Abstract
Nitrogen (N), phosphorus (P) and potassium (K) are the three important nutrients required by any plant for healthy growth. Among these, P stands as the second limiting nutrient next to nitrogen. Even though different forms of P are abundantly present in soil, its availability in plant-utilizable form is limited. This deficiency is usually compensated by adding chemical fertilizers. However, the chemical fertilizers are expensive and are not eco-friendly. Nonjudicious and irregular usage for a long time leads to decreased soil activity and soil microflora leading to imbalance in equilibrium. Usage of microorganisms to augment the P availability is the best alternative. Phosphate-solubilizing microorganisms (PSMs) when applied in appropriate numbers into the rhizosphere help the plant by supplementing P in plant-utilizable form by several mechanisms. In addition, few PSMs also possess added features as plant growth-promoting rhizobacteria (PGPR) and biocontrol agents conferring protection from phytopathogens. Improvement in soil characters by PSMs is an added advantage. Recent advances in technology paved the way for modifying PSMs with desired qualities. In spite of these, several areas in this area of research suffer different lacunae. Efforts are being made to discuss all major areas pertaining to PSMs in the present review.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microorganisms
- Phosphate solubilization
- Plant growth-promoting rhizobacteria
- Crop protection
- Phytopathogens
12.1 Introduction
Sustainable agriculture remains a stand-alone solution to scarcity of food and prevailing hunger. Statistical estimates of the Food and Agriculture Organization of the United Nations (FAO 2005) indicate that more than 923 million people face chronic hunger. Further this is expected to increase by 9.3 billion in 2050. In view of this, there is an urgent requirement for a revolution in agricultural productivity by bringing marginal and uncultivable lands (soils of low productivity) into the frontiers of agriculture. High yields in agriculture depend on healthy crops which in turn depend on plant health and soil fertility.
One of the major factors that deprive plant health is nutritional deficiency. Different metabolic processes of plants at any growth stage can be adversely affected by low or no availability of soil nutrients. Plants of different genotypes differ in their ability to uptake nutrients from soil by converting unavailable forms to assimilable form because of root surface area, root exudates and rhizosphere microflora (Sessitsch et al. 2013). Generally unavailability of nutrients is attributed to factors like low solubility, poor mobility or inherent low nutrient concentrations in different soils.
In soil bulk, plant essential nutrients are relatively high in total amounts but the concentration in soil solution (i.e. plant available form) of rhizosphere is not sufficient enough to meet the needs of healthy plant growth. Nutrients like phosphorus (P), potassium (K), iron (Fe), zinc (Zn), manganese (Mn) and copper (Cu) have limited mobility/solubility in soils. These are transported into the roots by a slow process called diffusion. Nitrogen (N), phosphorus (P) and potassium (K) are among the main macronutrients required for plant growth. Among them, N is abundantly available from atmospheric sources and biologically/chemically available to plants.
Phosphorus/phosphate (P) plays an important role in life acting as a backbone in molecules like DNA (deoxyribonucleic acid), RNA (ribonucleic acid) and phospholipids of animal and plant cells. After nitrogen, P is the second major growth-limiting nutrient in agricultural production. Directly or indirectly P nutrition in plants affects root surface area, crop yield and quality, N fixation, seed formation, crop maturity, stalk and stem strength and resistance against plant pathogens. Most of the P acquired by the plant is used in various plant metabolisms. Phosphorus absorption occurs mainly during vegetative plant growth and a major part of it is translocated to fruits and seeds. Phosphorus is also essential for cell division, photosynthesis, sugar breakdown and nutrient uptake and transport. Phosphorus deficiency leads to retarded growth and dark-green coloration in plants.
Many soils contain high amounts of P; however, most of it is unavailable to plants for utilization because of adsorption, precipitation and conversion to organic form. Plants prefer P in water-soluble form, i.e. (PO4)3−, (H2PO4)2−, (HPO4)2−. Concentrations of these forms of P are very low varying from 0.001 mg l−1 (poor soil) to 1 mg l−1 (highly fertile).
12.2 Forms of Soil P
Generally two forms of P exist in soils, namely, organic (Po) and inorganic (Pi), varying in terms of quality and quantity.
12.2.1 Organic P (Po)
More than 50 % of the total P is organic phosphorus. Chemically these are esters of orthophosphoric acid identified as inositol P, phospholipids and nucleic acids (Quiquam Poix and Mousain 2005). Inositol P (Ca-Mg salt of phytic acid) is the most abundant of total organic P ranging from 10 to 50 %. Water-insoluble fraction of total organic P includes phospholipids (1–5 %) (Dalal 1977) that is easily released and utilized by microorganisms in the soil. Nucleic acids (constituting 0.2–2.5 % of total organic P) are released from organism residues in two forms, viz. DNA and RNA, which are quickly broken down.
12.2.2 Inorganic P (Pi)
The natural source of P in soils is the mineral apatite, a calcium phosphate which is sparingly soluble. This mineral is found in very lower horizons of soil and is unavailable to plants. Mono- and dicalcium phosphates are simpler forms assimilable by the plants but their tendency to convert back to insoluble forms and their existence in extremely small quantities make them unavailable. Apart from apatite, P can also form minerals in combination with Fe (strengite) and Al (variscite) contributing little to the plant nutrition owing to their low solubility. Other common minerals of P in soil are represented in Table 12.1 in the order of decreasing solubility. Chemical dynamics of P and its constituents in soil were excellently reviewed (Jones and Oburger 2011).
12.3 Availability of P to Plants
Phosphates that are soluble in water or 2 % citric acid solution are known as available forms which can easily be assimilated by plants. Most of the rock phosphate forms present in soil are insoluble except for very low quantities of P from sedimentary origin. Plant root exudates contain organic acids (citric, malic, etc.) that can dissolve insoluble phosphates and assimilate through diffusion.
Scientists from England (Rothamsted Experimental Station) developed single super phosphate (SSP/water-soluble P) and later on introduced diammonium phosphate (DAP), mono-ammonium phosphate and NPK mixtures. This agricultural chemicalization, though increased world food production, has invited new problems. Excessive and nonjudicious application of chemicals over a long period leads to destruction of soil properties and microbiota.
Several strategies are being adopted to enhance the availability of P in different soils. One such strategy is application of high dose of soluble P fertilizers (1,000 mg/kg), followed by small amounts of application in subsequent years. Most of the added P will be fixed and this may be released over several years. Acidic soils have high P fixation capacity. These soils when amended with chemical fertilizers, soluble P is quickly fixed. In tropical countries, strategies like amendment of soils with rock phosphate in combination with organic materials like farmyard manure, compost and green manures are shown to increase plant growth and crop yields. Added manures were shown to assist desorption of sorbed phosphorus (Pi). Some researchers even tried processing of rock phosphates by grinding, heat treatment and fusion with Na, Mg and Si which showed some satisfactory result (Redding et al. 2006).
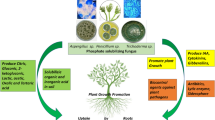
The added chemical phosphorus cannot satisfy plant requirements owing to soil pH. Tropical and subtropical soils are predominantly acidic and often extremely P deficient. Below pH 5.5, soil cations Fe, Al and Mn lock up P and make it unavailable to plants. Calcium and magnesium ions precipitate P at a pH range above 7. Due to phosphate fixation, the use efficiency of added chemical P by plants is just 15 % in the first year and 1–2 % in subsequent years (Mark Evans 2012) (Fig. 12.1).
Comparison of P-availability and plant growth promotion between soil fortified with PSM (left hand side) and soil amended with chemical P fertilizers (right hand side). Arrows in left side of figure indicate different mechanisms in which PSMs contribute to phosphate solubilization and plant growth promotion. Steps involved in isolation and mass multiplication of PSMs are shown as a flow chart (top left of figure). Abbreviations: P phosphorus, Pi inorganic P, Po organic phosphorus, PSM phosphate solubilizing microorganisms, PSB phosphate solubilizing bacteria, PSA phosphate solubilizing actinomycetes, IAA indole acetic acid, GA gibberellic acid, HCN hydrogen cyanide
12.4 Phosphate-Solubilizing Microorganisms (PSMs)
The soil which acts as a substratum for plant growth is an ecological hot zone with many constant biological activities. It is a dynamic system regulating organic matter decomposition and availability of plant nutrients. Soil microorganisms are significantly responsible for major global biogeochemical cycles. Microorganisms in soil influence health of the soil directly or indirectly through their beneficial and detrimental activities. The major microbial activity is confined to the aggregates with accumulated organic matter around the rhizosphere. The rhizosphere was defined in 1904 by Hiltner as being the volume of soil, influenced by the presence of living plant roots, whose extension may vary with soil type, plant species, age and other factors. Rhizospheric microorganisms are responsible for several activities like decomposition, nutrient mobilization and mineralization, storage and release of nutrients and water, N fixation and denitrification.
The concept of using pure cultures of soil microorganisms to increase P nutrition of plants through increased solubility of calcium phosphates is not new. Evidence of the involvement of microorganisms in solubilizing insoluble phosphates was shown as early as 1903. Since then several studies on solubilization of P by different kinds of microorganisms were extensively carried out. Phosphate-solubilizing microorganisms are ubiquitous in nature and vary from soil to soil in number (Lopez et al. 2012). Several microorganisms belonging to bacteria, fungi, actinomycetes, cyanobacteria and even protozoa were shown to be involved in making the soil P solution. The type and form of insoluble P present and type of soil determine the microbial community of PSB. Biodiversity of phosphate-solubilizing organisms in relation to plant host specificity and various rhizosphere soils was studied earlier. The capacity of bacterial isolates to solubilize phosphates depends upon the zone of their origin, those derived from the rhizoplane having the highest, the rhizosphere intermediate and non-rhizosphere soil the least capacity (Gurdeep Kaur and Sudhakara Reddy 2014).
Different properties of soil, viz. physical and chemical properties, organic matter and P content, determine the population of PSMs in soil. In general agricultural and range lands harbour high counts of PSMs. Most of the PSMs are effective in Ca-P containing calcareous soil compared to Fe-P and Al-P containing Alfisols. In total microbial population of soil, phosphate-solubilizing bacteria (PSB) constitute about 50 % and phosphate-solubilizing fungi (PSF) 0.1–0.5 %. In general, PSB outnumber PSF by 2–150-folds.
The interaction between microorganisms and soil constituents are vital to all terrestrial ecosystems. Plant and microbial ecosystems are often stressed in acquisition of P primarily due to low aqueous solubility of PO4 3−. Plants obtain P from soil in the form of HPO4 −1 and H2PO4 −1. Thus, the ability of microorganisms to solubilize and mineralize P in soil is vital. Crop plants require approximately 10–100 kg P ha−1.
