Abstract
Overexpansion of population and poor availability of resources in developing countries have resulted in serious problems of poverty, malnutrition, and poor health. Rapidly changing climate as a result of global warming is making the situation worse. Farmers are deviating from agriculture due to increased input costs and low benefits. Tools of plant biotechnology like GM crops have improved the present situation and undoubtedly have contributed to the increase of farmer’s income, nutrition and health, and poverty reduction leading to a step toward food and nutritional security in the developing world. As a result, GM crops have emerged as the fastest adopted crop technology in the history of modern agriculture in spite of a strong opposition initially, which is nullifying gradually.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Agriculture worldwide needs a significant increase in productivity to ensure sufficient availability of food and other raw materials for ongoing population expansion which is estimated to exceed 9 billion by the year 2050. More than 3 billion people suffer from diseases caused by inadequate nutrition in one or the other way (Gomez-Galera et al. 2010; Farre et al. 2010), and around 900 million people are undersupplied with calorie worldwide (FAO 2012). The conditions are worse in developing countries which have the largest proportion of undernourished people especially in sub-Saharan Africa and South Asia (FAO 2012). Poverty in this part of the world is usually linked to low agricultural productivity as a consequence of poor availability of resources, less agricultural land, and overgrowing population. Plant biotechnology is a potent tool that can improve the present situation and contribute to the poverty reduction and food security in the developing world (Qaim 2010). Conventional breeding and development of genetically engineered plants or GM crops are two widely used biotechnological strategies to resolve the widespread problems of malnutrition and health and for increasing farmers’ income and elimination of poverty in developing countries. Conventional breeding involves identifying parents with traits that complement each other and are sexually compatible. However, plant breeding has been successful but limited. It requires selection of offspring with desired traits over a long period of time, and production of improved crop lines can take as long as 10 years from the first parental crossing to generation and distribution of selected improved crops. Besides, strategies to overcome yield-limiting factors and hence enhancement in crop production by conventional breeding have been slow due to the lack of desirable level of genetic variability in germplasm (Sahoo and Jaiwal 2008). This leaves limited options of genetic improvement through selection since selection operates on existing genetic variability. Furthermore, the reproductive barriers limit the transfer of favorable alleles from interspecific and intergeneric sources. Moreover, the approach is time-consuming and labor intensive. Besides, this can also lead to crop vulnerability due to pests and disease epidemics and unpredictable climatic factors. Therefore, the development of genetically engineered plants by the introduction and/or overexpression of selected genes seems to be a viable option to enhance the tolerance to various stresses and hence stabilize yield (Kaur and Murphy 2012; Atif et al. 2013). In addition to widening the gene pool of useful genes, it also allows introgression of novel genes and traits from any living organism into elite agronomic background. Genetic engineering also avoids the complexities of linkage drag. Even for traits that can be improved by traditional breeding, genetic engineering may facilitate and speed up the process (Potrykus 2010). The first GM crop became commercially available in the mid-1990s (Qaim 2009) and was grown in 1.7 million hectares of land. Since then, farmers around the world have adopted genetically modified (GM) crops at a very rapid rate, and by the year 2013, 175.2 million hectares of GM crops were grown in 28 countries (Fig. 11.2). This GM crop revolution started in the USA where the adoption rate for soybean and maize is 95 % and 75 %, respectively (USDA 2013); however, now more than 50 % of this area is in developing countries (James 2013). These figures in themselves are indicative that GM crops have brought benefits to farmers and society by increasing agricultural productivity and reducing food costs while providing numerous economic, environmental, and nutritional benefits even in the era of rapidly changing climate.
11.2 GM Crops: An Overview of Plant Transformation
Genetic engineering is the process in which a desired gene is isolated, cloned, and inserted into a host organism. Initially, a plasmid vector is designed to transfer the candidate gene into the crop plant genome. The transformation vector often contains a cassette with a selectable marker along with the transgene expression cassette that allows for the selection of plant cells that contain the transgene. Transformed plant cells are finally regenerated into plants (Fig. 11.1). Two commonly used methods that can be employed to insert the transgene into the plant genome are Agrobacterium-mediated transformation and transformation via bombardment with DNA-coated particles (Altpeter et al. 2005; Tzfira and Citovsky 2006; Prado et al. 2014). The first GM plants were produced using either Agrobacterium-based or direct gene transfer techniques, such as particle bombardment. Agrobacterium tumefaciens infects wounded plant tissue(s) and inserts a short section of DNA, called the transfer DNA or T-DNA, into the host plant genome (Chilton et al. 1977). This work by Mary-Dell Chilton provided evidence that plant genomes could be manipulated more precisely, and hence very recently, she has been awarded the 2013 World Food Prize (World Food Prize 2013). This technology no doubt leads to overcome the bottlenecks faced during green revolution while using conventional breeding techniques.
Overview of primary methods used for plant transformation. During the transformation process, either Agrobacterium tumefaciens or particle bombardment is used to transfer the desired gene(s) into individual plant cells. These transferred genes then become integrated into the genome of some recipient cells. Whole new transgenic plants are regenerated from transformed cells, giving rise to a transgenic line (Source: modified after Mirkov 2003)
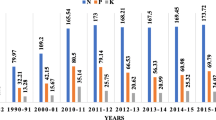
Global area of transgenic crops in millions of hectares, since 1996 to 2013 (Source: James 2013)
11.3 GM Crops in Developing Countries
GM crops are the fastest adopted crop technology in the history of modern agriculture with 100-fold increase from 1.7 million hectares in 1996 to over 175 million hectares in 2013. Developing countries have established the use of GM crops, with Brazil, Argentina, India, China, and South Africa being the prominent players among the 19 developing countries adopting the technology in 2013. These five countries together constitute 41 % of world population and are growing 47 % of global GM crops. Over 18 million farmers across the globe planted GM crops in 2013, among which16.5 million were small, poor farmers from developing countries. More than 7.5 million small farmers from China were growing 4.2 million hectares of Bt cotton; however, the trendsetters were 7.3 million farmers from India cultivating a record of 11.0 million hectares of Bt cotton (James 2013). Bt cotton resistance to bollworms and budworms is quite relevant in developing countries. Bt crops are based on the cry genes of a soil bacterium, Bacillus thuringiensis (Bt), which produces proteins that are specifically toxic to larvae of some lepidopteran and coleopteran insect species, whereas other insect pests, especially sucking pests, remain unaffected. Different strains of the bacterium have different cry genes classified into groups cryI–cryIV and subgroups A, B, C, etc., and each encodes a protein that is effective against a different type of insect (Maagd et al. 1999; Halford 2012). More advanced transgenic cotton varieties such as Bollgard II, which contains two Bt genes and expresses two Cry proteins (Cry1Ac and Cry2Ab2), are now available and are becoming widely used. Bollgard I technology involved the Cry1Ac Bt gene. The USA which accounts for 40 % of the global GM crops is the leading adopter with 70.1 million hectares among the developed world, with Canada at the second spot; however, a developing country like Brazil is emerging as a strong global leader and is only trailing by the USA with 40.3 million hectares (James 2013). Figure 11.3 shows the relative area of biotech crops in developed and developing countries in millions of hectares, since 1996 to 2013.
Adoption of GM crops in developed and developing countries in millions of hectares (Source: James 2013)
11.4 Transgenic Crops for Increase of Farmer’s Income
Agriculture in developing countries is mostly dependent on nature as there is a lack of appropriate agricultural technologies, the reason for which may be economic or the unavailability of apt techniques and their inefficient implementation. Farmers are threatened by extreme weather, crop pests, and hence unpredictable and low productivity which culminates into food scarcity and insecurity. The extreme weather conditions like frequent occurrences of drought and floods invariably result in low crop production and acute food shortages. As a result, poor people in developing countries suffer from different types of malnutrition. Tools of plant biotechnology provide opportunities for improving the economy of developing countries and the well-being of the people and offer a means for increasing agricultural production, improving human health, and minimizing environmental degradation by developing high-yield varieties, which requires less use of chemical pesticides and do not require mechanical tilling (Carpenter 2010).
