Abstract
Acute and chronic liver diseases are major causes of morbidity and mortality worldwide. Presently, orthotropic liver transplant is the only gold standard therapy available for such patients suffering from end-stage liver diseases. Availability of suitable liver for transplantation is very low. Thus, each year many patients die waiting for liver transplantation. Considering these facts, there is need of alternative or bridging therapy that will minimize mortality and morbidity of patients who are on transplant waiting list. Thus, stem cell population capable of differentiating to functional hepatocytes could be potential therapy in regenerating damaged liver. Stem cell sources such as embryonic, fetal, induced pluripotent or adult stem cells, or facilitator cells present in bone marrow-derived stem cells can mediate the regenerative process in the liver. This chapter summarizes the use of different sources of stem cells in regenerating damaged liver and an update on recent advances on the management of liver diseases using stem cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Liver disease is an exceptionally common cause of morbidity and mortality worldwide. The acute and chronic liver diseases are still treated with supportive rather than curative approaches. Orthotropic liver transplantation has so far been the only available therapy for patients with end-stage liver diseases. Unfortunately, the availability of donor organs is limited, and many patients die each year waiting for liver transplants. Prevalence rates and the demand for liver transplantation are rising annually worldwide. In Europe, nearly 5,500 orthotropic liver transplantations (OLTs) are performed [1]. In the UK, active transplantation list in March 2012 was 7,636 patients at the end of March 2012 and 508 patient died waiting for suitable transplant [2]. Similarly, in USA as of October 2013, 123,392 liver transplants had been reported and as of today 16,479 patients are on waiting list [3].
Cell-based therapies with stem cells and their progeny is a promising new approach to this largely unmet medical need. Recent advances in the liver repopulation field and the use of alternative cell sources are under investigation. The major drawbacks and the most important advantages of adult or fetal hepatocytes and hepatocytes generated from different stem cells in the wide range of experimental and clinical applications are published. Even after 40 years of extensive experimental research, none of the procedures represent a “gold standard” in the clinical practice. The achievement of positive outcomes in many experimental and clinical studies involving liver progenitor cells has been handicapped by the limited engraftment of cells that can be of therapeutic significance. If stem cells are to be used as an alternative or bridge to organ transplantation, it is very important to reduce the cell loss during the transplantation, repeated cell infusions, and large numbers of cells for transplantation.
Acute Liver Failure
Acute liver failure (ALF) or fulminant hepatic failure (FHP) is characterized by rapid deterioration of hepatocyte function that leads to hepatic encephalopathy, coagulopathy, cerebral edema, infection, and multiorgan dysfunction syndrome [4]. ALF still has high mortality rate, and orthotropic liver transplantation (OLT) is the only available treatment that gives satisfactory results [4, 5]. However, clinicians face difficulties in making a decision about the transplant due to limited patient history and rapid deterioration of the patient. Thus, delay results in a patient becoming un-transplantable due to other contraindications like multiorgan failure. Urgent OLT has become a standard treatment for ALF patients in USA where survival rates have shown improvement and 1-year survival exceeding 80 % [6].
The common causes of ALF are viral hepatitis, idiosyncratic drug reactions, acetaminophen, and mushroom ingestion [7]. Viral hepatitis B is the most common cause of ALF worldwide, responsible for about 70 % of cases, and it produces significant morbidity and mortality [8, 9]. Another causative factor for ALF in Western world is acetaminophen (APAP) overdose which is more commonly encountered. APAP intake leads to excessive production of its active metabolite N-acetyl-p-benzoquinone imine in the liver, causing depletion of the glutathione stores followed by centrilobular necrosis [10].
However, OLTs have numerous limitations like shortage of donor organs, the high costs, and the lifelong immunosuppressive treatments [11]. Various alternatives to OLT have been evaluated, such as split liver, cross circulation, plasma exchange, hemofiltration, hemodialysis, and hemoperfusion without any significant improvement [12, 13]. The alternatives such as stem cell transplant or artificial liver support systems can be helpful, as bridge to transplant that will increase the availability of suitable donor for patients who would otherwise have died might survive until transplantation.
Safety and efficacy of hepatocyte transplantation procedure has been studied in several animal models of ALF. The galactosamine-induced liver failure is the most commonly used models that include mice, rats, rabbits, guinea pigs, and dogs [14–16]; thioacetamide-induced liver injury in rabbits and rats [17, 18]; other models include complete hepatic devascularization [9, 19] and total [20] or subtotal (95 %) hepatectomy [21]. These models showed replacement of about 1–5 % of total hepatocyte mass, which is the limiting factor for treatment of ALF [22]. In experimental animal models, improved survival rate in ALF was documented by these studies.
Chronic Liver Failure
Chronic liver failure (CLF) death toll is about 1.4 million annually, and nearly 150,000 patients die due to CLF in India [23]. The predominant reasons for CLF were cirrhosis due to hepatitis C, B, and D viral infection, followed by HCC, and alcoholic cirrhosis with or without concomitant infection with HCV. Autoimmune causes include primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), and biliary atresia. Nonalcoholic steatohepatitis (NASH) is associated with diabetes, protein malnutrition, obesity, coronary artery disease, and corticosteroid treatment. Other inherited causes are alpha-1 antitrypsin deficiency, hemochromatosis, Wilson’s disease, galactosemia, and glycogen storage diseases [24].
Stem cell therapy for CLF patients has more limitations than acute or metabolic liver diseases. During the diseases progression, there is a major loss of hepatocytes and abnormal hepatic architecture due to scar formation in the liver.
Study of CLF in animal models is difficult due to lack of suitable animal model that can mimic the human situation. There are toxin-induced animal models such as carbon tetrachloride (CCl4) cirrhosis, phenobarbital, retrorsine, and end-to-side portacaval shunt [25–29]. In the animal experiments, liver toxins were injected to normal liver, and 4 weeks after the discontinuation of liver toxins, animals were subjected to cell therapy. During the experimental studies, different cell types applied intrasplenic, namely, fetal hepatocytes and mesenchymal, stem cells [29], rat or porcine hepatocytes [26], syngeneic rat hepatocytes [27], or immortalized rat hepatocytes [25]. These cell therapy experimental models clearly improved liver function and prolonged survival.
It is believed that at least 20 % of the normal hepatocyte mass is required to carry out normal physiological parameters [30]. Hepatocyte recovery from an average liver is about 2.8 × 1011 hepatocytes, occupying almost 80 % of the total liver volume. Considering the safety of the cell transplantation, only 2.4 × 106 hepatocytes per gram of liver can be transplanted, suggesting replacement of approximately 10 % of functional liver mass. The therapeutic mass of hepatocytes actually required to restore adequate liver function for CLF patient is extremely high and is not possible to transplant into the scar liver or spleen. In this situation, transplantation hepatocytes into other sites will accommodate the large number of therapeutic hepatocyte mass and carryout metabolic function for temporary support.
