Abstract
Beneficial plant-microbe interactions have utmost importance for enhancing plant growth, improving soil structure, and managing plant diseases. Not surprisingly, such mutual interactions, where plants provide nourishment to rhizospheric microbes and in return microbes help in facilitating plant growth and stress amelioration, actually lay the foundation of sustainable agriculture. To cope with the major challenge of pathogen attack, beneficial rhizospheric microbes have proven their efficacy by induced systemic resistance (ISR). Therefore, such microbes are increasingly used in the form of biofertilizers and biopesticides. Moreover, such plant-microbe interactions elicit a range of defense-responsive activities in order to combat the pathogen challenge. The main microbes-mediated defense strategies adopted by plants include activation of antioxidant status of the plant by reprogramming defense-related enzymes, modulation of quorum sensing phenomenon, and activation of phenylpropanoid pathway leading to phenolics production, lignin deposition, and transgenerational defense response. In this chapter, we highlight the relevance of beneficial interactions between plant and microbes in enhancing plants’ innate immune system against pathogen attack. This review provides a better understanding of the recent advances and major outcome of positive plant-microbe interactions and linking their relevance to plant defense response.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Plants are the basis of life on earth that provide carbon source for all non-photosynthetic organisms. However, ~300,000 plant species are attacked by a huge number of detrimental organisms including pathogens and insects (Pieterse and Dicke 2007). Such biotic factors tremendously influence the plant growth and limit total agricultural production. Hence, sustainable approach of enhancing plant growth and managing plant diseases is being integrated to achieve higher crop yield. Plants in nature interact with wide range of beneficial and detrimental microorganisms providing baseline for linking aboveground and belowground community members (Van der Putten et al. 2001; Pineda et al. 2010).
The successful establishment of plant-microbe interaction depends on the ability of roots to interact with microbes as rhizosphere is the main zone where such interactions take place. The rhizospheric interaction could directly affect the plant growth by exerting either beneficial, neutral, or detrimental effects. Beneficial plant-microbe interactions require involvement of plant growth-promoting rhizobacteria (PGPR), endophytes, and mycorrhizal fungi that enhance plant growth by improving nutritional status for plants and helping the plants to combat abiotic and biotic stresses (Harrison 2005; Berendsen et al. 2012). In contrary, the detrimental interaction is imparted by pathogenic microorganisms resulting into various kinds of plant diseases. In the crowd of mixed population of beneficial and detrimental microbes residing in the rhizospheric region, the selection of potent biocontrol microbes is necessary to eliminate the pathogens and to combat the challenge imposed by pathogens. Moreover, plants also develop strategies spontaneously to recognize biotic and abiotic interactions and further translate the signal into defense response (Pei et al. 2000; Jones and Dangl 2006; Dicke and Hilker 2003).
Interestingly, plant-microbe interaction is regulated through signal-transduction pathways allowing plants to prioritize defense responses following stress conditions. It is well known that plants’ response to pathogen attack is regulated by jasmonic acid (JA) and salicylic acid (SA)-dependent pathways effective against necrotrophic and biotrophic pathogens, respectively (Pieterse et al. 2012). The induced resistance imparted by these pathways represents two distinct responses: systemic acquired resistance (SAR) and ISR (van Loon et al. 1998). SAR is mediated by SA which is frequently produced following pathogen infection (Park and Kloepper 2000; Jeun et al. 2004). In contrary, JA- and ethylene (ET)-dependent ISRs are activated by beneficial microbes proven to produce antimicrobial compounds, siderophores, O-antigen of lipopolysaccharides (LPS) and salicylate. Moreover, ISR leads to the expression of pathogenesis-related (PR) proteins such as PR-1, PR-2, chitinases, and some peroxidases (POxs) (van Wees et al. 2000; Silva et al. 2004; Jetiyanon 2007). Considering the importance of ISR, recently, noteworthy consideration is being given to exploiting the beneficial soil microbes for enhancing plants’ immunity against pathogen attack. Moreover, microbe-mediated suppression of plant diseases provides eco-friendly and sustainable approach of plant disease management. It is believed that such beneficial microbes can enhance the plant’s innate immunity level against the invading pathogens by inducing an array of defense responses that include enhancement in antioxidant status of the plant by reprogramming defense-related enzymes, modulation of quorum sensing activities, and activation of phenylpropanoid pathway leading to phenolics production and lignin deposition.
