Abstract
Juvenile idiopathic arthritis (JIA) is a heterogeneous condition including all forms of chronic arthritis of unknown origin, duration ≥6 weeks and with onset before 16 years of age. It is characterized by chronic synovial inflammation with potential risk of developing progressive joint destruction and serious functional disability [1–4]. It affects around 1 in 1,000 children under the age of 16 [5–8]. Typically, the child presents with a history of morning stiffness, vague pain and one or more swollen joints.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Juvenile idiopathic arthritis (JIA) is a heterogeneous condition including all forms of chronic arthritis of unknown origin, duration ≥6 weeks and with onset before 16 years of age. It is characterized by chronic synovial inflammation with potential risk of developing progressive joint destruction and serious functional disability [1–4]. It affects around 1 in 1,000 children under the age of 16 [5–8]. Typically, the child presents with a history of morning stiffness, vague pain and one or more swollen joints.
Recent research has shown that adult disease is seen in up to 75% of those having suffered JIA during childhood [9]. Moreover, the temporomandibular joint (TMJ) is involved in up to 70% of patients; in a high proportion it is overt and thus not treated specifically [10]. This may lead to pubertal growth disturbances of the TMJs, including restricted mandibular growth [9, 11, 12], with development of malocclusion and facial deformities [13]. Assessment of the TMJs, with treatment of those testing positive, has therefore become an important issue in management of children with JIA. The new understanding of JIA being more aggressive than previously believed and the introduction of new disease-modifying drugs have led to earlier and more aggressive treatment, and have fuelled the search for more accurate disease markers to better monitor therapeutic response.
Classification
Based on clinical and laboratory findings, JIA is currently subdivided to include [14–16] (Table 1):
-
Oligoarthritis: one to four joints affected in the first 6 months of disease. This category is further divided into persistent oligoarthritis (with no more than four involved joints during the disease course) and extended oligoarthritis, involving more than four joints after the first 6 months.
-
Polyarthritis: more than four joints affected within the first 6 months.
-
Systemic arthritis: arthritis accompanied by systemic illness including fever.
-
Psoriatic arthritis: arthritis associated with psoriasis.
-
Enthesitis-related arthritis: these patients are often HLA-B27 positive.
This classification is unsatisfactory because many of the identified subgroups appear to be too inhomogeneous. Furthermore, it is difficult to distinguish, early in the disease course, between patients who are most likely to develop joint damage, and who therefore require a more aggressive treatment at an early stage, and patients who will have a mild disease course. Finally, in clinical trials, drug efficacy is judged only on clinical parameters, since measures that can allow the early identification of the progression of joint damage, and therefore of drug efficacy on disease progression, are not available in children.
Imaging
The role of imaging is to secure the diagnosis, to assess the extent, severity and activity of the disease, and to help monitor therapeutic response and potential complications to steroid therapy and immobilization, such as compression fractures and avascular necrosis. During the past decade there has been a shift from traditional radio — graphy towards newer techniques such as ultrasound and magnetic resonance imaging, thus without proper evaluation of their accuracy and validity.
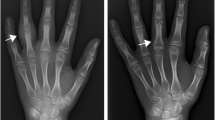
Radiographs can show periostitis, bone erosion, cartilage loss [indirectly, through joint space narrowing (JSN)], osteoporosis and joint misalignment (Figs. 1–4), but cannot visualize synovium, joint effusion, articular cartilage, bone marrow, or ligaments and tendons directly. Plain radiographs have particularly low sensitivity for disease in early stages (Table 2) [17–24].
Joint damage evaluation has traditionally been performed by radiographic scoring methods assessing JSN and erosions; however, they are quite inaccurate, in part due to the growing skeleton [25]. Wrist disease has been associated with a more severe course of arthritis and a poorer functional outcome, and is the only joint in which suitable radiographic measures of disease progression have been reported. Much effort has been spent recently on validating existing scoring systems or devising new ones, of which a modified version of the Sharp/van der Heijde wrist score has gained most acceptance (Table 2). The original Sharp/van der Heijde score is based on the assessment of JSN in 15 different locations, and bone erosion in 16 locations for each hand/wrist, separately. JSN is scored from 0 to 4, and bone erosion from 0 to 5, and the scores are summarized to make a total score ranging from 0 to 280 [26]. Notably, carpal/metacarpal changes in younger children tend to present as bony deformation, ranging from mild squaring to severe compression, rather than definite erosions as often seen in adults (Fig. 3). This has been accounted for in Sharp/van der Heijde’s scoring system in that both entities are considered pathological and scored in a similar fashion.