In an average soil only 0.05–0.01 % of the total P present is available to plant because of its chemical fixation and low solubility. A survey of Indian soils revealed that 98 % of these soils need P fertilization either in the form of chemical or biological fertilizer. Phosphate availability in soil is greatly enhanced through microbial production of metabolites leading to lowering of pH and release of P from organic and inorganic complexes. In Russia a commercial biofertilizer by the name “phosphobacterin” containing B. megaterium var. phosphaticum was produced and widely used with yield increases of 5–10 % over controls. A commercial formulation of Penicillium bilaii chalubuda has also been registered in Canada (Jumpstart ®) as biological enhancer of plant nutrients now sold by Novozyme (Jumpstart 2012).
12.4.1 Phosphate-Solubilizing Bacteria (PSB)
Bacteria are well known for their ability to release bound phosphorus from different sources. PSB occur in moist soils and may represent up to 40 % of cultivable population. Both aerobic and anaerobic P-solubilizing strains of bacteria are found prevalent in considerable numbers in soil, in plant rhizospheres and even in marine environments (Syed and Damare 2013). Keeping this in view management strategies need to be formulated with introduction of native efficient strains suitable for sustainability (Zhaoa et al. 2014).
Common groups of bacteria involved in phosphate solubilization are Bacillus, Pseudomonas, Azotobacter, Burkholderia and Rhizobium. Even with a single bacterium species of single phylogenetic lineage, significant variations exist in terms of source of isolation (Zhaoa et al. 2014). Phosphate solubilization in rhizobia accompanied with N fixation was reported earlier. About 60 % of Bradyrhizobium strains were reported to be capable of P solubilization (Hayat et al. 2010). Some strains of P-solubilizing rhizobia can colonize the roots like other plant growth-promoting rhizobacteria (PGPR) and increase the yield of legumes and nonlegumes.
Increase of plant growth in wheat by solubilization of inorganic phosphates from tricalcium phosphates (TCP) and Mussoorie rock phosphate (MRP) was observed using Azotobacter chroococcum (Kumar et al. 2001). Positive results were also observed in cotton and wheat varieties when inoculated with P-solubilizing A. chroococcum with greater NPK uptake (Narula et al. 2005).
Reports on phosphate solubilization by pseudomonads in general are scanty. It has been reported that 18 % of fluorescent pseudomonads were positive for solubilization of TCP as evident from visible dissolution halos on Pikovskaya’s agar. Carbon and nitrogen sources are important parameters for active proliferation and production of organic and inorganic acids for P solubilization (Scervino et al. 2011). Fluorescent pseudomonads facilitated increase in root surface area and mineral phosphate solubilization leading to increased nutrient uptake and seedling biomass. Fluorescent pseudomonads and rhizobia were shown to solubilize organic and inorganic (Antoun 2012) phosphates. Several species of fluorescent pseudomonads such as P. fluorescens NJ-101, P. fluorescens EM85, P. aeruginosa, Pseudomonas sp., P. chlororaphis, P. savastanoi, P. picketii, P. lulea OK2, P. rhizosphaerae LMG 1640, P. graminis DSM 11363, P. striata and P. corrugata have been reported as efficient P solubilizers. Bacteria exhibiting solubilizing activity and also colonizing mycorrhizal hyphae may contribute indirectly to uptake P by mycorrhiza. Investigations in this regard have shown that these bacteria are found in hyphal mucilage, hyphoplane, in hyphal wall layers and even inside hyphae and spores (Gonzalez-Chavez et al. 2008). These bacteria apart from P solubilization may execute other functions simultaneously. Selection of bacteria based on a single trait may not be promising for inoculation technology (Praveen Kumar et al. 2012). Few bacteria studied for their P-solubilizing ability are listed below.
Gram-positive bacteria: Bacillus brevis, B. cereus var. albolactis, B. circulans, B. coagulans, B. firmus, B. megaterium, B. megaterium var. phosphaticum, B. mesentricum, B. mycoides, B. polymyxa, B. pumilus, B. pulvifaciens, B. sphaericus, B. subtilis, Clostridium sp., B. licheniformis, B. amyloliquefaciens, A. atrophaeus.
Gram-negative bacteria: Acetobacter diazotrophicus, Achromobacter sp., Aerobacter aerogenes, Agrobacterium radiobacter, Agrobacterium sp., Alcaligenes sp., Arthrobacter mysorens, Bradyrhizobium sp., Brevibacterium sp., Burkholderia cepacia, Citrobacter freundii, Enterobacter aerogenes, Enterobacter agglomerans, Enterobacter asburiae, Enterobacter cloacae, Escherichia freundii, Escherichia intermedia, Erwinia herbicola, Flavobacterium sp., Gluconobacter diazotrophicus, Micrococcus sp., Mycobacterium sp., Nitrosomonas sp., Pseudomonas calcis, P. cepacia, P. fluorescens, P. putida, P. rathonia, P. striata, P. syringae, Serratia marcescens, S. phosphaticum, Thiobacillus ferrooxidans, T. thiooxidans, Rahnella aquatilis, Rhizobium meliloti, Xanthomonas sp., Azotobacter chroococcum, Kluyvera ascorbata, Azospirillum brasilense, A. lipoferum, Acinetobacter calcoaceticus.
12.4.2 Phosphate-Solubilizing Fungi (PSF)
Phosphate-solubilizing fungi are known for their ability to solubilize high amounts of bound P for plant growth promotion. Release of enzymes like acid and alkaline phosphatases, phytase and organic acids (citric, oxalate, gluconate, etc.) appears as strategies for dissolution of insoluble phosphates by PSF. Reports indicate that they are able to show 5–20 % increment in plant growth (Gunes et al. 2009). Out of the total fungal population, PSF constitute about 0.1–0.5 %. In general, the most commonly encountered genera of PSF are Aspergillus and Penicillium (Reyes et al. 2002). A nematophagous fungus, Arthrobotrys oligospora, was also shown to solubilize phosphate in vitro and in vivo (Duponnois et al. 2006). Recently, aluminium and rock phosphate solubilization by a specific Penicillium sp. and yeast was studied by Xiao et al. (2013b) and Narsian et al. (2010), respectively. Unlike their bacterial counterparts, PSF were able to retain P-solubilization trait even after several subcultures. Moreover, they were able to solubilize more amount of bound P because of their ability to secrete more acids. Although bacteria have been used as commercial preparations to improve the plant growth, fungi seem to be a better option for the same purpose. A list of few fungi which were studied for P dissolution phenotype is given below.
PSF: Achrothecium sp., Alternaria tenuis, Aspergillus aculeatus, A. awamori, A. carbonum, A. flavus, A. foetidus, A. fumigatus, A. japonicus, A. nidulans, A. nidulans var. acristatus, A. niger, A. rugulosus, A. terreus, A. wentii, Cephalosporium sp., Chaetomium globosum, Cladosporium herbarum, Cunninghamella sp., C. elegans, Curvularia lunata, Fusarium oxysporum, Helminthosporium sp., Humicola lanuginosa, H. inslens, Mortierella sp., Micromonospora sp., Mucor sp., Myrothecium roridum, Oidiodendron sp., Paecilomyces lilacinus, P. fusisporus, Penicillium aurantiogriseum, P. bilaji, P. digitatum, P. funiculosum, P. lilacinum, P. oxalicum, P. pinophilum, P. rubrum, P. rugulosum, P. simplicissimum, P. variabile, Phoma sp., Populospora mytilina, Pythium sp., Rhizoctonia solani, Rhizopus sp., Sclerotium rolfsii, Torulaspora globosa, Torula thermophila, Trichoderma harzianum, T. viridae, Schwanniomyces occidentalis, Emericella rugulosa, Penicillium camemberti, Colletotrichum sp.
Yeast: Yarrowia lipolytica, Schizosaccharomyces pombe, Pichia fermentas
12.4.3 Phosphate-Solubilizing Actinomycetes (PSA)
Actinomycetes are widely distributed in nature and stand second to bacteria in terms of population in soil. They constitute 10–50 % of the total soil microflora depending on soil conditions. These bacteria with their ability to secrete secondary metabolites including antibacterial, antifungal, insecticidal and antihelminthic compounds are helping the plants to survive from ailments. They are also endowed with many other properties like production of phytohormones, siderophores, etc., through which they promote the healthy plant growth (Franco-Correa et al. 2010). Actinomycetes are isolated from various sources and screened for their efficiency to enhance P availability to plants. According to one study, 20 % of the total actinomycete population is PSA and that predominantly belong to genera Streptomyces and Micromonospora (Hamdali et al. 2008). Unlike fungi, actinomycetes cannot acidify the external medium though they release several organic anions in large quantities. Solubilization of P in these organisms is thought to be because of production of acid anions or by some other mechanisms (Hamdali et al. 2010). Field trials have shown increased plant growth but the reason for the increment is not clearly attributed to P solubilization or other beneficial effects. Owing to their ability to withstand extreme environments, these organisms are studied for enhancing P availability during municipal and animal waste composting (Chang and Yang 2009). Few actinomycetes listed below are known to have an active role in P solubilization.
Actinomyces sp., Actinomyces coelicolor, Streptomyces sp., Streptomyces violascens, S. noboritoensis, S. cinereorectus, S. cinnabarinus, Microbacterium aurantiacum, M. kitamiense, Angustibacter luteus, Kocuria flava, Isoptericola hypogeus, Agromyces soli, Kocuria palustris, Microbacterium yannicii, Isoptericola variabilis, Nocardia sp., Streptoverticillium sp., Thermoactinomycetes sp., Micromonospora sp.
12.4.4 Cyanobacteria P Solubilization
The ability of cyanobacteria to solubilize bound P became evident from the work of Kaushik (1995). These organisms apart from P solubilization can extend many other benefits like N2 fixation, production of growth-promoting hormones and many secondary metabolites. Inoculation of plants with P-solubilizing cyanobacteria has improved plant growth by increasing the availability of P and N nutrients.
Like bacteria, cyanobacteria are also known to mobilize bound phosphates. They were observed to solubilize Ca3 (PO4)2, Fe PO4, Al PO4 and (Ca5 (PO4)3.OH). They are also known to solubilize organic sources of phosphorus. Different strategies were thought to be involved in the release of bound P including production of organic acids, chelators, dissimilatory reduction and enzymatic solubilization or simultaneous action of one or more of these. Despite these proposals, it is still ambiguous with regard to definite operative method of P solubilization by cyanobacteria. In a recent study, Westiellopsis prolifica and Anabaena variabilis were shown solubilize TCP and MRP (Yandigeri et al. 2011). Further, it was demonstrated that P solubilization was due to the production and action of phthalic acid without any decrease in pH. Few examples of cyanobacteria studied for their ability to release bound P are listed below.
Anabaena, Calothrix braunii, Tolypothrix, Scytonema, Hapalosiphon fontinalis, Nostoc sp., Scytonema cincinnatom, Tolypothrix tenuis, Tolypothrix ceylonica, Westiellopsis prolifica, Phormidium sp.