Microbe-Mediated Antioxidants Status in the Host
Successful pathogen infection in plants results into oxidative burst that lead to production of reactive oxygen species (ROS) causing oxidative destruction of the cell (Asada 1999; Dat et al. 2000). These ROS are formed as a result of excitation of O2 to singlet form of oxygen or via formation of a superoxide radical (O2 −), hydrogen peroxide (H2O2), or a hydroxyl radical (HO−). The enhanced production of ROS after pathogen infection acts as cellular indicator of stress conditions and is triggered by the activity of NADPH oxidases (Cazale et al. 1999; Pei et al. 2000). However, recently, it has been investigated that apart from NADPH oxidases, the other sources such as amine oxidases and cell wall-bound POxs also participate in the formation of ROS during programmed cell death and pathogen defense (Dat et al. 2000; Grant and Loake 2000). It is interesting to note that though the elevated level of ROS imposes threat condition to the cells, it also acts as a signal for commencement of stress response and defense pathways (Desikan et al. 2001). The steady-state level of ROS in plant cells should always be under control as their over accumulation eventually result in cell death as a consequence from various kinds of oxidative processes, viz., lipid peroxidation, protein oxidation, nucleic acid damage, and enzyme inactivation.
In order to cope with the detrimental effects of elevated level of ROS, plants are bestowed with efficient antioxidant enzymes that contribute to ROS-scavenging mechanisms of plants. Major ROS scavengers in plants are reported to be superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) (Mittler 2002) (Table 5.1). To maintain the balance of superoxide radicals in cells, the activities of SOD and APX or CAT enzymes play crucial role as this balance avert formation of hydroxyl radical via the metal-dependent Haber–Weiss or the Fenton reactions (Asada and Takahashi 1987; Bowler et al. 1991). Moreover, the key pathways of ROS scavengers in plants include SOD present in more or less all cellular compartments. The major threat to plant system is instigated by various kinds of plant pathogens which upon infection lead to rapid formation of ROS. Plants generally increase their tolerance against invading pathogens by elevating activities of antioxidant enzymes.
The major defense strategy adopted by plants is phenylpropanoid pathway catalyzing transformation of l-phenylalanine into trans-cinnamic acid (Dixon and Paiva 1995) which play an important role in the biosynthesis of phenolics having strong antimicrobial properties (Nicholson and Hammerschmidt 1992). Interestingly, activation of the phenylpropanoid pathway also leads to deposition of lignin and induction of antioxidant enzymes including SOD and peroxidase (POx) (Silva et al. 2004; Singhai et al. 2011). It is interesting to note that beneficial soil microflora in rhizosphere provide supportive environment for the plants by augmenting the antioxidant status. Such microbe-mediated ISR protects the plants from various plant pathogens. Earlier reports have revealed significant role of many microbes in enhancing antioxidant enzymes in plants contributing to their resistance against pathogens (Table 5.2). Previously, rhizobacterial strains and Serratia marcescens were reported to enhance activities of POx, phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO), and lipoxygenase (LOX) in betelvine and tomato after pathogen attack by Phytophthora nicotianae and Pseudomonas syringae, respectively. Modulation of such antioxidant status in plants treated with beneficial rhizobacteria enhanced disease resistance against pathogen attack (Diallo et al. 2011). Similar observation was recorded by Singhai et al. (2011) where pseudomonad strains were found to increase the level of POx and PAL in potato leading to tolerance against potato scab disease caused by Streptomyces scabies. Similarly, in another interesting study by Jain et al. (2012), the microbial consortium enhanced tolerance in pea plants against Sclerotinia sclerotiorum by inducing the level of PAL, POx, PPO, and SOD. Most recently, Singh et al. (2013) showed elevation of SOD and PO in Sclerotium rolfsii challenged chickpea plants treated with triple consortium of Pseudomonas, Trichoderma, and Rhizobium. Taken together, these reports clearly validate the importance of beneficial rhizosphere microbes in imparting tolerance to the plants against diverse range of pathogens by modulating their innate antioxidant status as shown in Fig. 5.1.
Microbe-Mediated Activation of Phenylpropanoid Pathway
Soil is the locale of numerous microorganisms and can aptly be referred to as the mine of microorganisms. The rhizospheric regions of the plants inhabit most of the microbial communities in its vicinity, be it having beneficial or deleterious effects on the plant. Beneficial microorganisms may include PGPR and mycorrhizal fungi that induce systemic defense response in the host to preclude it from the chronic impairment of phytopathogens comprising of bacteria, fungi, viruses, and nematodes. To avoid the perplexity in the use of different terms for denoting the beneficial microbes, two new terms were proposed by Bashan and Holguin (1998): “biocontrol plant growth-promoting bacteria (PGPB)” that suppress plant diseases by enhancing the plant defense responses and “PGPB” that specifically play eminent role in augmenting plant growth. The upgradation of the conventionally used “PGPR” term coined by Kloepper et al. (1980) to “PGPB” would thereby include the useful microbes that do not inhabit the rhizospheric region of the soil yet enhance the vigor of plants against the phytopathogens.
