Abstract
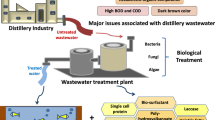
Distilleries are one of the most polluting industries generating enormous amount of wastewater from which an average of 10–15 L of effluent is released with the production of 1 L of alcohol. The distillery wastewater known as spent wash is characterized by its dark brown color, high temperature, low pH, and high percentage of dissolved organic and inorganic matter. It also contains nearly 2 % of the dark brown recalcitrant pigment called melanoidin which imparts dark brown color to the effluent. Various physical, chemical, and alternate treatment methods have been adopted for the removal of color from this wastewater. But these methods only change the form of contaminants rather than degrading them completely.
Biological methods produce relatively little amount of product after treatment by resolving a large amount of organism elements into carbon dioxide to be stabilized, or by removing organic matters contained in wastewater with the generation of methane gas. In the biological treatment methods, pollutants in wastewater can be resolved, detoxified, and separated by using mainly microorganisms. Due to the relatively low cost and the variations of work progress, the biological methods have been most widely used all over the world. A number of fungi, bacteria, yeast, and algae have been reported to have effluent treatment capabilities by the process of absorption, adsorption, and enzymatic degradation techniques. Toxicity studies of the biologically treated wastewaters also suggested that the process is efficient enough to reduce the toxicity of the spent wash by around 80 %. Hence, compared to the common and expensive physical or chemical ways for decolorization, an efficient bioremediation system has been found successful through biosorption and enzymatic ways of decolorization.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Alcohol distilleries in India are one of the most polluting industries; in addition, they are high consumers of raw water. In India, major distilleries are an agro-based industry with around 300 units located mainly in rural, sugarcane-growing regions. The total installed capacity is 3250 million L alcohol per annum with an estimated production of 2300.4 million L in 2006–2007 (Ethanol India 2007). Bioethanol is produced worldwide for beverage, industrial, chemical, and some fuel use, by fermenting agricultural products such as molasses, sucrose-containing juices from sugarcane or sugarbeets, potatoes, fruits, and grains (notably maize, wheat, grain sorghum, barley, and rye). With growing population, industrialization, and energy consumption, coupled with an increasing reliance on fossil fuels, the energy security needs of the world continue to escalate.
2.2 Critical Review
2.2.1 Process of Ethanol Production
Alcohol manufacture in distilleries consists of four main steps, viz., feed preparation, fermentation, distillation, and packaging (Fig. 2.1).
-
a.
Feed Preparation
Ethanol can be produced from a wide range of feedstock. These include sugar-based (cane and beet molasses, cane juice), starch-based (corn, wheat, cassava, rice, barley), and cellulosic (crop residues, sugarcane bagasse, wood, municipal solid wastes) materials. In general, sugar-based feedstock containing readily available fermentable sugars are preferred while Indian distilleries almost exclusively use sugarcane molasses The composition of molasses varies with the variety of cane, the agroclimatic conditions of the region, sugar manufacturing process, and handling and storage (Godbole 2002).
-
b.
Fermentation
Yeast culture is prepared in the laboratory and propagated in a series of fermenters. The feed is inoculated with about 10 % by volume of yeast ( Saccharomyces cerevisiae) inoculum. This is an anaerobic process carried out under controlled conditions of temperature and pH wherein reducing sugars are broken down to ethyl alcohol and carbon dioxide. The reaction is exothermic. To maintain the temperature between 25 and 32 °C, plate heat exchangers are used; alternatively some units spray cooling water on the fermenter walls. Fermentation can be carried out in either batch or continuous mode. Fermentation time for batch operation is typically 24–36 h with an efficiency of about 95 %. The resulting broth contains 6–8 % alcohol. The sludge (mainly yeast cells) is separated by settling and discharged from the bottom, while the cell free fermentation broth is sent for distillation.
-
c.
Distillation
Distillation is a two-stage process and is typically carried out in a series of bubble cap fractionating columns. The first stage consists of the analyzer column and is followed by rectification columns. The cell free fermentation broth (wash) is preheated to about 90 °C by heat exchange with the effluent (spent wash) and then sent to the degasifying section of the analyzer column. Here, the liquor is heated by live steam and fractionated to give about 40–45 % alcohol. The bottom discharge from the analyzer column is the spent wash. The alcohol vapors are led to the rectification column where by reflux action, 96 % alcohol is tapped, cooled, and collected. The condensed water from this stage, known as spent lees is usually pumped back to the analyzer column.
-
d.
Packaging
Rectified spirit (~ 96 % ethanol by volume) is marketed directly for the manufacture of chemicals such as acetic acid, acetone, oxalic acid, and absolute alcohol. Denatured ethanol for industrial and laboratory use typically contains 60–95 % ethanol as well as between 1–5 % each of methanol, isopropanol, methyl isobutyl ketone (MIBK), ethyl acetate, etc. (Skerratt 2004). For beverages, the alcohol is matured and blended with malt alcohol (for manufacture of whisky) and diluted to requisite strength to obtain the desired type of liquor. This is bottled appropriately in a bottling plant. Anhydrous ethanol for fuel-blending applications (power alcohol) requires concentration of the ethanol to > 99.5 wt % purity.