12.4.5 Protozoa and Other Mesofauna P Solubilization
Protozoa live in soil at the expense of bacteria of the genera Aerobacter, Agrobacterium, Bacillus, Escherichia, Micrococcus and Pseudomonas by ingesting them into their protoplasm. Protozoa are abundant in the upper layer of the soil and their number is directly dependent on bacterial population. Owing to inadequate studies on soil protozoa, it is difficult to define their role in P cycling. They directly or indirectly influence the P cycling by regulating the number of bacteria in the soil. Even they can reduce the affectivity of the added PSB by grazing (Rosenberg et al. 2009). Apart from this, protozoa are known to increase P bioavailability by assimilating soluble minerals. But this line of research received less attention because of the hindrances in mass cultivation and negative impacts on dynamics of the food webs.
Interactions between P and soil mesofauna have recently been extensively reviewed (Chapuis-Lardy et al. 2011). Different mesofaunal populations exert both positive and negative impacts on P availability. Nematodes reduce the inoculated PSMs and thereby reduce the P availability. Earthworms play an important role in P nutrition by the method clearly not elucidated. It was shown that they enhanced ability of P solubilization by fungi (A. awamori) (Sreenivas and Narayanasamy 2009) and bacteria (B. megaterium) (Wan and Wong 2004) resulting in increased soluble P (organic and inorganic) in soil. In another study, earthworm casts and furrows were reported as sites for proliferation of PSMs and their activity (Mba 1997).
Although several PSMs occur in soil, usually their numbers are not high enough to compete with other bacteria commonly established in the rhizosphere. Thus, the amount of P liberated by them is generally not sufficient for substantial increase in in situ plant growth. Therefore, inoculation by a target microorganism at a much higher concentration than that normally found in soil is necessary to take advantage of the property of P solubilization for plant growth enhancement.
12.5 Mechanism of P Solubilization
Microorganisms, mainly residing in the milieu of rhizosphere, have the ability to influence the change of chemical environment by uptake and release of organic and inorganic ions. They can drastically influence the availability of P from organic (Po) and inorganic (Pi) phosphates. Dissolution and availability of Pi depends on the properties of inorganic minerals available. Broadly three main mechanisms are adopted by soil microorganisms for P solubilizations by (1) releasing mineral-dissolving substances like organic acid anions, hydroxyl ions and CO2, siderophores and protons; (2) secreting extracellular enzymes (i.e. biochemical mineralization of Po); and (iii) substrate degradation (biological mineralization).
Phosphate immobilization and dissolution (i.e. P mineralization and solubilization) are two important processes deciding the fate of P in soil. Plants receive assimilable P if the rate of P solubilization (from minerals) and mineralization (bound organic P, disintegration of microbial biomass) exceeds P immobilization (by mineral formation, incorporation by uptake into microbial biomass). Phosphorus solution concentration in soil is affected by different processes of soil P cycle. Three different major processes described by Sims and Pierzynski (2005) that affect the solution P are (1) dissolution precipitation (mineral equilibria), (2) sorption desorption (interaction between P in solution and soil solid surfaces) and (3) mineralization immobilization (biologically mediated conversions of P between inorganic and organic forms). In all these processes of soil P cycle, microorganisms play an important role.
12.5.1 Solubilization of Inorganic Phosphates (Pi)
Many researchers have proposed different mechanisms responsible for release of P (in solution) from inorganic phosphates (Ca-P, Al-P, Fe-P, etc.). Some of them are (1) production of organic acids (chelation of P-bound cations), (2) production of inorganic acids, (3) H2S production, (4) lowering of pH through release of protons, (5) proton release from NH4 + (assimilation/respiration), (6) P assimilation from liquid (indirect dissolution), (7) production of siderophores, (8) production of exopolysaccharides and (9) direct oxidation pathway.
12.5.1.1 Production of Organic Acids
Several studies have shown the ability of PSMs to solubilize insoluble phosphates in pure liquid culture medium, and this was often due to the excretion of different organic acids (Sharma et al. 2013). These organic acids are known to act through (1) lowering the pH, (2) enhancing chelation of cations bound to P, (3) forming complexes with P-associated metal ions and (4) competing with P for adsorption. Organic acids are produced by direct oxidation pathway (at the outer face of the cytochrome membrane) or mostly by oxidative respiration and fermentation of organic C sources. These acids can either dissolve P directly or chelate Fe, Al and Ca ions associated with P. Organic acids like gluconic acid, oxalic acid, citric acid, lactic acid, tartaric acid, aspartic acid, etc., were detected during the course of P-solubilization study using paper chromatography, TLC and HPLC. However, these studies lack correlation between acids produced and amounts of P liberated.
The chelating ability of organic acids also plays a significant role in making the P available from insoluble inorganic phosphates. In a study conducted by Kucey (1988), it was shown that addition of 0.05 M EDTA into the culture medium exerted the same effect shown by Penicillium bilaji. In another study, the addition of NaOH arrested P-solubilizing activity of Rhizobium which was thought to be associated with 2-ketogluconic acid. These studies emphasize that P solubilization by these organisms is due to their ability to reduce the pH of the medium.
12.5.1.2 Lowering of pH
P solubilization by acidification was well studied in many bacterial and fungal species. However, reports on P solubilization by alkalization are scanty. The excretion of organic acids by PSMs is associated with release of protons and lowering of pH (Maliha et al. 2004). Release of protons or hydroxide ions by PSMs can also influence soil solution pH.
Acidification and lowering of pH are not the only mechanisms involved in solubilization as reduction in pH does not always correlate with the amount of P solubilized. Correlating to this, HPLC analysis of culture filtrate from P-solubilizing Pseudomonas sp. did not reveal any organic acid formation. Parks et al. (1990) proposed an alternative mechanism of P solubilization by proton excretion because of NH4 + assimilation.
12.5.1.3 Proton Release from NH4 + (Assimilation/Respiration)
The amount of protons released into the external medium influences the soil pH which in turn is influenced by the type of N sources used by microorganisms. Among different N sources, ammonium salts are reported to be the best followed by asparagine, sodium nitrate, potassium nitrate, urea and calcium nitrate. With NH4 + as sole N source, reduction in pH and amount of P solubilized was observed to be high compared to that of nitrate (NO3 −). This is due to extrusion of protons to compensate NH4 + uptake (Sharan et al. 2008). This was in contradiction with the findings of Reyes et al. (1999), who reported decrease in P solubilization when higher concentration of NH4 + was supplied to the medium.
Ammonium-driven proton release may stand as sole mechanism for solubilization in some microorganisms. But in some organisms no significant relation could be accounted for pH change and P mobilized. This indicates existence of additional solubilization mechanisms. Also, proton release depends on different mechanisms and only partly on NH4 + assimilation as evidenced from the work of Park et al. (2009).
12.5.1.4 P Solubilization by Inorganic Acids and H2S
Inorganic acids like HCl, H2SO4 and HNO3 were reported to solubilize less amounts of Pi. Enterobacter agglomerans was shown solubilizing P from hydroxyapatite mediated by HCl. A study by Kim et al. (1997) using E. agglomerans and a genetically modified E. coli showed that incorporation of culture media with known acids like HCl, citric acid, oxalic acid and lactic acid increased P solubilization from hydroxyapatite although citric acid was able to solubilize more P than HCL. Production of acids like HNO3 and H2SO4 and dissolution of phosphates were observed in bacterial genera Nitrosomonas and Thiobacillus.
Release of bound inorganic P by H2S is another mechanism. Certain bacteria produce H2S which reacts with ferric phosphate resulting in the formation of ferrous sulphate and P release. Microbial S oxidation is a mechanism increasing mineral phosphate solubility by production of H2SO4, nitrate and CO2. However, these mechanisms are less accepted than P solubilization by organic acids.
12.5.1.5 Indirect P Dissolution
The sink theory proposed by Halvorson et al. (1990) states that rhizospheric microorganisms assimilate large amounts of P from soil solution by P uptake system. As a result equilibrium between insoluble and soluble P is disturbed. Consequently, sparingly soluble phosphates would then be dissolved indirectly. Phosphorus content in PSMs was similar to those observed in non-P-solubilizing microorganisms due to the fact that the P content of the organisms is correlated with decomposition of P containing organic substrates. Although some P released by PSMs will be used by plants and other soil organisms, where most of the part remains immobilized with the biomass. This P is released when cells die due to changes in environmental conditions, starvation or predation. Environmental changes such as drying-rewetting or freezing-thawing can result in sudden increase in available P due to the unusual high proportion of microbial cell lysis (Butterly et al. 2009).
12.5.1.6 Direct Oxidation Pathway
Goldstein (1995) suggested the essential role played by extracellular oxidation in soils where calcium phosphate provides a significant pool of unavailable mineral phosphorus. This was confirmed by the biochemical analysis of lowering pH and insoluble P solubilization by Burkholderia cepacia DA 23 (Song et al. 2008).
12.5.1.7 Exopolysaccharide (EPS)-Mediated P Release
The role of low molecular weight organic acids in the solubilization of mineral P is well documented. But the knowledge on the role of high molecular weight microbial exudates (nonenzymatic mucilage, EPS) on P solubilization is limited. EPS and biosurfactants are produced by microorganisms largely in response to biofilm formation and stress. Microbial exopolysaccharides are polymers of carbohydrates (homo- or heteropolysaccharides) excreted by some bacteria and fungi on the outer side of their cell walls. Earlier studies have shown that the EPS have the ability to form complexes with metals in soil (order of affinity to form complexes Al3+ > Cu2+ > Zn2+ > Fe3+ > Mg2+ > K+) (Ochoa- Loza et al. 2001) implicating their role of P solubilization in soil.
Microbial EPS, under pure culture studies, have shown to stimulate dissolution of TCP in synergy with organic anions. In support of this, four bacterial strains (PSB) – Enterobacter sp. (EnHy-401), Arthrobacter sp. (ArHy-505), Azotobacter sp. (AzHy-510) and Enterobacter sp. (EnHy-402) – were evaluated for their role of EPS in dissolution of insoluble phosphates (Yi et al. 2008). Further the rate of dissolution was showed dependent on microbial source and concentration of EPS.
12.5.1.8 Siderophore-Mediated P Release
Siderophores are iron (Fe)-chelating agents produced by almost all microorganisms when subjected to Fe deficiency. They are low molecular weight (<10,000 D) virtually ferric-specific ligands produced as scavenging agents in order to combat low iron stress. Siderophore production is not widely being investigated as a method for phosphate solubilization. In view of dominance of mineral dissolution by organic acids as a method for P solubilization, ligand exchange and its role in enhancing available P are not obvious. These ligands were extensively studied with respect to their ability to mobilize Fe.