Diverse interactions prevail between the microbial commune and plant roots, for instance, the symbiotic associations by mycorrhizal fungi that aid in the uptake of water and minerals (Harrison 2005), and the nodule-inhabiting Rhizobium bacteria that fix the atmospheric nitrogen for the plant (Spaink 2000). Several other types of beneficial microbes like PGPR and fungi are reported to suppress plant diseases (Van Loon et al. 1998; Harman et al. 2004; Kloepper et al. 2004) or insect herbivory (Van Oosten et al. 2008) by enhancing the defense response of the plant thereby resulting in overall increment in the plant growth parameters. The plethora of benefits endowed to the plants by the microbes can be attributed to either direct effect through mycoparasitism of soil-borne pathogens or indirectly by eliciting the plant defense mechanisms thereby fortifying the plant immune system against the invading pathogens (Van Loon et al. 1998; Pozo and Azcon-Aguilar 2007).
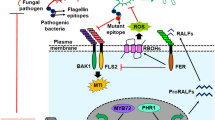
Several microbial determinants are related with the elicitation of defense responses in plant system. Most commonly studied and well associated as the inducers of host immune response conferred by rhizobacteria are the microbe-associated molecular patterns (MAMPs). Also, LPS and flagellin found at the cell surfaces of rhizobacteria are found to be potent in inducing defense response of the associated host against phytopathogens (Bakker et al. 2007). Another important mechanism employed by beneficial microbes in disease suppression is to create competition for iron by forming low molecular weight iron chelators known as siderophores (Meziane et al. 2005). Important chemicals that act as elicitors for the defense response are the variously secreted antibiotics by the rhizobacteria and fungi (Ran et al. 2005); surfactin, a lipoprotein secreted by Bacillus subtilis (Ongena et al. 2007); biosurfactant massetolide A from P. fluorescens (Tran et al. 2007); N-alkylated benzylamine (Ongena et al. 2005); N-acyl-l-homoserine lactone (Schuhegger et al. 2006); and volatile organic compound 2, 3-butanediol by Bacillus spp. (Ryu et al. 2004). Fungal proteins like endochitinase secreted by Trichoderma spp. have been shown to enhance plant defense-related proteins (Harman and Shoresh 2007; Keswani et al. 2014). A small protein SM1 produced by strains of Trichoderma virens can induce terpenoid phytoalexin biosynthesis and POx activity as studied in cotton plants and other systems as well (Djonovic et al. 2006, 2007). Another class of proteins associated with the immune system of the plants is the products of avirulence like (Avr) genes, produced not only by the phytopathogens but also by the beneficial microbes. They generally function as pathogen-specific elicitors of the hypersensitive responses in plants containing the corresponding resistance (R) gene (Woo et al. 2006). Small secondary metabolites produced by Trichoderma species have also been reported to possess defense response induction activity mainly via eliciting the expression of PR proteins on application to plants causing both local and systemic disease suppression (Vinale et al. 2008; Keswani et al. 2014).
Plant defense response gets activated by the metabolism of phenyl propanoid (PP) pathway in which PAL catalyzes the first important step of the general PP metabolism. Further, the pathway leads to the synthesis of other important compounds having indispensible role in providing plant defense including cell wall strengthening and repair (lignin and suberin), antimicrobial activity (pterocarpan, isoflavonoid phytoalexins), and signaling SA (Hummerschmidt 1999). Beneficial microbes like Trichoderma spp. have been reported to trigger the terpenoid phytoalexin defense compounds in cotton seedlings thereby controlling Rhizoctonia solani infestation apart from the conventional mycoparasitic mode exhibited by the species of the genus (Howell et al. 2000). Also, biocontrol of Pythium ultimum on Arabidopsis seedlings by T. harzianum strain T22 reported the elevated expression level of NPR1 gene which is the main gene involved in disease resistance (Shoresh et al. 2010). Recently, the concept of using two or three compatible beneficial microbes has been proposed for better management of disease. It has been shown that significant enhancement of PP activity is recorded in chickpea plants treated with the triple consortium developed using Trichoderma, Bacillus, and Rhizobium spp. when challenged with S. rolfsii (Singh et al. 2013). Similar results were previously reported by Jain et al. (2012) where the consortium of beneficial microbes (Trichoderma spp., Pseudomonas spp., Bacillus spp.) were reported for the increment of various defense-related enzymes in pea plants against S. sclerotinia challenge. Another class of beneficial fungi Piriformospora indica has also been reported to cause elevation of defense response in plants against soil-borne pathogens (Serfling et al. 2007; Stein et al. 2008) showing its mechanism to be related with the upregulation of JA-mediated pathway.