Types of Stem Cells Used for Liver Failures
Embryonic Stem Cells
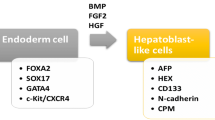
Embryonic stem cells (ES) are capable of generating unlimited hepatocytes, which can be used for transplantation. The study of ES is of great interest to clinicians, but it is also surrounded by controversies about its use. First critical step in generating ES from the definitive endoderm (DE) tissue can be achieved by treating the cultures with TGFβ family ligand activin [31]. Many investigators used DE as starting material to generate hepatocyte-like cells [32–34]. The hepatic maturation can be achieved by the combination of HGF, OSM, FGF, and dexamethasone to expand the hepatoblast population and to promote hepatic maturation. The mouse and human ES cells can be differentiated in to “hepatocyte-like” cells. These cells showed hepatocyte morphology, glycogen storage, express hepatic enzymes, and drug metabolism and secrete albumin [34–39].
Yamamoto et al. and Heo et al. demonstrated mouse ES have potential to differentiate in to hepatocyte-like cells. These cells, when transplanted in to mouse model of liver injury, engrafted and showed improved survival without malignancy [40, 41]. Bin et al. showed the therapeutic effect of embryonic hepatocytes in Wistar rats with CCl4-induced cirrhosis [42]. Transplantation of EC in different liver injury models showed evidence of repopulation of recipient liver; however, undifferentiated ES showed teratogenic property in immunodeficient mice [40, 43].
The major obstacle in the use of ES in the clinic is safety issue and ethical concerns. ECs are capable of unlimited proliferation and able to produce hepatocyte-like cells. They show reasonable functional capacity, although there is no consensus about which functional parameters are necessary for clinical use. ES-derived hepatocytes into animals with various liver injury models showed rescue of liver function, although engraftment of the cells was low.
Fetal Hepatocytes
Human fetal liver (FL) has alternative source of liver progenitor that can be procured from donated tissues after indicated abortions. The FL, between gestation weeks 5–18, contains a large number of actively dividing hepatic progenitor cells that are termed as hepatoblasts. FL cells are committed progenitors, and the development is directed to differentiate to liver cells. FL can be an ideal candidate for cell therapy without concern of teratoma or cancer formation after transplantation.
It has been hypothesized that FL arise from bipotential mesendoderm precursor from mesoderm and endoderm [44]. Cells from the ventral midline of the endoderm lip, probably originating from the mesendodermal prechordal plate, give rise to part of the liver bud [45]. Many studies have shown that mesodermal multipotent adult progenitor cells in both mice and humans can differentiate into hepatocytes [46, 47]. FL tissue obtained during the first trimester mainly exhibits hematopoietic and endothelial markers [48]. During the second trimester development, the hematopoietic cells (HSC) shift towards the hematopoietic cells. Using markers originally expressed on HSC, including c-Kit (CD117) and CD34, can be useful for the identification and isolation of FH. Isolated FH were found to co-express hepatocytic or biliary phenotypic markers implying lineage progression [48]. We have previously isolated and characterized FH from human FL expressing the markers CD34 and CD117 [29, 49, 50]. Flow cytometric and IHC analysis FH in early passages were positive for the hepatic stem cell markers EPCAM, CD133, and CD90, but not CD34 or CD45, indicating the non-hematopoietic origin of these cells. We have confirmed with IHC staining of CK19, CK18, and albumin the committed progenitors for biliary and hepatocytic lineages [29, 49, 50]. The isolation of fetal hepatocytes (FH) consists of mechanical disruptions into small fragment and then incubation with collagenase for digestion of connective tissue. Mechanical disruptions may yield about 104–106 cells per donation (unpublished data). Our own experiments have yielded an average of 5 × 104 cells (data not published) from 12 human fetal tissue isolations (gestation week 5–11) by mechanical disruption (Fig. 1).
There has been fair success in using FH in different animal models. These experiments show successful repopulation of immunodeficient mouse and rat liver with rodent fetal hepatocytes following liver injury [51–53]. In our previous study, we used retrorsine-induced liver injury mouse model, followed by 30 % partial hepatectomy. We infused 2 × 106 intrasplenic injection of hFH and/or MSC. Overall animal survival rates were 100 % during the experiments. We confirmed the presence of engraftment and repopulation in the experimental model by immunohistochemistry and PCR. Immunohistochemical analysis showed cell colonies positive for human albumin, alpha-feto protein, c-met, human hepatocyte antigen, and the human cytokeratins CK 8, 18, and 19. We also detected higher levels of human serum albumin and mouse serum. RT-PCR analysis showed intense expression of the important hepatic factors HNF 1α, β, HNF 4α which provides useful information of persistence hepatocyte function and differentiation [29].
Isolated FH under GMP (good manufacturing practices) compliance can be useful for clinical transplant. In several clinical studies, FH was assessed for infectious diseases such as HCV, HBV, HIV, EBV, HEV, HDV, toxoplasmosis, rubella, cytomegalovirus, parvovirus, herpes simplex type 1 and 2, TPHA, sterility, and endotoxin testing. Therapeutic advantage of FH is useful for a variety of genetic/metabolic liver diseases and acute and chronic liver failure. In recent studies, a significant improvement of all liver serum biochemistry has been achieved after transplantation of FH in a patient with Crigler–Najjar syndrome and biliary atresia [54, 55]. FH isolated from second trimester (EpCAM + cells) and labeled with Tcd, l-hexamethyl-propylene-amine oxime (Tc-HMPAO) were infused into the hepatic artery of 25 patients with end-stage liver cirrhosis. All patients showed marked improvement of clinical and biochemical parameters during 6 months follow-up [23]. In another study, FH was transplanted in a patient with end-stage chronic liver failure. The patient received 2 intrasplenic infusions (5 × 108) on day 0 and 80. The patient’s Model for End-Stage Liver Disease (MELD) score improved from 15 to 10 within the first 18 months of observation [56].
Presently available clinical case reports on use of FH for liver disease give very limited information. This may be due to ethical concerns in getting donors. Larger clinical studies are essential to prove the clinical efficacy of FH.
iPSC
Induced pluripotent stem cells (iPSCs) are adult cells that are genetically reprogrammed to an embryonic-like stem cell. This is achieved by introducing genes and factors important for maintaining the properties of ES. The reprogramming of adult cells into ES cells enables the generation of patient-specific stem cells and thus has enormous potential for the treatment of degenerative diseases.