11.4.1 The First Generation of GM Crops: Increasing Farmer’s Income
The first generations of GM crops were focused on traits which directly benefit the farmers. These were called as input traits and affect the husbandry and management of a crop. Input traits include herbicide tolerance; resistance to insects or pathogens like fungus, bacteria, and virus; and the ability to survive stress conditions, such as drought (Halford 2006). Insect resistance and herbicide tolerance are two primarily and most widely targeted traits, which directly benefit the growers although consumers may benefit indirectly through lower food prices (Chen and Lin 2013; Rommens 2010; Halford 2012). These first-generation GM crops have proved to be quite promising in enhancing agricultural productivity and reducing poverty in developing countries (Christou and Twyman 2004; Farre’ et al. 2010, 2011).
Development of the GM IR traits in crops has resulted in less expenditure on insecticides and lower costs of production and hence higher incomes through improved yields in all countries. Thus, gains from technology are direct, and farmers mostly in developing countries have been able to improve both their productivity and economic returns. The gains from GM HT traits on the other hand have come from a combination of effects including reduced costs of production, e.g., using low-cost, broad-spectrum herbicide (glyphosate), which is directly beneficial for farmers. Indirect gains have come by the facilitation of changes in farming systems, e.g., in both North and South America, it facilitated the moving away from conventional to low or no-tillage production systems and enabled many farmers to plant a second crop of soybeans after wheat in the same season (Brookes and Barfoot 2013). Adopting conservation tillage techniques reduces soil erosion and improves soil quality through a gradual accumulation of organic material in the soil (Park et al. 2011). Insecticide reduction and yield effects are closely related. Yield enhancement varies depending on the environment and the local intensity of pest and weed pressures. Pest pressure is often higher in the tropics and subtropics, and resource-poor farmers face more severe constraints in chemical pest control, so yield effects will be more pronounced in developing countries (Qaim and Zilberman 2003; Qaim 2009; Park et al. 2011). Adoption of Bt crops resulted in 65 % insecticide reduction and 24 % increase in effective yield in China. As a result, increase in gross margin in China reached US$470/ha (Pray et al. 2002). Similar results were later reported in India where adoption of Bt crops resulted in 41 % insecticide reduction and 37 % increase in effective yield leading to US$135/ha increase in gross margin (Qaim et al. 2006; Sadashivappa and Qaim 2009). Farmers in developing countries received $3.74 for each dollar invested in GM crop seeds in 2012 (the cost being equal to 21 % of total technology gains), while farmers in developed countries received $3.04 for each dollar invested in GM crop seed (the cost being equal to 25 % of the total technology gains) (Brookes and Barfoot 2014). The higher share of total technology gains realized by farmers in developing countries relative to farmers in developed countries mainly reflects weaker provision and enforcement of intellectual property rights coupled with higher average levels of benefits in developing countries.
Crop biotechnology undoubtedly helps farmers earn reasonable incomes for their work. The net economic benefit at the farm level in 2012 was $18.8 billion, equal to an average increase in income of $117/hectare. For the 17-year period (1996–2012), the global farm income gain has been $116.9 billion. The total farm income gain of $116.9 billion was divided equally between farmers in developing and developed countries. Fifty-eight percent (58 %) economic gains were due to reduced production costs (less plowing, fewer pesticide sprays, and less labor) and 42 % due to substantial yield gains of 377 million tons. The highest yield gains were obtained by farmers in developing countries, many of which are resource poor and farm small plots of land. The cost farmers paid for accessing crop biotechnology in 2012 ($5.6 billion payable to the seed supply chain) was equal to 23 % of the total gains (a total of $24.4 billion inclusive of the $18.8 billion income gains). Globally, farmers received an average of $3.33 for each dollar invested in GM crop seeds (Brookes and Barfoot 2014). So, crop biotechnology continues to be a good investment for farmers around the world.
11.4.2 Concern of Farmers about Planting Genetically Modified Crops
Interest of farmers lies in increased income and productivity; better allocation of labor, time, and resources: and safer practices and products for themselves and the environment. In view of these facts, major concerns of farmers while planting genetically modified crops are as follows:
-
1.
Seeds bred for particular soils, particular temperature, and rainfall zones do not perform to the optimum in other soils and zones; therefore, farmers must have access to seeds that are suitable for the agroecological conditions of their particular fields. Farmers must be able to coexist with their neighbors in neighborly ways so that each farmer can choose what is appropriate for his/her field (Kershen 2010).
-
2.
Laws should not be too stringent when it comes to using genetically modified seeds. They must not face discriminatory rules and regulations that limit their choices and inappropriately impose liability upon them simply because they desire to grow genetically modified crops. When laws allow farmers the choice, farmers have chosen quickly and broadly to grow genetically modified crops in their fields. In countries like India, Pakistan, and Brazil, farmers are defying the law to improve their lives and their farms (Rehman 2007; Roy et al. 2007). Illegal cultivation of Bt cotton in Pakistan forced the regulators to approve its cultivation in 2010 (Nazli et al. 2010).
Interestingly, a small country like Bangladesh approved Bt eggplant (brinjal) for planting for the first time in 2013 and released four varieties of Bt eggplant in January 2014 (BARI 2014). In the future, it may serve as an exemplary model for other small poor countries (James 2013).
11.5 Transgenic Crops for Poverty Alleviation
For developing world, poverty is the main cause of chronic food insecurity (Wijk 2002; Christou and Twyman 2004; Yuan et al. 2011). GM crops can contribute to the alleviation of poverty in developing countries through increased income for producers as well as addressing persistent problems of hunger (Juma 2011; Brookes and Barfoot 2009, 2013). The reasons for the increase in producer’s income may vary from region to region as well as from farm to farm (Finger et al. 2011). According to the UN reports of 2011, half the population in sub-Saharan Africa and in the least developed countries subsists on less than US$ 1 per day which comes to more than a quarter of the population in developing countries as a whole (UN 2011). On a larger scale, this translates into productivity losses that can account for 2–4 % of gross domestic product (GDP) as demonstrated for several countries in South Asia (FAO 2012). Developments in crop will have a direct impact on poverty alleviation as many of the poorest people and countries in the world are highly reliant on agriculture (DFID 2005). Hence, a remarkable gain in overall economic welfare can be achieved through adoption of technologies such as genetic engineering of plants. GM crops can contribute significantly to poverty reduction and rural development, when they are suited to the small farm sector and embedded in a contributory institutional environment. The effects of transgenic crops are considered in relation to crop yield, inputs such as pesticides, and their effects on overall profitability. Yield improvement, higher revenue, and lower pesticide costs are widely reported for Bt cotton, producing in most cases significant net benefit after accounting for higher seed prices.
So, GM technology has had a significant positive impact on farm income derived from a combination of enhanced productivity and efficiency gains. It was assumed that the productivity of unskilled labor would rise by 2 % following adoption of second-/next-generation GM crops. Even golden rice on its own could add $3.2 billion per year to developing countries economic welfare (Anderson 2010). Global value of GM rice has recently been estimated to be US$64 billion per year by aggregating the expected annual benefits (Demont and Stein 2013).