A complex cross talk subsists between the plant and the beneficial microbes in response to the pathogenic microbes. The advancement in the research related to the responses triggered in plants against the pathogens by the beneficial microbes demonstrates the involvement of specific MAMPs analogous to the MAMPs of the pathogen highlighting the extensive coordination prevailing between different defense pathways involved.
Microbe-Mediated-Induced Lignification
Tertiary structure of lignin results from the polymerization of polyphenols and free radicals p-coniferyl, coumaryl, and sinapyl alcohols within the plant cell wall. This polymerization also results in the formation of covalent cross links with polysaccharide and protein moieties framing a tremendously resistant cell wall towards mechanical and enzymatic disruption against various classes of plant pathogens, insects, and herbivores (Bernards and Lewis 1998; Sederoff et al. 1999; Davin and Lewis 2000; Hatfield and Vermerris as 2001; Boerjan et al. 2003). Thus, lignin serves as physical defense shield in plant defense. Modifications in lignin composition, content, and distribution affect the strength of the shield which ultimately influences the agro-industrial pertinence of the plant material (Lewis and Yamamoto 1990).
Lignification obstructs phytopathogen growth on plant surface by a pentagonal approach (Ride 1978) as mentioned below:
-
1.
Lignin deposition shields the plant tissue surface from enzymatic hydrolysis and mechanical penetration of phytopathogens by intensifying compressive forces between lignin layers preventing cellular penetration of phytopathogens.
-
2.
Lignification of walls hinders the mobility of water and electrolytes between plant cells and phytopathogens facilitating pathogen killing by starvation.
-
3.
Chemical modification of cell wall components is an effective strategy for disguising the pathogen enzymes. Coniferyl alcohol and ferulic acid covalently bound to cell wall glycoproteins and esterification of cell wall polysaccharides with cinnamic acid derivatives evidently reduces the cell wall damage due to unavailability of substrates (Friend 1976; Whitmore 1978).
-
4.
Generation of free radicals and low molecular weight phenolic precursors produced during polymerization of lignin may directly inactivate pathogens’ membranes, enzymes, toxins, and elicitors.
-
5.
Fungal walls contain chitin, cellulose, and hydroxyproline-rich proteins which can serve as matrices for lignin polymerization. Consequently, the hyphal tips become lignified and lose plasticity necessary for growth and penetration (Gottlieb and Pelczar 1951).
Among various mechanisms of plant defense, lignification is a strong structural defense strategy employed by plants to prevent pathogen penetration, and this relationship of lignification and disease resistance in plants is clearly witnessed in various studies. Comparatively, rapid lignin accumulation and deposition is observed in resistant cultivars than susceptible varieties (Vance et al. 1976; Yates et al. 1997; Durrant and Dong 2004). Not much is known about the role of rhizospheric microbes in strengthening plant’s cell wall towards various biotic stresses. A recent study aimed to determine the efficacy of a triple microbial consortium of fluorescent Pseudomonas PHU094, Trichoderma THU0816, and Rhizobium RL091 strain on physiological defense responses in chickpea against the collar rot pathogen S. rolfsii. The result clearly illustrates the profound variation of lignin deposition in chickpea infected with S. rolfsii, which is attributed to different combinations of plant beneficial microbes to trigger lignification process. Interestingly, on treatment with triple consortium, uniform and maximum lignin deposition in the intrafascicular cambial cells was clearly observed and the phloem cells also displayed an enhanced lignification in sclerenchyma cap. Thus, claiming that beneficial rhizospheric microbes when employed in synergistic consortium can enhance the physical strength and durability of the cell wall towards cell wall-degrading phytopathogen (Singh et al. 2013).
In another study, alterations in phenolic metabolism and lignin deposition were analyzed in the roots of tomato plants after elicitation with Fusarium mycelium extract (FME), Fusarium culture filtrate (FCF), chitosan (CHT), and Trichoderma mycelium extract (TME). Maximum lignin synthesis was observed in plants treated with FME followed by CHT. Lignin deposition in the root cell walls increased to 5.7 times within 24 h after elicitation with FME. Similarly, CHT increased lignin deposition to almost five times, 24 h after elicitation. Thus, it was concluded that cell wall strengthening by lignin deposition was preceded by elicitation of lignin synthesizing enzymes revealing its essential role in defense response of tomato plants in response to various elicitors including one derived from Fusarium oxysporum f. sp. lycopersici, the causal organism of Fusarium wilt of tomato (Mandal and Mitra 2007).