Rossi and colleagues noted that JSN tends to predominate over erosive change in JIA, and that erosion tends to involve locations other than those seen in adult rheumatoid arthritis [19]. For these reasons Ravelli et al. added another five locations to those described by Sharp/van der Heijde (second to fourth metacarpal bases, the capitate and the hamate), giving a total score of 330 [18]. When scoring is preceded by a meticulous standardization process, accuracy appears to be appropriate for clinical use.
Ultrasound (US) performs better than clinical examination in the diagnosis and localization of inflammatory change, such as joint effusion, bursal fluid collection and synovitis [27, 28] (Figs. 5–9). US can visualize inflammatory change relatively accurately, while assessment of chronic change is less feasible [29]. A structured assessment of synovitis and tenosynovitis, and classification of the findings, has been devised; however, the technique needs further validation [30] (Table 1). A few small uncontrolled studies have described improved sensitivity for detection of bone erosions in joints with the use of US as compared with conventional plain radiography; however, firm conclusions cannot be drawn from these studies. US may also demonstrate changes to cartilage.
Sagittal ultrasound view (standard view) of the dorsal wrist in a 6-year-old girl with juvenile idiopathic arthritis shows synovial hypertrophy and small effusions in the radiocarpal (white arrows) and midcarpal joints (black arrow). C capitate, E inflamed extensor tendons, L lunate, RAD distal radius (for color reproduction see p 317)
Axial ultrasound view (standard view) of the dorsal right wrist in a 7-year-old girl with juvenile idiopathic arthritis shows tenosynovitis of the extensors (compartments 2–4) (a) and verified by hyperemia on color Doppler (b). R distal radius, tubercle of Lister; U distal ulna (for color reproduction see p 317)
Magnetic resonance imaging (MRI) can visualize both active JIA change, such as soft tissue and synovial inflammation, effusion, bone marrow edema and chronic change. Additional contrast-enhanced series can help quantify the inflammation process, and also differentiate pannus from joint effusion [20]. Furthermore MRI is the only technique for assessment of bone marrow edema [21].
The protocol should include pulse sequences for assessment of the synovium (T2-weighted and fat-saturated/ gadolinium enhanced T1-weighted images) and cartilage and bone [T1/proton density weighted and three-dimensional (3D) spoiled gradient recalled echo sequences] in different planes (Figs. 10, 11).
Coronal magnetic resonance images of the wrist in a 12-year-old girl with juvenile idiopathic arthritis, including a T1-weighted image [three-dimensional (3D) spin echo] showing no erosion (a), a T2 fat-saturated image showing a sliver of fluid in the radioulnar joint (within normal variation) (b), a T1-weighted (3D spoiled gradient recalled echo sequence) before (c) and after (d) intravenous contrast administration, showing mildly increased synovial enhancement around the distal scaphoid, the trapezoid and the trapezium, consistent with focal inflammation
Sagittal magnetic resonance images of the right knee in a 12-year-old boy presenting with a swollen, painful knee. a T1 fat-saturated image (water exited) showing a swollen, prepatellar recess. b Contrastenhanced image showing vivid enhancement of a mildly thickened synovium, consistent with synovitis. The clinical picture suggested juvenile idiopathic arthritis
The value of MRI as an advanced method to evaluate disease activity and disease damage in adults with rheumatoid arthritis is under active investigation by a research consortium called Outcome Measures in Rheumatology Clinical Trials (OMERACT). However, the results drawn from OMERACT studies are not directly applicable to children, because adult rheumatoid arthritis is different from JIA and because the growing skeleton of children needs a different approach (Fig. 12). Indeed, in children ossification is incomplete and joint space width varies with age [16, 22]. Thus, despite technical progress in the imaging of cartilage, as development of ultrashort TE sequences, driven equilibrium, Fourier transform (DEFT) imaging, and steady-state free precession (SSFP) sequences for the detection of subtle surface irregularities and tiny focal defects of the articular cartilage, diffusion weighted techniques to assess degradation of collagen fibers, delayed gadolinium-enhanced cartilage imaging (dGEMRIC) to detect changes in cartilage proteoglycan content, and T2 relaxation time mapping to detect integrity of collagen in the extracellular matrix, the potential helpfulness of these techniques is yet to be seen. The same goes for sophisticated analysis of 3D image data to provide articular surface contour mapping, and 3D rendering as well as volumetric quantification of articular cartilage to evaluate the progression and response to treatment in patients with chronic arthritis. Progress in the assessment of synovitis, including dynamic contrast-enhanced MRI to assess the degree of inflammation, is also flawed with methodological difficulties, although voxel-by-voxel analysis of signal intensity versus time curves has proven more accurate than the region-of-interest approach in adults with rheumatoid arthritis. Other techniques for assessment of inflammation using semiautomated or automated segmentation program techniques of dynamic contrast-enhanced MRI have also been described [31, 32]. Thus, although MRI is a potential powerful imaging tool to assess joint inflammation and the progression of joint damage, standardized, validated, and feasible assessment systems are lacking.