The quantum and characteristics of wastewater generated at various stages in the manufacturing process are provided in Tables 2.1 and 2.2, respectively. The main source of wastewater generation is the distillation step wherein large volumes of dark brown effluent (termed as spent wash, stillage, slop, or vinasse) is generated in the temperature range of 71–81 °C (Yeoh 1997; Nandy et al. 2002; Patil et al. 2003). The characteristics of the spent wash depend on the raw material used (Mall and Kumar 1997), and also it is estimated that 88 % of the molasses constituents end up as waste (Jain et al. 2002).
The spent wash is the most polluting stream and contains practically all unfermentable soluble matter present in the molasses. Apart from the extremely high chemical oxygen demand (COD) and biochemical oxygen demand (BOD) load, the dark color is also a key concern. This dark color is mainly imparted by melanoidins that are low and high molecular weight polymers formed as one of the final products of Maillard reaction, which is a nonenzymatic browning reaction resulting from the reaction of reducing sugars and amino compounds (Martins and van Boekel 2004). This reaction proceeds effectively at temperatures above 50 °C and pH 4–7. These are complex organic compounds, when released in environment without treatment, react with a wide variety of other chemicals in presence of light and heat to form highly toxic and recalcitrant compounds (Kinae et al. 1981; Zacharewski et al. 1995). Thus, it is obligatory to treat the effluent before disposal into the environment.
2.3 Bioremediation
Generally, methods of treating wastewater include physical–chemical methods and biological methods. Methods such as sedimentation, flotation, screening, adsorption, coagulation, oxidation, ozonation, electrolysis, reverse osmosis, ultrafiltration, and nanofiltration technologies have been used for treatment of suspended solids, colloidal particles, floating matters, colors, and toxic compounds (Pokhrel and Viraraghavan 2004). The drawbacks of the physical–chemical methods include high costs and the need to re-treat the products, which further increases the cost of treatment. Biological method produces relatively little amount of product after treatment by resolving a large amount of organism elements into carbon dioxide to be stabilized, or by removing organic matters contained in wastewater with the generation of methane gas. In the biological treatment method, pollutants in wastewater can be resolved, detoxified, and separated by using mainly microorganisms. Due to the relatively low cost and the variations of work progress, the biological methods have been most widely used all over the world.
2.4 Treatment of Distillery Spent Wash
Biological treatment can be divided into aerobic and anaerobic depending on the availability of oxygen. Aerobic treatment involves activated sludge treatment, aerated lagoons, and aerobic biological reactors. Anaerobic filter, upflow sludge blanket (UASB), fluidized bed, anaerobic lagoon, and anaerobic contact reactors are anaerobic processes, that are commonly used to treat distillery mill effluents. Among these treatments one thing is common, use of microbes (Pokhrel and Viraraghavan 2004). A number of fungi, bacteria, yeast, and algae have been reported to have effluent-treatment capabilities.
2.4.1 Decolorization of Effluent by Fungi
In recent years, several basidiomycetes and ascomycetes type fungi have been used in the decolorization of wastewaters from distilleries. Filamentous fungi have lower sensitivity to variations in temperature, pH, nutrients, and aeration, and have lower nucleic acid content in the biomass (Knapp et al. 2001). Coriolus sp. no. 20, in class basidiomycetes, was the first strain for the application of its ability to remove melanoidins from molasses wastewater (Watanabe et al. 1982). Published papers report the use of wide variety of fungi like Aspergillus fumigatus G-2-6 (Ohmomo et al. 1987), Emericella nidulans var. lata (Kaushik and Thakur 2009a), Geotrichum candidum (Kim and Shoda 1999), Trametes sp. (González et al. 2000), Aspergillus niger (Patil et al. 2003), Citeromyces sp. (Sirianuntapiboon et al. 2003), Flavodon flavus (Raghukumar et al. 2004), and Phanerochaete chrysosporium (Thakkar et al. 2006) for decolorization of distillery mill effluent.
White rot fungi is another group of widely exploited microorganism in distillery effluent bioremediation. White rot fungi produce various isoforms of extracellular oxidases including laccases, manganese peroxidases and lignin peroxidase, which are involved in the degradation of various xenobiotic compounds and dyes. Another important mechanism involved in decolorization of the distillery mill effluent by fungi is adsorption.
2.4.2 Decolorization of Effluent by Bacteria
Different bacterial cultures capable of both bioremediation and decolorization of distillery spent wash have been isolated. Different researchers have reported isolation of various bacterial strains acclimatized on higher concentrations of distillery mill effluent. These are Lactobacillus hilgardii (Ohmomo et al. 1988), Bacillus sp. (Kambe et al. 1999; Kaushik and Thakur 2009b), Pseudomonas putida (Ghosh et al. 2002), Bacillus thuringiensis (Kumar and Chandra 2006), and Pseudomonas aeruginosa (Mohana et al. 2007). Some researchers carried out melanoidin decolorization by using immobilized whole cells. These strains were able to reduce significant levels of BOD and COD. The major products left after treatment were biomass, carbon dioxide, and volatile acids.
Besides fungi and bacteria, yeast (Moriya et al. 1990; Sirianuntapiboon et al. 2003) and algae (Valderrama et al. 2002; Kumar and Chandra 2004) have also been utilized widely since long back for biodegradation of complex, toxic, and recalcitrant compounds present in distillery spent wash.
