Abstract
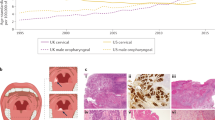
The demographics and prognosis of locally advanced head and neck squamous cell cancer (HNSCC) have changed dramatically over the past two decades. Epidemiological evidence has revealed a significant increase in the incidence of oropharyngeal cancer (OPC) in North America and Europe [1–3]. Molecular studies of oropharyngeal tumours have revealed that this increase is due to a rise in the incidence of tumours containing human papillomavirus (HPV), most specifically HPV16. Evidence shows that HPV16 is the molecular cause that mechanistically drives the development and viability of the cancer cells [4]. HPV-associated OPC (HPVOPC) presently accounts for ~70 % of OPC seen in the USA, and an increasing fraction of these malignancies is seen in Europe [1, 2, 5].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Overall Survival
- Radiation Therapy Oncology Group
- Poor Prognostic Feature
- Radiation Therapy Oncology Group Study
- Lower Level Node
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The demographics and prognosis of locally advanced head and neck squamous cell cancer (HNSCC) have changed dramatically over the past two decades. Epidemiological evidence has revealed a significant increase in the incidence of oropharyngeal cancer (OPC) in North America and Europe [1–3]. Molecular studies of oropharyngeal tumours have revealed that this increase is due to a rise in the incidence of tumours containing human papillomavirus (HPV), most specifically HPV16. Evidence shows that HPV16 is the molecular cause that mechanistically drives the development and viability of the cancer cells [4]. HPV-associated OPC (HPVOPC) presently accounts for ~70 % of OPC seen in the USA, and an increasing fraction of these malignancies is seen in Europe [1, 2, 5].
Our current understanding is that two clinically significant carcinogenic and biological pathways exist that cause OPC. One is environmentally and smoking-related OPC (EROPC), which is caused by smoking, alcohol and environmental carcinogens. Carcinogenesis in this entity is independent of HPV; tumours are HPV-negative and result from p53 mutations and loss of cell-cycle regulation, usually via p16 deletion, methylation or mutation. The other pathway is HPVOPC, in which carcinogenesis is driven by HPV. Carefully conducted studies have shown that high-risk HPVs are identified rarely outside the oropharynx [6, 7]. A small number of true larynx cancers occur which are identifiable as HPV16-positive; however, the biology of the rare HPV-positive tumours in the larynx may be different from HPVOPC. Some tumours classified as oral cancers, supraglottic cancers or nasopharynx cancers occur in watershed areas within and adjacent to the oropharynx and are probably misclassified because of local spread of an OPC. These are biologically-related OPCs and not oral cancer per se, having been misclassified. Thus, the vast majority of HPV-related HNCs are HPVOPC. In addition to having distinct biologies, HPVOPC and EROPC have responded differently to therapy [8, 9].
The Current State of the Art—Therapeutics
Existing data from clinical trials and retrospective analysis of patient materials suggests the two forms of OPC—HPVOPC and EROPC—are biologically distinct and have distinct prognoses. The relative paucity of genetic changes in HPV-positive HNC is in sharp contrast to what is observed in HPV-negative HNC, and is mechanistically related to the direct effects of viral proteins in inactivating regulators of key cellular processes [8–12]. In contrast to EROPCs, HPVOPCs usually do not contain p53 mutations [9, 11]. Similarly, p16, an inhibitor of mitosis in the Rb pathway of cell growth, is often upregulated in HPVOPC, whereas it is lost in EROPC, as a consequence of viral alterations in Rb function [9, 13, 14]. Up-regulation of p16 can be seen in about 20 % of non-HPV-related cancers, including other sites in the head and neck; however, p16 appears to be up-regulated in >95 % of HPVOPCs, making it a good screening tool and a potentially important diagnostic tool [7, 14, 15]. The biological differences in the carcinogenesis of these tumours are expressed in their distinctly different responses to therapeutic interventions.
