Abstract
Brain morphometric abnormalities have been extensively reported in schizophrenia. In this research report, we used a voxel-based morphometry (VBM) approach to identify the effect of various confounding factors on gray matter (GM) volume changes in patients with schizophrenia in comparison to healthy control subjects. Our findings highlight the importance of accounting for all possible confounding factors during study design and analyses, as well as setting appropriate statistical significance thresholds while reporting results in brain morphometric studies of schizophrenia.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Voxel-based morphometry
- Computerized tomography
- Structural magnetic resonance imaging
- GLM ANCOVA analysis using VBM
1 Introduction
Structural brain abnormalities have been widely reported in schizophrenia, using various imaging modalities such as computerized tomography (CT), structural magnetic resonance imaging (sMRI), and diffusion tensor imaging (DTI) [1]. However, other than whole brain volume reductions, no regional brain morphometric abnormality has emerged from these studies to be considered pathognomonic of the disorder. Confounding variables such as age, age of onset [2], illness chronicity [3], medication exposure [4], substance abuse [5], unequal gender distribution [6], or handedness of study samples [7] could contribute to the inconsistent findings, especially when they are not controlled for in the analyses.
Various techniques have been employed to examine brain morphometry in schizophrenia. Voxel-based morphometry (VBM) implemented in the Statistical Parametric Mapping 8 (SPM8) software (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm) is perhaps the most commonly used tool for identifying regional gray matter (GM) differences in schizophrenia by surveying the whole brain. It uses T1-weighted volumetric MRI scans, which are segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) volumes. These tissue maps will be spatially normalized to a standard template. Then, statistical tests will be applied across all voxels in the image, to identify volumetric differences between diseased and control subjects [8]. Thus, VBM being an automated, rater-independent method provides an unbiased whole-brain approach for brain morphometric analysis.
Most studies that have reported significant regional GM reductions in the frontal, temporal, and parietal cortices in schizophrenia have controlled for confounding factors such as demographic characteristics (age and gender) and whole brain volume [total brain volume (TBV) or intracranial volume (ICV)] [9–12]. However, clinical variables such as duration of illness and medication exposure could also affect brain volumes as mentioned above. Thus, inclusion of these clinical variables could be essential for obtaining reliable results from VBM analysis. The aim of the present study is to demonstrate the importance of including the above clinical variables in VBM comparisons between patients with schizophrenia and healthy control subjects. We performed VBM analysis between a sample of patients with schizophrenia and matched healthy control subjects initially using only the demographic (age, gender) and whole brain (TBV) confounding variables and then repeated the VBM analysis with further addition of the above 2 clinical variables (duration of illness, neuroleptic exposure in risperidone equivalents). We hypothesized that the above two VBM analyses would yield different results.
2 Methodology
2.1 Study Sample
The study was carried out at the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, India, with due approval from the NIMHANS Ethics Committee thus conforming to the ethical standards laid down in the 1964 Declaration of Helsinki. The study sample consists of 15 patients diagnosed with schizophrenia. The diagnosis for schizophrenia was ascertained with the Structured Clinical Interview for DSM-IV (Diagnostic Statistical Manual for Mental Disorders-Fourth edition) Axis I Disorders by a Psychiatrist. Eleven out of fifteen patients were not on neuroleptics. The remaining patients were on antipsychotics, the doses of which were converted to “risperidone equivalents” [13, 14]. Healthy control subjects (N = 15) with no neurological or psychiatric history matched for age, gender, and education with the schizophrenia sample were also recruited. Written informed consent was obtained from all participants prior to recruitment into the study.
2.2 Image Acquisition
MRI scans were performed on Siemens Magnetom Skyra 3.0 T scanner using a 20 channel head/neck coil. T1 anatomical images were acquired with Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with a resolution of 1 × 1 × 1 mm3. The image parameters were as follows: field of view = 200 mm, echo time, TE = 2.44 ms, repetition time, TR = 1,900 ms, flip angle = 9°, and bandwidth = 180 Hz/pixel. The GRAPPA technique with an acceleration factor of one was used for the experiment. The total acquisition time was 4 min 26 s. All scans were inspected for motion artifacts and gross pathology by an experienced neuroradiologist (J.S.).
