Abstract
Apart from two major components nitrogen and phosphorous, potassium is the third essential macronutrient required for the growth and metabolism of plant, and its deficiency in plants causes poorly developed roots, slow growth, low resistance to disease, delayed maturity, small seed production and lower yields. The concentration of soluble K in soil is very small as maximum part of K exists in insoluble form. Silt, clay and sand are important components of soil in earth and biggest reservoir of potassium. Most common deposits of potassium are feldspar and mica. The available K level in soil dropped in the last decade due to rapid development of agriculture and application of imbalanced fertilizers. Potassium is released when these minerals are slowly weathered, or, alternatively, it can be solubilized by some beneficial microorganisms and made available for plants. Several bacterial and fungal strains have been identified for their ability of high potassium solubilization. Various species of Pisolithus, Cenococcum, Piloderma, Bacillus, Paenibacillus, Acidithiobacillus, Pseudomonas, Burkholderia, Aspergillus and Clostridium have been reported to release large amount of potassium from different minerals and enhance the productivity of many crops. Co-inoculation of PSMs and KSMs in conjunction with direct application of rock P and K minerals into the soil has been reported to increase N, P and K uptake, photosynthesis and the yield of plants grown in P- and K-limited soils. Thus, identification of microbial strains capable of solubilizing potassium minerals can rapidly conserve our existing resources and escape environmental pollution hazards caused by heavy application of chemical fertilizers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 Introduction
Potassium (K) is a soft, silver-white metal, light in its pure form that reacts very violently with water. It is commonly called potash (K2O), a term that has been derived from an early production technique where K was leached from wood ashes and concentrated by evaporating the leachate in large iron pots (Mikkelsen and Bruulsema 2005). Soils contain varying quantities of K-bearing minerals that constitute a major K reserve. The K reservoir in the earth crust is associated with primary alumina silicates that are the most abundant K-bearing minerals such as K feldspar, mica, biotite, muscovite and nepheline. The secondary alumina silicates, however, comprise hydrous mica (illite) as well as a continuum of micaceous weathered or inherited products, viz. mixed-layer phyllosilicates (Bertsch and Thomas 1985). McAfee (2008) described feldspar and mica ranging from 90 % to 98 % as the most common soil components of potassium. The potassium content of Indian soils varies from less than 0.5 % to 3.00 % (Mengel and Kirkby 1987). Some of the mineral sources available in India containing 3–14 % K2O are glauconitic sand, feldspar, muscovite and nepheline syenite (Indian Minerals Yearbook 2011). Out of various feldspars, potassium feldspar is the most common one and contains up to 13 % K2O (Rao et al. 1998). Micas are important for plant nutrition because they represent a major source of K, whereas the K in biotite acts as a good fertilizer for plants (Arnold 1963).
17.1.1 Forms of Potassium
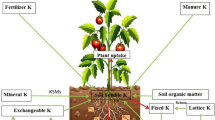
Potassium is available in four forms in the soil which are K+ ions in the soil solution, an exchangeable cation tightly held on the surfaces of clay minerals and organic matter, potassium fixed by weathered micaceous minerals and potassium present in the lattice of certain K-containing primary minerals. All forms of potassium are linked with each other. The amounts of exchangeable and readily available potassium do not provide the rate at which the non-exchangeable or fixed potassium can move into the exchangeable form. These are the reasons why some soils may have a relatively low level when tested and yet supply enough potassium for relatively high crop yields. It has been hypothesized that the lack of crop response on these soils may be attributed to K release from non-exchangeable soil K, particularly from K feldspars (Rehm and Sorensen 1985).
17.1.1.1 Soluble Potassium
Soluble potassium is the most available form but its contribution to total K is very small. According to a report of During (1984), it ranged from 3 to 30 μg/ml in most soils of New Zealand. This type of potassium does not form any chelates, complexes or ion pairs in the soil. Plants take up most of their potassium directly in this form and so deplete it very rapidly in soil.
17.1.1.2 Exchangeable Potassium
Exchangeable K is defined as the fraction that occupies sites in the soil colloidal complex (Malavolta 1985). It is a major bioavailable form of K in the soil, usually 0.1–2 % of total potassium, i.e. between 10 and 400 ppm (Schroeder 1974). The amount of K+ held by clay minerals at exchange sites depended on kinetic as well as thermodynamic factors (Parfitt 1992).
Release of non-exchangeable K to the exchangeable form occurred when levels of exchangeable and soluble K were decreased due to crop uptake or leaching and perhaps by increase in microbial activity (Sparks et al. 1980; Sparks 2000). Hence, the amount of the exchangeable K concentration determines the effectiveness of resupply. The exchangeable K is more related to the type of clay and its net negative charge. It was found that the exchangeable K levels of allophanic soils were relatively small, whereas soils with large amounts of vermiculite or mica had greater amounts of exchangeable K (Parfitt 1992). Potassium in this form is mostly contained by minerals as feldspar and mica. For optimal nutrition of a crop, the replenishment of a K-depleted soil solution was affected predominantly by the release of exchangeable K from clay minerals (Sheng and Huang 2002).
17.1.1.3 Fixed Potassium
Fixed K is held between the layers of micaceous clay minerals and not readily accessible for exchange with other solution cations. The K+ present in the wedge, edge, step and crack positions can be referred to as fixed K. Potassium in this form is temporarily trapped between the expanding layers of some clay minerals and most likely between structural layers in the soil of micas, intergraded hydrous micas (e.g. illites) and vermiculites or in the wedge zones during edge weathering of the micas (Kirkman et al. 1994). A fixed form of potassium in soils which is high in feldspars and volcanic glass where the K is structurally bonded is slowly available to plants over time and cannot be replaced by ordinary cation exchange process. This would contribute little to plant growth, because of the low levels or absence of micaceous 2:1 clay minerals (Fieldes and Swindale 1954).
17.1.1.4 Structural Potassium
Potassium within a soil also exists as structural potassium and is variously known as mineral K, unweathered K, native K, matrix K or inert K. It constitutes the largest amount of the total K in most soils (Metson 1980). It is mostly bound covalently within the crystal structure of the K-bearing minerals such as micas (biotite and muscovite), feldspars (orthoclase and microcline) and volcanic glasses (acidic and basic) (Metson 1968), and it only becomes available upon long-term weathering. Biotite and basic volcanic glasses were found to weather easily, whereas feldspars and acidic volcanic glass weathered slowly (Fieldes and Swindale 1954). Generally, highly weathered soils from humid and tropical areas have much less structural potassium remaining than newer soils or soils from cold arid climates.
17.2 Potassium as Fertilizer
Glauconite sand contains around 4–8 % K2O and has been used as a source of potash fertilizer worldwide (Majumder et al. 1995; Yadav et al. 2000). The need for potash in fertilizer can be determined by plant analysis and soil testing.
17.2.1 Potassium Chloride
It is a common source of fertilizer, and for the most part, it is mined as sylvite ore (KCl, the potassium analogue of halite, or rock salt, NaCl), mixed with NaCl, beneficiated to remove some of the contaminants and sold. It is highly soluble; hence, excessive rate can cause salt damage to plants. Further, KCl may also be the result of crystallization from brine, either from solution mining of KCl ore or precipitation from hypersaline waters, e.g. Searles Lake and Dead Sea, often with carnallite (KClMgCl2) as an intermediate precipitate. The final form of KCl from crystallization processes is white crystals. Typical fertilizer analysis of KCl consists of 60–63 % K2O and 46 % Cl.
17.2.2 Potassium Sulfate
Potassium sulfate has ~5 % market share and basically constitutes 48–53 % K2O and 17–18 % S. It is found rarely in pure form. This fertilizer is used particularly for horticultural crops in which chloride uptake is a problem, as in tobacco plant.
17.2.3 Potassium Magnesium Sulfate
This is the primary K fertilizer produced by German and French mines from langbeinite ore and historically was called ‘double manure salts’. The analysis comprises 20–22 % K2O with secondary nutrients like S (21–22 %) and Mg (10–11 %). Potassium magnesium sulfate is a good source of K when there is also a need for magnesium and sulfate in plant nutrition, though excess K is expected to induce magnesium deficiency.
17.2.4 Potassium Nitrate
In this fertilizer the formulated forms of potassium are KCl and nitric acid with 44 % K2O and 13 % N. It is expensive for agronomic use, mostly applied for horticulture and greenhouses. It was also known as saltpetre to the ancients and recognized by Glauber in the seventeenth century as the ‘principle of vegetation’. Besides above-cited fertilizers, potassium phosphate (KH2PO4) and potassium thiosulfate (K2S2O3) are also available. Kelp meal, plant residues and wood ash, which contain K mainly as a carbonate (K2CO3), hydroxide (KOH) and rock powder, e.g. granite and minerals such as alunite, orthoclase, microcline, etc., are some other sources of potassium sold as K fertilizer.