2.4.3 Decolorization of Effluent by Algae
Cyanobacteria are considered ideal for treatment of distillery effluent as they apart from degrading the polymers also oxygenate water bodies, thus reduce the BOD and COD levels. Kalavathi et al. (2001) explored the possibility of using a marine cyanobacterium for decolorization of distillery spent wash and its ability to use melanoidins as carbon and nitrogen source. A marine filamentous, nonheterocystous form Oscillatoria boryana BDU 92181 used the recalcitrant biopolymer melanoidin as nitrogen and carbon source leading to decolorization. The mechanism of color removal is postulated to be due to the production of hydrogen peroxide, hydroxyl anions, and molecular oxygen, released by the cyanobacterium during photosynthesis.
2.5 Role of Bioreactors in Effluent Treatment
-
a.
Anaerobic Reactors
Wastewater treatment using anaerobic process is a very promising reemerging technology, produces very little sludge, requires less energy, and can become profitable by cogeneration of useful biogas (Mailleret et al. 2003). However, these processes have been sensitive to organic shock loadings, low pH, and show slow growth rate of anaerobic microbes resulting in longer hydraulic retention times (HRT). This often results in poor performance of conventional mixed reactors. Biomethanation using biphasic system is most appropriate treatment method for high strength wastewater because of its multiple advantages viz., possibility of maintaining optimal conditions for buffering of imbalances between organic acid production and consumption, stable performance, and higher methane concentration in the biogas produced (Seth et al. 1995). In recent years, the UASB process has been successfully used for the treatment of various types of wastewaters (Lettinga and Hulshoff Pol 1991). Jhung and Choi (1995) performed a comparative study of UASB and anaerobic fixed film reactors for treatment of molasses wastewater. The UASB technology is well suited for high strength distillery wastewaters only when the process has been successfully started up and is in stable operation. However, the conventional UASB reactors showed severe limitations mainly related to mass transfer resistance or the appearance of concentration gradients inside the systems, slow primary startup requiring several weeks, and difficulty in controlling granulation process which depends upon a large number of parameters.
-
b.
Aerobic reactors
Anaerobically treated distillery spent wash still contains high concentrations of organic pollutants and as such cannot be discharged directly. Aerobic treatment of anaerobically treated distillery spent wash has been attempted for the decolorization of the major colorant, melanoidin and for further reduction of the COD and BOD. A large number of microorganisms such as bacteria (pure and mixed culture), cyanobacteria, yeast, fungi, etc. have been isolated in recent years that are capable of degrading melanoidin and ultimately decolorizing the wastewater.
2.6 Enzymatic Processes for Decolorization
A large number of enzymes (e.g., peroxidases, oxidoreductases, cellulolytic enzymes, proteases amylases, etc.) from a variety of different sources have been reported to play an important role in an array of waste treatment applications (Ferrer et al. 1991; Dec and Bollag 1994). Paper and pulp mills, textiles and dye-making industries, alcohol distilleries, and leather industries are some of the industries that discharge highly colored effluents. The ligninolytic system consists of two main groups of enzymes: peroxidases (lignin peroxidases and manganese peroxidases) and laccases (Leonowicz et al. 2001; Arana et al. 2004; Baldrian 2006). Although the enzymatic system associated with decolorization of melanoidin containing wastewater appears to be related to the presence and activity of fungal ligninolytic mechanisms, this relation is as yet not completely understood. Laccase is a multicopper blue oxidase capable of oxidizing ortho- and para diphenols and aromatic amines by removing an electron and proton from a hydroxyl group to form a free radical. These enzymes lack substrate specificity and are thus capable of degrading a wide range of xenobiotics including industrial colored wastewaters. The mechanism of action of these enzymes is as follows:
-
a.
Lignin Peroxidase (LiP)
LiP is a heme-containing glycoprotein, which requires hydrogen peroxide as an oxidant. LiP from different sources was shown to mineralize a variety of recalcitrant aromatic compounds and to oxidize a number of polycyclic aromatic and phenolic compounds (Karam and Nicell 1997).
Fungi secrete several isoenzymes into their cultivation medium, although the enzymes may also be cell wall-bound (Lackner et al. 1991). LiP oxidizes nonphenolic lignin substructures by abstracting one electron and generating cation radicals, which are then decomposed chemically (Fig. 2.2). LiP is secreted during secondary metabolism as a response to nitrogen limitation. They are strong oxidizers capable of catalyzing the oxidation of phenols, aromatic amines, aromatic ethers, and polycyclic aromatic hydrocarbons (Breen and Singleton 1999).
Mechanism of action for lignin peroxidase (LiP). ox oxidized state of enzyme. (Breen and Singleton 1999)
-
b.
Manganese Peroxidase (MnP)
MnP is also a heme-containing glycoprotein which requires hydrogen peroxide as an oxidant. MnP oxidizes Mn(II) to Mn(IIl) which then oxidizes phenol rings to phenoxy radicals, which lead to decomposition of compounds (Fig. 2.3). MnP catalyzes the oxidation of several monoaromatic phenols and aromatic dyes, but depends on both divalent manganese and certain types of buffers. The enzyme requirement for high concentrations of Mn(III) makes its feasibility for wastewater treatment application doubtful (Karam and Nicell 1997). Evidence for the crucial role of MnP in lignin biodegradation are accumulating, e.g., in depolymerization of lignin (Wariishi et al. 1991) and chlorolignin (Lackner et al. 1991), in demethylation of lignin and delignification and bleaching of pulp (Paice et al. 1993), and in mediating initial steps in the degradation of high-molecular mass lignin (Perez and Jeffries 1992).