2.3 Voxel-Based Morphometry (VBM)
VBM permits hypothesis-free whole brain, voxel-by-voxel between-group comparisons of GM volumes [15]. In our study, image processing and analysis was done using the VBM8 toolbox (Christian Gaser’sVBM8 toolbox; http://dbm.neuro.uni-jena.de/) of Statistical Parametric Mapping (SPM) version 8 algorithm (www.fil.ion.ucl.ac.uk/spm) running under MATLAB R2012a. The primary step of VBM analysis of MR images involves spatial matching of MRI scans from different individuals to the same stereotactic space, a process known as spatial normalization. This preprocessing step confirms that location in one subject’s MRI corresponds to the same location in other subject’s MRI scan. Inter-subject registration of brain images was done by creating a sample-specific DARTEL template (Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra). A high-dimensional spatial normalization with DARTEL was used to normalize images to the DARTEL template. MR images were segmented to GM, WM, and CSF using prior tissue probability maps. Finally, images were spatially smoothed with a Gaussian kernel of 8 mm full-width-at-half-maximum which makes data conform to the Gaussian field model. The TBVs were calculated as sum of GM and WM volumes.
2.4 Statistical Analysis
For VBM analysis, analysis of covariance (ANCOVA) within the framework of general linear model (GLM) was used to compare between the two groups (schizophrenia and healthy control subjects). In the first step, only structural (TBV) and demographic (age, gender) characteristics were included as covariates, while, in the subsequent analysis, all of the above 3 variables along with the clinical confounding factors, viz., duration of illness and neuroleptic dosage (in risperidone equivalents), were included as covariates. A voxel-level peak threshold of family-wise error correction (FWEv) p < 0.05 was set a priori to indicate significant volumetric differences between the two samples. However, in order to examine trend-level differences between the groups, VBM analyses were also performed using voxel-level peak threshold of false discovery rate correction (FDRv) p < 0.05, cluster-extent-corrected family-wise error correction (FWEc) p < 0.05, as well as at p < 0.001 (uncorrected, extent threshold (k) = 0 voxels) with 3 and 5 covariates, respectively.
3 Results
The mean age for schizophrenia and healthy participants was 29.06 ± 8.71 and 31.36 ± 12.24, respectively. The gender (male/female) ratio for schizophrenia sample was 10:5, and for healthy control subjects, it was 9:6. GM volumetric differences in patients with schizophrenia when compared to healthy control subjects at the different statistical significance thresholds (FWEv p < 0.05; FDRv p < 0.05; FWEc p < 0.05), using 3 (age, gender, TBV) and 5 covariates (age, gender, TBV, duration of illness, neuroleptic dosage), respectively, are given in Table 1.
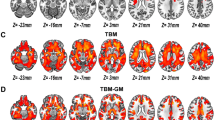
Voxel-based morphometric comparisons across groups at an uncorrected (p < 0.001) statistical threshold showed extensive volumetric reductions in the schizophrenia group when compared to the healthy control group with both 3 and 5 covariates (Figs. 3 and 5). When age, gender, and TBV were considered as covariates, GM volumes were significantly reduced in left inferior frontal gyrus (IFG) and right cerebellar posterior declive in patients with schizophrenia when compared to healthy control subjects after correcting for multiple comparisons using FWEv (p < 0.05) as shown in Fig. 1. At a threshold of FDRv (p < 0.05), significant regional GM volume reductions were observed in left IFG, right cerebellar posterior declive, right insula, right claustrum, and right fusiform gyrus in patients with schizophrenia compared to healthy control subjects as shown in Fig. 2. However, at an uncorrected threshold (p < 0.001; k = 0 voxels), diffuse GM volume deficits were observed in cortical and subcortical regions in schizophrenia patients when compared to healthy control subjects as shown in Fig. 3. When age, gender, TBV, duration of illness, and cumulative drug exposure were considered as covariates, no significant volume differences were observed at FWEv (p < 0.05) or FDRv (p < 0.05) thresholds. But at cluster-wise family-wise error (FWE)-corrected significance thresholding (FWEc; p < 0.05; k = 648 voxels), GM volume deficits in left claustrum and IFG were observed in patients with schizophrenia when compared to healthy control subjects as shown in Fig. 4. At an uncorrected threshold (p < 0.001; k = 0 voxels), widespread GM volume deficits were observed in cortical and subcortical regions in patients with schizophrenia in comparison to healthy control subjects as shown in Fig. 5.
Images depicting gray matter volume reductions in schizophrenia subjects, SZ (n = 15), when compared to healthy control subjects, HCS (n = 15), at a cluster-wise FWE-corrected significance threshold of p < 0.05 and an extent threshold of 648 voxels, with age, gender, TBV, duration of illness, and cumulative medication doses as covariates
Images depicting gray matter volume reductions in schizophrenia subjects, SZ (n = 15), when compared to healthy control subjects, HCS (n = 15), at an uncorrected significance threshold of p < 0.001 and an extent threshold of 0 voxels, with age, gender, total brain volume, duration of illness, and cumulative medication doses as covariates
4 Discussion
The findings of this exploratory study using VBM analyses highlight the variability of morphometric findings in schizophrenia at different statistical significance thresholds with or without inclusion of clinical confounding factors as covariates. When only demographic variables (age, gender) and whole brain volume (TBV) were included as covariates, significant morphometric reductions were noted in patients with schizophrenia even at the most stringent statistical significance threshold of FWEv p < 0.05. However, when clinical confounding factors (duration of illness and neuroleptic exposure) were included as covariates in addition to the above-mentioned covariates, volumetric reductions were noted only at more liberal statistical significance thresholds of FWEc (p < 0.05) and p < 0.001, uncorrected for multiple comparisons.