17.3 Functions of Potassium in Plants
Potassium is a major nutrient element found in the soil which is required by plants in greater amounts. Being essential or vital nutrient for plant growth, potassium (K+) plays an important role in plant regulatory development including osmoregulation, plant-water relation and internal cation/anion balance. It also has substantial effect on enzyme activation involved in the formation of organic substances, protein and starch synthesis, respiratory and photosynthetic metabolism (Lauchli and Pfluger 1979; Wyn Jones et al. 1979; Marschner 2010), stomatal movement and water relations (turgor regulation and osmotic adjustment) by increasing protein production in plants (Marschner 1995). Enzyme activation is also needed to metabolize carbohydrates for the manufacture of amino acids and proteins and tolerance of external stress such as frost, drought, heat and light. Potassium also functions in triggering the growth of young tissues and cell enlargement and, hence, improves early growth. Besides, potassium is important during plant ontogeny and in improving plant quality and oil content in plants. Hence, large amount of potassium is required to maintain plant health but it often receives less attention than N and P in many crop production systems. The crop yield can be positively and negatively influenced by favourable and unfavourable environmental conditions. Unfavourable environmental conditions which would create potentially damaging physiological changes within plants are known as stresses (Shao et al. 2008). Potassium has also been involved in various physiological functions related to plant health and resistance to biotic and abiotic stresses such as diseases, pests, drought, salinity, cold, frost and waterlogging (Wang et al. 2013) However, suitable application of potassium can improve insect and disease resistance in plants. The protective role of K+ in plants suffering from drought stress has been well documented (Pier and Berkowitz 1987; Sen Gupta et al. 1989). There are major challenges for agriculture to enhance crop yields and to stabilize plant development and yield formation under biotic and abiotic stress conditions (Reynolds et al. 2011).
17.3.1 Biotic Stress Resistance
Biotic components such as weeds caused the highest potential loss (~32 %), followed by animal pests (18 %), fungi, bacteria (15 %) and viruses (3 %) (Oerke and Dehne 2004). Recently, it was seen in K-deficient soil that plants were more susceptible to infection than those plants having adequate supply of K (Wang et al. 2013) and also the rate of rice borer infestation was greatest when there was no supply of K, but decreased rapidly as the K concentration increased (Sarwar 2012). It was found that the use of K in fields extensively decreased the occurrence of fungal diseases by 70 %, bacterial by 69 %, viral by 41 %, insect and mite infestation by 63 % and nematodes by 33 % (Perrenoud 1990). The synthesis of high-molecular-weight compounds (such as proteins, starch and cellulose) was markedly increased in K-sufficient plants, thus lowering the concentrations of low-molecular-weight compounds, such as soluble sugars, organic acids, amino acids and amides in the plant tissues which were generally responsible for the development of infections and insect infestations. Thereby, plants were protected from diseases and pest attacks in K-sufficient plants (Marschner 2012). Adequate K also increased phenol concentrations, which could also play a significant role in plant resistance (Prasad et al. 2010).
17.3.2 Abiotic Stress Resistance
Abiotic stresses can cause major damage to the crops as compared to yield losses from biotic stress (Oerke 2006). Major abiotic factors are drought stress (low moisture, aquaporins and water uptake, osmotic and stomatal regulation, detoxification of reactive oxygen species, etc.), salt stress, cold stress and waterlogging stress. The use of potassium triggers many plant activities, whereas depletion of potassium uptake can cause problem for plant growth. Potassium to a great extent contributes to the survival of the plants exposed to various abiotic stresses. It was documented by Wang et al. (2013) that abiotic stress factors such as heat, cold, drought and salinity had a huge impact on world agriculture, and they might reduce average yields by ~50 % for most major crop plants. Increased application of K+ has been shown to enhance photosynthetic rate, plant growth and yield and drought resistance in different crops under water stress conditions (Sharma et al. 1996; Tiwari et al. 1998; Yadav et al. 1999; Egilla et al. 2001).
17.3.2.1 Drought and Low Moisture Stress
Association between K stress and plant drought resistance has been demonstrated by many workers. Drought can be defeated by plants by inducing deeper rooting, larger absorption surfaces and greater water retention in plant tissues, which can also be overcome by application of K fertilizers with other nutrients like phosphorus and nitrogen (Kirkby et al. 2009). Sufficient amounts of K enhanced the total dry mass accumulation of crop plants under drought stress/low-moisture conditions in comparison to lower K concentrations (Egilla et al. 2001). Similar findings were documented by Lindhauer (1985) that not only plant dry mass was increased but also leaf area and water retention in plant tissues under drought-stressed conditions improved. It was found that plants that were continuously exposed to drought stress could form reactive oxygen species, which caused leaf damage (Cakmak et al. 2005; Foyer et al. 2002; Oerke and Dehne 2004). Wang et al. (2013) found a close relation of K in physiological and molecular mechanisms of plant drought resistance. For the period of drought stress, root growth and the rate of K+ diffusion in the soil towards the root got restricted and depressed the plant resistance as well as K absorption.
17.3.2.1.1 Aquaporins and Water Uptake
Potassium is also involved in plant-water relations by regulating the osmotic potential and hydraulic conductivity of membranes and altering water permeability (Heinen et al. 2009; Maurel and Chrispeels 2001). Aquaporin is water along with K+ ions which moves through specific channel protein present in the plasma and intracellular membranes of cells (Maathuis et al. 1997; Steudle 2000). K+ is found in the plant cell in two distinct compartments, the cytosol and the vacuole (Leigh 2001), hence transported through plant cell membranes with the help of specific protein channels (Maathuis et al. 1997). Under drought stress conditions, aquaporin gene expression could be regulated (Tyerman et al. 2002; Lian et al. 2004) to help the plants to maintain their water balance (Kaldenhoff et al. 2008). During water stress, roots regulated their water and ion uptake capacities by modifying PIPs (plasma membrane intrinsic proteins) and K+ channel at the transcription level to cope with the water deficiency (Smart et al. 2001; Galmes et al. 2007; Cuellar et al. 2010). Kanai et al. (2011) also observed close coupling between aquaporin activities and K channel transporters. They found that aquaporin activities might have been suppressed by K deficiency and resulted in a reduction of root hydraulic conductance and water supply to the growing stem for diameter expansion and the leaf for transpiration.
17.3.2.1.2 Osmotic and Stomatal Regulation
Under drought stress condition, the maintenance of enough water levels is important for plant survival; hence, osmotic regulation is the most important trait involved in maintaining high cellular turgor potential and water retention in response to drought stress. An adequate amount of K may help osmotic adjustment, which maintains higher turgor pressure, relative water content and lower osmotic potential, thus improving the ability of plants to tolerate drought stress (Kant and Kafkafi 2002; Egilla et al. 2005). Stomata are essential to control plant water loss via transpiration and quick stomatal closures are needed to survive during stressed conditions. Stomatal closure is preceded by rapid release of K+ from the guard cells into the leaf, thus improving the ability of plants to tolerate drought stress (Kant and Kafkafi 2002). When K+ is deficient, the stomata cannot function properly and water losses from plant may reach damaging levels (Gething 1990).
17.3.2.1.3 Detoxification of Reactive Oxygen Species (ROS)
Generally, drought stress or K-deficient plants induce ROS, e.g. superoxide radical (O• 2), hydrogen peroxide (H2O2) and hydroxyl radical (•HO), production (Cakmak 2005). Production of ROS is mainly responsible for impairment of cellular functions and growth depression in stress conditions. K requirement for drought-stressed plants could be related to the role of K in enhancing photosynthetic CO2 fixation and transporting photosynthates into sink organs and inhibiting the transfer of photosynthetic electrons to O2, thus reducing ROS production (Cakmak 2005). Besides the photosynthetic electron transports, nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase activation represents another major source for production of ROS in plant cells by a number of biotic and abiotic stress factors. Furthermore, abscisic acid has also been shown to be effective in increasing H2O2 and O2 − accumulations in roots or leaves (Lin and Kao 2001; Jiang and Zhang 2001). Hence, maintaining an adequate K nutritional status critical for plant osmotic adjustment and for mitigating ROS damage as induced by drought stress.
17.3.2.2 Salinity Stress
Salinity is another major abiotic stress that affects major part of the total land area on earth. Saline soils generally had higher concentrations of Na+ than K+ and Ca2+ which resulted in passive accumulation of Na+ in root and shoot (Bohra and Doerffling 1993). Due to the accumulation of salt, water uptake by plant roots in soil became difficult thus disturbing water balance, while high concentrations of salts in plant tissue were found to be toxic (Wang et al. 2013). Salinity inhibited seed germination and plant growth, affected the leaf anatomy and physiology of plants and, thereby, influenced plant-water relations, photosynthesis, protein synthesis, energy production and lipid metabolism (Parida and Das 2005).