Approximately, 500 different siderophores are known which are used by both plants and microorganisms. Several PSMs are also reported to produce siderophores (Collavino et al. 2010). Very few works have been carried out to evaluate siderophore production as a method of P solubilization. Reid et al. (1985) showed 13-fold increments in P diffusion when compared with water without knowledge about siderophore enhancing P solubilization. Here two siderophores (desferrioxamine-B, desferrichrome) and an iron-chelating agent EDDHA (ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid)) were compared to water for Fe and P diffusion using root simulating technique.
12.5.2 Solubilization of Organic P (Po)
Microorganisms adopt various mechanisms for making the available P from insoluble reserve in soil. Approximately, 4–90 % of the total soil P is organic P (Po). Generally, in order to solubilize this Po, microorganisms employ mechanisms involving secretion of several enzymes. These are either cell wall bound or freely excreted to the surrounding environment. Extracellular enzymes are thought to be more active inducing large changes in soil solution P concentration. However, experimental evidence of exo- and endoenzyme activity is still ambiguous.
The modes of action of enzymes vary with different types of enzymes secreted. They may involve in (1) dephosphorylation of esters and anhydrides of H3PO4 (Tabatabai 1994), in (2) release of P from phytate degradation (Singh et al. 2014) or by (3) cleaving C-P bond of organophosphates (Rodriguez et al. 2006).
Phosphatases or phosphohydrolases are a broad group of enzymes secreted by PSMs catalyzing dephosphorylation reaction. Among this, phosphomonoesterases (or phosphatases) are most abundant and well studied. Based on their pH optima, phosphomonoesterases are further classified as acid and alkaline phosphomonoesterases. Both of these phosphatases are produced by PSMs depending on external conditions (Jorquera et al. 2011). Acid phosphatases are typically predominant in acidic soils, while alkaline phosphatases are observed in neutral and alkaline soils (Singh and Reddy 2011).
Acid phosphatases are also secreted by plant roots apart from microorganisms. It is difficult to differentiate between root- and PSM-produced phosphatases (Richardson and Simpsom 2011). It was observed that phosphatases of microbial origin have more affinity towards Po compared to those derived from plant roots. Laboratory studies evidenced that gross mineralization processes produced 1–4 mg P Kg−1. However, it is not easy to distinguish between enzymatic (biochemical) and biological (microbial) mineralization. Lower concentration of divalent cations (Ca, Mg, Zn, Co) was observed to be acting as enzyme activators, whereas high concentration of several metals (Zn, Mn, Cu, Mn (II), Fe (II)), polyvalent anions (MoO4 2−, AsO4 3−) and orthophosphate (end product) were found to inhibit enzyme activities (Quiquampoix and Mousain 2005). Further, enzyme activities are not simply related to their release rate but are strongly influenced by soil properties.
Inositol phosphates are the most abundant of the total organic P in a soil. Microorganisms bring about degradation of phytate leading to the release of bound P. This process is assisted by secretion of enzyme phytase. Phytate is the major stored form of P in plant seed and pollen, although plants’ ability to obtain P from phytate is limited. In an experiment it was shown that genetically transformed Arabidopsis plant with phytase gene (Phy A) could assimilate phytate with significant increment in growth and P nutrition (Richardson et al. 2001). Here the P content of the plant was equivalent to control plant supplemented with inorganic phosphates. This confirms the role of microorganisms in mineralization of phytate and thus increasing the P nutrition (Richardson and Simpson 2011). Yet in another study, Rodriguez et al. (2006) demonstrated the role of special enzyme phosphonotases and C-P lyases in cleavage of C-P bond of organophosphates in the availability of soluble P in soil. Besides this, phytase-producing isolates of Advenella sp. and Cellulosimicrobium sp. were recently reported as PGPR increasing P content in Indian mustard (Kumar et al. 2013; Singh et al. 2014).
Each P solubilizer may adopt one or more than one mechanisms to solubilize insoluble P. Any one single mechanism as the sole method responsible for P solubilization cannot be pointed out, although organic acid production and pH reduction were witnessed in most of the occasions. Recently, biochar addition was shown alluviating toxicity caused by fluoride produced during the course of P solubilization using Aspergillus niger, thereby increasing the solubilization efficiency (Mendes et al. 2014).
12.6 Effect of PSMs on Soil Properties
In addition to P solubilization, PSMs also provide multiple beneficial effects on many soil properties including soil structure, soil enzymes and their activities and soil microbial community (Vassileva et al. 2010) (Table 12.2).
12.6.1 PSM-Soil Aggregate Stability
Microorganisms affect the soil properties either mechanically or by excretion of polysaccharides into the medium. Many investigators have suggested that production of polysaccharides by PSMs is responsible for soil structure improvement (Bearden and Petersen 2000). PSMs were shown to increase the soil aggregate stability, water-soluble C and carbohydrate C in rhizosphere.
12.6.2 PSM-Soil and Plant Enzymes
It is also interesting to note that phosphate solubilizers increased the enzyme content and activity in PSM-amended soils. They were shown to improve dehydrogenase, phosphatase, β-glucosidase (Medina et al. 2006) and antioxidant enzymes (ascorbate peroxidase (APOX), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT)) in plants grown in contaminated soils enriched with PSMs (Azcon et al. 2009b). Further, synergistic interaction between AM fungi and PSF was reported to improve enzyme activities in bulk soil at different salinities (Zhang et al. 2011). The increase of enzymatic activities in soils and plants confers increase in availability and acquisition of nutrients.
12.6.3 PSM-Soil Microbial Community
It is well known that AM fungi affect microbial community in both direct and indirect ways. Addition of PSMs enhanced the amount of biomarker fatty acids of all groups of microorganisms as a result of the increase in carbon sources, while the microbial community remained unaffected by inoculation with AM fungi alone. An increased AM fungal growth and activity was found in A. niger (a P solubilizer)-treated agro-waste (Medina et al. 2007). In another study, it was shown that A. niger-treated sugar beet/rock phosphate amendment is a suitable tool for increasing the bacterial community in the rhizosphere (Azcon et al. 2009a). This work emphasized on bacterial community profiles generated from DGGE of amplified soil DNA and clearly implies that application of PSMs seems to play a decisive role in improving microbial community structure and soil properties.
12.6.4 PSM-Soil Rehabilitation
Remediation of heavy metal-contaminated sites using phosphate amendments like soluble phosphate, insoluble P sources like rock phosphate (RP) is an interesting approach. Hydroxyapatite is shown to be a very efficient metal immobilizer. Through this approach, heavy metal stabilization was studied using hydroxyapatite, synthetic and natural apatites and rock phosphates. However, bioavailability of heavy metals in soil is affected by the presence of organic matter forming complexes and chelates of varying stability (Kiikila et al. 2002). In such cases, various microorganisms can mobilize metals through autotrophic and heterotrophic leaching, chelation and methylation. Acidification through organic acid production and siderophores can supply protons and metal-complexing anions leading to metal release (Gadd and Sayer 2000) (Table 12.2).
Medina et al. (2006) have demonstrated that A. niger-treated sugar beet and rock phosphate amendment improved growth and nutrition of white clover grown in heavy metal (Zn and Cd)-contaminated soil. Here microbially mineralized RP (simultaneously solubilized), sugar beet and fungal mycelium in combination with AM fungi (G. mosseae) increased the plant growth 28 times more than non-mycorrhizal control plants.
12.7 PSM Effect on Plant Growth Under Greenhouse and Field Conditions
New and novel solutions for plant growth enhancements are required to ease the burden imposed on environment and other resources. The major applications of bacteria for improved plant growth include agriculture, horticulture, forestry and environmental restoration. Certain cooperative microbial activities can be exploited as a low-input biotechnology and basis for a strategy to help sustainable and environmentally friendly practices fundamental to stability and productivity of agricultural systems. Phosphate reserves are likely to be depleted in about 500–600 years at the present mining rates (7,100 million tons/annum). In India, 98 % of crop lands are deficient in available soil P and hence it imports two million tons of rock phosphates annually. Different P management practices enforced are costlier and practically not feasible.
The use of microorganisms to increase crop yield has been limited due to the variability and inconsistency of results between laboratory, greenhouse and field studies. Soil is an unpredictable environment and intended results are sometimes difficult to obtain. The bulk of literature available on the subject indicates that the number of failures equals the number of successful trails as pointed out by Goldstein and Krishnaraj (2007). Nevertheless, microbially mediated P management is an eco-friendly and commercially viable strategy for sustainable crop production. The use of PSMs can increase crop yields up to 70 % and at the same time reduce the usage of chemical P by 25 %.
There are several reports indicating substantial increase in plant growth resulting from single, dual or three-member association of beneficial inoculants in the rhizosphere. Such syntropic associations could be of greater practical value for plant growth under different agroecosystems. In agriculture and forestry, inoculation practices, mixing of two or more microbial species often has a more positive effect on plant growth than the use of a single bacterium (Kishore 2007; Yu et al. 2012).
12.7.1 Inoculation Effects of Single Species of PSMs
Numerous reports are available indicating the beneficial effects of PSMs when inoculated as a single agent. A published account on the overall growth and development in different crops with consistent P uptake and crop yield increase was compiled and reviewed by Lucy et al. (2004). In spite of many technological developments, the performance of microbial inoculants within the vicinity of rhizosphere cannot be precisely predicted. The success of microbial inoculants is mainly determined by their root-colonizing ability and survivability to harness considerable benefits in competition with indigenous resident microbes. The application of single species of PSMs and the positive effects on plant growth were reported in many studies. Phosphate solubilization trait in microorganisms like Rhizobium, Azotobacter, etc., which are natural nitrogen fixers confer added advantages (Kumar et al. 2001). Such organisms with additional traits of plant growth promotion create confusion among the researchers in determining the actual role of P solubilization in plant growth. However, increased P levels within the plant tissues were reported even in field experiments with acid-tolerant PSB.
The effectiveness of PSF in enhancing plant Pi uptake and growth was controlled by the type of soil, particularly by the Pi-sorption capacity of soil. PSF are studied individually and in combination with other fungi for increment in growth parameters of many plants (chick pea, maize, wheat, faba bean, lentil, rice, soybean). PSF in combination with RP was proved to be more effective in P availability and growth promotion. PSF alone or in combination with rock phosphate (Mussoorie, Telesmi, etc.) was found responsible for increase in growth, seed production, shoot height, seed weight, N,P accumulation, dry matter yield, root length and yield.
Application of rock phosphate alone did not significantly increase the growth and plant P uptake (Cabello et al. 2005). Mollisols usually exhibit a low Pi-fixation capacity; for this reason it is not surprising that inoculation with PSMs alone increased P uptake. The effectiveness of PSM inoculation alone to enhance plant Pi uptake in subtropical and tropical acidic soils is relatively low and variable. By contrast, the effectiveness of PSM inoculation to enhance plant Pi uptake of mycorrhizal plants grown in tropical or subtropical soils can be relatively higher compared to data reported in temperate soils. Most of the soils like mollisols and calcareous and sandy soils are characterized by a low P-sorption capacity and relatively high soil Ca-P content. Therefore, freshly released Pi by PSMs can remain longer in soil solution until its absorption by the roots (Duponnois et al. 2006).
