21% survival with good neurological outcome in patients with asystole as first detected rhythm, and 23 % for those presenting in PEA.
Expert consensus opinion in guidelines has filled the gap where the evidence is slim, so that we are now suggested to consider the use of TH also: in comatose survivors of CA with non-shockable rhythms; following in-hospital arrest; or for paediatric patients, even if the lower level of evidence for these categories is acknowledged [18]. There is also growing appreciation that it is rational to think beyond the sole hypothermia process of induction, maintenance, and rewarming. Active temperature management after restoration of normothermia in patients who do not regain consciousness should be a priority at least for the initial 72 h that follow restoration of spontaneous circulation (ROSC). Indeed a large number of patients tend to become febrile after restoration of normothermia. A hyperthermic rebound should not be underestimated as fever after any acute neurological insult (including CA) is detrimental; however, it is still unknown until when it should be considered a hazard [21]. For this reason, a rigid maintenance of normothermia should be considered at least for the first 3 days after ROSC and particularly in those patients not regaining consciousness after restoration of normothermia and sedation hold.
It is precisely with the aim of moving beyond the cooling process that five scientific societies agreed on replacing the term TH with the more appropriate one of target temperature management [22].
The Hypothermia Network Registry sheds also some light on adverse events related to TH [20]. Bradycardia was the most common arrhythmia (13 %) but very rarely requiring pacing, regardless of whether pharmacological or electrical, while pneumonia was the most frequent infection (41%) [20]. Bleeding requiring transfusion occurred in 4% of all patients and the risk was significantly higher if angiography/coronary intervention was performed (2.8 % vs. 6.2 %, respectively, p = 0.02). Sustained hyperglycemia [defined as >8 mmol/l (144 mg/dl) for >4 h] was observed in 37 % of patients. Electrolyte disorders were also quite common (18–19 %), specifically hypokalemia, hypomagnesemia, and hypophosphatemia [20]. The incidence of sepsis was low (4 %) but higher in patients with intravascular devices for temperature management (OR 2.6), intra-aortic ballon pump (IABP, OR 3.2), or undergoing coronary angiography (OR 4.4) [23]. Of note bleeding, infection, arrhythmia, and electrolyte disorders were not associated with increased mortality. Only sustained hyperglycemia and seizures (despite treatment) were associated with worse outcome [23].
Yet there are many unanswered questions, among them when to start hypothermia. Cooling should be started as early as possible, stated the 2010 ERC guidelines [18]. The cooling process can be started on the field and during transportation leading to a reduction in core temperature at hospital or intensive care unit (ICU) admission [24]. Our 7 years’ experience is in keeping with such reports. The emergency medical service (EMS) of the province of Pordenone (north-east Italy) was able to start cooling in the out-of-hospital setting in 56 patients. Hypothermia was initiated with straightforward and inexpensive means without delaying hospital admission. This included ice-packs, means of inducing heat loss (i.e., turning off ambulance heating, opening the window, uncover patient as much as possible, and any other mean immediately at hand), and cold IV fluids. When comparing temperature at ICU admission with 117 patients that were not cooled (including in-hospital CA) there was a significant reduction in core temperature if cooling was started in the out-of-hospital setting (34.7 ± 2.1 °C vs. 35.4 ± 1.3 °C, p < 0.01).
Despite this strong logical and pathophysiological rationale, as yet there are no human data supporting that a reduced time to achieve the target temperature is associated with better outcomes. On the contrary, a recent Australian trial on early out-of-hospital cooling was stopped for futility after enrolling over 200 patients [24]. Also, an intra-arrest trial using transnasal evaporative cooling which allows for a very fast cooling rate, did not show improvements in ROSC or hospital discharge, despite the study not being really powered for such results [25]. Currently, four more trials are on the way, two in Australia, one in North America, and one in Europe.