Fig. 2.3 Mechanism of action for manganese peroxidase (MnP). ox oxidized state of enzyme. (Breen and Singleton 1999)
-
c.
Laccase
Laccase (EC 1.10.3.2, benzenediol:oxygen oxidoreductase) is a multicopper blue oxidase capable of oxidizing ortho- and para-diphenols and aromatic amines by removing an electron and proton from a hydroxyl group to form a free radical. Laccase in nature can be found in eukaryotes as fungi (principally by basidiomycetes), plants, and insects. However, in recent years, there is an increasing evidence for the existence in prokaryotes (Claus 2003). Corresponding genes have been found in gram-negative and gram-positive bacteria Azospirillum lipoferum (Bally et al. 1983), Marinomonas mediterranea (Sánchez-Amat and Solano 1997), and Bacillus subtilis (Martins et al. 2002).
Laccases not only catalyze the removal of a hydrogen atom from the hydroxyl group of methoxy-substituted monophenols, ortho- and para-diphenols, but can also oxidize other substrates such as aromatic amines, syringaldazine, and nonphenolic compounds to form free radicals (Bourbonnais et al. 1997; Li et al. 1999). After long reaction times there can be coupling reactions between the reaction products and even polymerization. It is known that laccases can catalyze the polymerization of various phenols and halogen, alkyl- and alkoxy-substituted anilines (Hoff et al. 1985). The laccase molecule, as an active holoenzyme form, is a dimeric or tetradimeric glycoprotein, usually containing four copper atoms per monomer, bound to three redox sites (Fig. 2.4). The molecular mass of the monomer ranges from about 50–100 kDa. Typical fungal laccase is a protein of approximately 60–70 kDa with acidic isoelectric point around pH 4.0. Several laccase isoenzymes have been detected in many fungal species. Several laccases, however, exhibit a homodimeric structure, the enzyme being composed of two identical subunits with a molecular weight typical for monomeric laccase.
Copper centers of the laccase. (Adapted from Claus 2004)
Application of Laccases
The interest in laccases as potential industrial biocatalysts has particularly increased after the discovery of their ability to oxidize recalcitrant nonphenolic lignin compounds (Li et al. 1999). This capability has later been shown to be generally applicable to a number of biotechnological problems; all of them are related to the degradation or chemical modification of structurally diverse compounds, being either xenobiotic or naturally occurring aromatic compounds. Laccase is currently being investigated by a number of research groups, e.g., with respect to litter mineralization (Dedeyan et al. 2000), dye detoxification, and decolorization (Abadulla et al. 2000; Kaushik and Thakur 2013). Laccases in both free and immobilized form as well as in organic solvents have found various biotechnological applications such as analytical tools—biosensors for phenols, development of oxygen cathodes in biofuel cells, organic synthesis, immunoassays labeling, delignification, demethylation, and thereby bleaching of craft pulp (Bourbonnais and Paice 1992; Bourbonnais et al. 1995) In addition, laccases have also shown to be useful for the removal of toxic compounds through oxidative enzymatic coupling of the contaminants, leading to insoluble complex structures (Wang et al. 2002). Laccase was found to be responsible for the transformation of 2,4,6-trichlorophenol to 2,6-dichloro-1,4-hydroquinol and 2,6-dichloro-1,4-benzoquinone (Leontievsky et al. 2000). Laccases from white rot fungi have been also used to oxidize alkenes, carbazole, N-ethylcarbazole, fluorene, and dibenzothiophene in the presence of hydroxybenzotriole (HBT) and 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as mediators (Niku-Paavola and Viikari 2000; Bressler et al. 2000). An isolate of the fungus Flavodon flavus was shown to be able to decolorize the effluent from a Kraft paper mill bleach plant. F. flavus decolorized several synthetic dyes like azure B, brilliant green, congo red, crystal violet, and Remazol brilliant blue R in low nitrogen medium (Raghukumar 2000). Partial decolorization of two azo dyes (orange G and amaranth) and complete decolorization of two triphenylmethane dyes (bromophenol blue and malachite green) was achieved by cultures of Pycnoporus sanguineus producing laccase as the sole phenoloxidase (Pointing et al. 2000). Laccase purified from Trametes hirsuta, was able to degrade triarylmethane, indigoid, azo, and athraquinonic dyes used in dyeing textiles (Abadulla et al. 2000) as well as 23 industrial dyes (Rodriguez et al. 1999).
2.7 Adsorption-Assisted Decolorization
Several methods for the treatment of colored wastewaters have been proposed in the literature. These include physicochemical treatment processes, chemical oxidation, and biological degradation. Among various physicochemical treatment processes, adsorption has been found to be an attractive technique for the removal of most organic compounds in wastewaters, especially at lower concentrations. Activated carbon has been the most commonly used adsorbent. However, high cost of activation, regeneration, and the disposal of the concentrate from the cleaning cycles pose problems in the use of activated carbon. Hence, the search of new low cost adsorbents has attracted a number of investigators. Several low cost adsorbents like wood, coir pith, coal fly ash, bagasse fly ash (BFA), and coal-fired boiler bottom ash have been used for the treatment of a wide variety of wastewaters.