Retrospective studies in unselected patients indicate that patients with HPVOPC have a significantly better prognosis than patients with EROPC, regardless of therapeutic intervention [16–18]. These differences are profound and only fractionally related to the improved demographics of HPVOPC patients who tend to be younger and have less exposure to smoking and alcohol [19–21]. In one retrospective study from Denmark in which radiotherapy (RT) was the sole therapy, p16 was used as a surrogate for HPV [22]. In this randomized study of a radiation sensitizer, the control arm of RT only was analysed for p16 expression. Five-year survival was 62 % among p16-positive patients compared with 26 % in p16-negative patients. Locoregional control (LRC) was 58 % versus 28 %, respectively. The data support the notion that p16-positive, and, hence by implication, HPV-positive, tumours are more responsive to RT and more likely to be cured. One caveat of the study relates to the determination of p16 positivity. A significant fraction of p16-positive tumours were not of oropharyngeal origin and HPV status was not obtained, hence the relationship of p16-positive and HPV positivity, especially HPV16 positivity, in this study is less strong than might be expected [22]. These results illustrate limitations with many studies; first because of using p16 as a surrogate marker for HPV carcinogenesis and second because of the need for uniform diagnostic criteria to help identify HPV in HPVOPC for therapeutic management.
In another trial reported by Licitra et al., a cohort of surgically treated patients were evaluated retrospectively to determine tumour HPV status [23]. Surgery alone was effective therapy for a small group of patients who received a surgical resection only and no RT. The HPVOPC surgery-only patients were spared the long-term consequences of radiation. However, selection for surgery only was not well explained [23]. New function sparing surgical technology has increased the rate of patients with OPCs receiving surgery as a first therapy. Prior to the development of transoral laser microdissection (TLM) and transoral robotic surgery (TORS), OPC patients were treated with non-surgical therapy to spare surgical morbidity [24]. These technologies substantially reduce surgical morbidity in OPC. Several retrospective studies have been published; however, it is difficult to tease out the role of postoperative treatments in this population as most of these studies are not protocol-driven trials with clear inclusion and exclusion criteria. What is evident in these studies is that those patients with p16-positive tumours have done very well in survival and functional outcomes and HPV16 positivity and/or p16 has been predictive of this quantitatively larger overall survival (OS) than might have been expected [25].
Eastern Cooperative Oncology Group (ECOG) 2399 is a phase II study of operable patients treated with an aggressive sequential therapy (ST) regimen of induction chemotherapy followed by chemoradiotherapy (CRT) for organ preservation [26]. This study prospectively evaluated HPV status and was the first prospective study of HPV and therapeutic outcome. The investigators reported a significant difference in survival for patients with HPVOPC compared with EROPC. Sixty-two patients with OPC were treated, all the 38 HPVOPC patients were HPV16-positive as opposed to other high-risk HPV. Even among this small group of patients, OS was significantly better for the HPVOPC group. An analysis of failure in this population revealed several important features. First, LRC was much better in the HPVOPC patients. Additionally, co-morbidities and noncancer deaths were much reduced. The impact of therapy on LRC was most striking: there was a 95 % versus 67 % LRC rate in HPVOPC versus EROPC, respectively [26, 27]. Thus, LRC seems to be significantly improved in HPVOPC patients treated with induction chemotherapy and CRT compared with EROPC patients. In a trial to identify patients with a good prognosis, the University of Michigan used a single cycle of induction chemotherapy to select operable OPC patients for RT or surgery [28, 29]. They analysed 42 informative cases for HPV and HPV copy number and found that responses to induction chemotherapy correlated with HPV status, as did disease-specific survival. The relationship to copy number of HPV in the tumours was less clear although there was a suggestion that increasing copy number was associated with a better prognosis [28].
Recently, results of retrospective analyses of survival and HPV status were reported from two large phase III trials comparing CRT regimens in locally advanced HNSCC [15, 30]. In both trials there were insufficient patient numbers to report a treatment effect; however, the impact of HPV on survival, regardless of therapeutic assignment, was highly significant. The Radiation Therapy Oncology Group (RTOG) study 0129 has the most extensive data and retrospectively analysed outcomes in 323 out of 433 OPC cases [15]. In RTOG 0129, patients were randomized between CRT with accelerated fractionation with cisplatin versus regular fractionation and cisplatin. The OS and progression-free survival (PFS) at 3 years were 82 % and 74 % in HPVOPC compared with 57 % and 43 % for EROPC, respectively. A careful analysis of failure and death revealed an LRC rate of 86 % versus 65 % for HPVOPC versus EROPC, and a second primary tumour rate of 6 % versus 15 %, respectively. Non-cancer deaths also occurred in 9 % and 19 %, respectively. All these data support a better outcome for HPVOPC, much of which is found in improved LRC, some fraction of which is explained by less co-morbidity.