Microbe-Mediated Quorum Sensing in Pathogen Management
The phenomenon of quorum sensing (QS) depicts the bacterial cell-cell communication and is generally cell density dependent. This cell-cell communication network is mediated by signal molecules (also called autoinducers), for example, oligopeptides and N-acylhomoserine lactones (AHL) in Gram-positive and Gram-negative bacteria, respectively. The QS plays a significant role in biofilm formation and in determining virulence factor in pathogenic bacterial species (Gram et al. 2002). In contrary, the phenomenon of antiquorum sensing (anti-QS) also exists where autoinducers interrupt with QS and thereby reducing the pathogenicity in several bacterial species (Truchado et al. 2012). Since QS contribute significantly in regulating virulence factor in many plant pathogens, anti-QS could be of great interest for decreasing pathogenic behavior and in developing innovative approach of disease control (Alvarez et al. 2012).
Considering the importance of anti-QS, presently, this phenomenon is getting noteworthy attention in plant disease management using bacterial biosensors and indicators. Interestingly, the cloning of aiiA gene (from Bacillus sp.) into transgenic potato and tobacco for enhancing disease resistance against Erwinia carotovora is perhaps the very first example (Dong et al. 2001). Expression of this gene resulted in the production of AHL-lactonase in transgenic plants that interrupt with the QS systems arresting the virulence factor of E. carotovora leading to reduced disease incidence. Likewise, another example is the generation of transgenic tobacco lines using expI (E. carotovora AHL gene) which, after expression in plants, trap the pathogen in premature stage when it is unable to cause infection (Mäe et al. 2001). It is interesting to note that the autoinducer molecule AHL also contributes to the production of antimicrobial compounds in nonpathogenic Pseudomonas chlororaphis which has been successfully employed in suppressing plant diseases (Pierson et al. 1998a, b). Molina et al. (2003) evaluated the biocontrol potential of AHL-degrading Bacillus sp. A24 and genetically engineered AHL-degrading strain P3/pME6863 against soft rot in potato caused by E. carotovora. Recently, the novel approach of disrupting QS using structural analogs stimulating AHL-degradative microflora has been investigated by some researchers (Crépin et al. 2012a, b). In conclusion, these studies altogether clearly validate that the QS inhibition of phytopathogenic bacteria could be successfully employed for plant disease management. Hence, more focused researches are needed towards this approach.
Microbe-Mediated Nutrient Uptake and Defense
Fifty years back, the drive required for feeding the surplus population gave birth to the much talked about “Green Revolution” in India, leading to tremendous increase in food production. The unremitting use of fertilizers and pesticides has undoubtedly contributed to the increment in the food production but had led to the slow death of the soil microflora and fertility as well. In order to resolve the burning issue of deposition of toxic residues leading to biomagnifications in the food web, urgent need of using eco-friendly alternatives is required. Biopesticides comprising of microbial inoculants have emerged as a silver line for the current scenario with multifaceted benefits like safer approach both to the environment and to human kind, more targeted activity, low dose effectiveness, easily decomposable, natural propagation, and multiplication along with fortifying the plants’ immune system (Berg 2009).
A constant conversation exists between the plants and the microbes in its vicinity. The plant-microbe interaction persists owing to the beneficial mutualism between the two partners which upshot various remuneration to plants as well as microbial commune. Positive effect on the growth and health of plants, enhanced stress tolerance, induction of disease reduction, biodiversity enrichment with ability to foster nutrient availability and uptake are the consequence of the interface between plant and microbes (Lugtenberg et al. 2002; Morrissey et al. 2004). These interactions are specific in terms of the host colonization due to the specific secondary metabolism and morphology (Berg and Smalla 2009). However, plant growth promotion and disease reduction or control have been the most noteworthy consequence of this interaction. Growth promotion in plants can be mediated through direct mechanisms by the microbes and also through indirect means through their antagonistic properties thereby reducing disease incidence allowing the healthy proliferation of the plants.
The competence of the microbes to colonize plant habitats is one of the crucial requirements for an effective plant-microbe interaction (Kamilova et al. 2005). Recognition, adherence, invasion (in case of endophytes and pathogens), colonization, and growth are the essential steps required for successful colonization apart from the various strategies employed by the microbial commune to establish the interaction. However, the initiation is executed by the plant itself by releasing signals recognized by the microbes which reciprocate the signals to initiate the colonization (Bais et al. 2006). Generally, motile organisms are preferred to participate and react in this cross talk (Lugtenberg et al. 2002).