An efficient, cost-effective, and environmentally friendly technique; biosorption is mainly a physicochemical process involving the use of biological material-live or nonviable, can be used to concentrate and recover or eliminate the pollutants from aqueous solutions. Various workers have investigated the biosorption of various organic pollutants and color from wastewaters (Tsezos and Bell 1989; Fu and Viraraghavan 2001). Biomass of some natural microbial species, including bacteria, fungi, and algae, is capable of removing the different textile dyes by biosorption, biodegradation, or mineralization (Carliell et al. 1995). Some low-cost fungal biomass has been used as biosorbent for the removal of dye and metal ions from or wastewater, which included Trametes versicolor (Bayramoglu et al. 2003), and Corynebacterium glutamicum (Won et al. 2004).
-
a.
Mechanism of Biosorption
According to the dependence on the cell’s metabolism, biosorption mechanisms can be divided into:
-
1.
Metabolism-dependent
-
2.
Nonmetabolism-dependent
-
1.
-
According to the location where the sorbate removed from solution is found, biosorption can be classified as
-
1.
Extracellular accumulation/precipitation
-
2.
Cell surface sorption/precipitation and
-
3.
Intracellular accumulation
Microbial biomass consists of small particles with low density, poor mechanical strength, and little rigidity. This phenomenon is generally based on a set of chemical and physical mechanisms (involving physicochemical interactions such as electrostatic interactions, ion exchange, complexation, chelation, and precipitation) leading to the immobilization of a solute component on the microbial cell wall components. The complexity of the microbial structure implies that there are many ways for the pollutant to be captured by the cells. Biosorption mechanisms are therefore various (physical adsorption, chemical binding of ionic groups, ion exchange, etc.) and in some cases they are still not very well understood (Veglio and Beolchini 1997). Cell surface sorption is a physicochemical interaction, which is not dependent on metabolism. Cell walls of microbial biomass mainly composed of polysaccharides, proteins, and lipids, offer abundant functional groups such as carboxyl, hydroxyl, phosphate, and amino groups, as well as hydrophobic adsorption sites such as aliphatic carbon chains and aromatic rings (Ringot et al. 2005). This physicochemical phenomenon is quick and can be reversible.
Physical Adsorption
If the attraction between the solid surface and the adsorbed molecules is physical in nature, the adsorption is referred to as physical adsorption (physiosorption). Generally, in physical adsorption the attractive forces between adsorbed molecules and the solid surface are van der Waals forces and they being weak in nature result in reversible adsorption. Electrostatic interactions have been demonstrated to be responsible for copper biosorption by bacterium Zoogloea ramigera and alga Chlorella vulgaris (Aksu et al. 1992), and for chromium biosorption by fungi Ganoderma lucidum and Aspergillus niger (Srivastava and Thakur 2006).
Chemical Adsorption
If the attraction forces are due to chemical bonding, the adsorption process is called chemisorption. In view of the higher strength of the bonding in chemisorption, it is difficult to remove chemisorbed species from the solid surface. Aksu et al. (1992) hypothesized that biosorption of copper by C. vulgaris and Z. ramigera takes place through both adsorption and formation of coordination bonds between metals and amino and carboxyl groups of cell wall polysaccharides. Microorganisms may also produce organic acids (e.g., citric, oxalic, gluonic, fumaric, lactic, and malic acids), which may chelate toxic metals resulting in the formation of metalloorganic molecules. These organic acids help in the solubilization of metal compounds and their leaching from the surfaces.
Ion Exchange
Ion exchange is basically a reversible chemical process wherein an ion from solution is exchanged for a similarly charged ion attached to an immobile solid particle. Ion exchange shares various common features along with adsorption, in regard to application in batch and fixed-bed processes and they can be grouped together as ‘‘sorption processes’’ for a unified treatment to have high water quality. Ion exchange has been fruitfully used too for the removal of colors. By far the largest application of ion exchange (Clifford 1999) to drinking water treatment is in the area of softening that is the removal of calcium, magnesium, and other polyvalent cations in exchange for sodium. Various studies have been carried out using ion exchange for the removal of dyes (Liu et al. 2007; Wu et al. 2008). Delval et al. (2005) prepared starch-based polymers by a crosslinking reaction of starch-enriched flour using epichlorohydrin as a crosslinking agent in the presence of NH4OH.
-
b.
Factors Affecting Biosorption
The following factors affect the biosorption process:
-
1.
Temperature seems not to influence the biosorption performances in the range of 20–35 °C (Aksu et al. 1992).
-
2.
pH seems to be the most important parameter in the biosorptive process: it affects the solution chemistry of the metals, the activity of the functional groups in the biomass (Galun et al. 1987).
-
3.
Biomass concentration in solution seems to influence the specific uptake: for lower values of biomass concentrations there is an increase in the specific uptake. Interference in between the binding sites due to increased biomass was suggested as a possible reason (Gadd et al. 1988).
-
1.
-
c.
Biosorption Equilibrium Models
One of the most important characteristics of an adsorbent is the quantity of adsorbate it can accumulate which is usually calculated from the adsorption isotherms. The adsorption isotherms are constant-temperature equilibrium relationship between the quantity of adsorbate per unit of adsorbent (qe) and its equilibrium solution concentration (Ce). Several equations or models are available that describe this function like the Freundlich and the Langmuir equations.
2.8 Future Prospects
The present status described in the chapter has allowed important information on the types of species involved in decolorization and degradation of distillery spent wash in the various lab scale to pilot scale studies but their interaction with the native microbial communities is still being questioned. Future studies should, therefore, focus not only on identification of other communities as observed in denaturing gradient gel electrophoresis (DGGE) band pattern but also their quantification using reliable quantitative methods. Assessment of activity and the interactions between the introduced organisms will also be important for the design and control of biological reactors. Another area of study scope lies with isolated and purified microbial enzymes and the focus lie on investigation of production strategies such as recombinant expression in another organism.