In 2012, the direct global farm income benefit from GM crops was $18.8 billion. This is equivalent to having added 5.6 % to the value of global production of the four main crops of soybean, maize, canola, and cotton. Positive yield impacts from the use of this technology have occurred in all user countries when compared to average yields derived from crops using conventional technology (such as application of insecticides and seed treatments). Because most of the farmers in developing countries are small-scale farmers, so any increase in their income can have a direct impact on poverty alleviation. The average yield impact across the total area planted to insect-resistant (IR) traits during the 1996–2012 period has been +10.4 % for insect-resistant corn and +16.1 % for insect-resistant cotton. In 2012, 46.2 % of the farm income benefits have been earned by developing country farmers, and the vast majority of these income gains have been from GM IR cotton and GM HT soybeans (Tables 11.1 and 11.2). In the absence of crop biotechnology, 17.3 million farmers using this technology in 2012 would not have maintained global production levels equivalent to 2012 levels and would have required additional plantings of 4.9 million ha of soybeans, 6.9 million ha of corn, 3.1 million ha of cotton, and 0.2 million ha of canola. This total area requirement is equivalent to 9 % of the arable land in the USA or 24 % of the arable land in Brazil (Brookes and Barfoot 2014). GM crops are, hence, allowing farmers to grow more without using additional land. It is, therefore, a land-saving technology (James 2010).
11.5.1 Role of Bt Cotton in Alleviating Poverty in India
India has the third largest area in the world under GM crops. There are more than 60 transgenic crops under research and more than 20 under field trials in India (ISAAA 2013). But only one crop is under cultivation, i.e., Bt cotton, since its approval in 2002 by the Genetic Engineering Approval Committee (GEAC) of the Government of India. Premature discontinuation of GM mustard in 2001 and the moratorium of Bt brinjal by the Ministry of Environment and Forests (MOEF) in 2010 raised a question on the regulatory system of GM crops in India. However, on a brighter side, Bt cotton is doing exceptionally well. In India, a case study was done, which compared the performance of over 9000 Bt and non-Bt cotton farm plots in Maharashtra, and it was found that Bt cotton varieties had a significant positive impact on average yields and on the economic performance of cotton growers. Between 2003–2004 and 2006–2007, cotton yields in India indicate a significant yield advantage of more than 30 % with Bt cotton compared with conventional varieties with corresponding increase in farm income (Karihaloo and Kumar 2009). Similar study was done on resource-poor small-scale cotton farmers of South Africa, and similar results were obtained (Bennett et al. 2006). Bt cotton produces 82 % higher aggregate incomes per hectare in India, and as a result annual consumption expenditures of Bt-adopting households increased by 18 %, during 2006–2008, in comparison with non-adopters (Qaim 2009; Klumper and Qaim 2014). The yield increases in the range of +30 % to +40 % have been confirmed later in India (Qaim 2009, 2010; Gruere and Sengupta 2011; Herring and Rao 2012). As a result, Bt cotton area increased from 0.05 million hectares in 2002 to 9.3 million hectares in 2011–2012, accounting for 88 % of total area (IGMORIS 2013), and the Indian cotton sector switched from a net import to a significant export situation. Despite this significant increase in cotton area, the use of insecticides on cotton decreased from 46 % of total insecticides used in agriculture during 2001–2002 to 20 % in 2011–2012 (Kranthi 2012). Bt technology further contributed to 24 % increase in cotton yield per acre through reduced pest damage and a 50 % increase in profit among cotton smallholders (Kathage and Qaim 2012). Bt cotton has significantly raised living standards of small farm households in India.
11.6 Transgenic Crops for Nutrition and Health
On the one hand, the world is facing newer challenges, such as the expansion of cultivated areas to less fertile fields and the adaptation of crops to a globally changing climate (Avni and Bla’zquez 2011), on the other hand the nutritional quality of food or balanced diet is emerging as a major problem in developing countries. In response to escalating food prices, poor households had to limit their food consumption, and poor people are unable to procure a balanced diet. Food prices throughout the world have increased sharply in the last decade. For example, prices of wheat and maize were three times higher in 2008 than at the beginning of 2003, and the price of rice was five times higher (Braun 2008, 2010). Majorities of the people living in developing countries are extremely poor and cannot afford combination of expensive foods like meat, fish, milk, pulses, etc., which usually forms essential balanced diets. According to an estimate, food production would have to be doubled by 2050 to overcome existing hunger, feed an additional 2 billion people, and accommodate rising demand from income growth (Braun 2010; Adenle et al. 2012).
Lack of balanced diet leads to micronutrient deficiencies and, hence, negative consequences on people’s nutrition and health. Micronutrients are involved in all aspects of development, growth, and physiology of the human body, and their deficiencies can cause birth defects, permanent physical and mental impairment, as well as an increased risk of death by infectious and chronic diseases. The long-term consequences of insufficient amounts of essential micronutrients in the human diet can be more devastating than low-energy intake (Murgia et al. 2013). The leading micronutrient deficiencies are iron deficiency, iodine deficiency, zinc deficiency, folic acid deficiency, and vitamin A deficiency. One or more of these affect almost half of the world’s population. Since children’s nutrition is crucial for their physical and cognitive development and for their productivity and earnings as adults, the health and economic consequences of insufficient food and poor diets are lifelong – for the individuals as well as for society. Besides, GM crops with insect-resistant genes may reduce the need for pesticides which improves the health of farmers, especially in developing countries where pesticides are still applied with handheld sprayers (Chrispeels 2014).
11.6.1 Next Generation GM Crops: Improving Nutrition and Health
Scientific knowledge has achieved breakthrough in the field of genomics, proteomics, and metabolomics in the recent times. It has broadened our understanding of the sources and nutritional values of the products of many of food crops (Arber 2010). Next-generation GM crops target traits which affect the composition of the crop product for quality improvements for nutrition and industrial purposes and are called as output traits. These traits include improved nutritional value like staple foods with enhanced contents of essential amino acids (especially lysine and methionine) and micronutrients (vitamins A and E, iron, folate, and ascorbate); oilseeds with improved fatty acid composition (oleic acid, omega-3 fatty acid); changes in starch quality, i.e., resistant starch and antioxidants (anthocyanins); etc. (Jefferson-Moore and Traxler 2005; Pew Initiative on Food and Biotechnology 2007).
In general, one or several key genes in metabolic pathways are introduced or knocked down by genetic modification to promote the accumulation of healthy metabolites, and nutritional requirements can be addressed directly by contributing to multipoint intervention strategies (Yuan et al. 2011). The main beneficiaries of these so-called next-generation GM crops are consumers and/or food processors. One famous example is golden rice, which can prevent vitamin A deficiency that prevails in poor populations solely dependent on rice as a staple food crop. Approximately 500,000 children in developing countries become blind each year owing to vitamin A deficiency. Africa accounts for almost 50 % of the children who are clinically or subclinically deficient in vitamin A, particularly under 5 years of age (FAO/WHO 1998; WHO 2010). Golden rice contains a high content of β-carotene by introduction of a previously absent biosynthetic pathway into rice endosperm. A GM line containing β-carotene was developed by Ingo Potrykus and coworkers in 2000 at the Swiss Federal Institute of Technology, Zurich (Potrykus 2003). Rice endosperm contains geranylgeranyl diphosphate, which is converted into β-carotene by three enzymes produced from different transgenes: phytoene synthase (psy), lycopene β-cyclase gene from daffodil (Narcissus pseudonarcissus), and a phytoene desaturase (crtI) gene from the bacterium Erwinia uredovora. The GM rice producing β-carotene was crossed with another line engineered with multiple genes to improve iron availability, including a phytase-encoding gene from Aspergillus fumigatus (Lucca et al. 2001). The high-β-carotene/high-availability iron hybrid was called golden rice (Halford 2012). Higher β-carotene intakes will improve the vitamin A status of individuals, thus reducing the incidence of adverse health outcomes (Qaim 2010). The replacement of the daffodil genes Zmppsy1 and EucrtI with its maize ortholog is the basis of Golden Rice 2, which produces up to 37 μg of carotenoids per gram dry weight (DW) of grain, of which 31 μg/g is β-carotene (Paine et al. 2005). The putative impact of golden rice was calculated as up to 40,000 lives saved per year for India alone (Khush 2012). β-Carotene in golden rice is as good as pure β-carotene in oil at providing vitamin A to children (Tang et al. 2012). It was the use of genetic engineering together with conventional breeding, i.e., combinatorial transformation method, which has enabled the production of provitamin A (PVA) in corn and rice plants as an alternative source of vitamin A to save millions of children who go blind every year (Avni and Bla’zquez 2011).