17.3.2.3 Cold Stress
Cold stress inhibited plant growth and development and resulted in reduced crop productivity (Wang et al. 2013). Devi et al. (2012) noticed that in Panax ginseng, a high K+ concentration activated the plant’s antioxidant system and increased the levels of ginsenoside-related secondary metabolite transcripts, which are associated with cold tolerance. Thus, cold stress might have destroyed photosynthetic processes and reduced the efficacy of antioxidant enzymes, resulting in ROS accumulation (Mittler 2002; Xiong et al. 2002; Suzuki and Mittler 2006). Potassium improved plant survival under cold stress by increasing antioxidant levels and reducing ROS production (Cakmak 2005; Devi et al. 2012).
High concentrations of K+ protect plant cell against freezing by lowering the freezing point of the cell solution. The plasma membrane is the prime site which is mainly affected when changes in temperature occur, and membrane fluidity is reduced by cold stress resulting in changes of fatty acid unsaturation and the lipid-protein composition of the cell membrane (Wang et al. 2006). Maximum growth response and chilling resistance in tomato, eggplant and pepper plants with the addition of K+ were associated with increase in phospholipids, membrane permeability and improvement in biophysical and biochemical properties of cell (Hakerlerker et al. 1997). Hence, higher K tissue concentrations reduced chilling damage and increased cold resistance, thereby increasing yield production (Mengel 2001; Kant and Kafkafi 2002).
17.3.2.4 Waterlogging Stress
Waterlogging, a serious obstacle for sustainable agriculture development, affected approximately 10 % of the global land area (Setter and Waters 2003). The main biological consequence that plant encounters during waterlogging stress is the respiration of roots, and microorganisms further deplete the residual oxygen, hence making the environment hypoxic (i.e. oxygen levels limit mitochondrial respiration) and later anoxic (i.e. respiration is completely inhibited). It resulted in a severe decline in energy status of root cells which affected important metabolic processes of plants (Bailey-Serres and Voesenek 2008; Wegner 2010). To overcome this difficulty, application of K could efficiently offset the adverse effects of waterlogging on plants. It was reported that K supplement under waterlogging not only increased plant growth, photosynthetic pigments and photosynthetic capacity but also improved plant nutrient uptake as a result of higher K+, Ca2+, N, Mn2+ and Fe2+ accumulation (Ashraf et al. 2011). Exogenous application of K in soil and as foliar spray alleviated the adverse effects of waterlogging on cotton and many other plants such as corn (Welch and Flannery 1985; Csatho 1991), rice (Datta and Mikkelsen 1985), wheat (Beaton and Sekhon 1985; Khurana and Bhaya 1990) and oilseed rape (Sharma and Kolte 1994). There are major challenges for agriculture to enhance crop yields and to stabilize plant development and yield formation under biotic and abiotic stress conditions (Reynolds et al. 2011).
17.4 Deficiency of Potassium
There are several factors that lead to the insufficient supply of nutrients in soil and as a result plant has to face deficiency. In addition, the presence of extreme amounts of reduced substances in poorly drained soils is also responsible for retarded root growth and reduced K uptake (Fairhurst et al. 2007). Fundamentally, K+ is water soluble and highly mobile and transported to the xylem in plants (Lack and Evans 2005). It acts as regulator in plants since it is constituent of 60 different enzyme systems of drought tolerance and water-use efficiency. K deficiency, also known as potash deficiency, occurs widely in plants and has a strong impact on plant metabolism. A plenty of deficiency symptoms have been reported which include chlorosis, poor growth, reduced yield and poor fibre quality with the increased susceptibility to diseases (Amtmann et al. 2008) and pests (Amtmann et al. 2006; Troufflard et al. 2010). It also affects photosynthesis process and plant growth; hence, purple spots may also appear on the leaf undersides. Besides, older leaves change from yellow to brown, leaf tips and margins dry up, root oxidation power declines, younger leaves decolourize, and root length and density reduce causing reduction of nutrient uptake and cytokine production in roots. Moreover, K-deficient plants are highly light sensitive and very rapidly become chlorotic and necrotic when exposed to high light intensity (Cakmak 2005). Potassium deficiency also inhibits evaporation which causes the temperature in the leaves to rise and results in burning of cells, which mainly occurs on the edges of the leaves. Stressed plants may be more sensitive to the cold injury. Sometimes rust-brown spots may appear in the leaf if K deficiency is severe, and leaves often turn or curl radially on the top and entire leaves become necrotic and eventually fall off. Flowering will be severely inhibited which ultimately affects crop production.
The reasons for potassium deficiency could be insufficient fertilization, excessive ‘table salt’ (sodium) in the root environment, unfavourable soil structure (e.g. sandy soils) and formation of depletion zones around roots (Kayser and Isselstein 2005; Moody and Bell 2006; Andrist-Rangel et al. 2007). Effects of K deficiency on plants depended on specific crop type such as corn becoming small in size and showing low yield and tomatoes exhibiting uneven fruit ripening, whereas leaves of cotton plants turned reddish brown and then black, appeared scorched and eventually fell, and yield and quality of forage crops were adversely affected (www.ncagr.gov). To overcome the deficiency, the prevention and cure can be achieved at great extent by adding potassium-specific fertilizer, e.g. potassium carbonate, rock potash, potassium nitrate, potassium sulfate, etc. Thus, study of potassium uptake and strategies to enhance uptake in plants is necessary.
17.5 Pathways and Mechanisms for Potassium Uptake
Plant cannot acquire nutrient directly unless it is present in available or dissolved form or released by weathering. There are several processes that contribute to the availability of potassium in the soil. Besides, the release of K from the rocks and minerals requires weathering over long periods of time, although calcinations of rocks can break the K out of the structure but makes the K more expensive than that from the evaporite ores. In addition to plant uptake of K from the soil solution, some of the exchangeable potassium on the soil colloids is also absorbed directly by roots. A plant root possesses a negative charge and attracts the positively charged potassium (K+) which is held on the clay mineral surfaces and edges. However, potassium uptake of plants can be increased by using potassium solubilizers as bioinoculants which further increased the crop productivity (Shanware et al. 2014). Lian et al. (2008) reported that there were three major reaction pathways utilized by Aspergillus fumigatus to release potassium from potassium minerals which were acid hydrolysis, secretion of insoluble macromolecules and polymers bound in the cell membrane and direct biophysical forces which could split mineral grains.
It was demonstrated that potassium-solubilizing microorganisms have the capacity to dissolve K from insoluble minerals (Alexander 1985). Potassium-solubilizing bacteria are able to solubilize rock K mineral powder, such as micas, illite and orthoclases through various processes such as acidolysis, enzymolysis, capsule absorption and complexation by extracellular polysaccharides (Avakyan 1984; Rozanova 1986; Malinovskaya 1988; Malinovskaya et al. 1990; Friedrich et al. 1991; Ullman et al. 1996; Welch et al. 1999). Mechanisms involved in degradation of potassium-bearing minerals can be divided into direct (bacterial cell wall) and indirect (bioleaching, mineral weathering, microbial weathering and mechanical fragmentation) mechanisms.
17.5.1 Bacterial Cell Wall
Prokaryotes have various cell wall types which secrete one or more metabolic products that react with ions or compounds in the environment resulting in the deposition of mineral particles. Bacterial surfaces such as cell walls or polymeric materials (exopolymers) exuded by bacteria, including slimes, sheaths or biofilms, and even dormant spores, acted as important sites for the adsorption of ions and mineral nucleation and growth (Konhauser 1998; Beveridge 1989; Banfield and Zhang 2001; Bäuerlein 2003). In gram-negative bacteria, the layers external to the bacterial cell wall that may be involved in mineral nucleation include S-layers, capsules, slimes and sheaths. S-layers are acidic and possess a net negative charge, thereby having an affinity for metal cations (Southam 2000). In some cases, capsules are also known to form in response to the presence of metal ions (Appanna and Preston 1987).
17.5.2 Bioleaching
The process of removal of soluble material from the rocks in solution by percolating water is termed as leaching. Halite (NaCl) and sylvite (KCl) are highly water soluble, while carbonates and sulfates are sparingly soluble. Bioleaching microbes are mainly Thiobacillus ferrooxidans, T. thiooxidans and Leptospirillum ferrooxidans.
17.5.3 Mineral Weathering
The weathering reactions contribute to nutrient cycling (Huntington et al. 2000). Chemical weathering of bedrock releases inorganic nutrients such as Ca, Mg, K, Fe and P, which are then cycled through the saprolite, soil and vegetation. Inorganic nutrients were cycled by microorganisms via uptake, release, biomineralization, oxidation and reduction (Berner and Berner 1996). Microorganisms also contributed to mineral weathering and soil formation by secreting organic acids and other ligands such as siderophores as indicated by many researchers (Richter and Markewitz 1995; Kalinowski et al. 2000; Liermann et al. 2000, 2005; Richter and Oh 2002). Within the rooting zone, plants take up mineral nutrients, which are recycled back into the soil when plants get decomposed.