Tumour HPV16 status, survival and demographics in subjects with OPC treated in TAX324, a large international randomized phase III clinical trial, were also evaluated retrospectively. TAX 324 compared survival between ST with TPF or PF followed by CRT with weekly carboplatin in patients with locally advanced HNSCC [30]. The data show a significant difference in survival outcome and patterns of failure between patients with HPVOPC and EROPC and significant differences in demographic characteristics in the populations. Of the 501 patients entered on TAX 324, 264 (53 %) were identified as having OPC. Of these 264 subjects, 119 had tissue prospectively collected and 111, or 42 % of all OPC cases, were analysable for HPV16 status and constituted the study population.
The demographic data and test results for group comparisons are shown in Table 6.1. Fifty-six (50 %) patients were identified as HPV-positive (HPVOPC) and 55 (50 %) as HPV negative (EROPC). Both HPVOPC and EROPC cases were divided evenly with regard to treatment assignment and sex. HPVOPC cases were significantly younger compared to EROPC cases (56 vs. 58 years, p = 0.02), performance status (PS) was also significantly different between the two populations, despite selection for good PS in patients for enrolment in this trial. Thus, 77 % of HPVOPC patients were PS 0 compared with 49 % of the EROPC patients (p = 0.003).
Results for OS, PFS and site of failure for the 111 patients analysed for HPV16 status, independent of the treatment arm, are also shown in Table 6.1 [30]. HPVOPC and EROPC surviving patients were followed for a median of 83 months and 82 months, respectively. At the time of analysis, 79 % of HPVOPC patients were still alive, and their PFS rate was 73 %, compared with the 31 % OS and 29 % PFS for the EROPC patients (both p < 0.0001). The median OS time for the EROPC patients is 21 months (95 % CI 13–49 months), whereas median survival has not been reached in the HPVOPC group after almost 7 years median follow-up. The reduction in mortality was 80 % in HPVOPC compared with EROPC (HR = 0.2; 95 % CI 0.10–0.38; p < 0.0001). Analysis of the site of failure, as shown in Table 6.1, revealed a significant reduction in LRF (13 % vs. 42 %, p = 0.0006) and slightly reduced distant failure in the HPVOPC patients compared with the EROPC patients. Total disease failures showed a significant difference (16 % vs. 49 %; p = 0.0002) and a borderline improvement in deaths without recurrence (p = 0.07). These data indicate that LRC is the major parameter contributing to improved survival and that PS and co-morbidities among EROPC patients account for another fraction of mortality.
The data presented here from multiple trials clearly shows that survival is significantly better for HPVOPC than with EROPC, and that improved survival is primarily a function of HPV status and improved LRC. A fraction of this improvement is also related to a reduced co-morbidity. In Table 6.2, comparable data on OS and PFS for TAX 324, RTOG 0129 is shown for a qualitative comparison and demonstrates that survival and PFS in RTOG 0129 and TAX 324 are similar at the 3-year analysis time point and significantly better for HPVOPC [15, 30, 31]. The RTOG re-analysed their data recently and suggested that HPVOPC can be divided into good prognosis and intermediate prognosis on the basis of smoking, stage and nodal involvement [32]. The RTOG study retrospectively showed that smoking is a significant prognostic factor and smoking history is an important component of decision-making for therapy and consideration for studies. In the original analysis of RTOG 0129, a history of smoking above and below 20 pack-years (PY) correlated to meaningfully different population outcomes [15]. More recently, a review of their data suggested that current smoking was a highly negative prognostic factor, regardless of HPV status, and that a 5 PY history had a significant impact on prognosis, although the original paper suggested 20 PY was a realistic cut-off [32]. Others have reported similar results [33]. Unreported long-term follow-up from the RTOG study beyond 3 years limits the reliability of the survival data. Further, the absolute differences in survival between a 20, 10 or 5 PY history are in the order of 2–5 % at the time of analysis. So, while the survival analysis based on smoking exposure results in statistically significant comparisons of high risks between exposures, the numeric impact on survival may be trivially small and unhelpful in making treatment selections in which toxicity of therapy is a major consideration.
Other prognostic features are hard to identify in the literature. The University of Michigan recently proposed that matted nodes in the neck were a poor prognostic feature indicative of a high risk of distant metastases and local regional failure, regardless of HPV status [34]. Earlier literature suggested that positive margins and extracapsular nodal extension in tumours were poor prognostic features as were bilateral and contralateral lymph node involvement and nodal involvement lower in the neck [35]. One troublesome difficulty in staging HPV-positive patients is a high rate of cystic nodal disease, which although large and hence technically of higher stage, is not believed to be as poor a prognostic finding as solid nodes or multiple solid nodes [34].