Bananas having levels of PVA greater than 15-fold higher than wild type have been developed through the overexpression of a single gene, phytoene synthase, using either constitutive promoters or fruit-preferred promoters. Two different phytoene synthase genes, one from a naturally high-PVA banana and other from maize gene used in Golden Rice 2, were differently expressed in bananas, and lines with elevated PVA have been identified (Dale et al. 2013). Anemia caused by iron deficiency is the world’s most common nutritional deficiency. It affects pregnant and nursing women and young children most commonly (Earl and Woteki 1998; Swaminathan 2002). Genetic enrichment of iron in Indian rice Pusa Basmati (Oryza sativa L.) has also been accomplished through recombinant DNA technology (Shivprakash et al. 2006). Co-expression of endosperm-specific recombinant soybean ferritin and Aspergillus phytase in maize resulted in significant increases in the levels of bioavailable iron (Drakakaki et al. 2005). A similar end was achieved earlier with lettuce (Goto et al. 2000). Transgenic rice plants expressing the NAS (nicotianamine synthase) genes Osnas1, Osnas2, or Osnas3 accumulated up to 19 lg/g of iron in the endosperm (Johnson et al. 2011). Recently, phosphate bioavailabilities of barley grains have been improved from 30 to 60 % using cisgenesis with an endogenous phytase gene (Holme et al. 2012). Barley grains are widely used for feeding monogastric animals such as chickens and pigs. A large number of rice or soybean ferritin overaccumulators in rice mega-variety IR64, including marker-free events, were generated and evaluated by introducing soybean or rice ferritin genes into the endosperm for product development. As much as a 37- and 19-fold increase in the expression of ferritin gene in single and co-transformed plants, respectively, and a 3.4-fold increase in Fe content in the grain over the IR64 wild type were achieved (Oliva et al. 2014).
Multivitamin maize expressing the rice dhar gene from the ascorbate recycling pathway accumulated six times the normal level of ascorbate (Naqvi et al. 2009). Similarly, the constitutive expression of two Arabidopsis cDNA clones encoding q-hydroxyphenylpyruvate dioxygenase (HPPD) and 2-methyl-6-phytylplastoquinol methyltransferase (MPBQ MT) increased the tocopherol content by threefold in transgenic maize (Naqvi et al. 2011). The essential fatty acids are abundant in fish, shellfish, nuts, and leafy vegetables, but they are not present in cereals (Farre et al. 2011). Genetic engineering can be used to produce oilseeds such as soybean and canola that have nutritional properties similar to fish oils (Damude and Kinney 2008). These include fatty acids associated with lowering risks of coronary heart disease (Haslam et al. 2013). For example, omega-3 fatty acid which reduces coronary heart disease and maintains heart health has been increased from 12 to 50 % in canola (Ursin 2003). The fatty acid biosynthesis pathway in plants was modulated to produce ω-3 and ω-6 PUFAs by introducing the microbial enzymes responsible for a sequence of fatty acid desaturation and elongation reactions (Domergue et al. 2005). Studies have also shown that the use of oil from transgenic soya in which the fatty acid metabolic pathways have been modified can increase the n-3 VLC-PUFAs of chicken meat (Rymer and Givens 2009).
Folate prevents neural tube defects and causes widespread megaloblastic anemia during pregnancy and often exacerbates already existing iron deficiency anemia (Rush 2000; Barber et al. 2000; Laurence et al. 1981; Rosenquist et al. 1991). Enhancing folate content in staple crops by metabolic engineering is a promising, cost-effective strategy to eradicate folate malnutrition worldwide (Blancquaert et al. 2014). The transformation of two pathway genes from Arabidopsis thaliana increased folate production from <1 mg/g to 17 mg/g in rice which is enough to meet the requirements necessary to combat its deficiency (Storozhenko et al. 2007). Several other traits are also under development, such as rice enriched with lactoferrin to reduce diarrhea in high-risk patients.
Similarly, biofortification is another important and widely used technique to nutritionally enhance the food crops at source (Zhu et al. 2007). Biofortification of staple food crops might be used as one of the possible strategies against micronutrient malnutrition in developing countries. Biofortification allows the poor to receive the necessary amounts of vitamin A, zinc, and iron via their regular staple food diets and, hence, delivers naturally fortified foods to people with limited access to commercially marketed fortified foods or supplements (Braun 2010). However, the desired traits for biofortification may not be present at all in a food crop; the best-known example is golden rice, in which the carotenoid biosynthetic pathway has been reconstituted in non-carotenogenic endosperm tissue, as a means to deliver provitamin A (Mayer et al. 2008). So, biofortification of staple crop plant tissues can be achieved through breeding where this is possible, while recombinant DNA technology must be applied in all other cases (Bayer 2010). Hence, the science of biotechnology, either through conventional breeding (often in conjunction with marker-assisted selection) or genetic modification approaches, has great potential to achieve biofortification for nutritional benefits (Table 11.3).
11.7 Transgenic Crops in the Era of Climate Change
A question that often comes in one’s mind is whether genetic engineering can contribute to food security, as well as enhancing human nutrition and farming under a changing climate. Global climate change is increasing temperatures worldwide resulting in global warming besides rapid climate variability and unscheduled expansion or shrinkage of the extreme climates (Keer 2007; IPCC 2001, 2007; Webb et al. 2012). In other words, as a result of climatic change, there is an increase in the frequency of extreme events that are likely to decrease crop yield affecting all dimensions of crop production (Singh et al. 2015). Human activities are hugely accelerating this change in global climate. Continuously increasing human population is hence making the situation even worse. It is expected to peak before the end of the century, with 10 billion people before 2100 (Lutz et al. 2001; Duhamel and Vandenkoornhuyse 2013). Food crises are exacerbated by global warming as agricultural productivity has declined worldwide as a consequence of the hot summers experienced in the recent past (Mittler 2006; Mittler and Blumwald 2010). It is estimated that global warming will reduce about 6 % and 5 % average yield per 1 °C rise when it comes to C3 and C4 crops, respectively (Yamori et al. 2013) Besides, current trends in yield increase are insufficient to double food production by 2050 (Ray et al. 2013). However, the impact of climate change will cut across all boundaries, and the most susceptible victims of this climate change are going to be the most food-insecure developing countries with a challenge posed to them to attain millennium development goals and achieve sustainable development by climate change (Singh et al. 2015).