In 2006, Kazutoshi Takahashi and Shinya Yamanaka first introduced factors for reprogramming of mouse fibroblasts through retroviral transduction with 24 transcription factors highly expressed in ES [57]. Similarly, Park et al. [58] generated iPSCs from human skin through ectopic expression of four genes (Oct3/4, Sox2, c-Myc, and Klf4). Human iPSCs and ES show similar morphologies, proliferation rates, and expression of a number of stem cell markers. However, the specific difference between ESCs and iPSCs is the origin of cell type. iPSCs are derived from adult tissue. In addition, genomic analyses of two types of cells show that hundreds of genes are differentially expressed [59]. Importantly, considering the adult origin, iPSCs can contain an epigenetic “memory” of the donor tissue, which restrict their differentiation capacity and therefore utility [60]. Jang and colleagues generated human iPSCs from a variety of adult human cells, including the liver cells, fibroblasts, bone marrow stem cells, and skin cells [61]. They found that though the iPSCs overall were molecularly similar to each other and to embryonic stem cells, they retained a distinct molecular “signature” inherited from the cell of origin. Regardless of their origin, the different iPSCs showed the same ability to develop into liver cells. A comprehensive study by Miura et al. using various mouse iPSC, has demonstrated that the origin of the iPSC has a profound influence on the tumor-forming propensities in a cell therapy animal model. Mouse tail-tip fibroblast iPSC cells have shown the highest tumorigenic propensity, whereas gastric epithelial cells and hepatocyte iPS cells have shown lower propensities [62].
Human iPSCs can be directed to differentiate into hepatocyte-like cells using different differentiation methods [63–66]. Jozefczuk et al. [67] demonstrated the 80 % similarities in gene expression responsible for normal liver physiology between human ES and iPSC. Hepatocyte-like cells generated from iPSC have been shown to secrete human albumin, synthesize urea, and express human cytochrome P450 enzymes [65]. Espejel et al. [68] demonstrated the iPSC-derived hepatocytes have both the functional and proliferative capabilities needed for liver regeneration in mice with fumarylacetoacetate hydrolase deficiency. Asgari et al. performed a growth factor-mediated differentiation of iPSCs and evaluated their potential for recovery of CCl4-injured mouse liver following transplantation [69]. In another study, transplantation iPSCs are engrafted, integrated, and proliferated in livers of an immune-deficient mouse model. iPSCs secreted human albumin and cells function was similar to primary human hepatocytes, including metabolic function [70].
The recent success in generating iPSC without viral vectors has brought iPSC one step closer to therapeutic application [71]. However, the suitability of individual iPSC for generating cell for therapy needs to be demonstrated. In spite of these, limitations iPSC-derived hepatocytes are a very promising population for cell therapies.
Adult Hepatocytes
Adult hepatocyte (AH) transplantation has led its scientific foundations over 40 years. Transplantation of AH in animals has shown effectiveness in defective hepatic enzymes in metabolic models, improving survival rate in acute hepatic and chronic liver failure [72–79]. Animal models have given insight of mechanism of AH engraftment. However, limitations of animal models reflected on the clinical AH transplant. Animal studies showed that the AH get engrafted in the recipient liver and function normally, even when the engraftment accounted for only 1–5 % of the total hepatocyte mass [22]. This low percentage of engraftment is unlikely to support acute or chronic damage unless a significant liver repopulation is achieved.
The source of AH for the experimental and clinic use is livers discarded for liver transplantation, liver biopsies, or livers after tumor resection [80–82]. Isolation of AH from liver biopsies is quite difficult due to size variations and the paucity of visible vessels available for catheterization. Nationwide study by Baccarani et al.[82] found that organs rejected have steatosis more than 30 %, resulting in reduced hepatocyte yield, decreased viability, and reduced hepatocyte engraftment. Overall cell yield 9.3 × 109 ± 8 × 109 hepatocytes, achieving 7.2 × 106 ± 7 × 106 hepatocytes/g of liver tissue digested with an average viability of 73 % ± 14 % (Figs. 2 and 3).
Over the years, many researchers have attempted to optimize a hepatocyte isolation protocol. Unfortunately, there is no consensus on a single isolation protocol for use in all laboratories, so it is difficult to compare among the outcomes of different studies [83]. AH isolation process starts from surgical removal to transportation to the laboratory and the isolation process. Hepatocytes are exposed to a number of variables that affect their functional ability and viability. The surgical technique of resection, for example, necessitates restriction of blood supply to the organ, leading to warm ischemia and hypoxia of the affected portion of the liver and potentially reducing the viability of hepatocytes. Thus, a protocol that preserves the liver, minimizing the damage to hepatocytes, is essential. Berry and Friend in 1969 demonstrated a protocol involving a two-step collagenase perfusion method. This protocol has become the basis of all current hepatocyte isolation protocol [84]. To standardize the protocol of human AH isolation and functionality testing, European Centre for Validation of Alternative Methods (ECVAM) has recommended the protocol. One of the most debited recommendations by ECVAM is the source of the liver, which will be never accepted by the clinicians.
Furthermore, culture of primary AH is difficult to maintain and cannot be efficiently expanded. Primary AH during the culture looses the metabolic and biotransformation capacity [85, 86]. Cryopreservation of hepatocytes allows the banking of hepatocytes that could be used upon need. Various cryopreservation protocols successfully used in animal experiments when applied human hepatocytes results in loss of 60–65 % hepatocyte viability and 5–90 % attachment rate [30, 87, 88].
Mito et al. in 1993 attempted the first human AH transplantation, till date more than 60 clinical cases have been treated with AH transplantation either to bridge patients to OLT or to improve hepatic metabolic deficiencies [30]. Strom et al. reported the results of AH transplantation into 25 patients diagnosed with acute liver failure from a number of clinical trials in USA. Complete recovery without organ transplantation occurred in 2 patients, 6 were bridged to OLT (within 1–10 days), and the remaining 10 died between 18 h and 52 days after the first hepatocyte transplantation [89, 90]. Fisher et al. reported a 37-year-old woman with FHF who was infused with 8.8 × 108 allogeneic AH into the liver through a catheter placed into the portal vein. This patient fully recovered, with a rapid fall in serum ammonia levels and was discharged from the hospital after 2 weeks [91]. Pareja et al. reported two patients received AH transplant as a bridge to whole-organ retransplantation. Patient already received a liver transplant (LT) in the past, with an end-stage liver disease due to recurrent hepatitis C virus cirrhosis while on the waiting list for an OLT. Both patients showed low blood ammonia levels and clinically improved the degree of hepatic encephalopathy, thus serving as a bridge to liver retransplantation in 1 patient [92].
The clinical use of hepatocyte transplantation is currently limited to support for the inborn errors of metabolism mainly because none of the “hepatocyte-like cells” were able to give metabolic support as AH. Importantly, treatment of acute and chronic liver failures required larger number of hepatocyte dose, which can be useful as temporary bridge to OLT. Successful cryopreservation protocol that could maintain the metabolic capacity after thawing is absolute clinical necessity.