When age, gender, and TBV were included as covariates in the GLM ANCOVA analysis using VBM, significant volumetric reductions were noted in the left inferior frontal gyrus (IFG) and right cerebellar declive in patients with schizophrenia in comparison to healthy control subjects at the stringent significance threshold of FWEv p < 0.05. At a more liberal threshold of FDRv p < 0.05, more brain regions showed volumetric reductions (Table 1). Even more brain regions showed volumetric reductions at an uncorrected (p < 0.001) significance threshold (Fig. 3). Our findings of volumetric reductions in schizophrenia are in agreement with a large volume of previous morphometric results [9, 10, 16, 17]. However, it is to be noted that the morphometric findings vary according to the statistical significance threshold employed. Moreover, only age, gender, and TBV were entered as covariates in the morphometric analyses.
When the clinical variables (duration of illness and neuroleptic exposure) were entered as covariates in the GLM ANCOVA in addition to age, gender, and TBV, there were no significant volumetric differences at the a priori decided significance threshold of FWEv p < 0.05. Only a trend toward volumetric reductions was noted in the left claustrum and left IFG at FWEc p < 0.05 (Table 1 and Fig. 4), with more brain regions showing trend toward volumetric reductions at a threshold of p < 0.001 (uncorrected) in patients with schizophrenia (Fig. 5).
In this exploratory analysis on a limited sample of patients with schizophrenia and healthy control subjects, the left inferior frontal gyrus seems to show robust volumetric reductions [FWEv p < 0.05 with 3 covariates; FWEc p < 0.05 with 5 covariates, trend] in patients with schizophrenia. Volumetric reduction in left IFG has been reported in many previous studies on patients with schizophrenia [18, 19]. Left IFG encompasses the Broca’s area responsible for word generation, which is the most consistently replicated cognitive dysfunction in schizophrenia [20]. However, it is to be noted that even the left IFG only showed a trend for volumetric reduction at FWEc p < 0.05, when 5 covariates were used in the GLM ANCOVA.
Overall, the results of the study highlight the importance of controlling for confounding variables in morphometric studies of schizophrenia using VBM. In order to obtain reliable results from morphometric studies, researchers should, therefore, control for all the various demographic, clinical, and other variables that might impact brain volumes, both at the sample recruitment stage and at the analysis stage. Another important issue that requires careful consideration is the statistical significance threshold that is used for making inferences regarding morphometric differences.
5 Conclusion
The present study demonstrates how the results of morphometric analyses vary according to the statistical significance threshold employed. Perhaps the inconsistency of previously reported morphometric findings in schizophrenia may reflect the variable extent to which the above methodological issues were given due consideration [21, 22]. Therefore, future morphometric studies need to carefully control for various socio-demographic and clinical confounding variables that affect brain structure and employ appropriate statistical significance thresholds, in order to generate more reliable results.