The climate change which is generally related to the abiotic stresses due to extreme environmental changes may result in melting of portions of the Himalayan glaciers, disturb the monsoon pattern, and increase flooding/drought in Asia. Increased uncertainty over the availability of water for irrigation and more frequent floods will affect 25 % of the world’s cereal production. Most of the abiotic stress tolerance mechanisms in plants are complex due to the involvement of multiple metabolic pathways. Hence, manipulating these characters through conventional breeding remains a big challenge. The genetic transformation of plants is an efficient alternative to this problem. The genetic transformation of plants with regulatory genes, e.g., transcription factors, is a promising method for genetic engineering because many of the ways in which plants can adapt to cold, drought, oxidative stress, and extreme temperatures is through transcriptional control (Mittler and Blumwald 2010). The genetic transformation studies to overcome various types of stresses have already been discussed in detail in this book in an article by Bauddh and coworkers (Table 11.4). Genetic engineering (GE) has already contributed to the reduction of greenhouse gas (GHG) emissions as a result of less fuel use and additional soil carbon storage from reduced tillage with GM crops. When global impact of biotech crops on environmental effects was estimated between 1996 and 2010, it was found that farming with transgenic crops since 1996 has led to additional soil carbon sequestered, equivalent to 133,639 million tons of CO2 (Brookes and Barfoot 2012). In 2012, this was equivalent to removing 27 billion kg of carbon dioxide from the atmosphere or equal to removing 11.9 million cars from the road for 1 year (Brookes and Barfoot 2014). Increased productivity from GM crops has decreased pressure for land conversion of non-cropland to cropland which is a major contributor to the greenhouse gas increase in the atmosphere. This indicates that it can play a large role in both the mitigation of and adaptation to climate change.
11.8 Conclusions and Future Prospects
In spite of the strong opposition, farmers around the world have adopted GM crops at an unprecedented rate (Herring 2008; James 2013) especially in some major developing countries like India and China. The principal beneficiaries of agricultural development from GM crops are likely to be poor farmers and poor consumers (Weale 2010). GM crops have the potential to improve food security in developing countries by improving incomes of farmers and availability of lower-priced and better-quality food for consumers (Qaim and Kouser 2013). There are several constraints to the research and application of biotechnology in developing countries like financial resources, lack of policies and absence of systems for the delivery of technologies to potential users, and finally lack of awareness, leading to misconceptions about the potential of and risks posed by biotechnology. Hence, public controversies about the risks and benefits of GM crops continue (Gilbert 2013; Fernandez-Cornejo et al. 2014). However, there is no scientific evidence that the process of transferring genes from one kind of organism to another possesses intrinsic problems. Further, there are no such reports that anyone has become ill as a result of eating GM foods. Hundreds of millions of people are regularly consuming foods produced by GM crops (Raven 2010). There is an increasing scientific consensus, even in Europe, that the GM foods and crops currently on the market have brought no documented new risks either to human health or to the environment (Paarlberg 2010; European Commission 2010; European Academies Science Advisory Council 2013; DeFrancesco 2013). However, the existing negative public attitude toward GM crops, especially in Europe, has contributed to a stringent complex regulatory framework and has limited public and private investments into GM crop research, increasing the cost of technologies making it difficult for developing countries to continue the research on large scale and, hence, reap its benefits (Qaim 2010). Besides, investments should be more in the areas of R&D, rural infrastructure, rural institutions, and information monitoring and sharing to enhance agricultural productivity (Braun 2010). The public sector needs to resource these developing country-targeted projects as they do not represent commercially valuable targets and therefore cannot be a commercial priority for the private sector (Bayer 2010). However, to develop novel traits and to distribute it systematically at a wider scale would require the expertise and resources of both public and private sector institutions. In the immediate future, the research should focus on development of methods avoiding antibiotic- or herbicide-resistant genes as selectable marker or use of positive selectable markers such as phosphomannose isomerase (pmi), xylose isomerase (xyl A), etc., to widen the acceptability of GM crops and selection of genes for the desirable traits for the transfer and strategies for the seed distribution system, where the end user in the developing countries is benefited and not only industries in developed countries. There is a wide scope to produce nutritionally enhanced crops such as finger millet, cassava, etc., which are widely grown in sub-Saharan countries like Africa and are nutritionally poorer crops. Once nutritionally enhanced, these crops can be easily available and, hence, beneficial to poorer local populations. The new tools of genomics, proteomics, and metabolomics would allow better understanding of vital processes and metabolic pathways for their improvement. Generation of more number of next-generation GM crops in the near future and development of new biotechnologies and non-targeted safety assessment approaches may improve public perception about the potential risk of GM crops (Chen and Lin 2013). The focus should now be on the use of new techniques like RNA interference, agro-infiltration, cisgenesis, oligonucleotide-directed mutagenesis, and zinc finger nuclease technology that may or may not come under the strict GM regulations (Halford 2012). Crops created through genome engineering might prove to be more acceptable to the public than plants that carry foreign DNA in their genomes. Crops with enhanced nutritional value can be created by altering only a few nucleotides. The use of gene knockouts to disrupt biochemical pathways should make it possible to create plants that accumulate a variety of valuable biosynthetic intermediates (Voytas and Gao 2014).
GM crops hold a significant potential to contribute to poverty reduction, better nutrition and health, and sustainable development in developing countries especially in the present scenario of climate change. So, risk–benefit analysis approach should be considered over risk assessment. Saving lives by curbing malnutrition and food security should be the first priority. Hence, the “if’s and but’s” should be in waiting, when it comes to saving lives. Commercialization of GM crops will have substantial implications for the alleviation of poverty, hunger, and malnutrition. They have much more to offer the developing world than the developed because when it comes to food developing world needs more and has fewer alternatives.
References
Adenle AA, Aworh OC, Akromah R, Govindan P (2012) Developing GM super cassava for improved health and food security: future challenges in Africa. Agric Food Secur 1:11. http://www.agricultureandfoodsecurity.com/content/1/1/11