Bone Marrow
Petersen et al. first described the contribution of bone marrow-derived stem cells (BMSC) to liver regeneration [93]. Cell populations in BMSC that contribute to regeneration are hematopoietic stem cells, mesenchymal stem cells, multipotent adult progenitor cells, and very small embryonic-like cells.
Lagasse et al. reported that intravenous injection of adult bone marrow cells in the FAH−/− mouse (animal model of tyrosinemia type I) rescued the mouse and restored the biochemical function of its liver. Moreover, hematopoietic stem cells gave rise to donor-derived hematopoietic and hepatic regeneration [94]. There is increasing evidence in the literature, suggesting BMS transplantation can be useful in liver rescue after acute of chronic injury [93–100]. Jang et al. reported that transplantation of BMS via the portal vein promoted functional improvement in mice with CCl4-induced acute liver injury. Liver function was restored 2–7 days after transplantation and fibrosis reduction was also reported [101]. Shizhu et al. transplanted BMS cells via tail veins of mice. BMSCs were found to populate the damaged liver around the portal and centrilobular regions, and they appeared to differentiate into albumin-producing hepatocyte-like cells. Animals showed toward improved liver enzymes as well as enhanced survival rates [102]. Many investigators showed successful BMSC engraftment in recipient liver, but repopulation was low.
Several clinical trials found that BMSCs were beneficial in the treatment of the patients with end-stage liver failure. Autologous BMSCs transplantation resulted in improvement of liver function tests [103–108]. Gasbarrini et al. reported the use of autologous unsorted BMSCs as rescue treatment for hepatic failure in a 67-year-old man who was ineligible for liver transplantation. Patient showed rapid improvement in hepatic synthetic function after the portal venous infusion of the cells. A liver biopsy performed 20 days after cell transplant was reported to show increased hepatocyte replication around necrotic foci [109]. Salama et al. conducted a study of 90 patients with end-stage liver disease. The patients received G-CSF for 5 days followed by autologous CD34+ and CD133+ stem cell infusion in the portal vein. Study reported that 54 % patient showed near-normal levels of liver enzymes. Couto et al. investigated BMSC therapy in patients with severe liver disease. BMMCs were isolated from autologous bone marrow and 10 % of the cells were labeled with 99mTc-SnCl2. Eight patients received 2.0–15.0 × 108 cells. A patient developed a cutaneous immunomediated disorder, and another patient developed hepatocellular carcinoma (HCC) 12 months after infusion. A reduction in bilirubin was shown at 1 week, while serum albumin increased above baseline up to 1 month after infusion. Couto et al. pointed out that the early improvement of liver function should be interpreted with caution, and controlled studies are needed to determine whether BMMCs infusion affects HCC development in cirrhosis [105].
Spahr et al. reported the feasibility of autologous bone marrow mononuclear cells (BMMNCs) for the treatment of patients with decompensated alcoholic liver disease (ALD). Administration of G-CSF and followed by the CD34+ cells from BMSC showed significant improvement in liver function in many clinical trials [103, 110, 111]. In the study, 58 patients (mean age 54 years, mean MELD score 19, all with cirrhosis, 81 % with alcoholic steatohepatitis at baseline liver biopsy) were randomized. The procedure includes the combination of G-CSF injections and autologous BMMNCs into the hepatic artery. Adverse events were observed in BMNCs and standard medical treatment (SMT) groups. After 3 months, 2 and 4 patients died, respectively, in the BMNCs and standard medical treatment groups, respectively. The MELD score improved in parallel in both groups during follow-up with 18 patients (64 %) from the BMMCT group and 18 patients (53 %) from the SMT group [112].
Vast amount of experimental and clinical data denotes that transplantation of BMSC brings functional outcome into liver parenchyma, either by fusion or transdifferentiation of MSCs, though the amount of fusion or transdifferentiation is extremely low. One of the mechanisms behind transient hepatoprotective effect is different soluble growth factors produced by MSCs. Considering the outcome of many studies, the knowledge of biological properties and plasticity of BMSC is incomplete. In clinical settings, controlled studies are needed to determine the effectiveness of BMMNCs. Presently, 108 clinical trials are going on for the evaluation of therapeutic efficacy of BMSCs for end-stage liver diseases [113]. Overall, accessibility and availability of HSCs for transplant is an attractive tool for the liver regenerative therapies.
Umbilical Cord Blood
Umbilical cord blood (UCB) cells are rich source of hematopoietic stem cells without any ethical concerns. Recent studies on UCB-derived stem cells (UCBSc) showed that these cells are capable of differentiating into adipocytes, osteocytes, chondrocytes, cardiomyocytes, neurons, and hepatocytes in vitro [114].
Newsome et al. demonstrated that human UCBSc could differentiate into hepatocytes after transplantation into immunodeficient mice without evidence of cell fusion. The percentage of human hepatocytes reached an average of 0.011 % after 16 weeks compared with mouse [115]. Kögler et al. reported that UCBSc differentiate into hepatocytes after transplantation into a pre-immune fetal sheep model. In vitro UCBSs lack HLA class II and costimulatory molecule expression [116]. Many experimental animal models of liver injury by carbon tetrachloride (CCl4), 2-acetylaminofluorene (AAF), and the Fas ligand showed that the repopulation of recipient liver by UCBSc is extremely low [117–123]. Two studies do report that UCBSc transplantation significantly reduces the mortality caused by induced liver injury [117, 118]. Burra et al. evaluated the therapeutic potential of UCB-derived mesenchymal stem cells (UCMSCs) in a murine model of acute liver injury using CCl4. UCMSCs-transplanted mice showed a more rapid damage resolution, lower inflammation level, and an increased catalase activity compared to CCl4-treated mice alone [124]. Chen Li demonstrated the therapeutic potential of human umbilical cord matrix stem cells (hUCMSCs) into nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice with CCl4-induced ALF. hUCMSCs were engrafted in to recipient liver and showed the survival benefit and prevented the release of liver injury biomarkers. These data suggest that direct transplantation of native hUCMSCs can rescue ALF and repopulate livers of mice through paracrine effects to stimulate endogenous liver regeneration rather than hepatic differentiation for compensated liver function [125].
Zhang et al. evaluated the therapeutic use of hUCMSCs in 45 chronic hepatitis B patients with decompensated LC. During the study, 30 patients who received hUCMSCs showed significant reduction in the volume of ascites and improved liver function indicated by the increase of serum albumin levels, decrease in total serum bilirubin levels, and decrease in the sodium model for end-stage liver disease scores [126]. Presently, there are 4 ongoing clinical trials (phase I and II) using UCBSc, investigators thus studying the safety and efficacy of UCBSc on patients with liver cirrhosis.