References
M.E. Shenton, T.J. Whitford, M. Kubicki, Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin. Neurosci. 12, 317–332 (2010)
L. Burke, C. Androutsos, J. Jogia, P. Byrne, S. Frangou, The Maudsley early onset schizophrenia study: the effect of age of onset and illness duration on fronto-parietal gray matter. Eur. Psychiatry. 23, 233–236 (2008)
S.B. Schwarzkopf, S.C. Olson, J.A. Coffman, H.A. Nasrallah, Third and lateral ventricular volumes in schizophrenia: support for progressive enlargement of both structures. Psychopharmacol. Bull. 26, 385–391 (1990)
M.S. Keshavan, W.W. Bagwell, G.L. Haas, J.A. Sweeney, N.R. Schooler, J.W. Pettegrew, Changes in caudate volume with neuroleptic treatment. Lancet 344, 1434 (1994)
J. Borne, R. Riascos, H. Cuellar, D. Vargas, R. Rojas, Neuroimaging in drug and substance abuse part II: opioids and solvents. Top. Magn. Reson. Imaging 16, 239–245 (2005)
J. Barnes, G.R. Ridgway, J. Bartlett, S.M. Henley, M. Lehmann, N. Hobbs, M.J. Clarkson, D.G. MacManus, S. Ourselin, N.C. Fox, Head size, age and gender adjustment in MRI studies: a necessary nuisance. Neuroimage 53, 1244–1255 (2010)
C.D. Good, I. Johnsrude, J. Ashburner, R.N. Henson, K.J. Friston, R.S. Frackowiak, Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 14, 685–700 (2001)
J.L. Whitwell, Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J. Neurosci. 29, 9661–9664 (2009)
R.A. Honea, A. Meyer-Lindenberg, K.B. Hobbs, L. Pezawas, V.S. Mattay, M.F. Egan, B. Verchinski, R.E. Passingham, D.R. Weinberger, H. Callicott, Is gray matter volume an intermediate phenotype for schizophrenia. A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol. Psychiatry 63, 465–474 (2008)
E.M. Meisenzahl, N. Koutsouleris, R. Bottlender, J. Scheuerecker, M. Jäger, S.J. Teipel, S. Holzinger, T. Frodl, U. Preuss, G. Schmitt, B. Burgermeister, M. Reiser, C. Born, H.J. Möller, Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr. Res. 104, 44–60 (2008)
V. Molina, G. Galindo, B. Cortés, A.G. de Herrera, A. Ledo, J. Sanz, C. Montes, J.A. Hernández-Tamames, Different gray matter patterns in chronic schizophrenia and chronic bipolar disorder patients identified using voxel-based morphometry. Eur. Arch. Psychiatry Clin. Neurosci. 261, 313–322 (2011)
D.R. Watson, J.M. Anderson, F. Bai, S.L. Barrett, T.M. McGinnity, C.C. Mulholland, T.M. Rushe, S.J. Cooper, A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav. Brain Res. 227, 91–99 (2012)
S.W. Woods, Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry 64, 663–667 (2003)
R.A. Kroken, E. Johnsen, T. Ruud, T. Wentzel-Larsen, H.A. Jorgensen, Treatment of schizophrenia with antipsychotics in Norwegian emergency wards, a cross-sectional national study. BMC Psychiatry 9, 24 (2009)
J. Ashburner, K.J. Friston, Voxel-based morphometry-the methods. Neuroimage 11, 805–821 (2000)
I. Harvey, M.A. Ron, G. Du Boulay, D. Wicks, S.W. Lewis, R.M. Murray, Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol. Med. 23, 591–604 (1993)
K.O. Lim, W. Tew, M. Kushner, K. Chow, B. Matsumoto, L.E. DeLisi, Cortical gray matter volume deficit in patients with first-episode schizophrenia. Am. J. Psychiatry 153, 1548–1553 (1996)
T. Ohtani, J.J. Levitt, P.G. Nestor, T. Kawashima, T. Asami, M.E. Shenton, M. Niznikiewicz, R.W. McCarley, Prefrontal cortex volume deficit in schizophrenia: A new look using 3T MRI with manual parcellation. Schizophr. Res. 152, 184–190 (2014)
U.S. Torres, E. Portela-Oliveira, S. Borgwardt, G.F. Busatto, Structural brain changes associated with antipsychotic treatment in schizophrenia as revealed by voxel-based morphometric MRI: an activation likelihood estimation meta-analysis. BMC Psychiatry 13, 342 (2013)
A. Szöke, F. Schürhoff, F. Mathieu, A. Meary, S. Ionescu, M. Leboyer, Tests of executive functions in executive functions in first degree relatives of schizophrenia patients: a meta-analysis. Psychol. Med. 35, 771–782 (2005)
C.M. Bennett, G.L. Wolford, M.B. Miller, The principled control of false positives in neuroimaging. Soc Cogn Affect Neurosci. 4, 417–422 (2009)
J.P. Ioannidis, Excess significance bias in the literature on brain volume abnormalities. Arch. Gen. Psychiatry 68, 773–780 (2011)
Acknowledgments
This study was supported by the Cognitive Science Research Initiative (CSI), Department of Science and Technology (DST), Government of India (Grant No. SR/CSI/79/2010).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this paper
Cite this paper
Vijayakumari, A.A. et al. (2015). Exploration of the Effect of Demographic and Clinical Confounding Variables on Results of Voxel-Based Morphometric Analysis in Schizophrenia. In: Suresh, L., Dash, S., Panigrahi, B. (eds) Artificial Intelligence and Evolutionary Algorithms in Engineering Systems. Advances in Intelligent Systems and Computing, vol 324. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2126-5_16
Download citation
DOI: https://doi.org/10.1007/978-81-322-2126-5_16
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2125-8
Online ISBN: 978-81-322-2126-5
eBook Packages: EngineeringEngineering (R0)