Almeida AM, Silva AB, Araujo SS, Cardoso LA, Santos DMJ, Torne JM, Silva JM, Paul MJ, Fevereiro PS (2007) Responses to water withdrawal of tobacco plants genetically engineered with the AtTPS1 gene: a special reference to photosynthetic parameters. Euphytica 154:113–126
Altpeter F, Niranjan B, Beachy R, Bock R, Capell T et al (2005) Particle bombardment and the genetic enhancement of crops: myths and realities. Mol Breed 15:305–327
Anderson K (2010) Economic impacts of policies affecting crop biotechnology and trade. New Biotechnol 27:558–564
Arber W (2010) Genetic engineering compared to natural genetic variations. New Biotechnol 27:518–521
Atif RM, Patat-Ochatt EM, Svabova L, Ondrej V, Klenoticova H, Jacas L, Griga M, Ochatt SJ (2013) Gene transfer in legumes. Progress Bot 74:37–100
Avni A, Bla’ zquez MA (2011) Can plant biotechnology help in solving our food and energy shortage in the future? Editorial overview. Curr Opin Biotechnol 22:220–223
Bangladesh Agricultural Research Institute (BARI) (2014) Bt brinjal set for release to farmers in Bangladesh. www.bari.gov.bd/userfiles/BARI_Bt%20Brinjal%20in%20Bangladesh.doc
Barber R et al (2000) Investigation of folate pathway gene polymorphisms and the incidence of neural tube defects in a Texas Hispanic population. Mol Genet Metab 70:45–52
Bennett R, Kambhampati U, Morse S, Ismael Y (2006) Farm-level economic performance of genetically modified cotton in Maharashtra. India Rev Agric Econ 28:59–71
Beyer P (2010) Golden rice and ‘Golden’ crops for human nutrition. New Biotechnol 27:478–481
Blancquaert D, Steur HD, Gellynck X, Straeten DVD (2014) Present and future of folate biofortification of crop plants. J Exp Bot 65:895–906
Braun VW (2008) Food and financial crises: implications for agriculture and the poor. In: Food policy report. IFPRI, Washington, DC
Braun JV (2010) Food insecurity, hunger and malnutrition: necessary policy and technology changes. New Biotechnol 27:449–452
Brookes G, Barfoot P (2009) GM crops: global socio-economic and environmental impacts 1996–2007. PG Economics Ltd, Dorchester
Brookes G, Barfoot P (2012) Global impact of biotech crops- environmental effects, 1996–2010. GM Crops Food Biotechnol Agric Food Chain 3:129–137
Brookes G, Barfoot P (2013) The global income and production effects of genetically modified (GM) crops 1996–2011. GM Crops Food 4:74–83
Brookes G, Barfoot P (2014) GM crops: global socio-economic and environmental impacts 1996–2012. PG Economics Ltd, Dorchester
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EGWM, Hall RD, Bovy AG, Luo J et al (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26:1301–1308
Carpenter JE (2010) Peer-reviewed surveys indicate positive impact of commercialized GM crops. Nat Biotechnol 28:219–221
Chen H, Lin Y (2013) Promise and issues of genetically modified crops. Curr Opin Plant Biol 16(2):255–260
Chen M, Wang QY, Cheng XG, Xu ZS, Li LC, Ye XG, Xia LQ, Ma YZ (2007) GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem Biophy Res Co 353:299–305
Chilton MD, Drummond MH, Merlo DJ, Sciaky D, Montoya AL, Gordon MP et al (1977) Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell 11:263–271
Chrispeels MJ (2014) Yes indeed, most Americans do eat GMOs every day! J Integr Plant Biol 56:4–6
Christou P, Twyman R (2004) The potential of genetically enhanced plants to address food insecurity. Nutr Res Rev 17:23–42
Dale J, Harding R, Tushemeirwe W, Paul J, Namanya P, Kleidon J (2013) Use of transgene expression to elevate micronutrients in banana. In: Abstracts Plant and Animal Genome. PAG Asia 2013, Singapore, March 17–19, 2013. https://pag.confex.com/pag/asia2013/webprogram/paper8905.html
Damude HG, Kinney AJ (2008) Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids 42:179–185
DeFrancesco L (2013) How safe does transgenic food need to be? Nat Biotechnol 31:794–802
Demont M, Stein AJ (2013) Global value of GM rice: a review of expected agronomic and consumer benefits. New Biotechnol 30:426–436
DFID (2005) Growth and poverty reduction: the role of agriculture. http://www.dfid.gov.uk/Documents/publications/growthpoverty-agriculture.pdf
Domergue F, Abbadi A, Heinz E (2005) Relief for fish stocks: oceanic fatty acids in transgenic oilseeds. Trends Plant Sci 10:112–116
Drakakaki G, Marcel M, Glahn R, Lund L, Periagh S, Christou P, Stoger E (2005) Endosperm-specific co-expression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol Biol 59:869–880
Duhamel M, Vandenkoornhuyse P (2013) Sustainable agriculture: possible trajectories from mutualistic symbiosis and plant neodomestication. Trends Plant Sci 18:597–600
Earl R, Woteki CE (eds) (1998) Recommended guidelines for the prevention, detection and management of iron deficiency anemia among U.S. children and women of childbearing age, Institute of Medicine, National Academy Press, Washington, DC
European Academies Science Advisory Council (2013) Planting the future: opportunities and challenges for using crop genetic improvement technologies for sustainable agriculture. EASAC, Halle
European Commission (2010) A decade of EU-funded GMO research 2001–2010. European Commission, Brussels
FAO (2012) The state of food insecurity in the world. Food and Agriculture Organization of the United Nations, Rome
FAO/WHO (1998) Vitamin and mineral requirements in human nutrition. Report of a joint FAO/WHO expert consultation, Bangkok, Thailand
Farre G, Ramessar K, Twyman RM, Capell T, Christou P (2010) The humanitarian impact of plant biotechnology: recent breakthroughs vs bottlenecks for adoption. Curr Opin Plant Biol 13:219–225
Farre G, Twyman RM, Zhu C, Capell T, Christou P (2011) Nutritionally enhanced crops and food security: scientific achievements versus political expediency. Curr Opin Biotechnol 22:245–251
Fernandez-Cornejo J, Wechsler JJ, Livingston M, Mitchell L (2014) Genetically engineered crops in the United States. Economic research report ERR-162, United States Department of Agriculture, Washington, DC
Finger R, Benni E, Kaphengst T, Evans C, Herbert S, Lehmann B, Morse S, Stupak N (2011) A meta-analysis on farm-level costs and benefits of GM crops. Sustainability 3:743–762
Gilbert N (2013) A hard look at GM crops. Nature 497:24–26
Go’mez-Galera S, Rojas E, Durajalagraja S, Zhu C, Pelacho AM, Capell T, Christou P (2010) Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res 19:165–180
Goto F, Yoshihara T, Saiki H (2000) Iron accumulation and enhanced growth in transgenic lettuce plants expressing the iron-binding protein ferritin. Theor Appl Genet 100:658–664
Gruere G, Sengupta D (2011) Bt cotton and farmer suicides in India: an evidence-based assessment. J Dev Stud 47:316–337
Halford NG (ed) (2006) Plant biotechnology: current and future uses of genetically modified crops. Wiley, Chichester
Halford NG (2012) Toward two decades of plant biotechnology: successes, failures, and prospects. Food Energy Secur 1:9–28
Haslam RP, Ruiz-Lopez N, Eastmond P, Moloney M, Sayanova O, Napier JA (2013) The modification of plant oil composition via metabolic engineering-better nutrition by design. Plant Biotechnol J 11:157–168
Herring RJ (2008) Whose numbers count? Probing discrepant evidence on transgenic cotton in Warangal district of India. Int J Mult Res Approach 2:145–159
Herring R, Rao C (2012) On the ‘failure of Bt cotton’: analysing a decade of experience. Econ Polit Wkly XLVII(18):45–53
Holme IB, Dionisio G, Brinch-Pedersen H, Wendt T, Madsen CK, Vincze E, BachHolm P (2012) A cisgenic approach for improving the bioavailability of phosphate in the barley grain. ISB News report
Hong B, Ma C, Yang Y, Wang T, Yamaguchi-Shinozaki K, Gao J (2009) Over-expression of AtDREB1A in chrysanthemum enhances tolerance to heat stress. Plant Mol Biol 70:231–240
IGMORIS (2013) Indian GMO Research Information System. http://igmoris.nic.in/