Thus, there will be much excitement about the use of the UCBSc for the treatment of acute and chronic liver diseases. Collation of UCBSc is done by noninvasive methods, and there is no problem in the availability of donor. Importantly, UCBSc can be cryopreserved without major loss during cryopreservation. Furthermore, UCBSc are nonimmunogenic, which make them more suitable for allogeneic transplant.
Route of Administration
There are different approaches used for hepatocyte transplant in animal models. The most accepted approach for hepatocyte transplantation is intrasplenic transplantation. Hepatocytes injected in splenic pulp migrate through hepatic artery in liver parenchyma. The flow of the injection helps hepatocyte to invade the sinusoidal boundaries and entrap in vascular spaces [127]. Entrapped hepatocytes can integrate and proliferate into hepatic lobule. During intrasplenic transplantation, about 40 % of hepatocytes are entrapped in splenic pulp, and this serves as extra hepatic site for hepatocytes. The hepatocytes showed synthetic, metabolic, and biliary transport function [128] (Figs. 4 and 5).
Major limiting factors in using intrasplenic or intraportal route are portal vein thrombosis, portal hypertension, and pulmonary embolism due to transplanted cells [129]. Importantly, there are limitation of cellular dose that can be transplanted single time; thus, repetitive infusion of cells is not possible. However, portal hypertension usually resolves within hours after transplantation, and other measures taken to reduce these complications are by hepatic artery ligation to decrease the sinusoidal blood flow prior to hepatocytes infusion or the slow infusion of hepatocytes over a longer period [119, 130]. In our studies, we have used 70 % partial hepatectomy to mouse model of liver injury followed by 2 × 106 fetal hepatocytes. During the experiments, we have seen portal hypertension and lung embolism as a result of cell infusion. We could achieve survival rate of 50 % (unpublished data). Therefore, to overcome the limitation, we followed 30–40 % partial hepatectomy followed by cell infusion. From this modification, we could get 100 % survival. We have observed transient hypertension due to lung embolism, but it was transient [29].
Presently, different alternative sites to encourage hepatocyte attachment, proliferation, and survival are under investigation. In several animal studies, cells were directly injected into the liver parenchyma to avoid cell loss during the transplantation. But transplanted cells were observed in central veins indicating an increased risk of lung embolism [131–133]. Therefore, in the clinical setting, direct injection of cells into the liver may not be feasible. Ectopic hepatocyte transplantation is defined as a transplantation site for hepatocytes other than the liver or spleen. Cell therapy directed towards the liver may not be feasible in cirrhotic and fibrotic liver during end-stage disease. Thus, transplantation of cells in ectopic sites may give temporary relief for the metabolic and synthetic stress on hepatocytes. Researchers have evaluated different ectopic sites for hepatocyte transplant, including the intraperitoneal cavity, pancreas, mesenteric leaves, intrapulmonary artery, lung parenchyma, kidney capsule, interscapular fat pads, and lymph nodes [134–141]. Unfortunately, transplanted cells survive for a short time. The specific reason for short-term survival of cells is unknown; it is likely due to lack of liver specific growth factors or lack of neovascularization. To overcome this limitation, the use of growth factors, like SDF-1, HGF, EGF, or VEGFR, can play a crucial role for initial support for hepatocyte survival and proliferation. Yokoyama et al. injected fibroblast growth factor (bFGF) in subcutaneous space followed by transplantation of rat and mouse hepatocytes by providing a polyethylene terephthalate matrix. Transplanted hepatocytes survived from 4–8 months and retained their albumin synthetic and drug metabolizing capacity. Furthermore, the authors were able to transplant ten times the usual number of hepatocytes into the subcutaneous cavity [136].
Future Strategies
Stem cell therapy holds promises for acute and chronic liver diseases, but extensive research for the last 2 decades could not improve the outcome. Major limitation of the therapy is the availability of good quality hepatocytes for transplantation. Typically, hepatocytes are complex cells able to do multiple metabolic and secretary functions. The term “hepatocyte-like cells” fails to show major metabolic and secretory characteristics of adult hepatocyte [30]. There are ongoing effects to make “hepatocyte-like cells” more functional like adult hepatocytes. One of the important problem clinicians face during the fibrotic or chronic liver failure is the availability of space to accommodate functional hepatocyte. To overcome this issue, study of ectopic sites where large number of functional hepatocytes can be transplanted without any other complication is ideal need to cell therapy. Considering the variables in the pathogenesis of acute and chronic liver diseases, it is highly important to tailor made the therapies to specific patient using different stem cells (ES, iPSCs, FH, BMMNCs, CBSCs).
Development in the strategies of generating hepatocytes, banking of hepatocytes, hepatocyte engraftment, therapeutic cell number, and functionality of hepatocytes will change the present scenario of liver-related cell transplant.
Conclusion
Available data on clinical hepatocyte transplantation indicated the limitations of cell therapy. Realistically, cell therapy can be advantageous to acute liver failure or metabolic support where therapeutic cell dose is less as compared to chronic liver failure. Considering the limitations on availability and storage of adult hepatocytes, alternative sources of hepatocytes generated from stem cell can be available for treatment. There are numerous advantages of stem cells generated hepatocyte transplant: (1) unlimited supply of hepatocytes; (2) cryopreservation and banking of hepatocytes; (3) complete metabolic and secretory profile; (4) noninvasive treatment, without much hospitalization, and economical; (5) no need for posttransplant medication or immunosuppressant.
Many of the stem cell sources such as iPSCs, FH, BMMSCs can be alternative sources of hepatocytes and should be evaluated in larger clinical study. The present experimental and clinical studies demonstrate the advantages and limitations of each cell types used in therapy. Considering the information, revised clinical trials can be conducted in larger study groups; and this will take hepatocyte transplant in to clinical reality.