During weathering, physical, chemical and biological forces act on the parent materials and break them down into finer fractions, largely sand-, silt- and clay-size particles. This breakdown results in the release of several chemical elements, including potassium, and the formation of different clay minerals. Most of the total potassium inherited from the parent material during the soil-forming processes will be in the non-exchangeable and exchangeable forms. The relative amounts of sand, silt and clay fractions found in a soil depend on the kind of parent material (sandstone, limestone, shale or mica) from which the soil was derived. Potassium fixation and release is greatly influenced by the relative amounts of these fractions and the kinds of clay minerals present in the soil. Mineral dissolution studies with cultures of bacteria and fungi showed a dramatic increase in the dissolution rates of feldspar, biotite, quartz, apatite and other minerals (Berthelin and Belgy 1979; Callot et al. 1987; Thorseth et al. 1995; Ullman et al. 1996; Barker et al. 1997; Paris et al. 1996).
17.5.4 Microbial Weathering
The known and potential mechanisms of microbial weathering included redox reactions through the production of organic acids which led to weakening of chemical bonds in minerals for promoting mineral dissolution (Banfield et al. 1999; Harley and Gilkes 2000) and chelating molecules for mineral degradation (Uroz et al. 2007, 2009; Lian et al. 2008). Bacteria produced a wide range of low-molecular-weight organic acids such as citric, malic, oxalic, succinic and tartaric acid (Jones 1998; Neaman et al. 2005). Han and Lee (2005) concluded in a study that KSB solubilized potassium rock through production and secretion of organic acids. Similar were the observations of Prajapati and Modi (2012), which attributed the solubilization to reduction in pH due to organic acids. Few other reports are also available for feldspar solubilization by Bacillus mucilaginosus and Bacillus edaphicus due to acid production (Malinovskaya et al. 1990; Sheng and Huang 2002). In addition to organic acids, microorganisms (such as bacteria, algae, fungi and protozoa) used carbonic acid formed from carbon dioxide to attack the mineral surface, promoting the chemical weathering of rocks and minerals (Gadd 2007; Park et al. 2009).
Microbes played a key role in the weathering of major type of rocks, releasing various elements they needed as nutrients (Calvaruso et al. 2006). Many rock-inhabiting fungi were melanized, and melanin pigmentation conferred extra-mechanical strength to the hyphae to penetrate the rock surface and crevices (Dornieden et al. 1997; Sterflinger and Krumbein 1997) and also offered protection from metal toxicity (Gadd 1993). Fungi have been reported from a wide range of rock types, including rocks from extreme environments (Staley et al. 1983; Nienow and Fridman 1993; Sterflinger 2000; Etienne and Dupont 2002; Gorbushina 2007). In an experimental study conducted by Puente et al. (2004), fluorescent pseudomonads and bacilli were found to weather igneous rock, limestone and marble.
17.5.5 Biofilm
Biofilm helps to accelerate weathering of minerals like biotite and anorthite. Biotite weathering occurs in two stages: by oxidation within the rindlet zone and by alteration to kaolinite within the saprolite. Biofilms and biocrusts were normally supposed to cause higher weathering rates due to biodegradation (Warscheid and Braams 2000). It was accepted that the microbial biofilms not only accelerated the weathering process but also regulated denudation losses by acting as a protective layer covering the mineral-water-hyphal/root hair interface in the mycorrhizosphere and rhizosphere of vascular plants. Besides, biofilm formation on mineral surface promoted the corrosion of potassium-rich shale and the release of K, Si and Al in the bacteria-mineral contact model (Li-yang et al. 2014).
17.5.6 Mechanical Fragmentation
Fragmentation of the mineral caused by root activity increases the reactive surfaces, so having direct positive effect of the bacteria on mineral weathering. Mechanical fragmentation of rock particles also occurred when there was extension of hyphae (unique for fungal organisms) into the interior of minerals to acquire nutrition (Jongmans et al. 1997).
17.6 Potassium-Solubilizing Microorganisms (KSMs)
Potassium is the third major essential macronutrient for plant growth and development. It constitutes ~2.5 % of the lithosphere but actual soil concentrations of this nutrient vary widely ranging from 0.04 % to 3.0 % (Sparks and Huang 1985). Plants absorb K only in soluble form from soil, and its availability to crop plants is generally as low as 90–98 % of total K in soil in the unavailable mineral forms (Sparks 1987) such as feldspar and mica (McAfee 2008). The addition of chemical fertilizers causes environmental pollution and has many deteriorating impacts such as global warming, alteration of soil microbial diversity, etc. Moreover, they also influence soil-plant dynamics with its microbial distribution (Meena et al. 2013; Maurya et al. 2014).
Microorganisms on solid and liquid medium were tested, and it was observed that bacterium Enterobacter hormaechei (KSB-8) was more viable in liquid broth as compared to solid medium containing carrier lignite, while fungus Aspergillus terreus (KSF-1) was good at both liquid and solid medium (Kalawati and Modi 2014). Rhizosphere microorganisms play an important role in solubilization of bound form of soil minerals and enhancing the availability of plant nutrients in the soil. Increasing the bioavailability of phosphorus (P) and potassium in soils with inoculation of plant growth-promoting rhizobacteria (PGPR) singly or in consortium with or without rock materials has been reported by many researchers (Lin et al. 2002; Sahin et al. 2004; Girgis 2006; Eweda et al. 2007; Jha et al. 2012; Meena et al. 2015b; Singh et al. 2015), which may lead to increasing P uptake and plant growth. The mould A. niger has been well documented for its ability to solubilize P in rocks due to organic acid production, especially citric acid (Nahas et al. 1990; Vassileva et al. 1998; Jain et al. 2014).
Different microorganisms are used to supply different kind of nutrients in the soil such as symbiotic and non-symbiotic nitrogen-fixing bacteria which can supply nitrogen to plants by fixing the atmospheric nitrogen and converting the nitrogen into ammonium ion. Bacillus megaterium and Pseudomonas sp. are the most common phosphate-solubilizing bacteria that are used as a biofertilizer to solubilize phosphorus in soil. Solubilization of potassium from aluminosilicate minerals has also been observed by some fungi (Wallander and Tonie 1999; Glowa et al. 2003). It was proposed by Yuan et al. (2000) that ectomycorrhizae could mobilize potassium from clay minerals and thus enhanced its uptake by plants. Further, Yuan et al. (2004) studied the effect of four fungal strains, Pisolithus XC1, Pisolithus sp., P. microcarpus and Cenococcum geophilum SIV, collected from the roots of eucalyptus on the degradation of phlogopite and vermiculite; the results revealed that all four strains were able to weather the mineral phases and release elemental K. Moreover, Glowa et al. (2003) evaluated the ability of fungus Piloderma in extracting potassium from biotite, microcline and chlorite and found that the fungus species was able to acquire potassium from all three minerals, out of which biotite was more biodegradable (Meena et al. 2014a, 2015a).
Muentz (1890) showed the first evidence of microbial involvement in solubilization of rock potassium. Since then a diverse group of soil microflora has been reported to be involved in the solubilization of insoluble and fixed forms of K into available forms, which can easily be absorbed by plants (Li et al. 2006; Gundala et al. 2013; Zarjani et al. 2013). A wide range of potassium-solubilizing bacteria (KSB), namely, Bacillus edaphicus, B. circulans, Paenibacillus spp., Acidithiobacillus ferrooxidans, Pseudomonas spp., Burkholderia spp., etc., have been reported to release potassium from K-bearing minerals in soil (Sheng et al. 2008; Lian et al. 2002; Rajawat et al. 2012; Liu et al. 2012; Basak and Biswas 2012; Singh et al. 2010).
KSB such as Bacillus mucilaginosus solubilized potassium rock and stimulated plant growth through synthesis of growth-promoting substances via their biological activities. Similarly, silicate-solubilizing bacteria were found to dissolve potassium, silicon and aluminium from insoluble minerals (Aleksandrov et al. 1967). KSB have capacity to dissolve K from insoluble minerals (Alexander 1985). Many microorganisms in the soil are able to solubilize ‘unavailable’ forms of K-bearing minerals, such as micas, illite and orthoclases, by excreting organic acids which either directly dissolved rock K or chelated silicon ions to bring the K into solution (Groudev 1987; Friedrich et al. 1991; Ullman et al. 1996; Bennett et al. 1998). Therefore, the application of K-solubilizing microorganisms (KSMs) is a promising approach for increasing K availability in soils (Zahra et al. 1984; Vandevivere et al. 1994; Barker et al. 1998; Meena et al. 2014b; Kumar et al. 2015).