12.7.2 Co- and Multiple Agent Inoculations
As both N and P are two major plant nutrients, combined inoculation of nitrogen fixers and P solubilizers may benefit plants better than either group of organisms alone. Inoculation of nitrogen-fixing organisms like Rhizobium and PSF was shown to have significant impact on wheat, chick pea, faba beans, beans, peas, green gram, etc., in increasing grain yield, growth, nutrient uptake (N and P), grain protein, etc., when compared to controls. However, in contrast, few studies imply that this combination did not show increase in dry matter or total P uptake (Kucey 1987). Further, a decrease in total N fixation is explained by high acidic conditions caused by PSF hindering rhizobial root colonization. Therefore, before going for field experiments, the compatibility between the two associate members must be checked in vitro.
12.7.3 Arbuscular Mycorrhizal Fungi (AMF) and PSMs
Mycorrhizal symbiosis is found in almost all ecosystems worldwide to improve plant fitness and soil quality through key ecological processes. Most of the major plant families form AM associations, the most common mycorrhizal type. Their origin and divergence has been dated back to more than 450 million years. Plant hormones as produced by soil microorganisms are known to affect AM establishment. The rhizosphere of a mycorrhizal plant can have features that differ from those of a non-mycorrhizal plant (Johansson et al. 2004).
The primary effect of AMF is the improvement of P uptake by plants due to the ability of external mycelium of AMF to act as bridge between roots and the surrounding soil microhabitats. This provides access to the phosphate ions from soil beyond the phosphate depletion zone surrounding the roots. The phosphate made available by PSMs may not reach the root surface due to limited diffusion. It was proposed that if the solubilized phosphates were taken up by AM mycelium, there will be increase in P supply to the plant. In particular AM inoculations improve the establishment of both inoculated and indigenous phosphate-solubilizing microorganisms (Medina et al. 2007).
The dual inoculation of PSMs and AMF may overcome the limitations imposed on the effectiveness of PSMs to enhance plant Pi uptake in soils with high Pi-fixation capacity. During this interaction, mycorrhizal plants release higher amounts of carbonaceous materials into the rhizosphere which is used as C source by PSMs. Recently, crop yield and N concentration in wheat plants were reported to have increased by >50 and 90 %, respectively, by the synergistic effect of Glomus etunicatum and Burkholderia cepacia BAM-6 (PSB) (Saxena et al. 2014).
12.7.4 PSMs as PGPR and Biocontrol Agents
Beneficial plant-microbe interactions in the rhizosphere are the determinants of plant health and soil fertility. Beneficial soil bacteria from the rhizosphere which have been shown to improve plant health or increase yield are usually referred to as plant growth-promoting rhizobacteria (PGPR) (Ahemad and Kibret 2014). The PGPR and the mechanisms by which it promotes the plant growth are ambiguous and are not fully understood but are thought to include the following characters (PGPR traits): (1) the ability to produce or change the concentration of plant hormones like indole acetic acid (IAA), gibberellic acid (GA), cytokinins and ethylene, (2) the asymbiotic N2 fixation and symbiotic N2 fixation, (3) the solubilization of mineral phosphates and other nutrients and (4) the antagonism against phytopathogenic microorganism by the production of siderophores, β-1,3-glucanases, chitinases, antibiotics and cyanide (Figueiredo et al. 2011; Bhattacharyya and Jha 2012).
Apart from P solubilization, PSMs are also known to produce amino acids, vitamins and growth-promoting substances like IAA and GA (GA3) which help in better growth of plants (Kishore 2007; Kishore et al. 2012). Production of plant growth regulators and biocontrol substances by PSMs in addition to P solubilization is an added advantage for being used as an efficient bioinoculants. Although there is a growing evidence that PSB and PSF augment plant growth due to biosynthesis of growth-promoting substances, future research in this direction is needed.
Naumova et al. (1962) reported that A. chroococcum, B. megaterium var. phosphaticum and P. fluorescens have accumulated in the medium some biologically active compounds such as auxin, gibberellins, vitamins, etc., which can stimulate plant growth and inhibit growth of fungi like Fusarium and Alternaria. Barea et al. (1976) reported that out of 50 isolates of PSB, 20 synthesized 3 types of plant hormones, and 43 produced cytokinin-like substances. Sattar and Gaur (1987) tested 8 PSB and fungal isolates such as B. polymyxa, B. pulvifaciens, P. striata, A. awamori, A. niger, and P. digitatum for synthesis of auxins and gibberellins.
At present, there is evidence supporting the role of this mechanism in plant growth enhancement. For example, several soil microorganisms, including bacteria, improve the supply of P to plants as a consequence of their capability for inorganic and organic P solubilization considering the fact that P availability is a limiting step in plant nutrition. This evidence suggests a fundamental contribution of P-solubilizing bacteria to plant nutrition and, therefore, to the improvement of plant growth performance. Several reports indicating plant growth promotion by PSMs using various PGPR traits are listed in Table 12.3. However, the intrinsic ability of PSMs for synthesizing the growth-promoting substances varies considerably under different ecological niches.
Biocontrol microorganisms adapt different mechanisms to inhibit plant pathogens. These mechanisms generally involve competition for nutrients, production of bacterial metabolites such as iron-chelating siderophores, etc. (Hussein and Joo 2014). The siderophore hypothesis postulates that PGPR exert their plant growth-promotion activity by depriving pathogen of iron. Siderophores from microorganisms can also induce systemic resistance in plants. In one study, P-solubilizing fungi P. oxalicum showed a strong antibiotic activity against a pathogenic fungus that severely attacks rape seed (Brassica napus) (Lipping et al. 2008). The combination of P-solubilization traits and biocontrol activity of PSMs was shown effective in promoting plant growth both in conventional and stressed soils of different agroecosystems (Jog et al. 2014).
Similarly, the ability of the most common rhizosphere microorganisms to produce cyanide is apparent. Cyanide production in one study was reported to be detrimental to plant growth. However, in a standardized gnotobiotic system, cyanide has been shown to be involved in the suppression of black root rot and several other pathogens like Gaeumannomyces graminis causing diseases in cereals. The contribution of this compound in the disease-controlling ability varies among different species and strains. The effect of enzymes like β-1,3-glucanases, chitinases, acyl homoserine lactones (AHL), ACC deaminase, proteases, cellulases and pectinases produced by PSMs and their role in inhibition of plant pathogens were proved in many studies (Table 12.4).
While the mechanisms of biocontrol activity have been well investigated, those responsible for the plant growth promotion by T. harzianum (a PSF) have not been extensively studied. In one study, Altomare et al. (1999) investigated the capability of T. harzianum T-22 in vitro (with plant growth-promotion and biocontrol activity) to solubilize insoluble minerals including RP. Apart from this, a PSF, P. variabile P16, was observed to increase glucose oxidase (GOD) production in the presence of polysaccharides, which were found to serve as activators of defensive system in this fungus (Petruccioli et al. 1999). In fact, GOD activity can play a significant role in antibiosis in soil environment by the production H2O2 (enzymatically produced) which is cytotoxic to other microorganisms. Biocontrol abilities of some PSMs available are listed in Table 12.4.
12.8 PSM Biofertilizer-Production Technology
It is advised to isolate PSMs from selective soil samples depending on local needs. For example, acid-proficient PSMs are isolated from acidic soils. The visual detection of PSMs is done generally on Pikovskaya’s agar medium amended with TCP or other insoluble phosphates (Pikovskaya 1948). NBRIP and bromophenol blue agar-assisted isolation was later proposed. The presence of clear halos around the colonies indicates P solubilization (qualitative assay). Colonies showing higher zone of solubilization are selected for isolation, but this does not indicate the intrinsic ability of the organism as the property is lost after frequent subcultures. Therefore, isolates are further evaluated in liquid media containing insoluble phosphate by calorimetry and spectrophotometry (quantitative assay) (Morales et al. 2011). The detection of the presence of organic acids in cell-free extracts further confirms the selection of efficient organisms (Bashan et al. 2013). The efficient organisms selected in laboratory are identified by conventional (colony morphology, cell structure, biochemical tests) and molecular methods (PCR, RT-PCR, ARDRA, etc.) (Jaharamma et al. 2009). Greenhouse pot experiments, on selected plant species, using identified PSMs, are to be conducted for confirming increase in soil solution P and uptake by plant. Further thinning of isolates is done by checking the compatibility to indigenous microflora and other adverse environmental factors encountered during the course of fertilization (Bashan et al. 2013). Different inert carrier materials (lignite, talc, vermiculite, etc.) are employed for formulation of the selected PSM inoculants for easy dispersal. The quality evaluation of formulated PSM biofertilizers includes estimation of the number of cells per gram of the carrier material and permitted contamination levels as per country norms. Also, the moisture-holding capacity and shelf life of the final product are estimated. After fulfilling all criteria as PSM inoculants, the fermenter-level mass production followed by carrier formulation and packaging is done for usage in the farmer’s field.
12.9 Reasons for Failure of PSMs in the Field
In the general and more often the initial results obtained from plant experiments carried out in the greenhouse and laboratory contrast with those obtained in the farmer’s field. This inconsistency is primarily due to the lack of fundamental microbiological knowledge. Commercial PSMs often fail, the reasons for which are attributed to several factors (Yarzabal 2010). In the carrier-based formulation, the nature of carrier material, number of cell population per gram and effectiveness of inoculants used play an important role.
The PSM inoculations are made by various methods like seedling root dip, seed coating and spraying. The survival and efficiency of inoculated bacteria depend on its root-colonizing ability. Hence, appropriate methods of application need to be employed. Inoculants used are often non-native and tend to be affected by local environmental factors. More or less, these inoculants show host and locality specificity (Gyaneshwar et al. 1998a). The nature of soil including temperature, moisture, pH and porosity can adversely affect the bacterial P-solubilization ability. Even if the bacteria are an efficient P solubilizer and if it fails to colonize the root, it can lead to solubilized P refixation. Therefore, before using a PSM as inoculants, thorough experimental analysis in field conditions apart from lab and greenhouse conditions is needed. Most of the PSMs are isolated using neutral and unbuffered media, although it is well known that both the acidity and buffering capacity of soils could limit microbially mediated P solubilization (Gyaneshwar et al. 1998a). PSM survival in a microcosm of the field environment should be examined during the initial stages of laboratory testing (Tang et al. 1995).
Antagonism and competition of the introduced PSMs in soil with other indigenous microflora is another factor affecting the P-solubilization efficiency. Introduced PSMs are vulnerable to reduced ability to survive, multiply and colonize in the rhizosphere. They are prone to competition for nutrients and predation with other microflora. These factors rapidly affect the introduced and native PSMs in soil. Hence, the selection of native and endemic efficient isolates is needed to harness maximum benefits.