During the years 2006 to 2010 we, as part of a large European-Union-funded multicenter study, devised a new scoring system for wrist involvement in JIA, assessing both active and chronic change, based on MRI and radio — graphy combined. Based on a large cohort of around 350 patients aged from 5 to 15 years, we have examined feasibility and accuracy for all the different components within a scoring system, namely bone erosions, bone marrow edema, synovitis (effusion, synovial hypertrophy, increased enhancement) and tenosynovitis. In general, the accuracy, i.e., inter- and intraobserver agreement, seems to be better for active than for chronic change (Table 2), and thorough standardization of the scoring method prior to assessment appears to be crucial. One major problem in assessing bone erosions and bone marrow change on MRI in particular was the lack of normal references (Fig. 10). Thus, during the project, such standards were created based on a population of 89 healthy children aged 5–15 years [16, 22]. Informed by these findings, we had to adjust some of the definitions used for pathological change.
In conclusion, imaging is crucial for the assessment, grading and follow-up of children with JIA. Plain radio - graphy still plays an important role in the diagnostic work-up and in monitoring chronic change. US performs better than clinical examination in the diagnosis and localization of inflammatory change, but accurate scoring systems are lacking. US can also guide joint injections. MRI is an important tool for the detection and grading of active change, and is a promising technique for future assessment of chronic change. At present, the assessment of chronic change is flawed by the wide variation of normal bone size and shape, and the results drawn from the „adult OMERACT studies” are not directly applicable to children.
References
Ruperto N, Lovell DJ, Cuttica R et al (2009) Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis 69:718–722
Ruperto N, Lovell DJ, Cuttica R et al (2007) A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum 56:3096–3106
Ringold S, Wallace CA (2007) Measuring clinical response and remission in juvenile idiopathic arthritis. Curr Opin Rheumatolol 19:471–476
Oen K, Malleson PN, Cabral DA et al (2002) Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol 29:1989–1999
Gabriel SE, Michaud K (2009) Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 11:229
Kaipiainen-Seppanen O, Savolainen A (2001) Changes in the incidence of juvenile rheumatoid arthritis in Finland. Rheumatology (Oxford) 40:928–932
Riise OR, Handeland KS, Cvancarova M et al (2008) Incidence and characteristics of arthritis in Norwegian children: a population-based study. Pediatrics 121:e299–e306
Symmons DP, Jones M, Osborne J et al (1996) Pediatric rheumatology in the United Kingdom: data from the British Pediatric Rheumatology Group National Diagnostic Register. J Rheumatol 23:1975–1980
Arvidsson LZ, Smith HJ, Flato B, Larheim TA (2010) Temporomandibular joint findings in adults with long-standing juvenile idiopathic arthritis: CT and MR imaging assessment. Radiology 256:191–200
Weiss PF, Arabshahi B, Johnson A et al (2008) High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum 58:1189–1196
Arvidsson LZ, Fjeld MG, Smith HJ et al (2010) Craniofacial growth disturbance is related to temporomandibular joint abnormality in patients with juvenile idiopathic arthritis, but normal facial profile was also found at the 27-year follow-up. Scand J Rheumatol 39:373–379
Fjeld MG, Arvidsson LZ, Stabrun AE (2009) Average craniofacial development from 6 to 35 years of age in a mixed group of patients with juvenile idiopathic arthritis. Acta Odontol Scand 67:153–160
Dahllof G, Martens L (2001) Children with chronic health conditions — implications for oral health. In: Koch A, Poulsen A (Eds) Pediatric dentistry. A clinical approach. Munksgaard, Copenhagen, pp 421–444
Petty RE, Southwood TR, Manners P et al (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