KSMs can be isolated from many sources in in vitro conditions using different media. Prajapati and Modi (2012) isolated 14 bacterial strains from samples collected from ceramic industry using feldspar on a solid media, out of which five strains showed higher potassium solubilization. Recently, Parmar and Sindhu (2013) used Aleksandrov medium supplemented with mica to isolate 137 K-solubilizing bacteria from soil samples collected from wheat rhizosphere. Among isolated strains, 20 strains were found to solubilize potassium from mica. The amount of K released by the strains ranged from 15 to 48 mg L−1. Further examination for optimization of conditions for K release revealed that maximum solubilization occurred with glucose as carbon source at 25 °C temperature and 7.0 pH. Potassium solubilization was maximum when KCl was used as potassium source, followed by K2SO4, and least solubilization was found in mica powder. It was suggested that efficient potassium-solubilizing bacterial strains could be further exploited for plant growth improvement under field conditions. Sheng (2005) used sucrose minimal salt medium with illite as K source for the isolation of B. edaphicus strain NBT from rhizosphere soil of cotton. Wu et al. (2005) used the same medium with glass powder for the growth of B. mucilaginosus, a vigorous K solubilizer. Further, it was found to have great K-releasing capability and could promote the release of potassium through weathering of silicate minerals (Hu et al. 2006; Lian 1998; Zhao et al. 2006, 2008). Sangeeth et al. (2012) identified potassium-solubilizing bacterium, Paenibacillus glucanolyticus IISRK2, isolated from rhizosphere of black pepper plant. It was further evaluated for plant growth, and after the studies, it was documented that the strain efficiently promoted the shoot and dry matter of wheat plants promoting the overall plant growth. Commercially available microbial inoculants that are able to dissolve K from minerals and rocks not only enhance plant growth and yield but are also eco-friendly; microbes Aspergillus niger, Bacillus extorquens and Clostridium pasteurianum were found to grow on muscovite, biotite, orthoclase microclase and mica in vitro (Archana et al. 2013).
17.7 KSM Role in Sustainable Agriculture
Potassium is an essential and major nutrient for crop production (Alfaro et al. 2003; Zhang et al. 2011). The role of potassium is well known for improving shelf life of crops and disease resistance (Khawilkar and Ramteke 1993). Potassium is useful in agriculture land to increase crop yield as proper amount of potassium in soil can enhance root growth, improve drought resistance, activate many enzyme systems, maintain turgor pressure, reduce water loss and wilting, aid in photosynthesis and food formation, reduce respiration, prevent energy losses, enhance translocation of sugars and starch, produce grain rich in starch, increase protein content of plants, reduce waterlogging and retard crop diseases. Therefore, it is essential for the growth and metabolism of plants; the deficiency of potassium in plants causes poorly developed roots, slow growth and low resistance to disease, delayed maturity, small seeds and lower yields. With rapid development of agriculture or due to application of imbalanced fertilizers, available K level in soils has dropped. The concentration of soluble K is very small and the maximum part of K exists in insoluble form. Silt, clay and sand are biggest reservoirs of potassium and important component of soil. The most common component of potassium is feldspar and mica, and fortunately, India has the largest deposits of mica mines distributed in some districts of Bihar and Jharkhand. In such conditions the application of KSMs can be an alternative approach for increasing K availability (Krishnamurti and Huang 1988; Prajapati and Modi 2014; Zhang and Kong 2014).
It has been studied that by introducing potassium-solubilizing bacteria B. mucilaginosus and phosphate-solubilizing bacteria Bacillus megaterium var. phosphaticum, simultaneously, macronutrient (nitrogen, phosphate and potassium) uptake was increased in eggplant, pepper and cucumber leading to higher yields (Han et al. 2006). B. edaphicus strain was also examined for the growth promotion and increased potassium uptake on cotton and rape plants, and increased plant growth was observed in the soil treated with insoluble potassium with strain NBT. The shoot and root dry weight increased from 25 % to 33 % (cotton) and from 24 % to 27 % (rape), whereas K content was increased from 31 % to 34 % (P < 0.05) (cotton) and 28–31 % (rape) when the soil with insoluble K source and bioinoculant was compared to the uninoculated soil (Sheng 2005). Experiments conducted with tobacco seedlings, inoculated with Klebsiella variicola strains JM3, XF4 and XF11, showed greater height and dry weight than uninoculated seedlings. The GL7 significantly increased plant dry weight. Inoculation with the strains JM3, GL7, XF4 and XF11 significantly increased seedling absorption of N, P and K. Seedlings exposed to K feldspar absorbed significantly more N and K than those not exposed to added K feldspar (Jhang and Kong 2014). Krishnamurthy (1990) reported that the potassium content in tobacco leaf was strongly and positively correlated with available K status in sandy and sandy loam soils.
Nowadays, biofertilizer is a substitute to chemical fertilizer to increase soil fertility and crop production in sustainable farming. The use of plant growth-promoting microorganisms, including phosphate-solubilizing and potassium-mobilizing bacteria as biofertilizers, was suggested as a possible solution to improve plant nutrient and production (Vessey 2003). Beneficial microorganisms in the form of biofertilizers are applied on seeds/roots or in soil which mobilizes the availability of nutrients, especially N-P-K by their biological activity, thereby helping in the build-up of positive microflora and enhancing the soil health. The use of bioinoculants can also improve the physical properties and enhance water-holding capacity of soil. Moreover, microorganisms that are applied as biofertilizer can prevent nutrient leaching and lead to soil enrichment with nutrients. They are low in cost, compatible with long-term sustainability, and eco-friendly as compared to chemical fertilizers. Besides, the nutrient supply is constant and sustainable through these microorganisms’ activities. Though the use of biofertilizer has gained momentum in recent years since chemical fertilizers are high in cost and can cause hazardous effect (Aseri et al. 2008), unfortunately not much attention has been given to the manufacturing of K biofertilizers.
17.8 Concluding Remarks
Potassium being the third major nutrient for plants is vital for plant growth. However, majority of potassium in soils is available in insoluble forms. Therefore, potassium-solubilizing microorganisms, the component of soil microbial community, play an important role in K solubilization to provide available form to plants. K solubilization benefits crop growth and improves soil fertility in an eco-friendly manner. Potassium-solubilizing strains are able to colonize the rhizosphere, promote crop yield and enhance plant stress response during stress conditions and K uptake. Unfortunately, very little attention has been paid to K-solubilizing microorganisms and K biofertilizers as most of the researches are focused on nitrogen and phosphorus biofertilizers. Hence, it is requisite to study the efficient K-solubilizing microorganisms to improve sustainable agriculture and to keep the soil productive.
References
Aleksandrov VG, Blagodyr RN, Iiiev IP (1967) Liberation of phosphoric acid from apatite by silicate bacteria. Mikrobiol Zh (Kiev) 29:111–114
Alexander M (1985) Introduction to soil microbiology. Wiley, New York, pp 382–385
Alfaro MA, Jarvis SC, Gregory PJ (2003) The effect of grassland soil managements on soil potassium availability. J Soil Sci Plant Nutr 3(2):31–41
Amtmann A, Hammond JP, Armengaud P, White PJ (2006) Nutrient sensing and signalling in plants: potassium and phosphorus. Adv Bot Res 43:209–257
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133:682–691
Andrist-Rangel Y, Edwards AC, Hillier S, Oborn I (2007) Long-term K dynamics in organic and conventional mixed cropping systems as related to management and soil properties. Agric Ecosyst Environ 122:413–426
Appanna VD, Preston CM (1987) Manganese elicits the synthesis of a novel exopolysaccharide in an arctic Rhizobium. FEBS Lett 215:79–82
Archana DS, Nandish MS, Savalagi VP, Alagawadi AR (2013) Characterization of potassium solubilizing bacteria (KSB) from rhizosphere soil. Bioinfolet 10:248–257
Arnold PW (1963) Potassium in partially weathered soils. In: Proceedings of the first regional conference of the International Potash Institute, Berne, pp 11–17