Basically, three main types of interactions are related to the increment of plant growth mediated by beneficial microbes (Fig. 5.2). The most commonly known type of interaction is the symbiosis by Rhizobium species in fixing atmospheric nitrogen into ammonia in specific organs called nodules, found in leguminous plants (Van Rhijn and Vanderleyden 1995). Other important interaction is that of higher plants, commonly terrestrial flowering plants, with arbuscular mycorrhiza (AM) fungi which facilitate the absorption and translocation of phosphate from the soil (Harrison 1999). Lastly, the outcome of the numerous beneficial microbes that aid in mineralization of organic matter, thereby producing available nitrogen and phosphorous forms along with numerous micronutrients, provides the platform for the third type of interaction prevailing between plants and microbes (Hayatsu et al. 2008).
Phytohormones like indole-3-acetic acid (IAA), ET, cytokinins, and gibberellins are crucial for plant growth. Plants can obtain these hormones by either synthesizing themselves or by obtaining these from the microbes which can even alter the hormonal balance of the plant thereby causing alterations in the growth of the plants. Plant-associated bacteria have been shown to decrease the endogenous ACC levels thereby leading to increased root growth (Glick 2005). ACC deaminase-producing bacteria have also been reported to provide abiotic and biotic stress tolerance to plants thereby protecting them from the unfavorable conditions (Saleem et al. 2007). Seed treatment with auxin-producing Pseudomonas fluorescens has been reported to show stimulation of root growth, due to the production of nine times more tryptophan in root exudates of radish plants (Kamilova et al. 2006). This could be directly related to the growth promotion ability as the root growth-promoting hormone auxin is generally found in the root exudate which is synthesized from the amino acid tryptophan. Certain microbes like B. subtilis, B. amyloliquefaciens, and Enterobacter cloacae are known to enhance plant growth by releasing volatiles like 2, 3-butanediol and acetoin (Ryu et al. 2003). Increased photosynthetic efficiency and chlorophyll content were recorded in A. thaliana on treatment with B. subtilis GB03 which could be possibly related with the modulation of glucose and abscisic acid signaling (Zhang et al. 2008). Apart from auxin-producing bacteria, beneficial fungi like Trichoderma have been shown to be responsible for plant growth increment via auxin signaling (Contreras-Cornejo et al. 2009). Nitrogen fixation by the symbiotically associated Rhizobium species in the root nodules of leguminous plants or by free living bacteria like Azospirillum, Burkholderia, and Stenotrophomonas (Dobbelare et al. 2003) has been a major example for nutrient acquisition to plants by microbes. Bacterial indirect contribution to plant growth by liberating phosphorous from organic compounds such as phytates play an important role in providing the necessary phosphorous required for proper growth of the plant (Unno et al. 2005). Another important nutrient, sulfate, is also made available to plants through oxidation by bacteria (Banerjee and Yesmin 2002). Siderophores have also been an important mode for the uptake of important microelements like Fe and other poorly soluble inorganic nutrients. Also, relation of siderophore production has been reported with the antagonistic activity against pathogens. P. fluorescens CHA0 has been reported to produce gluconic acid which acidifies the surrounding environment and thereby solubilizes the mineral phosphate in the soil creating a nutrient-limited condition for the plant pathogens (De Werra et al. 2009). Fungal biocontrol agent Trichoderma and mycorrhizal fungus P. indica have been reported to produce siderophore as a mechanism to check the growth of pathogens by creating a competitive environment for the availability of iron (Shoresh et al. 2010). Phosphate absorption to plants by AM has been well reported (Smith et al. 2011; Balakrishnan and Subramanian 2012). Also, increment in Cu, Zn, B, Mn, and Fe uptake in plants has been attributed to AM (Lambert et al. 1980; Clark et al. 1999; Liu et al. 2000).
Other important microbes that have been well studied for their mode of action and regulation in promoting plant growth and antagonism are members of the genera Azospirillum (Cassan and Garcia 2008), Serratia (De Vleeschauwer and Hofte 2007), Stenotrophomonas (Ryan et al. 2009), and Streptomyces (Schrey and Tarkka 2008) along with fungal genera Ampelomyces, Coniothyrium, and Trichoderma (Harman et al. 2004).