2.9 Conclusion
Ethanol manufacture from molasses based industries generates large volumes of high strength wastewater, which is of serious environmental concern. It is estimated that in a large scale unit approximately 0.2 million L of molasses spent wash (MSW) is generated each day. The main source of wastewater generation is the distillation step wherein large volumes of dark brown effluent (termed as spent wash) is generated in the temperature range of 71–81 °C. This spent wash is dark brown colored polluting stream and contains practically all unfermentable soluble matter apart from the extremely high COD and BOD load. This dark color is mainly imparted by melanoidin, that are low and high molecular weight polymers formed as one of the final products of Maillard reaction. This colored waste stream contains highly toxic and recalcitrant compounds and when released untreated in any nearby water stream causes eutrophication and blocks the sunlight (due to color), ultimately creating a toxic environment to the aquatic biota. Therefore, a comprehensive treatment strategy is required for decolorization and detoxification of distillery spent wash before its disposal into the environment. Compared to the common and expensive physical or chemical ways for decolorization, an efficient bioremediation system has been found successful through biosorption and enzymatic ways of decolorization. However, pollution from distillery effluents is a complex environmental problem; its permanent solution will require comprehensive system considerations as well as multidisciplinary and holistic approaches.
References
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM (2000) Decolorization and detoxification of texile dyes with laccase from Trametes hirsute. Appl Environ Microbiol 66:3357–3362
Aksu Z, Sag Y, Kutsal T (1992) The biosorption copper (II) by C. vulgaris and Z. ramigera. Environ Technol 13:579–586
Arana A, Roda A, Téllez A, Loera O, Carbajo JM, Terrón MC, et al (2004) Comparative analysis of laccase-isozymes patterns of several related Polyporaceae species under different culture conditions. J Basic Microbiol 44:79–87
Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30:215–242
Bally R, Thomas-Bauzon D, Heulin T, Balandreau J, Richard C, De-Ley J (1983) Determination of the most frequent N2 fixing bacteria in the rice rhizosphere. Can J Microbiol 29:881–887
Bayramoglu G, Bektas S, Arica MY (2003) Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater 101:285–300
Bourbonnais R, Paice MG (1992) Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2ʹ-azinobis-(3-ethylbenzthiazoline-6-sulphonate). Appl Environ Microbiol 36:823–827
Bourbonnais R, Paice MG, Reid I, Lanthier P, Yaguchi M (1995) Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2-azinobis (3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol 61:1876–1880
Bourbonnais R, Paice MG, Freiermuth B, Bodie E, Borneman S (1997) Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol 63:4627–1632
Breen A, Singleton FL (1999) Fungi in lignocellulose breakdown and biopulping. Curr Opin Biotechnol 10:252–258
Bressler DC, Fedorak PM, Pickard MA (2000) Oxidation of carbazole, N-ethylcarbazole, fluorene, and dibenzothiophene by the laccase of Coriolopsis gallica. Biotechnol Lett 22:1119–1125
Carliell CM, Barclay SJ, Naidoo N, Buckley CA, Mulholland DA, Senio E (1995) Microbial decolourization of a reactive azo dye under anaerobic conditions. Water SA 21:61–69
Claus H (2003) Laccases and their occurrence in prokaryotes. Arch Microbiol 179:145–150
Claus H (2004) Laccases: structure, reactions, distribution. Micron 35:93–96
Clifford DA (1999) Ion exchange and inorganic adsorption, In: Letterman RD (ed), Water quality and treatment, 5th edn. McGraw-Hill, New York
Dec J, Bollag JM (1994) Use of plant-material for the decontamination of water polluted with phenols. Biotechnol Bioeng 44:1132–1139
Dedeyan B, Klonowska A, Tagger S, Tron T, Iacazio G, Gil G, Petit LJ (2000) Biochemical and molecular characterization of a laccase from Marasmius quercophilus. Appl Environ Microbiol 6:925–929
Delval F, Crini G, Bertini S, Filiatre C, Torri G (2005) Preparation characterization and sorption properties of crosslinked starch-based exchangers. Carbohydr Polym 60:67–75
Ethanol India (2007) A sugar industry perspective & ethanol production (Ethanol Information India). http://www.ethanolindia.net/sugarind.html. Accessed July 2012
Ferrer I, Dezotti M, Durán N (1991) Decolorization of kraft effluent by free and immobilized lignin peroxidase and horseradish peroxidase. Biotechnol Lett 13:577–582
Fu YZ, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. Bioresour Technol 79:251–262
Gadd GM, White C, De-Rome L (1988) Heavy metal and radionuclide by fungi and yeasts. In: Norris PR, Kelly DP (eds) Biohydrometallurgy A. Rowe, Chippenham
Galun M, Galun E, Siegel B, Séller E, Leer H, Siegel S (1987) Removal of metal ions from aqueous solutions by Pencillium biomass: kinetic and uptake parameters. Water Air Soil Pollut 33:359–371