Current Therapeutic Recommendations for HPVOPC
Patients with HPVOPC have more options for curative therapy than patients with EROPC because of improved prognosis and new technologies. Early-and intermediate-stage patients can be treated with surgery if the primary tumours are lateralized. When primary tumours involve the lateral base of tongue, exploration of the contralateral neck can provide evidence that allows a reduction of the radiation fields and sparing of the opposite neck. Standard therapy calls for adjuvant RT in stage 3 and stage 4 patients and adjuvant CRT in those with poor prognostic features [36]. The value of surgery in this setting is a reduction in radiation and chemotherapy if no poor prognostic features (e.g. multiple nodes, matted nodes extracapsular extension [ECE], lower level nodes) are identified. Preoperative testing should eliminate a clinically advanced population from surgery. Surgical therapy for midline tongue lesions remains morbid because of a bigger impact on function, early lymphatic spread and difficult margin control compared with lateralized lesions. For these tumours, adjuvant radiation must be more extensive, bilateral and morbid despite surgery; therefore, these patients should be advised non-surgical therapy. The value of surgery in HPVOPC is to provide part of the curative therapy and reduce the amount and field of radiation leading to less morbidity and late consequences for the patients [24].
For patients with advanced primary tumours or midline tongue involvement, the impact of surgery and radiation on function is less clear. A primary CRT approach with cisplatin-based CRT appears to be best. Evidence suggests that erbitux may be equivalent to cisplatin-based CRT, but this has not been established to date and a comparison trial is under way (RTOG 1016). Weekly cisplatin treatments would be more tolerable, less toxic and more likely to be completed and are a reasonable alternative to bolus cisplatin. Similarly, for patients with poor pathological findings, such as ECE, positive margin, or multiple positive lymph nodes, or lower level nodes, a postoperative CRT course is indicated on the basis of current evidence [35, 37]. Lymphovascular invasion (LVI) and perineural invasion (PNI), historically markers of poor prognosis, are lesser indications for CRT in HPVOPC and in non-smokers [35–37]. In smokers, CRT might be more likely to be helpful with these more minor indications.
In patients with poor prognostic findings, or with clinical and radiographic indications for extensive RT fields, CRT and/or high-dose RT, surgical resection may not be advantageous. Despite these patients having a technically resectable primary tumour, they should proceed with non-surgical combined modality therapy. Both ST and CRT are indicated and treatment should result in a high rate of cure in otherwise healthy patients. Evidence of advanced nodal diseases, matted nodes, low neck nodes or T4 tumours might sway decision-making towards a sequential approach because of a higher rate of distant metastases; however, evidence is lacking to support one approach over the other. For these patients, treatment decisions are based on the experience of the treating physicians and a multidisciplinary team approach.
Anecdotal evidence suggests that patients presenting with primary disease and metastases or with an early recurrence after curative CRT may be cured by a combined modality approach. Aggressive systemic chemotherapy and localized therapy to bulk disease areas or boney metastases with CRT may be effective in primary presentations. Systemic chemotherapy after removal of oligometastases or induction followed by CRT may lead to curative outcomes in first recurrences. Otherwise there is no specific therapy today for HPV-positive recurrent and/or metastatic patients. Before embarking on a curative course it is important to confirm HPV status. p16 is not adequate when assessing potential metastatic lesions as deriving from the original HPV-positive primary tumour. For example, many squamous cancers of the lung and oesophagus are p16-positive and HPV-negative [38].
Current Therapeutic Trials
In general, patients with HPVOPC are young and will live for prolonged periods. They are at high risk for long-term toxicity and mortality from therapy [39]. While the long-term consequences of chemotherapy and modern surgery for HNC are relatively constrained, high-dose RT and CRT substantially impact on local tissues and organ function and result in a significant rate of late mortality and morbidity in patients [40–44]. Studies are now being designed to reduce the impact of RT and CRT for patients. Identifying appropriate end-points and study arms which will allow an early assessment of outcomes will be problematic, particularly for equivalence studies wherein survival differences are small, and in which prolonged time periods and large patient numbers are necessary to accurately assess outcomes. For ST as given with TAX 324, 3-year PFS might be an appropriate end-point. The same may not be possible for CRT. The best example of changing outcomes in CRT trials would be R91-11, in which a premature negative conclusion regarding the efficacy of induction therapy was published with the early analysis. Late failures, toxicity and morbidity, a hallmark of upfront cisplatin-based CRT trials, led to equivalence between induction therapy and CRT for laryngectomy-free survival at 5 years, and more importantly a non-significant relative 10 % improvement in OS in the PF induction arm compared with the CRT arm, which included an every 3-week bolus cisplatin treatment for three cycles during RT [15, 30, 41, 45].