Kahn P (2011) Juvenile idiopathic arthritis — an update on pharmacotherapy. Bull NYU Hosp Jt Dis 69:264–276
Müller Ording LS (2012) Establishment of Normative MRI Standards for the Paediatric Skeleton to better outline Pathology. Focused on Juvenile Idiopathic Arthritis. A dissertation for the degree of Philosophiae Doctor. University of Tromsø, Faculty of Health Sciences, Tromsø
Poznanski AK, Hernandez RJ, Guire KE et al (1978) Carpal length in children—a useful measurement in the diagnosis of rheumatoid arthritis and some congenital malformation syndromes. Radiology 129:661–668
Ravelli A, Ioseliani M, Norambuena X et al (2007) Adapted versions of the Sharp/van der Heijde score are reliable and valid for assessment of radiographic progression in juvenile idiopathic arthritis. Arthritis Rheum 56:3087–3095
Rossi F, Di DF, Galipo O et al (2006) Use of the Sharp and Larsen scoring methods in the assessment of radiographic progression in juvenile idiopathic arthritis. Arthritis Rheum 55:717–723
Damasio MB, Malattia C, Tanturri de HL et al (2012) MRI of the wrist in juvenile idiopathic arthritis: proposal of a paediatric synovitis score by a consensus of an international working group. Results of a multicentre reliability study. Pediatr Radiol 42:1047–1055
Tanturri de HL, Damasio MB, Barbuti D et al (2012) MRI assessment of bone marrow in children with juvenile idiopathic arthritis: intra-and inter-observer variability. Pediatr Radiol 42:714–720
Avenarius DM, Ording Muller LS, Eldevik P et al (2012) The paediatric wrist revisited—findings of bony depressions in healthy children on radiographs compared to MRI. Pediatr Radiol 42:791–798
Boavida P, Hargunani R, Owens CM, Rosendahl K (2012) Magnetic resonance imaging and radiographic assessment of carpal depressions in children with juvenile idiopathic arthritis: normal variants or erosions? J Rheumatol 39:645–650
Muller LS, Avenarius D, Damasio B et al (2011) The paediatric wrist revisited: redefining MR findings in healthy children. Ann Rheum Dis 70:605–610
Johnson K (2006) Imaging of juvenile idiopathic arthritis. Pediatr Radiol 36:743–758
van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27:261–263
Breton S, Jousse-Joulin S, Cangemi C et al (2011) Comparison of clinical and ultrasonographic evaluations for peripheral synovitis in juvenile idiopathic arthritis. Semin Arthritis Rheum 41:272–278
Janow GL, Panghaal V, Trinh A et al (2011) Detection of active disease in juvenile idiopathic arthritis: sensitivity and specificity of the physical examination vs ultrasound. J Rheumatol 38:2671–2674
Muller L, Kellenberger CJ, Cannizzaro E et al (2009) Early diagnosis of temporomandibular joint involvement in juvenileidiopathic arthritis: a pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology (Oxford) 48:680–685
Malattia C, Damasio MB, Magnaguagno F et al (2008) Magnetic resonance imaging, ultrasonography, and conventional radiography in the assessment of bone erosions in juvenile idiopathic arthritis. Arthritis Rheum 59:1764–1772
Malattia C, Damasio MB, Basso C et al (2012) A novel automated system for MRI quantification of the inflamed synovial membrane volume in patients with juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 64:1657–1664
Malattia C, Damasio MB, Basso C et al (2010) Dynamic contrast-enhanced magnetic resonance imaging in the assessment of disease activity in patients with juvenile idiopathic arthritis. Rheumatology (Oxford) 49:178–185
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Italia
About this chapter
Cite this chapter
Rosendahl, K. (2013). Imaging of Juvenile Idiopathic Arthritis. In: Hodler, J., von Schulthess, G.K., Zollikofer, C.L. (eds) Musculoskeletal Diseases 2013–2016. Springer, Milano. https://doi.org/10.1007/978-88-470-5292-5_34
Download citation
DOI: https://doi.org/10.1007/978-88-470-5292-5_34
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-5291-8
Online ISBN: 978-88-470-5292-5
eBook Packages: MedicineMedicine (R0)