The inoculum size of the added PSMs affects the efficiency and P-solubilizing ability (Lucy et al. 2004). Also in some instances, negative impacts were observed when inoculants were applied in large numbers (Harris et al. 2006). The initial inoculum’s density and appropriate carrier formulation and dispersion determine delivery of the right number of viable cells and rhizosphere survivability.
12.10 Genetics of P Solubilization
Solubilization of mineral phosphates is predominantly by organic acids that is either by pH reduction or by phosphorus-associated cation chelation. Little is known about the genetic basis of the release of organic acids and mineral P-solubilization trait. A better understanding could pave the way for isolation, characterization and manipulation of genes involved in mineral and organic P solubilization. With an aim for the development of PSM strains (with enhanced P-solubilizing ability), few attempts were made to manipulate P-solubilizing genes through genetic engineering and molecular biotechnology followed by their expression in selected rhizobacterial strains (Table 12.5).
The rhizosphere contains a variety of carbon sources in varying amounts. The heterogenous microbial community in the vicinity produces different kinds of organic acids involved in nutrient mobility. Among the various organic acids, gluconic acid derived from direct glucose oxidation has been reported to be the major mechanism of P solubilization by Gram-negative bacteria and certain fungi (Scervino et al. 2011). Biosynthesis of gluconic acid is mediated by oxidative metabolism of glucose by glucose dehydrogenase (GDH) and the reaction requires pyrroloquinoline quinine (PQQ) as a cofactor. The involvement of genetic/biochemical mechanisms for synthesis of GDH-PQQ holozyme varies on constitutive and inducible phenotypes among bacterial species (Goldstein 1994). Glucose, gluconate, mannitol and glycerol are the possible inducers of holozyme activity (Van Schie et al. 1987). Therefore, genes involved in biosynthesis/transport of PQQ can be cloned from various bacteria and transferred to other bacteria (Babu Khan et al. 1995). Many such genes responsible for organic acid synthesis might be involved in P solubilization. Even though a few genes are reported to be involved in P solubilization, the evaluation of actual genetic basis responsible is still remote.
The strategy of introduction of genes in natural rhizosphere-competent bacteria for over-expression of P-solubilization (both organic and inorganic) phenotype was proved successful in a few cases. With this approach, the need for mixing of P solubilizer and N fixer can be avoided by insertion of genes of required trait. For instance, the apo-GDH gene containing Rhizobium can be inserted with genes of PQQ biosynthesis making it an effective PSM in addition to its natural N2-fixing ability. In an another approach, as adopted by Gyaneshwar et al. (1998b), MPS genes are screened directly in target bacteria by over-/under-expression of genes, followed by selection of transformants with MPS ability.
The genetical modification of PSMs has several advantages over transgenic plants owing to their ease of modification, adding several traits (PGPR) in a single organism, and their utilization for several crops instead of engineering crop by crop (Armarger 2002). All the available evidences indicate that the regulation of phosphatic enzymes is a complex process that requires considerable additional research. In any event, the existing knowledge about Enterobacteriaceae phosphatases constitutes a basis for better understanding and for further exploration of rules governing phosphatase expression in soil bacteria. The genes studied in different organisms for P solubilization are listed in Table 12.5.
12.11 Future Prospects and Conclusion
Further investigations are needed for evaluation of PSMs as biological inoculants for sustainable agriculture. As they play an important role in P nutrition of plants, several new stable combinations with other PGPR need to be studied. The biochemical/molecular basis of synergistic interactions within combinations is the subject of future research. The combination of PSMs and AMF proved to help withstand transplant shock in tissue culture plants and agroforestry seedlings are further needed to study in terms of molecular aspects. The response of different crops varies with PSM application basing on prevailing environmental factors that affect their survivability. PSMs resistant to different adverse environmental conditions can benefit plant growth in high temperature, drought, salinity and acidic conditions. The effect of such PSMs in improving antioxidant status of the plants mitigating adverse effects was recently studied (Ali et al. 2009; Xiao et al. 2013a).
Apart from high P-solubilization trait, PSMs with multiple PGP traits (siderophores, HCN, IAA, GA, β-glucanase, ACC deaminase, etc.) could confer better plant growth (Praveen Kumar et al. 2012). Reports on the application and dispersal of added PSMs on the plant and in the soil are very few. Efforts are under way to develop methodology of inoculants’ application as liquid inoculants avoiding conventional carrier-based formulations (Leo Daniel Amalraj et al. 2010). Multiple inoculants with interspecific compatibility, survivability and root-colonizing ability have recently been evaluated in few agroforestry tree species (Kishore et al. 2012).
On the other hand, the genetic manipulation of PSMs to improve P-solubilizing capabilities and introduction of new PGP traits by rDNA technology offer a feasible approach for obtaining improved strains. Cloning of genes involved in the synthesis of organic acids and phosphatases would be the first step in such genetic manipulation programmes. Sub-cloning of these genes into appropriate vectors followed by transfer and expression in target host strains along with some other important traits could result in new and efficient strains. Future research should concentrate on stability and performance of such genetically modified organisms once inoculated in soil. However, the putative risk involved in the release of GMO in soil is a matter of controversy with regard to possible horizontal transfer of inserted DNA to other soil microorganisms.
Several microorganisms are involved in P cycling in soils, but only 1 % can be cultivated under lab conditions, the remaining 99 % remain unstudied. Culture-independent methods developed in the recent past pave the way for the study of microbial ecology of P cycling microbes in soils. Development of PCR techniques making use of nucleic acid composition recently enabled consistent, precise culture media and growth-independent results (Peix et al. 2007) for detecting presence and quantifying expression of target genes in soil and rhizosphere. For example, specific primers are designed based on conserved regions directed at traits such as bacterial phytases (Jorquera et al. 2011).
References
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26:1–20
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Ali SZ, Sandhya V, Grover M, Kishore N, Venkateswar RL, Venkateswarlu B (2009) Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol Fertil Soils 46:45–55
Altomare C, Noevell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphates and micronutrients by the plant growth promoting and biocontrol fungus Trichoderma harzianum rifai. Appl Environ Microbiol 65(7):2926–2933
Anandham R, Indira GP, Madhaiyan M, Sa TM (2008) Potential plant growth promoting traits and bioacidulation of rock phosphate by thiosulfate oxidizing bacteria isolated from crop plants. J Basic Microbiol 48:439–447
Antoun H (2012) Beneficial microorganisms for sustainable use of phosphates in agriculture. Procedia Eng 46:62–67
Armarger N (2002) Genetically modified bacteria in agriculture. Biochimie 84:1061–1072
Azcon R, Medina A, Roldan A, Biro B, Vivas A (2009a) Significance of treated agro-waste residue and autochthonous inoculates (arbuscular mycorrhizal fungi and Bacillus cereus) on bacterial community structure and phytoextraction to remediate soils contaminated with heavy metals. Chemosphere 75:327–334
Azcon R, Peralvarez M, Biro B, Roldan A, Ruiz-Lonsano JM (2009b) Antioxidant activities and metal acquisition in mycorrhizal plants growing in a heavy metal multi-contaminated soil amended with treated lignocellulosic agrowaste. Appl Soil Ecol 41:168–177
Babu-Khan S, Yeo CT, Martin WL, Duron MR, Rogers RD, Goldstein A (1995) Cloning of a mineral phosphate-solubilizing gene from Pseudomonas cepacia. Appl Environ Microbiol 61:972–978
Barea JM, Navare E, Montoya E (1976) Production of plant growth regulators by rhizosphere phosphate-solubilizing bacteria. J Appl Bacteriol 40:129–134
Bashan Y, Kamnev AA, de Bashan LE (2013) A proposal for isolating and testing phosphate-solubilizing bacteria that enhance plant growth. Biol Fertil Soils 49:1–2
Bearden BN, Petersen L (2000) Influence of arbuscular mycorrhizal fungi on soil-structure and aggregate stability. Plant Soil 218:173–183
Bhargava T, Datta S, Ramakrishnan V, Roy RK, Sankaran K, Subrahmanyam YVBK (1995) Virulent Shigella codes for a soluble apyrase: identification, characterization and cloning of the gene. Curr Sci 68:293–300
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Burini JF, Gugi B, Merieau A, Guespin-Michel JF (1994) Lipase and acidic phosphatase from the psychrotrophic bacterium Pseudomonas fluorescens: two enzymes whose synthesis is regulated by growth temperature. FEMS Microbiol Lett 122:13–18
Butterly CR, Bunemann EK, McNeill AM, Baldock JA, Marschner P (2009) Carbon pulses but not phosphorus pulses are related to decrease in microbial biomass during repeated drying and rewetting of soils. Soil Biol Biochem 41:1406–1416
Cabello M, Irrazabal G, Bucsinszky AM, Saparrat M, Schalamuck S (2005) Effect of an arbuscular mycorrhizal fungus, G. mosseae and a rock-phosphate-solubilizing fungus, P. thomii in Mentha piperita growth in a soilless medium. J Basic Microbiol 45:182–189
Chandra S, Choure K, Dubey RC, Maheshwari DK (2007) Rhizosphere competent Mesorhizobium loti mp6 induce root hair curling, inhibit Sclerotinia sclerotiorum and enhances growth of Indian mustard (Brassica campestris). Braz J Microbiol 38:124–130
Chang CH, Yang SS (2009) Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour Technol 100:1648–1658
Chapuis-Lardy L, Le Bayon RC, Brossard M, Lopez-Hernandez D, Blanchart E (2011) Role of soil macrofauna in phosphorus cycling. In: Beunemann E, Oberson A, Frossard E (eds) Phosphorus in action: biological processes in soil phosphorus cycling, vol 26, Soil biology. Springer, Heidelberg