Intergovernmental Panel on Climate Change (IPCC) (2007) Summary for policymakers. In: Parry ML, Canziani OF, Palutiko JP, van der Linden PJ, Hanson CE (eds) Climate change: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Intergovernmental Panel on Climate Change (IPCC): synthesis report (2001) Contribution of working group I, II and III to the third assessment report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge
International Service for the Acquisition of Agri-biotech Applications (ISAAA) (2013) Global status of commercialized biotech/GM crops 2013. http://isaaa.org/resources/publications/briefs/46/executivesummary/default.asp
Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol 47:141–153
James C (2010) Global status of commercialized biotech/GM crops: 2010 – executive summary – ISAAA Brief No. 42, ISAAA, Ithaca
James C (2013) Global Status of Commercialized Biotech/GM Crops: 2013. ISAAA Brief No. 46. ISAAA, Ithaca
Jefferson-Moore KY, Traxler G (2005) Second-generation GMOs: where to from here? AgBioForum 8:143–150
Johnson AAT, Kyriacou B, Callahan DL, Carruthers L, Stangoulis J, Lombi E, Tester M (2011) Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLOSone 6:e24476
Juma C (2011) Preventing hunger: biotechnology is key. Nature 479:471–472
Karihaloo JL, Kumar PA (2009) Bt cotton in India – a status report, 2nd edn. Asia-Pacific Consortium on Agricultural Biotechnology (APCoBA), New Delhi
Karim S, Aronsson H, Ericson H, Pirhonen M, Leyman B, Welin B, Mantyla E, Palva ET, van Dijck P, Holstrom KO (2007) Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. Plant Mol Biol 64:371–386
Kathage J, Qaim M (2012) Economic impacts and impact dynamics of Bt (Bacillus thuringiensis) cotton in India. Proc Natl Acad Sci U S A 11652–11656. www.pnas.org/cgi/doi/10.1073/pnas.1203647109
Kathuria H, Giri J, Tyagi H, Tyagi AK (2009) Advances in transgenic rice biotechnology. Crit Rev Plant Sci 26:65–103
Kaur N, Murphy JB (2012) Enhanced isoflavone biosynthesis in transgenic cowpea (Vigna unguiculata l.) Callus. Plant Mol Biol Biotechnol 3:1–8
Keer RA (2007) Global warming is changing the world. Science 316:188–190
Kershen DL (2010) Trade and commerce in improved crops and food. An essay in food security. New Biotechnol 27:623–627
Khush GS (2012) Genetically modified crops: the fastest adopted crop technology in the history of modern agriculture. Agric Food Secur 1:14
Klumper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS One 9:e111629. doi:10.1371/journal.pone.0111629
Ko C, Woo Y, Lee DJ, Lee M, Kim CS (2007) Enhanced tolerance to heat stress in transgenic plants expressing the GASA4 gene. Plant Physiol Biochem 45:722–728
Kranthi KR (2012) Bt cotton-questions and answers. Indian Society for Cotton Improvement, Mumbai
Laurence KM et al (1981) Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br Med J (Clin Res Ed) 282:1509–1511
Lucca P, Hurrell R, Potrykus I (2001) Genetic engineering approach to improve bioavailability and the level of iron in rice grains. Theor Appl Genet 102:392–397
Lutz W et al (2001) The end of world population growth. Nature 412:543–545
Lv S, Yang A, Zhang K, Wang L, Zhang J (2007) Increase of glycine betaine synthesis improves drought tolerance in cotton. Mol Breed 20:233–248
Maagd DRA, Bosch D, Stiekema W (1999) Bacillus thuringiensis toxin-mediated insect resistance in plants. Trends Plant Sci 4:9–13
Mayer JE, Pfeiffer WF, Beyer P (2008) Biofortified crops to alleviate micronutrient malnutrition. Curr Opin Plant Biol 11:166–170
Miranda JA, Avonce N, Suarez R, Thevelein JM, Dijck PV, Iturriaga G (2007) A bifunctional TPS–TPP enzyme from yeast confers tolerance to multiple and extreme abiotic conditions in transgenic Arabidopsis. Planta 226:1411–1421
Mirkov TE (2003) The molecular basis of genetic modification and improvement of crops. In: Chrispeels MJ, Sadava DE (eds) Plants, genes, and crop biotechnology, 2nd edn. Jones and Bartlett, Boston, pp 124–151
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Mittler R, Blumwald E (2010) Genetic engineering for modern agriculture: challenges and perspectives. Annu Rev Plant Biol 61:443–462
Molinari HBC, Marur CJ, Filho JCB, Kobayashi AK, Pileggi M, Junior RPL, Pereira LFP, Vieira LGE (2007) Osmotic adjustment in transgenic citrus rootstock Carrizo citrange (Citrus sinensis Osb. Poncirus trifoliata L. Raf.) overproducing proline. Plant Sci 167:1375–1381
Mroczka A, Roberts PD, Fillatti JJ, Wiggins BE, Ulmasov T, Voelker T (2010) An intron sense suppression construct targeting soybean FAD2-1 requires a double-stranded RNA-producing inverted repeat T-DNA insert. Plant Physiol 153:882–891
Murgia I, De Gara L, Grusak MA (2013) Biofortification: how can we exploit plant science and biotechnology to reduce micronutrient deficiencies? Front Physiol 4:429. doi:10.3389/fpls.00429
Naqvi S, Zhu C, Farre’ G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci U S A 106:7762–7767
Naqvi S, Zhu C, Farre G, Sandmann G, Capell T, Christou P (2011) Synergistic metabolism in hybrid corn reveals bottlenecks in the carotenoid pathway and leads to the accumulation of extraordinary levels of the nutritionally important carotenoid zeaxanthin. Plant Biotechnol J 9:384–393
Nazli H, Sarela R, Meilke KD, Orden D (2010) Economic performance of Bt cotton varieties in Pakistan. Contributed paper at the Agricultural and Applied Economics Association (AAEA) Annual meetings, Denver, CO, July 25–27
Oliva N, Mohanty PC, Poletti S, Abrigo E, Atienza G, Torrizo L, Garcia R et al (2014) Large-scale production and evaluation of marker-free indica rice IR64 expressing phytoferritin genes. Mol Breed 33:23–37
Paarlberg R (2010) GMO foods and crops: Africa’s choice. New Biotechnol 27:609–613
Paine J, Shipton C, Chaggar S, Howells R, Kennedy M, Vernon G, Wright S, Hinchliffe E, Adams J, Silverstone A, Drake R (2005) Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat Biotechnol 23:482–487
Park JR, McFarlane I, Phipps RH, Ceddia G (2011) The role of transgenic crops in sustainable development. Plant Biotechnol J 9:2–21
Pew Initiative on Food and Biotechnology (2007) Applications of biotechnology for functional foods-a report. Pew initiative on food biotechnology Washington, DC
Potrykus I (2003) Nutritionally enhanced rice to combat malnutrition disorders of the poor. Nutr Rev 61:101–104
Potrykus I (2010) Lessons from the ‘Humanitarian Golden Rice’ project: regulation prevents development of public good genetically engineered crop products. New Biotechnol 27:466–472
Prado JR, Segers G, Voelker T, Carson D, Dobert R, Phillips J, Cook K, Cornejo C, Monken J, Grapes L, Reynolds T, Martino-Catt S (2014) Biotech crop development: from idea to product. Annu Rev Plant Biol 65:21.1–21.22. doi:10.1146/annurev-arplant-050213-040039
Pray CE, Huang J, Hu R, Rozelle S (2002) Five years of Bt cotton in China-the benefits continue. Plant J 31:423–430
Qaim M (2009) The economics of genetically modified crops. Annu Rev Resour Econ 1(665):693
Qaim M (2010) Benefits of genetically modified crops for the poor: household income, nutrition, and health. New Biotechnol 27:552–557. doi:10.1016/j.nbt.2010.07.009
Qaim M, Kouser S (2013) Genetically modified crops and food security. PLOSOne 8:e64879. doi:10.1371/journal.pone.0064879
Qaim M, Zilberman D (2003) Yield effects of genetically modified crops in developing countries. Science 299:900–902
Qaim M, Subramanian A, Naik G, Zilberman D (2006) Adoption of Bt cotton and impact variability: insights from India. Rev Agric Econ 28:48–58
Qin Q, Liu J, Zhang Z, Peng R, Xiong A, Yao Q, Chen J (2007) Isolation, optimization, and functional analysis of the cDNA encoding transcription factor OsDREB1B in Oryza Sativa L. Mol Breed 19:329–340