Aseri GK, Jain N, Panwar J, Rao AV, Meghwalc PR (2008) Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar Desert. Sci Hortic 117(2):130–135
Ashraf MA, Ahmad MSA, Ashraf M, Al-Qurainy F, Ashraf MY (2011) Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop Pasture Sci 62:25–38
Avakyan ZA (1984) Silicon compounds in solution bacteria quartz degradation. Mikrobiology 54:301–307
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Banfield JF, Zhang H (2001) Nanoparticles in the environment. Rev Mineral Geochem 44:1–58
Banfield J, Barker W, Welch S, Taunton A (1999) Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc Natl Acad Sci 96:3404–3411
Barker WW, Welch SA, Banfield JF (1997) Geomicrobiology of silicate mineral weathering. Min Soc Am Rev Mineral 35:391–428
Barker WW, Welch SA, Chu S, Banfield JF (1998) Experimental observations of the effects of bacteria on aluminosilicate weathering. Am Min 83:1551–1563
Basak B, Biswas D (2012) Modification of waste mica for alternative source of potassium: evaluation of potassium release in soil from waste mica treated with potassium solubilizing bacteria (KSB). LAMBERT Academic Publishing, Germany, ISBN-13:978-3659298424
Bauerlein E (2003) Biomineralization of unicellular organisms: an unusual membrane biochemistry for the production of inorganic nano- and microstructures. Angew Chem Int 42:614–641
Beaton JD, Sekhon GS (1985) Potassium nutrition of wheat and other small grains. In: Munson RD (ed) Potassium in agriculture. ASA, Madison
Bennett PC, Choi WJ, Rogera JR (1998) Microbial destruction of feldspars. Mineral Manag 8(62A):149–150
Berner EK, Berner RA (1996) Global environment: water, air, and geochemical cycles. Prentice Hall, Upper Saddle River, N.J: Prentice Hall
Berthelin J, Belgy G (1979) Microbial degradation of phyllosilicates during simulated podzolization. Geoderma 21:297–310
Bertsch PM, Thomas GW (1985) Potassium status of temperate region soils. In: Munson RD (ed) Potassium in agriculture. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, pp 131–162
Beveridge TJ (1989) Role of cellular design in bacterial metal accumulation and mineralization. Annu Rev Microbiol 43:147–171
Bohra JS, Doerffling K (1993) Potassium nutrition of rice (Oryza sativa L.) varieties under NaCl salinity. Plant Soil 152:299–303
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Callot G, Maurette M, Pottier L, Dubois A (1987) Biogenic etching of microfractures in amorphous and crystalline silicates. Nat (Lond) 328:147–149
Calvaruso C, Turpault MP, Frey-Klett P (2006) Root-associated bacteria contribute to mineral-weathering and to mineral nutrition in trees, a budgeting analysis. Appl Environ Microbiol 72:1258–1266
Csatho P (1991) Effect of NPK-fertilization and split application of nitrogen on lodging due to windstorm and harvestable grain yield of maize. Acta Agron Hung 40(3–4):281–294
Cuellar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I (2010) A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 61:58–69
Datta SK, Mikkelsen DS (1985) Potassium nutrition of rice. In: Munson RD (ed) Potassium in agriculture. ASA, Madison
Devi BSR, Kim YJ, Selvi SK, Gayathri S, Altanzul K, Parvin S, Yang DU, Lee OR, Lee S, Yang DC (2012) Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in Panax ginseng. Russ J Plant Physiol 59:318–325
Dornieden T, Gorbushina AA, Krumbein WE (1997) Changes in physical properties of marble by fungal growth. Int J Restor Build Mon 3:441–456
During C (1984) Fertilizers and soils in New Zealand, 3rd revised edn. Government Printer, Wellington, pp 355
Egilla JN, Davies FT, Drew MC (2001) Effect of potassium on drought resistance of Hibiscus rosa-sinensis cv. Leprechaun: plant growth, leaf macro- and micronutrient content and root longevity. Plant Soil 229:213–224
Egilla JN, Davies FT, Boutton TW (2005) Drought stress influences leaf water content, photosynthesis, and water-use efficiency of Hibiscus rosa-sinensis at three potassium concentrations. Photosynthetica 43:135–140
Etienne S, Dupont J (2002) Fungal weathering of basaltic rocks in a cold oceanic environment (Iceland): comparison between experimental and field observations. Earth Surf Proc Land 27:737–748
Eweda WE, Selim SM, Mostafa MI, Dalia A, El-Fattah A (2007) Use of Bacillus circulans as bio-accelerator enriching composted agricultural wastes É- identification and utilization of the Aust. J Basic Appl Sci 2(1):68–81
Fairhurst TH, Witt C, Buresh RJ, Dobermann A (2007) Rice: a practical guide to nutrient management. International Rice Research Institute, International Plant Nutrition Institute, and International Potash Institute, pp 89
Fieldes M, Swindale LD (1954) Chemical weathering of silicates in soil formation. N Z J Sci Tech B36:140–154
Foyer CH, Vanacker H, Gomez LD, Harbinson J (2002) Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiol Biochem 40:659–668
Friedrich S, Platonova NP, Karavaiko GI, Stichel E, Glombitza F (1991) Chemical and microbiological solubilization of silicates. Acta Biotechnol 11:187–196
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res 111:3–49
Galmes J, Pou A, Alsina MM, Tomas M, Medrano H, Flexas J (2007) Aquaporin expression in response to different water stress intensities and recovery in Richter-110 (Vitis sp.): relationship with ecophysiological status. Planta 226:671–681
Gething PA (1990) Potassium and water relationships. In: Potash facts. IPI, Bern Girgis, MGZ
Girgis MGZ (2006) Response of wheat to inoculation with phosphate and potassium mobilizers and organic amendment. Ann Agric Sci Ain Shams Univ Cairo 51(1):85–100
Glowa KR, Arocena JM, Massicotte HB (2003) Extraction of potassium and/or magnesium from selected soil minerals by Piloderma. Geomicrobiol J 20:99–112
Gorbushina AA (2007) Life on rocks. Environ Microbiol 9(7):1613–1631
Groudev SN (1987) Use of heterotrophic microorganisms in mineral biotechnology. Acta Biotechnol 7:299–306
Gundala PB, Chinthala P, Sreenivasulu B (2013) A new facultative alkaliphilic, potassium solubilizing, Bacillus sp. SVUNM9 isolated from mica cores of Nellore District, Andhra Pradesh. India Res Rev J Microbiol Biotechnol 2(1):1–7
Hakerlerker H, Oktay M, Eryuce N, Yagmur B (1997) Effect of potassium sources on the chilling tolerance of some vegetable seedlings grown in hotbeds. In: Proceedings of Regional Workshop of IPI, held at Bornova, Izmir, Turkey. IPI, Basel, pp 353–359
Han HS, Lee KD (2005) Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Res J Agric Biol Sci 1(2):176–180
Han HS, Supanjani E, Lee KD (2006) Effect of co-inoculation with phosphate and potassium solubilizing bacteria on mineral uptake and growth of pepper and cucumber. Plant Soil Environ 52:130–136
Harley AD, Gilkes RJ (2000) Factors influencing the release of plant nutrient elements from silicates rock powder: a geochemical overview. Nutr Cycl Agroecosyst 56:11–36
Heinen RB, Ye Q, Chaumont F (2009) Role of aquaporins in leaf physiology. J Exp Bot 60:2971–2985
Hu X, Chen J, Guo J (2006) Two phosphate and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990
Huntington TG, Hooper RP, Johnson CE, Aulenbach BT, Cappellato R, Blum AE (2000) Calcium depletion in a south eastern United States forest ecosystem. Soil Sci Soc Am J 64:1845–1858
Indian Minerals Yearbook (2011) (Part-II) 50th Edition, Potash, Indian Bureau of Mines
Jain R, Saxena J, Sharma V (2014) Differential effects of immobilized and free forms of phosphate solubilizing fungal strains on the growth and phosphorus uptake of mung bean plants. Ann Microbiol 64(4):1523–1534
Jha A, Sharma D, Saxena J (2012) Effect of single and dual phosphate solubilizing bacterial strain inoculations on overall growth of mung bean plants. Arch Agron Soil Sci 58:967–981
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage. Plant Cell Physiol 42:1265–1273
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44
Jongmans AG, Breemen N, Lundstrom US, van Hees PAW, Finlay RD, Srinivasan M, Unestam T, Giesler R, Melkerud PA, Olsson M (1997) Rock eating fungi. Nature 389:682–683
Kaldenhoff R, Ribas-Carbo M, Flexas J, Lovisolo C, Heckwolf M, Uehlein N (2008) Aquaporins and plant water balance. Plant Cell Environ 31:658–666
Kalinowski BE, Liermann LJ, Givens S, Brantley SL (2000) Rates of bacteria-promoted solubilization of Fe from minerals: a review of problems and approaches. Chem Geol 169:357–370
Kanai S, Moghaieb RE, El-Shemy HA, Panigrahi R, Mohapatra PK, Ito J, Nguyen NT, Saneoka H, Fujita K (2011) Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci 180:368–374
Kant S, Kafkafi U (2002) Potassium and abiotic stresses in plants. In: Pasricha NS, Bansal SK (eds) Potassium for sustainable crop production. Potash Institute of India, Gurgaon, pp 233–251
Kayser M, Isselstein J (2005) Potassium cycling and losses in grassland systems: a review. Grass Forage Sci 60:213–224
Khawilkar SA, Ramteke JR (1993) Response of applied K in cereals in Maharashtra. Agric: 5;84–96
Khurana GP, Bhaya GP (1990) Effect of potash on wheat irrigated with nitrate waters. Indian J Agron 35:429–431