References
Evolution of liver transplantations in Europe from 05/1968 to 12/2009. European Liver Transplant Registry. Available from: http://www.eltr.org
NHS Blood and Transplant (2012) Organ donation and transplantation: activity report 2011/12. http://www.organdonation.nhs.uk/statistics/transplant_activity_report/archive_activity_reports/pdf/ukt/activity_report_2011_12.pdf
Organ Procurement and Transplantation Network (2014) Waiting list candidates: liver. transplants: liver. Organ Procurement and Transplantation Network [Web site]. Accessed Jan 2014
Meier M, Woywodt A, Hoeper MM, Schneider A, Manns MP, Strassburg CP (2005) Acute liver failure: a message found under the skin. Postgrad Med J 81:269–270
Rajiv J (2005) Acute liver failure: current management and future prospects. J Hepatol 42(Suppl 1):S115–S123
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH et al (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137:947–954
Ezzat T, Dhar DK, Malago M, Olde Damink SW (2012) Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol 18(6):507–516
Lee WM (2008) Etiologies of acute liver failure. Semin Liver Dis 28:142–152
van den Broek MA, van Dam RM, Malagó M, Dejong CH, van Breukelen GJ, OldeDamink SW (2009) Feasibility of randomized controlled trials in liver surgery using surgery-related mortality or morbidity as endpoint. Br J Surg 96:1005–1014
Martin-Murphy BV, Holt MP, Ju C (2010) The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett 192:387–394
Fiegel HC, Lange C, Kneser U, Lambrecht W, Zander AR, Rogiers X, Kluth D (2006) Fetal and adult liver stem cells for liver regeneration and tissue engineering. J Cell Mol Med 10(3):577–587
Chamukean RAFM (1997) Hepatocyte transplantation for acute hepatic failure. In: Mito M, Sawa M (eds) Hepatocyte transplantation. Karger Landes Systems, New York, pp 157–167
Di Campli C, Nestola M, Piscaglia AC et al (2003) Cell-based therapy for liver diseases. Eur Rev Med Pharmacol Sci 7(2):41–44
Patil PB, Begum S, Joshi M et al (2014) Phenotypic and in vivo functional characterization of immortalized human fetal liver cells. Scand J Gastroenterol 49(6):705–714
Zenoroli MI (1985) Hepatic encephalopathy. Experimental studies in a rat model of fulminant hepatic failure. J Hepatol 2:301–312
Dixit V, Chang TMS (1990) Brain edema and the blood brain barrier in galactosamine-induced fulminant hepatic failure rats. An animal model for evaluation of liver support systems. ASAIO Trans 36:21–27
Peeling J, Schoemaker L, Gauthier T (1993) Cerebral metabolic and histologic effects of thiocetamide-induced liver failure. Am J Physiol 265:G572–G578
Yurdaydin C, Hortnagl H, Steindl P (1990) Increased serotoninergic and noradrnergic activity in hepatic encephalopathy in rats with thioacetamid-induced acute liver failure. Hepatology 12:695–700
Maziotti A, Bernardi M, Antonini L et al (1981) Plasma amino acid patterns in experimental acute hepatic failure: comparison between hepatectomy and liver devascularization in pigs. Surgery 90:527–534
Vogels BAPM, Maas MAW, Bosma A et al (1996) Significant improvement of survival by intrasplenic hepatocyte transplantation in totally hepatactomised rats. Cell Transplant 5:369–378
Roger V, Balladur P, Honiger J et al (1995) A good model of experimental acute hepatic failure: 95 % hepatectomy; treatment by transplantation of hepatocytes. Transplant Proc 27:2504–2505
Gupta S, Bhargava KB, Novikoff PM (1973) Hepatocyte transplantation: emerging insights into mechanisms of liver repopulation and their relevance to potential therapies. J Hepatol 30:162–170
Khan AA, Shaik MV, Parveen N et al (2010) Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant 19:409–418
2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008 (2010) U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation
Cai J, Ito M, Nagata H, Westerman KA, LaFleur D, Chowdhury JR et al (2002) Treatment of liver failure in rats with end-stage cirrhosis by transplantation of immortalized hepatocytes. Hepatology 36:386–394
Nagata H, Ito M, Cai J, Edge AS, Platt JL, Fox IJ (2003) Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology 124:422–431
Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM et al (2000) Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology 31:851–857
Lauterbourg BH, Sautter V, Preising R et al (1976) Hepatic functional deterioration after portacaval shunt rat. Gastroenterology 71:221–227
Joshi M, Patil PB, He Z et al (2012) Fetal liver derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy 14(6):657–669
Akhter J, Johnson LA, Morris DL (2009) Hepatocyte transplantation: a new approach to treat liver disorders. In: Dionigi R (ed) Recent advances in liver surgery. Landes Bioscience, Austin, pp 331–351
Yasunaga MT, Nishikawa ST, Nakano S et al (2005) Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol 23:1542–1550
Lavon N, Yanuka O, Benvenisty N (2004) Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation 72:230–238
Cai J, Zhao Y, Liu Y et al (2007) Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 45:1229–1239
Oertel M, Shafritz DA (2008) Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta 1782:61–74
Dan YY, Yeoh GC (2008) Liver stem cells: a scientific and clinical perspective. J Gastroenterol Hepatol 23:687–698
Agarwal S, Holton KL, Lanza R (2008) Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells 26:1117–1127
Hay DC, Zhao D, Ross A et al (2007) Direct differentiation of human embryonic stem cells to hepatocyte-like cells exhibiting functional activities. Cloning Stem Cells 9:51–62
Zhao CJ, Liu Y, Ye Y et al (2007) Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology 45:1229–1239
Baharvand H, Hashemi SM, Shahsavani M (2007) Differentiation of human embryonic stem cells into functional hepatocyte-like cells in a serum-free adherent culture condition. Differentiation 76(5):465–477
Yamamoto H, Quinn G, Asari A et al (2003) Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology 37:983–993
Heo J, Factor VM, Uren T et al (2006) Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology 44:1478–1486
Bin WT, Ma LM, Xu Q (2012) Embryonic hepatocyte transplantation for hepatic cirrhosis: efficacy and mechanism of action. World J Gastroenterol 28(4):309–322
Choi D, Oh HJ, Chang UJ et al (2002) In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant 11:359–368
Rodaway A, Patient R (2001) Mesendoderm: an ancient germ layer? Cell 105:169–172
Tremblay KD, Zaret KS (2005) Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol 280:87–99
Schwartz RE, Reyes M, Koodie L et al (2002) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 109:1291–1302
Lee KD, Kuo TK, Whang-Peng J et al (2004) In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 40:1275–1284
Nava S, Westgren M, Jaksch M (2005) Characterization of cells in the developing human liver. Differentiation 73:249–260
Nowak G, Ericzon BG, Nava S et al (2005) Identification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivo. Gut 54:972–979
Begum S, Joshi M, Ek M et al (2009) Characterization and engraftment of long-term serum-free human fetal liver cell cultures. Cytotherapy 24:201–211