Radiation dose reduction trials are either being planned or have been completed by the ECOG. ECOG 1308 is a phase II trial treating patients with p16-positive resectable OPC with an aggressive regimen of induction chemotherapy using weekly paclitaxel, cisplatin every 3 weeks, and cetuximab weekly for three cycles, followed by cetuximab + RT to a total radiation dose of 5400 cGy for responders. Non-responders receive standard RT with cetuximab. This trial completed accrual and is currently being analysed. Unfortunately, without a control arm evidence will be lacking to support this regimen as being equivalent to standard therapy and it should not be used in the community. ECOG is opening a randomized phase II trial in operable patients with early- and intermediate-stage disease to assess a reduced dose of RT versus a reduced dose of CRT for LRC. A similar trial of surgery with TLM is opening at Washington University and with TORS at Mount Sinai Medical Center. These studies are aimed at reducing RT-associated early and late morbidity through reduced doses.
The Mount Sinai School of Medicine surgical/radiation dose reduction trial explores a very different hypothesis. The Sinai Robotic Surgery (SIRS) trial is a surgical study in which operable patients are assessed pathologically after a TORS resection, and those with good prognostic features are followed without RT or CRT. CRT or systemic therapy is reserved for salvage therapy for those who relapse. Because of the excellent responses of HPVOPC and local control, it is hypothesized that survival will be equivalent to upfront RT and that at least 50 % patients will avoid radiation. Those with varying poor prognostic features will receive either reduced dose RT for modest features, such as LVI or PNI, or reduced RT with chemotherapy for ECE or positive margin. This is a radical departure from standard practice, which will be carefully monitored during the study and over the first 5 years of follow up. The Mount Sinai Medical Center is also leading the Quarterback Trial. This is a randomized trial in which patients presenting with localized HPV-positive disease who are inoperable, have poor prognostic features, or would not be spared CRT with an operation are treated with a course of dose-reduced TPF induction chemotherapy, followed by randomization 2:1 to CRT with 5600 cGy plus carboplatin, or 7000 cGy and carboplatin—the control arm. This is the only randomized dose reduction trial for this population. The end-points of this randomized trial are equivalence of the reduced dose RT for PFS at 3 years and reduced morbidity from the lower RT dose.
The RTOG has initiated a randomized trial to compare cisplatin to erbitux-based CRT, with full-dose RT in both arms. Although this is called a dose-reduction trial, it does not address the substantial morbidity of full-dose radiation given in both arms. Patients are likely to show little improvement in their long-term toxicity in either arm, although this will answer the question of equivalence between cisplatin and erbitux as CRT for this disease.
Future therapeutic trials will include therapeutic vaccines and immune modulators to alter or boost the immune response to HPV. It is also likely that there will be HPV-specific therapeutics developed to attack viral-specific processes, such as p53 binding, which are necessary for cancer cell survival. Finally, molecular antiviral approaches with anti-sense DNA or silencing RNA therapies may be envisaged. Much needs to be learned before we understand how best to apply any of these approaches.
Whereas it is tempting to reduce therapy for HPVOPC, clinicians are urged not to unilaterally lower radiation doses for HPV-positive patients outside of a clinical trial. Harmful differences in outcomes with dose reduction will not be discernible in a single practice or academic centre. It is only with protocol-driven prospective clinical trials with adequate numbers that sufficient evidence will be available to make confident, evidence-based recommendations for therapy. In addition, all of the trials described are gathering tissue and biomarker data, which will inform the next generation of studies. We would urge clinicians to participate in clinical trials so that these important questions can be answered as quickly and accurately as possible for our patients.
Summary Points
-
Patients with HPVOPCs are younger and healthier than those with traditionally EROPCs.
-
HPVOPCs are more responsive to almost any therapy than EROPCs and have much better local and regional control. The majority of patients with HPVOPC will survive their cancer and live longer, with the consequences of curative therapy.