Kirkby EA, LeBot J, Adamowicz S, Romheld V (2009) Nitrogen in physiology – an agronomic perspective and implications for the use of different nitrogen forms. International Fertiliser Society, Cambridge
Kirkman JH, Basker A, Surapaneni A, MacGregor AN (1994) Potassium in the soils of New Zealand – a review. N Z J Agric Res 37:207–227
Konhauser KO (1998) Diversity of bacterial iron mineralization. Earth Sci Rev 43:91–121
Krishnamurti GSR, Huang PM (1988) Kinetics of manganese released from selected manganese oxide minerals as influenced by potassium chloride. Soil Sci 146:326–334
Kumar A, Bahadur I, Maurya BR, Raghuwanshi R, Meena VS, Singh DK, Dixit J (2015) Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J Pure Appl Microbiol 9(1):715–724
Lack AJ, Evans DE (2005) Instant notes in plant biology, 2nd edn. Taylor and Francis, Oxford
Lauchli A, Pflueger R (1979) Potassium transport through plant cell membranes and metabolic role of potassium in plants. In: Proceedings of the 11th congress on potassium research – review and trends, pp 111–163
Leigh RA (2001) Potassium homeostasis and membrane transport. J Plant Nutr Soil Sci 164:193–198
Li FC, Li S, Yang YZ, Cheng LJ (2006) Advances in the study of weathering products of primary silicate minerals, exemplified by mica and feldspar. Acta Petrol Min 25:440–448
Lian BA (1998) A study on how silicate bacteria GY92 dissolves potassium from illite. Acta Min Sin 18(2):234–238
Lian B, Fu PQ, Mo DM, Liu CQ (2002) A comprehensive review of the mechanism of potassium release by silicate bacteria. Acta Min Sin 22:179–183
Lian HL, Yu X, Ye Q, Ding XS, Kitagawa Y, Kwak SS, Su WA, Tang ZC (2004) The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol 45:481–489
Lian B, Wang B, Pan M, Liu C, Teng HH (2008) Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim Cosmochim Acta 72(1):87–98
Liermann LJ, Kalinowski BE, Brantley SL, Ferry JG (2000) Role of bacterial siderophores in dissolution of hornblende. Geochim Cosmochim Acta 64:587–602
Liermann LJ, Guynn RL, Anbar A, Brantley SL (2005) Production of a molybdophore during metal-targeted dissolution of silicates by soil bacteria. Chem Geol 220(3–4):285–302
Lin CC, Kao CH (2001) Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci 160:323–329
Lin QM, Rao ZH, Sun YX, Yao J, Xing LJ (2002) Identification and practical application of silicate dissolving bacteria. Agric Sci China 1:81–85
Lindhauer MG (1985) Influence of K nutrition and drought on water relations and growth of sunflower (Helianthus-annuus L.). J Plant Nutr Soil Sci 148:654–669
Liu D, Lian B, Dong H (2012) Isolation of Paenibacillus sp. and assessment of its potential for enhancing mineral weathering. Geomicrobiol J 29:413–421
Li-yang M, Xiao-yan CAO, De-si S (2014) Effect of potassium solubilizing bacteria-mineral contact mode on decomposition behavior of potassium-rich shale. C J Non Ferr Metal 24:1099–1109
Maathuis JM, Ichida AM, Sanders D, Schroeder JI (1997) Role of higher plant K+ channels. Plant Physiol 114:1141–1149
Majumder AK, Govindarajan B, Sharma T, Ray HS, Rao TC (1995) An empirical model for chloridising-roasting of potassium in glauconitic sandstone. Int J Miner Process 43:81–89
Malavolta E (1985) Potassium status of tropical and subtropical region soils. In: Munson RD (ed) Potassium in agriculture. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Madison, pp 163–200
Malinovskaya IM (1988) A method for the determination of the ability of bacterial polysaccharides to sorb silicic acid ions. Mikrobiol Zhurn 57:84–86
Malinovskaya IM, Kosenko LV, Votselko SK, Podgorskii VS (1990) Role of Bacillus Mucilaginosus polysaccharide in degradation of silicate minerals. Mikrobiology 59:49–55
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, San Diego
Marschner H (2010) Mineral nutrition of higher plants. Academic, London
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic, London, pp 178–189
Maurel C, Chrispeels MJ (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125:135–138
Maurya BR, Meena VS, Meena OP (2014) Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos 27(1):181–187
McAfee J (2008) Potassium, a key nutrient for plant growth. Department of Soil and Crop Sciences: http://jimmcafee.tamu.edu/files/potassium
Meena OP, Maurya BR, Meena VS (2013) Influence of K- solubilizing bacteria on release of potassium from waste mica. Agric Sustain Dev 1(1):53–56
Meena VS, Maurya BR, Bahadur I (2014a) Potassium solubilization by bacterial strain in waste mica. Bangladesh J Bot 43(2):235–237
Meena VS, Maurya BR, Verma JP (2014b) Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res 169:337–347
Meena RK, Singh RK, Singh NP, Meena SK, Meena VS (2015a) Isolation of low temperature surviving plant growth-promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal Agric Biotechnol doi:10.1016/j.bcab.2015.08.006
Meena VS, Maurya BR, Verma JP, Aeron A, Kumar A, Kim K, Bajpai VK (2015a) Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecol Eng 81:340–347
Mengel K (2001) Principles of plant nutrition, 5th edn. Kluwer Academic Publishers, Dordrecht, pp 481–509
Mengel K, Kirkby EA (1987) Principles of plant nutrition. International Potash Inst, Bern, pp 200–210
Metson AJ (1968) Potassium in: soils of New Zealand. Part 2, New Zealand soil bureau bulletin 26. Government Printer, Wellington, pp 82–95
Metson AJ (1980) Potassium in New Zealand soils. Department of Scientific and Industrial Research. New Zealand Soil Bureau report no. 38, pp 61. New Zeland
Mikkelsen RL, Bruulsema TW (2005) Fertilizer use for horticultural crops in the US during the 20th century. Hort Technol 15:24–30
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moody PW, Bell MJ (2006) Availability of soil potassium and diagnostic soil tests. Aust J Soil Res 44:265–275
Muentz A (1890) Surla decomposition desroches etla formation de la terre arable. C R Acad Sci 110:1370–1372
Nahas E, Banzatto DA, Assis LC (1990) Fluorapatite solubilization by Aspergillus niger in vinasse medium. Soil Biol Biochem 22:1097–1101
Neaman A, Chorover J, Brantley SL (2005) Implication of the evolution of organic acid moieties for basalt weathering over ecological time. Am J Sci 305:147–185
Nienow JA, Friedman EI (1993) Terrestrial lithophytic (rock) communities. In: Friedman EI (ed) Antarctic microbiology, Wiley series in ecological and applied microbiology (Wiley series in ecological and applied microbiology). Wiley-Liss, pp 342–412, 644 pp
Oerke EC (2006) Crop losses to pests. J Agric Sci 144:31–43
Oerke EC, Dehne HW (2004) Safeguarding production-losses in major crops and the role of crop protection. Crop Prot 23:275–285
Parfitt RL (1992) Potassium-calcium exchange in some New Zealand soils. Aust J Soil Res 30:145–158
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Safe 60:324–349
Paris F, Botton B, Lapeyrie F (1996) In vitro weathering of phlogopite by ectomycorrhizal fungi. II. The effect of K+ and Mg2+ deficiency and N sources on accumulation of oxalate and H+. Plant Soil 179:141–150
Park J, Sanford RA, Bethke CM (2009) Microbial activity and chemical weathering in the Middendorf Aquifer, South Carolina. Chem Geol 258:232–241
Parmar P, Sindhu SS (2013) Potassium solubilization by rhizosphere bacteria: influence of nutritional and environmental conditions. J Microbiol Res 3(1):25–31
Perrenoud S (1990) Potassium and plant health, 2nd edn. International Potash Institute, Bern, pp 8–10
Pier PA, Berkowitz GA (1987) Modulation of water stress effects on photosynthesis by altered leaf K+. Plant Physiol 85:655–661
Prajapati KB, Modi HA (2012) Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIB Tech J Microbiol 1:8–14
Prajapati K, Modi HA (2014) The study of shelf life of potassium solubilizing microorganisms for liquid biofertilizer. Paripex Ind J Res 3:13–14
Prasad D, Singh R, Singh A (2010) Management of sheath blight of rice with integrated nutrients. Indian Phytopathol 63:11–15
Puente ME, Bashan Y, Li CY, Lebsky VK (2004) Microbial populations and activities in the rhizoplane of rock-weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biol 6:629–642
Rajawat MVS, Singh S, Singh G, Saxena AK (2012) Isolation and characterization of K-solubilizing bacteria isolated from different rhizospheric soil. In: Proceeding of 53rd annual conference of association of microbiologists of India, pp 124
Rao SC, Bansal SK, Subba Rao A, Takkar PN (1998) Potassium desorption kinetics of major benchmark soil series of India. J Ind Soc Soil Sci 46(3):357–362
Rehm GW, Sorensen RC (1985) Effects of potassium and magnesium applied for corn grown on an irrigated sandy soil. Soil Sci Soc Am J 49:1446–1450
Reynolds M, Bonnett D, Chapman SC, Furbank RT, Manes Y, Mather DE (2011) Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J Exp Bot 62:439–452
Richter DD, Markewitz D (1995) How deep is soil? Bioscience 45:600–609
Richter DD, Oh NH (2002) Biological factors affecting saprolite formation. Abstracts with Programs – Geological Society of America, Paper No. 48-4