Collavino MM, Sansberro PA, Mroginski LA, Aguilar OM (2010) Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol Fertil Soils 46:727–738
Dalal RC (1977) Soil organic phosphorus. Adv Agron 29:83–117
Duponnois R, Kisa M, Assigbetse K, Prin Y, Thioulouse J, Issartel M, Moulin P, Lepage M (2006) Fluorescent pseudomonads occurring in Macrotermes subhyalinus mound structures decrease Cd toxicity and improve its accumulation in sorghum plants. Sci Total Environ 370:391–400
Evans M (2012) Enhancing nutrient use efficiency. Arab Fertilizer 63:51–53
Figueiredo MVB, Seldin L, Araujo FF, Mariano RLR (2011) Plant growth promoting rhizobacteria: fundamentals and applications. In: Maheshwari DK (ed) Plant growth and health promoting bacteria. Springer, Berlin, pp 21–42
Food and Agriculture Organization of the United Nations (FAO) (2005) The state of food insecurity in the world: eradicating world hunger – key to achieving the Millenium development goals. FAO Corporate Document Repository. http://www.fao.org/docrep/008/a0200e/a0200e00.html. Accessed 20 Nov 2008
Fraga R, Rodriguez H, Gonzalez T (2001) Transfer of the gene encoding the Nap A acid phosphatase of Morganella morganii to a Burkholderia cepacia strain. Acta Biotechnol 21:359–369
Franco-Correa M, Quintana A, Duque C, Suarez C, Rodríguez MX, Barea JM (2010) Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol 45:209–217
Gadd GM, Sayer JA (2000) Fungal transformations of metals and metalloids. In: Lovley DR (ed) Environmental-metal interactions. American Society for Microbiology, Washington, DC, pp 237–256
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56:403–407
Goldstein AH (1994) Involvement of the quinoprotein glucose dehydrogenase in the solubilization of exogenous phosphates by gram-negative bacteria. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphate in microorganisms: cellular and molecular biology. ASM Press, Washington, DC, pp 197–203
Goldstein AH (1995) Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol Agric Hort 12:185–193
Goldstein AH, Krishnaraj PU (2007) Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: what separates a phenotype from a trait? In: Velazquez E, Rodríguez-Barrueco C (eds) First international meeting on Microbial Phosphate Solubilization. Springer Netherlands, pp 203–213
Goldstein AH, Liu ST (1987) Molecular cloning and regulation of a mineral phosphate solubilizing gene from Erwinia herbicola. Biotechnology 5:72–74
Gonzalez-Chavez MDA, Newsam R, Linderman R, Dodd J, Valdez-Carrasco JM (2008) Bacteria associated with the extraradical mycelium of an arbuscular mycorrhizal fungus in an As/Cu polluted soil. Agrociencia 42:1–10
Gunes A, Ataoglu N, Turan M, Esitken A, Ketterings QM (2009) Effects of phosphate-solubilizing microorganisms on strawberry yield and nutrient concentrations. J Plant Nutr Soil Sci 172:385–392
Gyaneshwar P, Kumar GN, Parekh LJ (1998a) Effect of buffering on the phosphate solubilization ability of microorganisms. World J Microbiol Biotechnol 14:669–673
Gyaneshwar P, Kumar GN, Parekh LJ (1998b) Cloning of mineral phosphate solubilizing genes from Synechocystis PCC 6803. Curr Sci 74:1097–1109
Halvorson HO, Keynan A, Kornberg HL (1990) Utilization of calcium phosphates for microbial growth at alkaline pH. Soil Biol Biochem 22:887–890
Hamdali H, Bouizgarne B, Hafidi M, Lebrihi A, Virolle MJ, Ouhdouch Y (2008) Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38:12–19
Hamdali H, Smirnov A, Esnault C, Ouhdouch Y, Virolle MJ (2010) Physiological studies and comparative analysis of rock phosphate solubilization abilities of Actinomycetales originating from Moroccan phosphate mines and of Streptomyces lividans. Appl Soil Ecol 44:24–31
Harris JN, New PB, Martin PM (2006) Laboratory tests can predict beneficial effects of phosphate-solubilizing bacteria on plants. Soil Biol Biochem 38:1521–1526
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60:579–598
Hussein KA, Joo JH (2014) Potential of siderophore production by bacteria isolated from heavy metal: polluted and rhizosphere soils. Curr Microbiol 68(6):717–723
Indiragandhi P, Anandham R, Madhaiyan M, Sa TM (2008) Characterization of plant growth-promoting traits of bacteria isolated from larval guts of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Curr Microbiol 56:327–333
Jaharamma M, Badri Narayanan K, Sakthivel N (2009) Genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads and their simultaneous role in promotion of plant growth and soil health. In: Mahoney CL, Springer DA (eds) Genetic diversity. Nova Science Publishers, Inc., New York, pp 195–212
Jha BK, Pragash MB, Cletus J, Raman G, Sakthivel N (2009) Simultaneous phosphate solubilization potential and antifungal activity of new fluorescent pseudomonad strain, P. aeruginosa, P. plecoglossicida and P. mosselii. World J Microbiol Biotechnol 25:573–581
Jiang C, Sheng X, Qian M, Wang Q (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal-polluted soil. Chemosphere 72:157–164
Jog R, Pandya M, Nareshkumar G, Rajkumar S (2014) Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 160(4):778–788
Johansson J, Paul L, Finlay RD (2004) Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol Ecol 48:1–12
Jones DL, Oburger E (2011) Solubilization of phosphorus by soil microorganisms: in phosphorus in action. Soil Biol 26:169–198
Joo GJ, Kim YM, Kim JT, Rhee IK, Kim JH, Lee IJ (2005) Gibberellins producing rhizobacteria increase endogenous gibberellins content and promote growth of red peppers. J Microbiol 43(6):510–515
Jorquera MA, Crowley DE, Marschner P, Greiner R, Ferna’ndez MT, Romero D, Menezes-Blackburn D, De La Luz Mora M (2011) Identification of β-propeller phytase-encoding genes in culturable Paenibacillus and Bacillus sp. From the rhizosphere of pasture plants on volcanic soils. FEMS Microbiol Ecol 75:163–172
JumpStart®. http://www.bioag.novozymes.com/en/products/canada/biofertility/JumpStart/Pages/default.aspx. Consulted 16 Apr 2012
Kaur G, Sudhakara RM (2014) Role of phosphate-solubilizing bacteria in improving the soil fertility and crop productivity in organic farming. Arch Agron Soil Sci 60(4):549–564
Kaushik BD (1995) Blue green algae for improvement in rice production. Seminar on natural resource management, HAU, Hisar, 11–13 Dec 1995
Khan MR, Khan SM (2001) Biomanagement of Fusarium wilt of Tomato by the soil application of certain phosphate solubilizing microorganisms. Int J Pest Manag 47:227–231
Kiikila O, Pennanen T, Perkiomaki J, Derome J (2002) Organic material as a copper immobilizing agent: a microcosm study on remediation. Basic Appl Ecol 3:245–253
Kim KY, McDonald GA, Jordan D (1997) Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol Fertil Soils 24:347–352
Kim KY, Jordan D, McDonald GA (1998) Enterobacter agglomerans, phosphate solubilizing bacteria, and microbial activity in soil: effect of carbon sources. Soil Biol Biochem 30:995–1003
Kishore N (2007) Formulation evaluation and mass production of multiagent bioinoculants for agroforestry tree nurseries. PhD dissertation, Kakatiya University, Warangal
Kishore N, Ramesh M, Ram reddy S (2012) Evaluation of PGPR traits of some phosphate solubilizing microorganisms associated with four agroforestry tree species. Asian J Microbiol Biotechnol Environ Sci 14(2):193–204
Krishnaraj PU, Goldstein AH (2001) Cloning of a Serratia marcescens DNA fragment that induces quinoprotein glucose-dehydrogenase-mediated gluconic acid production in Escherichia coli in the presence of stationary phase Serratia marcescens. FEMS Microbiol Lett 205:215–220
Kucey RMN (1987) Increased phosphorus uptake by wheat and field beans inoculated with a phosphate solubilizing Penicillium bilaii strain and with vesicular-arbuscular mycorrhizal fungi. Appl Environ Microbiol 55:2699–2703
Kucey RMN (1988) Effect of Penicillium bilaji on the solubility and uptake of P and micronutrients from soil by wheat. Can J Soil Sci 68:261–270
Kumar V, Behl RK, Narula N (2001) Establishment of phosphate solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under greenhouse conditions. Microbiol Res 156:87–93
Kumar KV, Singh N, Behl HM, Srivastava S (2008) Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 72:678–683
Kumar V, Singh P, Jorquera MA (2013) Isolation of phytase producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World J Microbiol Biotechnol 29(8):1361–1369
Leo Daniel Amalraj E, Kishore N, Desai S, Venkateswarlu B (2010) Growth, shelf life and bioefficacy of liquid inoculants (PSB, Azospirillum spp and Azotobacter spp) formulated with polymeric additives. In: Sayyed RZ, Reddy MS, Sharma YR, Reddy KRK, Desai S, Rao VK, Podile AR, Reddy BC, Kloepper JW (eds) Plant growth promotion by rhizobacteria for sustainable agriculture. Scientific Publishers, New Delhi, p 609
Lipping Y, Jiatao X, Daohong J, Yanping F, Guoqing L, Fangcan L (2008) Antifungal substances produced by Penicillium oxalicum strain PY-1-potential antibiotics against plant pathogenic fungi. World J Microbiol Biotechnol 24:909–915
Lopez BR, Tinoco-Ojanguren C, Bacilio M, Mendoz A, Bashan Y (2012) Endophytic bacteria of the rock-dwelling cactus Mammillaria fraileana affect plant growth and mobilization of elements from rocks. Environ Exp Bot 81:26–36
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Anton Leeuw Int J Gen 86(1):1–25
Maliha R, Samina K, Najma A, Sadia A, Farooq L (2004) Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms under in vitro conditions. Pak J Biol Sci 7:187–196
Mba CC (1997) Rock phosphate solubilizing Streptosporangium isolates from casts of tropical earthworms. Soil Biol Biochem 29:381–385
Medina A, Vassileva M, Barea JM, Azcon R (2006) The growth enhancement of clover by Aspergillus-treated sugar beet waste and Glomus mosseae inoculation in Zn contaminated soil. Appl Soil Ecol 33:87–98
Medina A, Jakobsen I, Vassilev N, Azcon R, Larsen J (2007) Fermentation of sugar beet waste by Aspergillus niger facilitates growth and P uptake of external mycelium of mixed populations of arbuscular mycorrhizal fungi. Soil Biol Biochem 39:485–492
Mendes GO, Zafra DL, Vassilev NB, da Silva IR, Ribeiro JI Jr, Costa MD (2014) Biochar enhances Aspergillus niger rock phosphate solubilization by increasing organic acid production and alleviating fluoride toxicity. Appl Environ Microbiol. doi:10.1128/AEM. 00241-14, AEM.00241–14; published ahead of print 7 March 2014
Morales A, Alvear M, Valenzuela E, Castillo CE, Borie F (2011) Screening, evaluation and selection of phosphate-solubilising fungi as potential biofertiliser. J Soil Sci Plant Nutr 11(4):89–103
Murphy JF, Zehnder GW (2000) Plant growth-promoting rhizobacterial mediated protection in tomato against tomato mottle virus. Plant Dis 84(7):779–784