-
Clinical investigation today is focused on improving treatment-related morbidity using new technologies and reducing long-term RT-associated toxicities. Future therapies, which are in development, will be directed at vaccines, immune modulation and anti-HPV-specific molecular targeting.
References
Chaturvedi AK, Engels EA, Anderson WF, et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–9.
Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301.
Dahlstrand HM, Dalianis T. Presence and influence of human papillomaviruses (HPV) in tonsillar cancer. Adv Cancer Res. 2005;93:59–89.
Rampias T, Sasaki C, Weinberger P, et al. E6 and E7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101:412–23.
Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–6.
Dahlgren L, Dahlstrand HM, Lindquist D, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–9.
Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1–8.
Psyrri A, DeFilippis RA, Edwards AP, et al. Role of the retinoblastoma pathway in senescence triggered by repression of the human papillomavirus E7 protein in cervical carcinoma cells. Cancer Res. 2004;64:3079–86.
Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–47.
Andl T, Kahn T, Pfuhl A, et al. Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res. 1998;58:5–13.
Münger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–60.
McLaughlin-Drubin ME, Münger K. Oncogenic activities of human papillomaviruses. Virus Res. 2009;143:195–208.
Hasegawa M, Nelson HH, Peters E, et al. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21:4231–6.
Mellin Dahlstrand H, Lindquist D, Björnestål L, et al. P16(INK4a) correlates to human papillomavirus presence, response to radiotherapy and clinical outcome in tonsillar carcinoma. Anticancer Res. 2005;25:4375–83.
Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Gillison M, Koch WM, Capone R, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20.
Gillison ML. Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: Implications for clinical research in head and neck cancers. J Clin Oncol. 2006;24:5623–5.
Ringström E, Peters E, Hasegawa M, et al. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res. 2002;8:3187–92.
Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–10.
D’Souza G, Kreimer AR, Viscidi R, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56.
Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20.
Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8.
Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–6.
Adelstein DJ, Ridge JA, Brizel DM, et al. Transoral resection of pharyngeal cancer: summary of a national cancer institute head and neck cancer steering committee clinical trials planning meeting, 6–7 November 2011, Arlington, Virginia. Head Neck. 2012;34:1681–703.
Haughey BH, Hinni ML, Salassa JR, et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck. 2011;33:1683–94.
Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oropharynx: results of eastern cooperative oncology group study E2399. J Clin Oncol. 2007;25:3971–7.
Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9.
Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–46.
Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–37.
Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: A subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–7.
Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–8.
Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–11.
Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–35.
Spector ME, Gallagher KK, Light E, et al. Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34:1727–33.
Cooper J, Pajak TF, Forastiere A, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44.
Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84:1198–205.
Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52.
Yanagawa N, Wang A, Kohler D, et al. Human papilloma virus genome is rare in North American non-small cell lung carcinoma patients. Lung Cancer. 2013;79:215–20.
Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–9.
Adelstein D, Li Y, Adams G, et al. An Intergroup Phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8.
Forastiere A, Maor M, Weber R, et al. Long term results of Intergroup RTOG 91–11: a phase III trial to preserve the larynx—induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. Proc Am Soc Clin Oncol. 2006;5517.
Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherap—results of a multicentric randomized german trial in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:1161–71.
Taylor S, Murthy A, Vannetzel J, et al. Randomized comparison of neoadjuvant cisplatin and fluorouracil infusion followed by radiation versus concomitant treatment in advanced head and neck cancer. J Clin Oncol. 1994;12:385–95.
Best SR, Ha PK, Blanco RG, et al. Factors associated with pharyngoesophageal stricture in patients treated with concurrent chemotherapy and radiation therapy for oropharyngeal squamous cell carcinoma. Head Neck. 2011;33:1727–34.
Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Carole Fakhry, Gypsyamber D’Souza, Rehan Kazi and Raghav C. Dwivedi
About this chapter
Cite this chapter
Posner, M.R. (2015). Clinical Management of HPV-Related Oropharyngeal Cancer. In: Fakhry, C., D’Souza, G. (eds) HPV and Head and Neck Cancers. Head and Neck Cancer Clinics. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2413-6_6
Download citation
DOI: https://doi.org/10.1007/978-81-322-2413-6_6
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2412-9
Online ISBN: 978-81-322-2413-6
eBook Packages: MedicineMedicine (R0)