What could the reasons be for this lack of benefit? One hypothesis is that if neuroprotection induced by hypothermia is not an on–off phenomenon but a spectrum with higher intensity at lower temperatures, then a patient who is difficult to cool or is cooled slowly would be exposed to a greater area under the curve of hypothermia—and thereby of neuroprotection—than a patient who achieves rapidly the target temperature. Another reason recently proposed by Oddo et al. [26], is that the impaired thermoregulation that follows ROSC is an indicator of the severity of injury, hence a determinant of post-resuscitation disease and CA prognosis. So future studies when assessing the benefit of early cooling on outcome should adjust for patient admission temperature and use the cooling rate rather than time to target temperature.
Another matter of debate is whether cooling should be extended to children and for how long. There are currently three trials and one Cochrane review on neonates, all indicating that hypothermia for 72 h at 33.5 °C is beneficial [27–29]. Once again it seems rational to use hypothermia after CA in children and, based on expert consensus, current guidelines recommend it although the length of treatment is not specified [18]. So, while waiting for the results of ongoing clinical trials, companies are now competing to produce devices that allow strict control by automatic temperature management in children of different sizes.
There is also a growing literature that suggests it might be worth regionalizing CA patients bypassing local hospitals in order to admit them directly to high-volume CA centers with percutaneous coronary intervention facilities “24/7” [30–33].
However, there are still many other areas of uncertainty, like: (1) the appropriate duration of hypothermia (does one size fit all?), (2) should we tailor the duration on an indicator of brain injury? (3) what is the optimal rewarming rate, and most of all, (4) what is the optimal depth of hypothermia?
4 The Target Temperature Management Trial
A critical review of the landmark studies easily reveals that they are not flawless and carry a potential risk of systematic error and random error design.
The Bernard trial was actually not a randomized trial but a quasi-randomized study as allocation was based according to odd and even days [2]. Of the 82 eligible patients only 77 were included, no justification for the missing 6 % was provided. Unscheduled interim analysis on 62 patients was performed, and no statistical correction was included in the final analysis. The two groups were uneven (43 vs. 34 patients). The discharge facility was used as a surrogate of neurological outcome. To make things worse the final result of the study depends on one single patient.
If we consider the largest study, the HACA trial, which enrolled 275 patients there are still areas of uncertainty: the study was prematurely stopped, there was no power calculation (a reasonable size would have probably included 600–700 patients), and the study lasted as long as 7 years [1]. Neurological status before CA was not investigated, early withdrawal of care was neither standardized nor reported; finally the study was highly selective since it included only 8 % of screened patients admitted to the emergency department following CA (275 of 3,551). Hence the results can be hardly generalized to the average CA population, and most of all, the controls were actually “not temperature-controlled,” since the majority of them were febrile.
In conclusion, the overall quality of evidence is low at least in humans and the optimal target temperature is not yet defined at least based on high quality evidence. Therefore, although promising, hypothermia should be further investigated. These precise conclusions were drawn by a systematic review that assessed the evidence using the GRADE-methodology [34].
With the attempt of improving our knowledge on hypothermia, several ICU have joined in the attempt to investigate two subfebrile temperatures in a large general population by means of a randomized, parallel-group, and assessor-blinded clinical trial: the target temperature management (TTM) trial [35]. A core temperature of 36 °C was chosen as comparator to 33 °C since it is safely below febrile temperatures with allowance for temperature fluctuations and because, based on the Hypothermia Network Registry data, the median temperature at hospital admission is 36 °C. Active temperature management (36 vs. 33 °C) was continued in patients who did not regain consciousness. A total of 36 centers from 10 different countries in Europe and Australia joined this study led by the principal investigator Niklas Nielsen (Sweden).
The main objective was to assess the efficacy and safety of two TTM strategies after resuscitation from out-of-hospital CA. Enrolment of 950 patients was completed in January 2013. The intervention phase lasted 36 h, while 72 h later (that is 5 days after ROSC) a blinded neurological evaluation was performed. Patients were followed until the end of the trial.