Ghosh M, Ganguli A, Tripathi AK (2002) Treatment of anaerobically digested distillery spentwash in a two-stage bioreactor using Pseudomonas putida and Aeromonas sp. Process Biochem 37:857–862
Godbole J (2002) Ethanol from cane molasses, Fuel Ethanol Workshop, Honululu, Hawaii. http://www.hawaii.gov/dbedt/ert/new-fuel/files/ethanol-workshop/10-Godbole-DOE-HI. Accessed 14 Nov 2002
González T, Terrón MC, Yagüe S, Zapico E, Galletti GC, González AE (2000) Pyrolysis/gas chromatography/mass spectrometry monitoring of fungal-biotreated distillery wastewater using Trametes sp. I-62 (CECT 20197). Rapid Commun Mass Spectrom 14:1417–1424
Hoff T, Liu SY, Bollag JM (1985) Transformation of halogen, alkyl, and alkoxy-substituted anilines by a laccase of Trametes versicolor. Appl Environ Microbiol 49:1040–1045
Jain N, Minocha AK, Verma CL (2002) Degradation of predigested distillery effluent by isolated bacterial strains. Indian J Exp Biol 40:101–105
Jhung JK, Choi E (1995) A comparative study of UASB and anaerobic fixed film reactors with development of sludge granulation. Water Res 29:271–277
Kalavathi DF, Uma L, Subramanian G (2001) Degradation and metabolization of the pigment-melanoidin in distillery effluent by the marine cyanobacterium Oscillatoria boryana BDU 92181. Enzyme Microb Technol 29:246–251
Kambe TN, Shimomura M, Nomura N, Chanpornpong T, Nakahara T (1999) Decolourization of molasses wastewater by Bacillus sp. under thermophilic and anaerobic conditions. J Biosci Bioeng 87:119–121
Karam J, Nicell JA (1997) Potential applications of enzymes in waste treatment. J Chem Technol Biotechnol 69:141–153
Kaushik G, Thakur IS (2009a) Isolation of fungi and optimization of process parameters for decolorization of distillery mill effluent. World J Microbiol Biotechnol 25:955–964
Kaushik G, Thakur IS (2009b) Isolation and characterization of distillery spent wash color reducing bacteria and process optimization by Taguchi approach. Int Biodeterior Biodegrad 63:420–426
Kaushik G, Thakur IS (2013) Biodegradation of synthetic dyes and purification, characterization and Mass spectroscopic analysis of thermotolerant laccase by Bacillus sp. Appl Biochem Microbiol 49:352–359
Kim SJ, Shoda M (1999) Batch decolourization of molasses by suspended and immobilizes fungus of Geotrichum candidum. J Biosci Bioeng 88:586–589
Kinae N, Hashu T, Makita T, Tomita I, Kimura I, Kanamori H (1981) Studies on the toxicity of pulp and paper mill effluents: mutagenicity of the sediment samples derived from kraft paper mills. Water Res 15:17–24
Knapp JS, Vantoch-Wood EJ, Zhang F (2001) Use of wood-rotting fungi for the decolourisation of dyes and industrial effluents. In: Gadd GM (ed) Fungi in bioremediation. British mycological society. Cambridge University Press, Cambridge, p 242
Kumar P, Chandra R (2004) Detoxification of distillery effluent through Bacillus thuringiensis (MTCC 4714) enhanced phytoremediation potential of Spirodela polyrrhiza (L.) Schliden. Bull Environ Contam Toxicol 73:903–910
Kumar P, Chandra R (2006) Decolurisation and detoxification of synthetic molasses melanoidins by individual and mixed cultures of Bacillus spp. Bioresour Technol 97:2096–2102
Lackner R, Srebotnik E, Messner K (1991) Oxidative degradation of high molecular weight chlorolignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun 178:1092–1098
Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas- Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D (2001) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41:185–227
Leontievsky AA, Myasoedova NM, Baskunov BP, Evans CS, Golovleva LA (2000) Transformation of 2,4,6-trichlorophenol by the white rot fungi Panus tigrinus and Coriolus versicolor. Biodegradation 11:331–340
Lettinga G, Hulshoff-Pol LW (1991) UASB process design for various types of wastewaters. Water Sci Technol 24:87–107
Li K, Xu F, Eriksson KEL (1999) Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 65:2654–2660
Liu CH, Wu JS, Chiu HC, Suen SY, Chu KH (2007) Removal of anionic reactive dyes from water using anion exchange membranes as adsorbers. Water Res 41:1491–1500
Mailleret L, Bernard O, Steyer JP (2003) Robust regulation of anaerobic digestion process. Water Sci Technol 48:87–94
Mall ID, Kumar V (1997) Removal of organic matter from distillery effluent using low cost adsorbent. Chem Eng World 32(7):89–96
Martins SIFS, van-Boekel MAJS (2004) A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem 90(1–2):257–269
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277:18849–18859
Mohana S, Desai C, Madamwar D (2007) Biodegradation and decolourization of anaerobically treated distillery spent wash by a novel bacterial consortium. Bioresour Technol 98:333–339
Moriya K, Iefuji H, Shimoi H, Sato S, Tadenuma M (1990) Treatment of distillery wastewater discharged from beet molasses spirits production using yeast. J Ferment Bioeng 69:138–140
Nandy T, Shastry S, Kaul SN (2002) Wastewater management in cane molasses distillery involving bioresource recovery. J Environ Manag 65(1):25–38