Raven PH (2010) Does the use of transgenic plants diminish or promote biodiversity? New Biotechnol 27:528–533
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS One 8(6):e66428. doi:10.1371/journal.pone.0066428
Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T, Kosar-Hashemi B, Li Z, Rahman S, Morell M (2006) High amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci U S A 103:3546–3551
Rehman UMS (2007) Pakistan agricultural situation cotton update. USDA GAIN report no. PK 7026, U.S. Embassy, Washington, DC
Rommens CM (2010) Barriers and paths to market for genetically engineered crops. Plant Biotechnol J 8:101–111
Rosenquist TH, Anne Ratashak S, Selhub J et al (1991) Homocysteine induces congenital defects of the heart and neural tube: effect of folic acid. Proc Natl Acad Sci U S A 93:15227–15232
Roy D, Herring RJ, Geisler C (2007) Naturalizing transgenics: loose seeds, official seeds, and risk in the decision matrix of Gujarati cotton farmers. J Dev Stud 43:158–176
Rush D (2000) Nutrition and maternal mortality in the developing world. Am J Clin Nutr 72:212–240
Rymer C, Givens DI (2009) The effect of feeding steariodonic acid enriched soya oil to broilers on the fatty acid composition and sensory characteristics of chicken meat. Br Poult 5:44
Sadashivappa P, Qaim M (2009) Effects of Bt cotton in India during the first five years of adoption. Presented at international association of agricultural economics triennial conference, Beijing
Sahoo L, Jaiwal PK (2008) Asiatic beans. In: Kole C, Hall T (eds) A compendium of transgenic crop plants. Blackwell Publication, Oxford, 115–132
Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K (2006) Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc Natl Acad Sci U S A 103:18822–18827
Savitch LV, Allard G, Seki M, Robert LS, Tinker NA, Huner NPA, Shinozaki K, Singh J (2005) The effect of over-expression of two Brassica CBF/DREB1-like transcription factors on photosynthetic capacity and frost tolerance in Brassica napus. Plant Cell Physiol 46:1525–1539
Singh RP, Varaprasad PV, Reddy KR (2015) Climate change: implications for stakeholders in genetic resources and seed sector. Adv Agron 129:117–180. doi:10.1016/bs.agron.2014.09.002
Sivaprakash KR et al (2006) Tissue-specific histochemical localization of iron and ferritin gene expression in transgenic indica rice Pusa Basmati (Oryza sativa L.). J Genet 85:157–160
Storozhenko S, Brouwer VD, Volckaert M, Navarrete O, Blancquaert D, Zhang GF, Lambert W, Van Der Straeten D (2007) Folate fortification of rice by metabolic engineering. Nat Biotechnol 25:1277–1279
Swaminathan MS (2002) Building a national nutrition security system. India- ASEAN eminent persons lecture series. FAO, Bangkok
Tang W, Hu Y, S-a Y, Wang Y, Dallal GE, Grusak MA, Russell RM (2012) β-carotene in golden rice is as good as β-carotene in oil at providing vitamin to children. Am J Clin Nutr 96:658–664
Tzfira T, Citovsky V (2006) Agrobacterium-mediated genetic transformation of plants: biology and biotechnology. Curr Opin Biotechnol 17:147–154
UN (2011) The millennium development goals report .United Nations, New York
Ursin VM (2003) Modification of plant lipids for human health: development of functional land-based omega-3 fatty acids. J Nutr 133:4271–4274
USDA-ERS (2013) Economic research service – Data products. United States Department of Agriculture ERS, Washington DC. http://www.ers.usda.gov/data-products.aspx#.UoqklKJ7yA
Vendruscolo ECG, Schuster I, Pileggi M, Scapim CA, Molinari HBC, Marur CJ, Vieira LGE (2007) Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J Plant Physiol 164:1367–1376
Voytas DF, Gao C (2014) Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol 12:e1001877. doi:10.1371/journal.pbio.1001877
Waditee R, Bhuiyan MNH, Rai V, Aoki K, Tanaka Y, Hibino T, Suzuki S, Takano J, Jagenodorf AT, Takabe T, Takabe T (2005) Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc Natl Acad Sci-U S A 102:1318–1323
Wagner N, Mroczka A, Roberts PD, Schreckengost W, Voelker T (2011) RNAi trigger fragment truncation attenuates soybean FAD2-1 transcript suppression and yields intermediate oil phenotypes. Plant Biotechnol J 9:723–728
Wang X, Cai J, Liu F, Dai T, Cao W, Wollenweber B, Jiang D (2014) Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol Biochem 74:185–192
Weale A (2010) Ethical arguments relevent to the GM crops. New Biotechnol 27:583–587
Webb NP, Stokes CJ, Scanlan JC (2012) Interacting effects of vegetation, soils and management on the sensitivity of savanna rangelands to climate change. Clim Change 112:925–943
WHO (2010) Micronutrient deficiencies: iron deficiency anaemia. WHO, Geneva. http://www.who.int/nutrition/topics/ida/en/
Wijk JV (2002) Food insecurity: prevalence, causes, and the potential of transgenic ‘Golden Rice’. Phytochem Rev 1:141–151
Wirth J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B, Osorio S, Tohge T, Fernie AR, Gunther D, Gunther W, Sautter C (2009) Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol J 7:631–644
World Food Prize (2013) Three biotechnology scientists awarded 2013 world food prize, press release. World Food Prize Foundation, Iowa
Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi-Shinozaki K, Shinozaki K, Yoshiba Y (2005) Effects of free proline accumulation in petunias under drought stress. J Exp Bot 56:1975–1981
Yamamoto K, Sakamoto H, Momonoki YS (2011) Maize acetylcholinesterase is a positive regulator of heat tolerance in plants. J Plant Physiol 168:1987–1992
Yamori W, Hikosaka K, Way DA (2013) Temperature response of photosynthesis in C3, C4 and CAM plants. Photosynth Res 119:101–117
Yuan D, Bassie L, Sabalza M, Miralpeix B, Dashevskaya S, Farre’ G, Rivera SM, Banakar R, Bai C, Sanahuja G, Arjo’ G, Avilla E, Zorrilla-Lo’pez U, Ugidos-Damboriena N, Lo’pez A, Almacellas D, Zhu C, Capell T, Hahne G, Twyman RM, Christou P (2011) The potential impact of plant biotechnology on the millennium development goals. Plant Cell Rep 30:249–265
Zhao J, Ren W, Zhi D, Wang L, Xia G (2007) Arabidopsis DREB1A/CBF3 bestowed transgenic tall fescue increased tolerance to drought stress. Plant Cell Rep 26:1521–1528
Zhu C, Naqvi S, Gomez-Galera S, Pelacho AM, Capell T, Christou P (2007) Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci 12:548–555
Zhu L, Meng GM, Cheung SCK, Yu H, Huang J, Sun Y, Shi Y, Liu Q (2012) High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol J 10(353):362
Zhu XF, Jin YH, Yoo CY, Lin XL, Kim WY, Yun DJ et al (2013) CYCLINH;1 regulates drought stress responses and blue light-induced stomatal opening by inhibiting Reactive Oxygen Species accumulation in Arabidopsis. Plant Physiol 162:1030–1041
Acknowledgements
PKJ is grateful to HSCST, Chandigarh, and UGC, Department of Biotechnology and Department of Science and Technology, New Delhi, for financial support to his laboratory for improvement of grain legumes and other crops. MS is thankful to HSCST, Chandigarh, and Department of Biotechnology, New Delhi, for JRF; Council of Scientific and Industrial Research, New Delhi, for SRF; and Department of Science and Technology, New Delhi, for the Young Scientist Award.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Sainger, M. et al. (2015). GM Crops for Developing World in the Era of Climate Change: For Increase of Farmer’s Income, Poverty Alleviation, Nutrition and Health. In: Jaiwal, P., Singh, R., Dhankher, O. (eds) Genetic Manipulation in Plants for Mitigation of Climate Change. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2662-8_11
Download citation
DOI: https://doi.org/10.1007/978-81-322-2662-8_11
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2660-4
Online ISBN: 978-81-322-2662-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)