Murty MG, Ladha JK (1987) Differential colonization of Azospirillum lipoferum on roots of two varieties of rice (Oryza sativa L.). Biol Fertil Soils 4(1–2):3–7
Narsian V, Samaha SM, Patel HH (2010) Rock phosphate dissolution by specific yeast. Indian J Microbiol 50:57–62
Narula N, Saharan BS, Kumar V, Bhatia R, Bishnoi LK, Lather BPS, Lakshminarayana K (2005) Impact of the use of biofertilizers on cotton (Gossypium hirsutum) crop under irrigated agroecosystem. Arch Agron Soil Sci 51(1):69–77
Naumova AN, Mishustin EN, Marienko VM (1962) On the nature of action of bacterial fertilisers (Azotobacterin, Phosphobacterin), upon agricultural crops. Bull Acad Sci USSR 5:709–717
Ochoa-Loza FJ, Artiola JF, Maier RM (2001) Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J Environ Qual 30:479–485
Pandey A, Trivedi P, Palini LMS (2006) Characterization of phosphate solubilizing and antagonistic strain of Pseudomonas putida (BO) isolated from sub-alpine location in the Indian central Himalaya. Curr Microbiol 53:102–107
Park KH, Lee CY, Son HJ (2009) Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett Appl Microbiol 49:222–228
Parks EJ, Olson GJ, Brickman FE, Baladi F (1990) Characterization of high performance liquid chromatography (HPLC) of the solubilization of phosphorus in iron ore by a fungus. Indian J Microbiol 5:18–19
Peix A, Velazquez E, Martynez-Molina E (2007) Molecular methods for biodiversity analysis of phosphate solubilizing microorganisms (PSM). In: Velazquez E, Rodrguez-Barrueco C (eds) First international meeting on microbial phosphate solubilization. Springer, Berlin, pp 97–100
Petruccioli M, Federici F, Bucke C, Keshavarz T (1999) Enhancement of glucose oxidase production by Penicillium variabile P16. Enzym Microb Technol 24:397–401
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiology 17:362–370
Poonguzhali S, Madhaiyan M, Sa T (2008) Isolation and identification of phosphate solubilizing bacteria from Chinese cabbage and their effect on growth and phosphorus utilization of plants. J Microbiol Biotechnol 18:773–777
Praveen KG, Kishore N, Leo Daniel Amalraj E, Mir Hassan Ahmed SK, Rasul A, Desai S (2012) Evaluation of fluorescent Pseudomonas spp. with single and multiple PGPR traits for plant growth promotion of sorghum in combination with AM fungi. Plant Growth Regul 67(2):133–140
Quiquampoix H, Mousain D (2005) Enzymatic hydrolysis of organic phosphorus. In: Turner BL, Frossardand E, Baldwin DS (eds) Organic phosphorus in the environment. CAB International, Wallingford, pp 89–112
Rajkumar M, Freitas H (2008) Effects of inoculation of plant growth promoting bacteria on Ni uptake by Indian mustard. Bioresour Technol 99:3491–3498
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr 6+ on the growth of Indian mustard. Chemosphere 62:741–748
Rane MR, Sarode PD, Chaudhari BL, Chincholkar SB (2008) Exploring antagonistic metabolites of established biocontrol agent of marine origin. Appl Biochem Biotechnol 151:665–675
Redding MR, Shatte T, Bell K (2006) Soil-sorption-desorption of phosphorus from piggery effluent compared with inorganic sources. Eur J Soil Sci 57:134–146
Reid RK, Reid CPP, Szaniszlo PJ (1985) Effects of synthetic and microbially produced chelates on the diffusion of iron and phosphorus to a simulated root in soil. Biol Fertil Soils 1:45–52
Reilly TJ, Baron GS, Nano F, Kuhlenschmidt MS (1996) Characterization and sequencing of a respiratory burst inhibiting acid phosphatase from Francisella tularensis. J Biol Chem 271:10973–10983
Reyes I, Bernier L, Simard R, Antoun H (1999) Effect of nitrogen source on solubilization of different inorganic phosphates by an isolate of Penicillium rugulosum and two UV-induced mutants. FEMS Microbiol Ecol 28:281–290
Reyes I, Bernier L, Antoun H (2002) Rock phosphate solubilization and colonization of maize rhizosphere by wild and genetically modified strains of Penicillium rugulosum. Microb Ecol 44:39–48
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability. Plant Physiol 156:989–996
Richardson AE, Hadobas PA, Hayes JE (2001) Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J 25:641–649
Rodriguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21
Rosenberg K, Bertaux J, Krome K, Hartmann A, Scheu S, Bonkowski M (2009) Soil amoebae rapidly change bacterial community composition in the rhizosphere of Arabidopsis thaliana. ISME J 3:675–684
Sattar MA, Gaur AC (1987) Production of auxins and Gibberellins by phosphate dissolving microorganisms. Zbl Mikrobiol 142:393–395
Saxena J, Minaxi, Jha A (2014) Impact of a phosphate solubilizing bacterium and an arbuscular mycorrhizal fungus (Glomus etunicatum) on growth, yield and P concentration in wheat plants. Clean Soil Air Water. doi:10.1002/clen.201300492
Scervino JM, Papinutti VL, Godoy MS, Rodriguez MA, Della Monica I, Recchi M, Pettinari MJ, Godeas AM (2011) Medium pH, carbon and nitrogen concentrations modulate the phosphate solubilization efficiency of Penicillium purpurogenum through organic acid production. J Appl Microbiol 110:1215–1223
Selvakumar G, Mohan M, Kundu S, Gupta AD, Joshi P, Nazim S, Gupta HS (2008) Cold tolerance and plant growth promotion potential of Serratia marcescens strain SRM (MTCC 8708) isolated from flowers of summer squash (Cucurbita pepo). Lett Appl Microbiol 46:171–175
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Walter WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Sharan A, Shikha, Darmwal NS (2008) Efficient phosphorus solubilization by mutant strain of Xanthomonas campestris using different carbon, nitrogen and phosphorus sources. World J Microbiol Biotechnol 24:3087–3090
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2:587
Siddiqui IA, Haque SE, Shaukat SS (2001) Use of rhizobacteria in the control of root rot-root knot disease complex of mungbean. J Phytopathol 149(6):337–346
Sims JT, Pierzynski GM (2005) Chemistry of phosphorus in soil. In: Tabatabai AM, Sparks DL (eds) Chemical processes in soil, SSSA book series 8. SSSA, Madison, pp 151–192
Singh H, Reddy MS (2011) Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils [J]. Eur J Soil Biol 47:30–34
Singh N, Pandey P, Dubey RC, Maheshwar DK (2008) Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (Sarg.) by rhizosphere competent Bacillus subtilis BN1. World J Microbiol Biotechnol 24:1669–1679
Singh P, Kumar V, Agrawal S (2014) Evaluation of phytase producing bacteria for their plant growth promoting activities. Int J Microbiol 2014, Article ID 426483, 7 p. doi:10.1155/2014/426483
Song OR, Lee SJ, Lee YS, Lee SC, Kim KK, Choi YL (2008) Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA 23 isolated from cultivated soil. Braz J Microbiol 39:151–156
Sreenivas C, Narayanasamy G (2009) Role of earthworm (Eisenia fetida) and phosphate solubilising microorganism (Aspergillus awamori) in vermi-phosphocomposting. Res Crops 10:293–300
Syed GD, Damare S (2013) Marine actinobacteria showing phosphate solubilizing efficiency in Chorao Island, Goa, India. Curr Microbiol 66(5):421–427
Tabatabai MA (1994) Soil enzymes In: Weaver RW, Angle JS, Bottomley PS (eds) Methods of soil analysis, part 2. Microbiological and biochemical properties. SSSA Book Series No. 5. Soil Science Society of America, Madison, pp 775–833
Tang WZ, Pasternak JJ, Glick BR (1995) Persistence in soil of the plant growth promoting rhizobacterium Pseudomonas putida GR12–2 and genetically manipulated derived strains. Can J Microbiol 4:445–451
Torriani-Gorini A, Yagil E, Silver S (1993) Phosphate in microorganisms: cellular and molecular biology. ASM Press, Washington, DC, p 427
Touati E, Danchin A (1987) The structure of the promoter and amino terminal region of the pH 2.5 acid phosphatase structural gene (appA) of E. coli: a negative control of transcription mediated by cyclic AMP. Biochimie 69(3):215–221
Van Schie BJ, Hellingwerf KJ, van Dijken JP, Elferink MGL, van Dijl JM, Kuenen JG, Konigns WN (1987) Energy transduction by electron transfer via a pyrrolo-quinoline quinone-dependent glucose dehydrogenase in Escherichia coli, Pseudomonas putida, and Acinetobacter calcoaceticus (var. lwoffii). J Bacteriol 163:493–499
Vassileva M, Serrano M, Bravo V, Jurado E, Nikolaeva I, Martos V, Vassilev N (2010) Multifunctional properties of phosphate solubilizing microorganisms grown on agro industrial wastes in fermentation and soil conditions. Appl Microbiol Biotechnol 85:1287–1299
Wan JHC, Wong MH (2004) Effects of earthworm activity and P-solubilizing bacteria on P availability in soil. J Plant Nutr Soil Sci 167:209–213
Xiao C, Zhang H, Fang Y, Chi R (2013a) Evaluation for rock phosphate solubilization in fermentation and soil–plant system using a stress-tolerant phosphate-solubilizing Aspergillus niger WHAK1. Appl Biochem Biotechnol 169(1):123–133
Xiao CQ, Chi RA, Hu LH (2013b) Solubilization of aluminum phosphate by specific Penicillium spp. J Cent South Univ Technol 20(8):2109–2114
Yandigeri MS, Meena KK, Srinivasan R, Pabbi S (2011) Effect of mineral phosphate solubilization on biological nitrogen fixation by diazotrophic cyanobacteria. Indian J Microbiol 51(1):48–53
Yarzabal LA (2010) Agricultural development in tropical acidic soils: potential and limits of phosphate solubilizing bacteria, chapter 10. In: Dion P (ed) Soil biology and agriculture in tropics. Soil biology, vol 21. Springer, Berlin/Heidelberg, pp 209–233
Yi Y, Huang W, Ying G (2008) Exopolysaccharide: a novel important factor in the microbial dissolution of tricalcium phosphate. World J Microbiol Biotechnol 24:1059–1065
Yu X, Liu X, Zhu TH, Liu GH, Mao C (2012) Co-inoculation with phosphate-solubilizing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur J Soil Biol 50:112–117
Zhang S, Reddy MS, Kloepper JW (2004) Tobacco growth enhancement and blue mold disease protection by rhizobacteria: relation between plant growth promotion and systemic disease protection by PGPR strain 90–166. Plant Soil 262:277–288
Zhang H, Wu X, Li G, Qin P (2011) Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol Fertil Soils 47:543–554
Zhaoa K, Penttinen P, Zhang X, Xiaoling AO, Liu M, Yu X, Chen Q (2014) Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol Res 169(1):76–82
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Kishore, N., Pindi, P.K., Ram Reddy, S. (2015). Phosphate-Solubilizing Microorganisms: A Critical Review. In: Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K. (eds) Plant Biology and Biotechnology. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2286-6_12
Download citation
DOI: https://doi.org/10.1007/978-81-322-2286-6_12
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2285-9
Online ISBN: 978-81-322-2286-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)