Niku-Paavola ML, Viikari L (2000) Enzymatic oxidation of alkenes. J Mol Catal B Enzym 10:435–444
Ohmomo S, Kaneko Y, Sirianuntapiboon S, Somachi P, Atthasampunna P, Nakamura I (1987) Decolorization of molasses wastewater by a thermophilic strain Aspergillus fumigatus G-2-6. Agric Biol Chem 52:3339–3346
Ohmomo S, Daengsabha W, Yoshikawa H, Yui M, Nozaki K, Nakajima T, Nakamura I (1988) Screening of anaerobic bacteria with the ability to decolourize molasses melanoidin. Agric Biol Chem 57:2429–2435
Paice MG, Reid ID, Boubonnais R, Archibald FS, Jurasek L (1993) Manganese peroxidase, produced by Trametes cersicolor during pulp bleaching, demethylates and delignifies kraft pulp. Appl Environ Microbiol 59:260–265
Patil PU, Kapadnis BP, Dhamankar VS (2003) Decolorization of synthetic melanoidin and biogas effluent by immobilized fungal isolate of Aspergillus niger UM2. All India Distiller’s Association (AIDA) Newsletter 53–56
Perez J, Jeffries TW (1992) Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol 58:2402–2409
Pointing SB, Jones EBG, Vrijmoed LLP (2000) Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia 92:139–44
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater: a review. Sci Total Environ 333:37–58
Raghukumar C (2000) Fungi from marine habitats: an application in bioremediation. Mycol Res 104:1222–1226
Raghukumar C, Mohandass C, Kamat S, Shailaja MS (2004) Simultaneous detoxification and decolorization of molasses spent wash by the immobilized white-rot fungus Flavodon flavus isolated from a marine habitat. Enzyme Microb Technol 35:197–202
Ringot D, Lerzy B, Bonhoure JP, Auclair E, Oriol E, Larondelle Y (2005) Effect of temperature on in vitro ochratoxin a biosorption onto yeast cell derivatives. Process Biochem 40:3008–3016
Rodriguez E, Pickard MA, Vazquez DR (1999) Industrial dye decolorization by laccases from ligninolytic fungi. Curr Microbiol 38:27–32
Sánchez-Amat A, Solano F (1997) A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem Biophys Res Commun 240:787–792
Seth R, Goyal SK, Handa BK (1995) Fixed film biomethanation of distillery spentwash using low cost porous media. Resour Conserv Recycl 14:79–89
Sirianuntapiboon S., Zohsalam P., Ohmomo S. 2003. Decolourization of molasses wastewater by Citeromyces sp. WR-43-6. Process Biochem 39:917–924
Skerratt G (2004) European distilleries: an overview. In: Tewari PK (ed) Liquid asset. Proceedings of the Indo-EU workshop on promoting efficient water use in agro-based industries. TERI, New Delhi, pp 1–11
Srivastava S, Thakur IS (2006) Biosorption potency of Aspergillus niger for removal of chromium (VI). Curr Microbiol 53:232–237
Tewari PK, Batra VS, Balakrishnan M (2007) Water management initiatives in sugarcane molasses based distilleries in India. Resour Conserv Recycl 52:351–367
Thakkar AP, Dhamankar VS, Kapadnis BP (2006) Biocatalytic decolourisation of molasses by Phanerochaete chrysosporium. Bioresour Technol 97:1377–1381
Tsezos M, Bell JP (1989) Comparison of the biosorption and desorption of hazardous organic pollutants by live and dead biomass. Water Res 23:561–568
Valderrama LT, Del-Campo CM, Rodriguez CM, Bashan LE, Bashan Y (2002) Treatment of recalcitrant wastewater from ethanol and citric acid using the microalga Chlorella vulgaris and the macrophyte Lemna minuscula. Water Res 36:4185–4192
Veglio F, Beolchini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44:301–316
Wang CJ, Thiele S, Bollag JM (2002) Interaction of 2,4,6-trinitrotoluene (TNT) and 4-amino-2,6-dinitrotoluene with humic monomers in the presence of oxidative enzymes. Arch Environ Contam Toxicol 42:1–8
Wariishi H, Valli K, Gold MH (1991) In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun 176:269–275
Watanabe Y, Sugi R, Tanaka Y, Hayashida S (1982) Enzymatic decolorization of melanoidin by Coriolus sp. No. 20. Agric Biol Chem 46:1623–1630
Won SW, Choi SB, Chung BW, Park D, Park JM, Yun YS (2004) Biosorptive decolorization of reactive orange 16 using the waste biomass of Corynebacterium glutamicum. Ind Eng Chem Res 43:7865–7869
Wu JS, Liu CH, Chu KH, Suen SY (2008) Removal of cationic dye methyl violet 2B from water by cation exchange membranes. J Membr Sci 309:239–245
Yeoh BG (1997) Two-phase anaerobic treatment of cane-molasses alcohol stillage. Water Sci Technol 36:(6-7):441–448
Zacharewski T, Berhane K, Gillesby B (1995) Detection of estrogen. and dioxin-like activity in pulp and paper MI black liquor and effluent using in Vitro recombinant receptor (reporter): I gene assays. Environ Sci Technol 29:2140–2146
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Kaushik, G. (2015). Bioremediation of Industrial Effluents: Distillery Effluent. In: Kaushik, G. (eds) Applied Environmental Biotechnology: Present Scenario and Future Trends. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2123-4_2
Download citation
DOI: https://doi.org/10.1007/978-81-322-2123-4_2
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2122-7
Online ISBN: 978-81-322-2123-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)