Inclusion criteria were: unconscious adult patients with out-of-hospital CA of presumed cardiac cause with sustained ROSC. Patients too cold on admission (core temperature <30 °C) were not included, as well as those with known limitations in therapy, unlikely to survive at 6 months, or with severe preexisting neurological dysfunction. Patients could not be screened any longer than 4 h from ROSC, and if they remained in shock in spite of maximum therapeutic efforts. All presenting rhythms were included with the exception of unwitnessed asystole.
The primary endpoint was survival at the end of the trial, while the secondary was the post-CA neurological function—blindly and thoroughly evaluated not only cerebral performance categories but also modified Rankin Scale and Informant Questionnaire on Cognitive Decline in the Elderly—at 6 months, as well as safety issues (i.e., bleeding, pneumonia, etc.). Withdrawal of care was not allowed for the first 5 days, unless for predefined ethical reasons, and had to be always motivated [35]. Further details are available online at http://clinicaltrials.gov/show/NCT01020916. Publication of the trial results is expected by late 2013.
In conclusion the TTM-Trial holds promises to be a large clinical randomized and monitored trial evaluating two subfebrile temperatures, with a pragmatic design in accordance with clinical practice, and hopefully its results will apply to the majority of out-of-hospital CA patients admitted to the intensive care.
References
HACA (2002) Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346(8):549–556
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G et al (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346(8):557–563
Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L (2001) Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation 51(3):275–281
Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M (2005) Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med 33(2):414–418
Hasegawa K, Hiraide A, Chang Y, Brown DF (2013) Association of pre hospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. J Am Med Assoc 309(3):257–266 (Epub 2013/01/17)
Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL (2011) Effect of adrenaline on survival in out-of-hospital cardiac arrest: a randomised double-blind placebo-controlled trial. Resuscitation 82(9):1138–1143 (Epub 2011/07/13)
Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L (2009) Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. J Am Med Assoc 302(20):2222–2229 (Epub 2009/11/26)
Brain Resuscitation Clinical Trial II Study Group (1991) A randomized clinical study of a calcium-entry blocker (lidoflazine) in the treatment of comatose survivors of cardiac arrest. N Engl J Med 324(18):1225–1231
Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T (2012) The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation (Epub 2012/09/25) 2013, 84(3):337–342
Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C et al (2007) Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation 73:29–39
Busch M, Soreide E, Lossius HM, Lexow K, Dickstein K (2006) Rapid implementation of therapeutic hypothermia in comatose out-of-hospital cardiac arrest survivors. Acta Anaesthesiol Scand 50(10):1277–1283
Oddo M, Schaller MD, Feihl F, Ribordy V, Liaudet L (2006) From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med 34(7):1865–1873
Knafelj R, Radsel P, Ploj T, Noc M (2007) Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation 74(2):227–34 (Epub 2007/03/27)
Belliard G, Catez E, Charron C, Caille V, Aegerter P, Dubourg O et al (2007) Efficacy of therapeutic hypothermia after out-of-hospital cardiac arrest due to ventricular fibrillation. Resuscitation 75(2):252–259 (Epub 2007/06/08)
Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bottiger BW et al (2008) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 79(3):350–379 (Epub 2008/10/31)
Negovsky VA (1972) The second step in resuscitation—the treatment of the ‘post-resuscitation disease’. Resuscitation 1(1):1–7
International Liaison Commitee on Resuscitation (2005) International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 4: advanced life support. Resuscitation 67(2–3):213–247