Microbe-Mediated Transgenerational Defense
Plant-recruited beneficial microbes can also prime the plants for enhanced defense responses, and the effect of priming could be passed on to the next generations as well. Priming of plants leads to enhanced perception of MAMPs, recognition of effector molecules secreted by pathogen, and recruitment of beneficial rhizospheric microbes (Conrath 2011). Recent understanding in the subject reflects that similar to animals, epigenetic inheritance in plants also takes place, and the epigenetic modifications of the chromatin as well as DNA methylation in plants could be well preserved in several subsequent generations (Pieterse 2012). Being sessile organisms, plants communicate with their offspring through this mechanism to “inform” the offsprings about the potential threats in their environment. Since, plants encounter potential biotic and abiotic threats from the environment at one or the other point of their life span, they are adapted to such mechanisms through the process of evolution for passing on the information to the next generation. Slaughter et al. (2012) demonstrated that the descendants of A. thaliana plants primed with an avirulent strain of P. syringae pv. tomato (PstavrRpt2) showed enhanced and rapid accumulation of defense-related gene transcripts associated with the SA signaling pathway. Further, the descendents also showed enhanced disease resistance against a virulent isolate of P. syringae and another oomycete pathogen Hyaloperonospora arabidopsidis. Interestingly, the progeny of transgenerationally primed plants when treated again with the priming agent displayed an even stronger primed phenotype. Recent evidences suggest that SA-mediated systemic resistance in plants also require chromatin remodeling and DNA methylation (Luna et al. 2012). Luna and Ton (2012) showed that transgenerationally acquired resistance was sustained through one stress-free generation, confirming epigenetic basis of the phenomenon. Failure of non expressor of PR 1 (npr1) mutants to sustain transgenerationally acquired resistance in the progenies further signifies the SA-inducible pathway in this phenomenon and the central role of NPR1. Further, transgenerationally acquired systemic resistance was also demonstrated against biotrophic pathogens. A study on progenies obtained from diseased Arabidopsis also resulted in enhanced resistance towards the downy mildew pathogen (Luna and Ton 2012). Histone deacetylase 6 (HDA6) is a well-studied histone deacetylase that has a prominent role in the silencing of genes. It was reported that HDA6 has also a significant role to play in the process of DNA methylation on its direct target locus. Thus, elucidation of the functions of HDA6 provided some very important clues of epigenetic regulation in plants (Kim et al. 2012). All these findings suggest the importance of transgenerationally acquired systemic resistance in plants and their potential role in managing plant diseases.
Future Prospects
The major challenge in the form of plant pathogens imposed to plant growth in natural and agricultural ecosystems urges for exploiting beneficial plant-microbe interactions. It is a well-known fact that microbial approach for plant disease management is necessary for maintaining the sustainability in agroecosystems. Therefore, it has been an emerging topic and gained considerable attention by many researchers. Though, various facets of plant-microbe interactions have long been studied, there is still a long way to go for achieving greater knowledge. The better understanding of plants’ rhizosphere components is necessary to know the cross talk between plants and microbes. Linking this information to stress conditions would certainly provide a clue about developing a favorable and friendly environment for plant growth. Moreover, molecular approaches of studying the regulatory components of plant-microbe interactions can provide better understanding of improving such relationships by their manipulation.
References
Alvarez MV, Moreira MR, Ponce A (2012) Antiquorum sensing and antimicrobial activity of natural agents with potential use in food. J Food Saf 32:379–387
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–287
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:234–266
Bakker PAHM, Pieterse CMJ, Van Loon LC (2007) Induced systemic resistance by fluorescent pseudomonas spp. Phytopathology 97:239–243
Balakrishnan N, Subramanian KS (2012) Mycorrhizal symbiosis and bioavailability of micronutrients in maize grain. Maydica 57:129–138
Banerjee M, Yesmin L (2002) Sulfur-oxidizing plant growth promoting rhizobacteria for enhanced canola performance. US Patent 07491535
Bashan Y, Holguin G (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Berg G (2009) Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18
Berg G, Smalla K (2009) Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol 68:1–13
Bernards MA, Lewis NG (1998) The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry 47:915–933
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inzé D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10:1723–1732
Cassán FD, García de Salamone I (2008) Azospirillum sp.: cell physiology, plant interactions and agronomic research in Argentina. Asociación Argentina de Microbiología, Argentina, p 266
Cazale AC, Droillard MJ, Wilson C, Heberle-Bors E, Barbier-Brygoo H, Laurière C (1999) MAP kinase activation by hypo-osmotic stress of tobacco cell suspensions: towards the oxidative burst response? Plant J 19:297–307