Rozanova EP (1986) Leaching of glass and during microbiological oxidation of oil. Microbiology 55:787–791
Sahin F, Cakmakci R, Kantar F (2004) Sugar beet and barley yields in relation to inoculation with N -fixing and phosphate solubilizing bacteria. Plant Soil 2(265):123–129
Sangeeth KP, Bhai RS, Srinivasan V (2012) Paenibacillus glucanolyticus, a promising potassium solubilizing bacterium isolated from black pepper (Piper nigrum L.) rhizosphere. J Spic Aromat Crops 21(2):118–124
Sarwar M (2012) Effects of potassium fertilization on population build up of rice stem borers (lepidopteron pests) and rice (Oryza sativa L.) yield. J Cereal Oilseeds 3:6–9
Schroeder D (1974) Relationships between soil potassium and the potassium nutrition of the plant. In: Potassium research and agricultural production. Proceedings of the 10th congress of the international Potash Institue, pp 53–63
Sen Gupta A, Berkowitz GA, Pier PA (1989) Maintenance of photosynthesis at low leaf water potential in wheat. Plant Physiol 89:1358–1365
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Shanware AS, Kalkar SA, Trivedi MM (2014) Potassium solublisers: occurrence, mechanism and their role as competent biofertilizers. Int J Curr Microbiol Appl Sci 3(9):622–629
Shao HB, Chu LY, Jaleel CA, Zhao CX (2008) Water-deficit stress-induced anatomical changes in higher plants. C R Biol 331:215–225
Sharma SR, Kolte SJ (1994) Effect of soil-applied NPK fertilizers on severity of black spot disease (Alternaria brassicae) and yield of oilseed rape. Plant Soil 167:313–320
Sharma KD, Nandwal AS, Kuhad MS (1996) Potassium effects on CO2 exchange, ARA and yield of cluster bean cultivars under water stress. J Pot Res 12:412–423
Sheng XF (2005) Growth promotion and increased potassium uptake of cotton and rape by a potassium releasing strain of Bacillus edaphicus. Soil Biol Biochem 37:1918–1922
Sheng XF, Huang WY (2002) Mechanism of potassium release from feldspar affected by the strain NBT of silicate bacterium. Acta Pedol Sin 39(6):863–871
Sheng XF, Zhao F, He H, Qiu G, Chen L (2008) Isolation, characterization of silicate mineral solubilizing Bacillus globisporus Q12 from the surface of weathered feldspar. Can J Microbiol 54:1064–1068
Singh G, Biswas DR, Marwah TS (2010) Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.). J Plant Nutr 33:1236–1251
Singh NP, Singh RK, Meena VS, Meena RK (2015) Can we use maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos 28(1):86–99
Smart LB, Moskal WA, Cameron KD, Bennett AB (2001) MIP genes are down-regulated under drought stress in Nicotiana glauca. Plant Cell Physiol 42:686–693
Southam G (2000) Bacterial surface-mediated mineral formation. In: Lovley DR (ed) Environmental microbe-mineral interactions. ASM Press, Washington, DC, pp 257–276
Sparks DL (1987) Potassium dynamics in soils. Adv Soil Sci 6:1–63
Sparks DL (2000) Bioavailability of soil potassium. In: Sumner ME (ed) Handbook of soil science. CRC Press, Boca Raton, pp D38–D52
Sparks DL, Huang PM (1985) Physical chemistry of soil potassium. In: Munson RD et al (eds) Potassium in agriculture. ASA, Madison, pp 201–276
Sparks DL, Martens DC, Zelazny LW (1980) Plant uptake and leaching of applied and indigenous potassium in Dothan soils. Agron J 72:551–555
Staley JT, Jackson MJ, Palmer FE, Adams JB, Borns DJ, Curtiss B, Taylor-George S (1983) Desert varnish coatings and microcolonial fungi on rocks of the Gibson and Great Victorian deserts, Australia. BMR J Aust Geol Geophys 8:83–87
Sterflinger K (2000) Fungi as geologic agents. Geomicrobiol J 17:97–124
Sterflinger K, Krumbein WE (1997) Dematiaceous fungi as a major agent for biopitting Mediterranean marbles and limestones. Geomicrobiol J 14(3):219–230
Steudle E (2000) Water uptake by plant roots: an integration of views. Plant Soil 226:45–56
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plantarum 126:45–51
Thorseth IH, Furnes H, Tumyr O (1995) Textual and chemical effects of bacterial activity on basaltic glass: an experimental approach. Chem Geol 119:139–160
Tiwari HS, Agarwal RM, Bhatt RK (1998) Photosynthesis, stomatal resistance and related characters as influenced by potassium under normal water supply and water stress conditions in rice (Oryza sativa L.). Indian J Plant Physi 3(4):314–316
Troufflard S, Mullen W, Larson TR, Graham IA, Crozier A, Amtmann A, Armengaud P (2010) Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biol 10(1):172
Tyerman SD, Niemietz CM, Bramley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25:173–194
Ullman WJ, Kirchman DL, Welch SA (1996) Laboratory evidence by microbially mediated silicate mineral dissolution in nature. Chem Geol 132:11–17
Uroz S, Calvaruso C, Turpault MP, Pierrat JC, Mustin C, Frey-Klett P (2007) Effect of the mycorrhizosphere on the genotypic and metabolic diversity of the bacterial communities involved in mineral weathering in a forest soil. Appl Environ Microbiol 73:3019–3027
Uroz S, Calvaruso C, Turpault MP, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 17:378–387
Vandevivere P, Welch SA, Ullman WJ, Kirchman DL (1994) Enhanced dissolution of silicate minerals by bacteria at near-neutral pH. Microb Ecol 27:241–251
Vassileva M, Vassilev N, Azcon R (1998) Rock phosphate solubilization by Aspergillus niger on olive cake-based medium and its further application in a soil-plant system. World J Microb Biot 14:281–284
Vessey JK (2003) Plant growth-promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wallander H, Tonie W (1999) Biotite and microcline as potassium sources in ectomycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Mycorrhiza 9:25–32
Wang XM, Li WQ, Li MY, Welti R (2006) Profiling lipid changes in plant response to low temperatures. Physiol Plantarum 126:90–96
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Warscheid T, Braams J (2000) Biodeterioration of stone: a review. Int Biodeterior Biodegrad 46:343–368
Wegner L (2010) Oxygen transport in waterlogged plants. In: Mancuso S, Shabala S (eds) Waterlogging signaling and tolerance in plants. Springer, Berlin/Heidelberg, pp 3–22
Welch LF, Flannery RL (1985) Potassium nutrition of corn. In: Munson RD (ed) Potassium in agriculture. ASA, Madison
Welch SA, Barker WW, Barfield JF (1999) Microbial extracellular polysaccharides and plagioclase dissolution. Geochim Cosmochim Acta 63:1405–1419
Wu SC, Cao ZH, Li ZG, Chung KC, Wong MH (2005) Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma 125:155–166
Wyn Jones RG, Brady CJ, Speirs J (1979) Ionic and osmotic relations in plant cells. In: Laidman DC, Wyn Jones RG (eds) Recent advances in the biochemistry of cereals. Academic, New York, pp 63–103
Xiong LM, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Yadav DS, Goyal AK, Vats BK (1999) Effect of potassium in Eleusine coracana (L.) Gaertn under moisture stress conditions. J Potassium Res 15(1–4):131–134
Yadav VP, Sharma T, Saxena VK (2000) Dissolution kinetics of potassium from glauconitic sandstone in acid lixiviant. Int J Miner Process 60:15–36
Yuan L, Fang DH, Wang ZH, Shun H, Huang JG (2000) Bio-mobilization of potassium from clay minerals: I. By ectomycorrhizas. Pedosphere 10:339–346
Yuan L, Huang JG, Li XL, Christie P (2004) Biological mobilization of potassium from clay minerals by ectomycorrhizal fungi and eucalypt seedling roots. Plant Soil 262:351–361
Zahra MK, Monib MS, Abdel-Al I, Heggo A (1984) Significance of soil inoculation with silicate bacteria. Zentralbl Mikrobiol 139(5):349–357
Zarjani JK, Aliasgharzad N, Oustan S, Emadi M, Ahmadi A (2013) Isolation and characterization of potassium solubilizing bacteria in some Iranian soils. Arch Agro Soil Sci 77:7569
Zhang C, Kong F (2014) Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol 82:18–25
Zhang QC, Wang GH, Feng YK, Qian P, Schoe-nau JJ (2011) Effect of potassium fertilization on soil potassium pools and rice response in an intensive cropping system in China. J Plant Nutr Soil Sci 174:73–80
Zhao TJ, Sun S, Liu Y, Liu JM, Liu Q, Yan YB, Zhou HM (2006) Regulating the drought responsive element (DRE)-mediated signaling pathway by synergic functions of trans-active and transinactive DRE binding factors in Brassica napus. J Biol Chem 281:10752–10759
Zhao F, Sheng X, Huang Z, He L (2008) Isolation of mineral potassium-solubilizing bacterial strains from agricultural soils in Shandong Province. Biodivers Sci 16:593–600
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Rawat, J., Sanwal, P., Saxena, J. (2016). Potassium and Its Role in Sustainable Agriculture. In: Meena, V., Maurya, B., Verma, J., Meena, R. (eds) Potassium Solubilizing Microorganisms for Sustainable Agriculture. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2776-2_17
Download citation
DOI: https://doi.org/10.1007/978-81-322-2776-2_17
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2774-8
Online ISBN: 978-81-322-2776-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)