Deakin CD, Nolan JP, Soar J, Sunde K, Koster RW, Smith GB et al (2010) European resuscitation council guidelines for resuscitation Section 4: adult advanced life support. Resuscitation 81(10):1305–1352 (Epub 2010/10/20)
Peberdy MA, Callaway CW, Neumar RW, Geocadin RG, Zimmerman JL, Donnino M et al (2010) Part 9: post-cardiac arrest care: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 122(18 Suppl 3):S768–786 (Epub 2010/10/22)
Nielsen N, Hovdenes J, Nilsson F, Rubertsson S, Stammet P, Sunde K et al (2009) Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand 53(7):926–934 (Epub 2009/06/25)
Zeiner A, Holzer M, Sterz F, Schorkhuber W, Eisenburger P, Havel C et al (2001) Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med 161(16):2007–2012 (Epub 2001/09/26)
Nunnally ME, Jaeschke R, Bellingan GJ, Lacroix J, Mourvillier B, Rodriguez-Vega GM et al (2011) Targeted temperature management in critical care: a report and recommendations from five professional societies. Crit Care Med 39(5):1113–1125 (Epub 2010/12/29)
Nielsen N, Sunde K, Hovdenes J, Riker RR, Rubertsson S, Stammet P et al (2011) Adverse events and their relation to mortality in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia. Crit Care Med 39(1):57–64 (Epub 2010/10/21)
Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ et al (2010) Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation 122(7):737–742 (Epub 2010/08/04)
Castren M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D et al (2010) Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 122(7):729–736 (Epub 2010/08/04)
Benz-Woerner J, Delodder F, Benz R, Cueni-Villoz N, Feihl F, Rossetti AO et al (2012) Body temperature regulation and outcome after cardiac arrest and therapeutic hypothermia. Resuscitation 83(3):338–342 (Epub 2011/11/15)
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA et al (2005) Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol 32(1):11–17 (Epub 2004/12/21)
Gluckman PD, Gunn AJ, Wyatt JS (2006) Hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 354(15):1643–1645 (Epub 2006/04/15)
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF et al (2005) Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353(15):1574–1584 (Epub 2005/10/14)
Carr BG, Goyal M, Band RA, Gaieski DF, Abella BS, Merchant RM et al (2009) A national analysis of the relationship between hospital factors and post-cardiac arrest mortality. Intensive Care Med 35(3):505–511 (Epub 2008/10/22)
Davis DP, Fisher R, Aguilar S, Metz M, Ochs G, McCallum-Brown L et al (2007) The feasibility of a regional cardiac arrest receiving system. Resuscitation 74(1):44–51 (Epub 2007/03/10)
Spaite DW, Bobrow BJ, Vadeboncoeur TF, Chikani V, Clark L, Mullins T et al (2008) The impact of prehospital transport interval on survival in out-of-hospital cardiac arrest: implications for regionalization of post-resuscitation care. Resuscitation 79(1):61–66 (Epub 2008/07/12)
Spaite DW, Stiell IG, Bobrow BJ, de Boer M, Maloney J, Denninghoff K et al (2009) Effect of transport interval on out-of-hospital cardiac arrest survival in the OPALS study: implications for triaging patients to specialized cardiac arrest centers. Ann Emerg Med 54(2):248–255 (Epub 2009/01/27)
Nielsen N, Friberg H, Gluud C, Herlitz J, Wetterslev J (2011) Hypothermia after cardiac arrest should be further evaluated—a systematic review of randomised trials with meta-analysis and trial sequential analysis. Int J Cardiol 151(3):333–341 (Epub 2010/07/02)
Nielsen N, Wetterslev J, al-Subaie N, Andersson B, Bro-Jeppesen J, Bishop G et al (2012) Target temperature management after out-of-hospital cardiac arrest–a randomized, parallel-group, assessor-blinded clinical trial–rationale and design. Am Heart J 163(4):541–548 (Epub 2012/04/24)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Italia
About this chapter
Cite this chapter
Pellis, T., Sanfilippo, F., Roncarati, A., Mione, V. (2014). Effectiveness of Hypothermia in Human Cardiac Arrest and Update on the Target Temperature Management-Trial. In: Gullo, A., Ristagno, G. (eds) Resuscitation. Springer, Milano. https://doi.org/10.1007/978-88-470-5507-0_16
Download citation
DOI: https://doi.org/10.1007/978-88-470-5507-0_16
Published:
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-5506-3
Online ISBN: 978-88-470-5507-0
eBook Packages: MedicineMedicine (R0)