Mahieu-Caputo D, Allain JE, Branger J et al (2004) Repopulation of athymic mouse liver by cryopreserved early human fetal hepatoblasts. Hum Gene Ther 15:1219–1228
Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA (2001) Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am J Pathol 159:1323–1334
Dabeva MD, Petkov PM, Sandhu J et al (2000) Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol 156:2017–2031
Khan AA, Parveen N, Mahaboob VS et al (2008) Treatment of Crigler-Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant Proc 40:1148–1150
Khan AA, Parveen N, Mahaboob VS et al (2008) Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: a case report. Transplant Proc 40:1153–1155
Gridelli B, Vizzini G, Pietrosi G et al (2012) Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl 18:226–237
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Park IH, Zhao R, West JA et al (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146
Chin MH, Mason MJ, Xie W et al (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5:111–123
Kim K, Doi A, Wen B et al (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467:285–290
Liu H, Ye Z, Kim Y et al (2010) Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology 51(5):1810–1819
Miura K, Okada Y, Aoi T et al (2009) Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27:743–745
Sullivan GJ, Hay DC, Park IH et al (2010) Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology 51:329–335
Rashid ST, Corbineau S, Hannan N et al (2010) Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest 120:3127–3136
Si-Tayeb K, Noto FK, Nagaoka M et al (2010) Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51:297–305
Zhou WL, Medine CN, Zhu L et al (2012) Stem cell differentiation and human liver disease. World J Gastroenterol 18(17):2018–2025
Jozefczuk J, Prigione A, Chavez L et al (2011) Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev 20:1259–1275
Espejel S, Roll GR, McLaughlin KJ et al (2010) Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest 120(9):3120–3126
Asgari S, Moslem M, Bagheri-Lankarani K et al (2013) Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells. Stem Cell Rev Rep 9(4):493–504
Ma X, Duan Y, Tschudy-Seney B, Roll G et al (2013) Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl Med 2(6):409–419
Huang P, He Z, Ji S et al (2011) Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475:386–389
Rajvanshi P, Kerr A, Bhargava KK et al (1996) Studies of liver repopulation using the dipeptidyl peptidase IV – deficient rat and other rodent recipients: cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology 23:482
Groth CG, Arborgh B, Bjorken C et al (1977) Correction of hyperbilirubinemia in the glucuronyltransferase-deficient rats by intraportal hepatocyte transplantation. Transplant Proc 9:313–316
Yoshida Y, Tokusashi Y, Lee GH et al (1996) Intrahepatic transplantation of normal hepatocytes prevents Wilson’s disease in Long—Evans cinnamon rats. Gastroenterology 11:1654–1660
De Vree JM, Ottenhoff R, Bosma PJ et al (2000) Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology 119:1720–1730
Michel JL, Rabier D, Rambaud C et al (1993) Intrasplenic transplantation of hepatocytes in spf-ash mice with congenital ornithine transcarbamylase deficiency. Chirurgie 119:666–671
Wiederkehr JC, Pollak R (1990) Hepatocyte transplantation for the low-density lipoprotein receptor-deficient state. A study in the Watanabe rabbit. Transplantation 50:466–471
Sugiyama K, Emori T, Nagase S (1982) Synthesis and secretion of plasma proteins by isolated hepatocytes of analbuminemic rats. J Biochem 92:775–779
Gagandeep S, Sokhi R, Slehria S et al (2000) Hepatocyte transplantation improves survival in mice with liver toxicity induced by hepatic over expression of Mad 1 transcription factor. Mol Ther 1:358–365
David P, Viollon C, Alexandre E et al (1998) Metabolic capacities in cultured human hepatocytes obtained from non-wedge small liver biopsies. Hum Exp Toxicol 17:544–553
Ballet F, Bouma M, Wang S et al (1984) Isolation, culture and characterization of adult human hepatocytes from surgical liver biopsies. Hepatology 4:849–854
Baccarani U, Sanna A, Cariani A et al (2003) Isolation of human hepatocytes from livers rejected for liver transplantation on a national basis: results of a 2 year experience. Liver Transpl 9:506–512
Richert L, Alexandre E, Lloyd T et al (2004) Tissue collection, transport and isolation procedures required to optimize human hepatocyte isolation from waste liver surgical resections. A multi-laboratory study. Liver Int 24:371–378
Berry MN, Friend DS (1969) High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol 43:506–520
de Sousa G, Dou M, Barbe D et al (1991) Freshly isolated or cryopreserved human hepatocytes in primary culture: influence of drug metabolism on hepatotoxicity. Toxicol In Vitro 5:483–486
Ruegg CE, Silber PM, Mughal RA (1997) Cytochrome P-450 induction and conjugated metabolism in primary human hepatocytes after cryopreservation. In Vitro Toxicol 10:217–222
Chesne C, Guillouzo A (1988) Cryopreservation of isolated rat hepatocytes: a critical evaluation of freezing and thawing conditions. Cryobiology 25:323–330
Ostrowska A, Bode CD, Pruss J et al (2000) Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes. Cell Tissue Bank 1:55–68
Strom SC, Chowdhury JR, Fox IJ (1999) Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 19:39–48
Strom SC, Fisher RA, Rubinstein WS et al (1997) Transplantation of human hepatocytes. Transplant Proc 29:2103–2106
Fisher RA, Bu D, Thompson M et al (2000) Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation 69:303–307
Pareja E, Gomez-Lechon MJ, Cortes M et al (2013) Human hepatocyte transplantation in patients with hepatic failure awaiting a graft. Eur Surg Res 50:273–281
Petersen BE, Bowen WC, Patrene KD et al (1999) Bone marrow as a potential source of hepatic oval cells. Science 284(5417):1168–1170
Lagasse E, Connors H, Al-Dhalimy M et al (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6(11):1229–1234
Alison MR, Poulsom R, Jeffery R et al (2000) Hepatocytes from non-hepatic adult stem cells. Nature 406(6793):257
Theise ND, Badve S, Saxena R et al (2000) Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 31(1):235–240
Quintana-Bustamante O, Alvarez-Barrientos A, Kofman AV et al (2006) Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology 43:108–116
Theise ND, Nimmakayalu M, Gardner R (2000) Liver from bone marrow in humans. Hepatology 32(1):11–16
Cantz T, Sharma AD, Jochheim-Richter A et al (2004) Reevaluation of bone marrow-derived cells as a source for hepatocyte regeneration. Cell Transplant 13(6):659–666
Kanazawa Y, Verma IM (2003) Little evidence of bone marrow-derived hepatocytes in the replacement of injured liver. Proc Natl Acad Sci U S A 100(1):11850–11853
Jang YY, Collector MI, Baylin SB et al (2004) Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 6(6):532–539
Shizhu J, Xiangwei M, Xun S et al (2012) Bone marrow mononuclear cell transplant therapy in mice with CCl4-induced acute liver failure. Turk J Gastroenterol 23:344–352
Gordon MY, Levicar N, Pai M et al (2006) Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells 24:1822–1830
Salama H, Zekri AR, Zern M et al (2010) Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant 19:1475–1486
Couto BG, Goldenberg RC, da Fonseca LM et al (2011) Bone marrow mononuclear cell therapy for patients with cirrhosis: a Phase 1 study. Liver Int 31(3):391–400
Lyra AC, Soares MB, da Silva LF, Fortes MF, Silva AG, Mota AC, Oliveira SA, Braga EL, de Carvalho WA, Genser B et al (2007) Feasibility and safety of autologous bone marrow mononuclear cell transplantation in patients with advanced chronic liver disease. World J Gastroenterol 13:1067–1073
Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K et al (2008) Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol 103:1952–1958
Lyra AC, Soares MB, da Silva LF, Braga EL, Oliveira SA, Fortes MF, Silva AG, Brustolim D, Genser B, Dos Santos RR et al (2010) Infusion of autologous bone marrow mononuclear cells through hepatic artery results in a short-term improvement of liver function in patients with chronic liver disease: a pilot randomized controlled study. Eur J Gastroenterol Hepatol 22:33–42
Gasbarrini A, Rapaccini GL, Rutella S, Zocco MA, Tittoto P, Leone G, Pola P, Gasbarrini G, Di Campli C (2007) Rescue therapy by portal infusion of autologous stem cells in a case of drug-induced hepatitis. Dig Liver Dis 39:878–882
Terai S, Ishikawa T, Omori K et al (2006) Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells 24:2292–2298
Evicar N, Pai M, Habib NA et al (2008) Long-term clinical results of autologous infusion of mobilized adult bone marrow derived CD34+ cells in patients with chronic liver disease. Cell Prolif 41 Suppl 1:115–125
Spahr L, Chalandon Y, Terraz S et al (2013) Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One 8(1):e53719
Bone marrow stem cells and liver http://www.clinicaltrials.gov/ct2/results?term=bone+marrow+stem+cells+AND+liver. Accessed on 17 Jan 2014
van de Ven C, Collins D, Bradley MB et al (2007) The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp Hematol 35:1753–1765
Newsome PN, Johannessen I, Boyle S et al (2003) Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology 124:1891–1900
Kögler G, Sensken S, Airey JA et al (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med 200:123–135
Di Campli C, Piscaglia AC, Rutella S et al (2005) Improvement of mortality rate and decrease in histologic hepatic injury after human cord blood stem cell infusion in a murine model of hepatotoxicity. Transplant Proc 37:2707–2710
Nonome K, Li XK, Takahara T et al (2005) Human umbilical cord blood-derived cells differentiate into hepatocyte-like cells in the Fas-mediated liver injury model. Am J Physiol Gastrointest Liver Physiol 289:G1091–G1099
Beerheide W, von Mach MA, Ringel M et al (2002) Downregulation of beta2-microglobulin in human cord blood somatic stem cells after transplantation into livers of SCID-mice: an escape mechanism of stem cells? Biochem Biophys Res Commun 294:1052–1063
Brulport M, Schormann W, Bauer A et al (2007) Fate of extrahepatic human stem and precursor cells after transplantation into mouse livers. Hepatology 46:861–870
Shyu MK, Yuan RH, Shih JC et al (2007) Kinetics and functional assay of liver repopulation after human cord blood transplantation. Dig Liver Dis 39:455–465
Wulf-Goldenberg A, Eckert K, Fichtner I (2008) Cytokine-pretreatment of CD34(+) cord blood stem cells in vitro reduces long-term cell engraftment in NOD/SCID mice. Eur J Cell Biol 87:69–80
Burra P, Arcidiacono D, Bizzaro D et al (2012) Systemic administration of a novel human umbilical cord mesenchymal stem cells population accelerates the resolution of acute liver injury. BMC Gastroenterol 12:88
Chen Li, Liu T, Zhang B et al (2012) Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A 18(13–14):1352–1364
Zhang Z, Lin H, Shi M et al (2012) Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 27(Suppl 2):112–120
Umbilical Cord Blood Therapy in Liver Cirrhosis http://www.clinicaltrials.gov/ct2/results?term=Umbilical+Cord+Blood+Therapy+in+Liver+Cirrhosis&Search=Search. Accessed 17 Jan 2014
Mito M, Ebata H, Kusano M et al (1979) Morphology and function of isolated hepatocytes transplanted into rat spleen. Transplantation 28:499–505
Strom SC, Jirtle RL, Jones RS et al (1982) Isolation, culture and transplantation of human hepatocytes. J Natl Cancer Inst 68:771–778
Rajvanshi P, Kerr A, Bhargava KK et al (1996) Efficacy and safety of repeated hepatocyte transplantation for significant liver repopulation in rodents. Gastroenterology 111:1092–1102
Soriano HE, Wood RP, Kang DC et al (1997) Hepatocellular transplantation in children with fulminant liver failure. J Hepatol 26:239A
von Mach MA, Hengstler JG, Brulport M et al (2004) In vitro cultured islet-derived progenitor cells of human origin express human albumin in severe combined immunodeficiency mouse liver in vivo. Stem Cells 22:1134–1141
Ruhnke M, Ungefroren H, Nussler A et al (2005) Reprogramming of human peripheral blood monocytes into functional hepatocyte and pancreatic islet-like cells. Gastroenterology 128:1774–1786
Yokoyama T, Ohashi K, Kuge H et al (2005) In vivo engineering of metabolically active hepatic tissue in a neovascularised subcutaneous cavity. Am J Transplant 6:50–59
Demetriou AA, Levenson SM, Novikoff PM et al (1986) Survival, organization and function of microcarrier-attached hepatocytes transplanted in rats. Proc Natl Acad Sci U S A 83:7475–7479
Jaffe V, Darby H, Selden C et al (1988) The growth of transplanted liver cells within the pancreas. Transplantation 45:497–498
Sano K, Cusick RA, Lee H et al (1996) Regenerative signals for heterotopic hepatocyte transplantation. Transplant Proc 28:1857–1858
Selden C, Gypta S, Johnstone R et al (1984) The pulmonary vascular bed as a site for implantation of isolated liver cells in inbred rats. Transplantation 38:81–83
Then P, Sandbichler P, Erhart R et al (1991) Hepatocyte transplantation into lung for treatment of acute hepatic failure in the rat. Transplant Proc 23:892–893
Ricordi C, Lacy PE, Callery MP et al (1989) Trophic factors from pancreatic islets in combined hepatocyte-islet allografts enhance hepatocellular survival. Surgery 105:218–223
Jirtle RL, Biles C, Michalopoulos G (1980) Morphologic and histochemical analysis of hepatocytes transplanted into syngeneic hosts. Am J Pathol 101:115–126
Komori J, Boone L, DeWard A et al (2012) The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol 30(10):976–983
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Joshi, M.G., Gadgil, A., Bhonde, R.R. (2014). Stem Cell Therapy for Acute and Chronic Liver Failure. In: Somasundaram, I. (eds) Stem Cell Therapy for Organ Failure. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2110-4_16
Download citation
DOI: https://doi.org/10.1007/978-81-322-2110-4_16
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2109-8
Online ISBN: 978-81-322-2110-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)