Clark RB, Zobel RW, Zeto SK (1999) Effects of mycorrhizal fungus isolates on mineral acquisition by Panicum virgatum acidic soils. Mycorrhiza 9:167–176
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16(10):524–531
Contreras-Cornejo HA, Macias-Rodriguez L, Cortes-Penagos C, Lopez-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592
Crépin A, Barbey C, Beury-Cirou A, Hélias V, Taupin L, Reverchon S, Nasser W, Faure D, Dufour A, Orange N, Feuilloley M, Heurlier K, Burini JF, Latour X (2012a) Quorum sensing signaling molecules produced by reference and emerging soft-rot bacteria (Dickeya and Pectobacterium spp.). PLoS One 7(4):e35176
Crépin A, Barbey C, Cirou A, Tanniéres M, Orange N, Orange N, Feuilloley M, Dessaux Y, Burini JF, Faure D, Latour X (2012b) Biological control of pathogen communication in the rhizosphere: a novel approach applied to potato soft rot due to Pectobacterium atrosepticum. Plant Soil 358:27–37
Dat J, Vandenabeele S, Vranová E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Davin LB, Lewis NG (2000) Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol 123:453–462
De Vleeschauwer D, Höfte M (2007) Using Serratia plymuthica to control fungal pathogens of plant. CAB Rev 2:46
De Werra P, Péchy-Tarr M, Keel C, Maurhofer M (2009) Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl Environ Microbiol 75:4162–4174
Desikan R, A-HMackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptosome by oxidative stress. Plant Physiol 127:159–172
Diallo S, Crépin A, Barbey C, Orange N, Burini JF, Latour X (2011) Mechanisms and recent advances in biological control mediated through the potato rhizosphere. FEMS Microbiol Ecol 75:351–364
Dicke M, Hilker M (2003) Induced plant defences: from molecular biology to evolutionary ecology. Basic Appl Ecol 4:3–14
Dixon RA, Paiva N (1995) Stress induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM (2006) Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant-Microbe Interact 8:838–853
Djonovic S, Vargas WA, Kolomiets MV, Horndeski M, Wiest A, Kenerley CM (2007) A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol 145:875–889
Dobbelare S, Vanderleydern J, Okon Y (2003) Plant-growth promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149
Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, Zhang LH (2001) Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813–817
Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42:185–209
Friend J (1976) Lignification in infected tissue. In: Friend J, Threfall DR (eds) Biochemical aspects of plantparasite relationships. Academic, London, pp 291–303
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 252:1–7
Gottlieb S, Pelczar MJ (1951) Microbiological aspects of lignin degradation. Bacteriol Rev 15:55–76
Gram L, Grossart H, Schlingloff A, Kiørboe T (2002) Possible quorum sensing in marine snow bacteria: production of acylated homoserine lactones by roseobacter strains isolated from marine snow. Appl Environ Microbiol 8(68):4111–4116
Grant JJ, Loake GJ (2000) Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol 124:21–29
Harman G, Shoresh M (2007) The mechanisms and applications of opportunistic plant symbionts. In: Vurro M, Gressel J (eds) Novel biotechnologies for biocontrol agent enhancement and management. Springer, Amsterdam, pp 131–155
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species – opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Harrison MJ (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol 50:361–389
Harrison MJ (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42
Hatfield R, Vermerris W (2001) Lignin formation in plants. The dilemma of linkage specificity. Plant Physiol 126:1351–1357
Hayatsu M, Tago K, Saito M (2008) Various players in the nitrogen cycle: diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci Plant Nutr 54:33–45
Howell CR, Hanson LE, Stipanovic RD, Puckhaber LS (2000) Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 90:248–252
Hummerschmidt R (1999) Phytoalexins: what have we learned after 60 years? Annu Rev Phytopathol 37:285–306
Jain A, Singh S, Sarma BK, Singh HB (2012) Microbial consortium mediated reprogramming of defence network in pea to enhance tolerance against Sclerotinia sclerotiorum. J Appl Microbiol 112:537–550
Jetiyanon K (2007) Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biol Control 42:178–185
Jeun YC, Park KS, Kim CH, Fowler WD, Kloepper JW (2004) Cytological observations of cucumber plants during induced resistance elicited by rhizobacteria. Biol Control 29:34–42
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kamilova F, Validov S, Azarova T, Mulders I, Lugtenberg B (2005) Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol 7:1809–1817
Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg BJJ (2006) Effects of the tomato pathogen Fusarium oxysporum f. sp. radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365on the composition of organic acids and sugars in tomato root exudate. Mol Plant-Microbe Interact 19:1121–1126
Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB (2014) Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Appl Microbiol Biotechnol 98:533–544
Kim JM, To TK, Seki M (2012) An epigenetic integrator: new insights into genome regulation, environmental stress responses and developmental controls by histone deacetylase 6. Plant Cell Physiol 53(5):794–800
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
Kloepper JW, Ryu CM, Zhang SA (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Lambert DH, Cole H, Baker DE (1980) The role of boron in plant response to mycorrhizal infection. Plant Soil 57:431–438
Lavania M, Chauhan PS, Chauhan SVS, Singh HB, Nautiyal CS (2006) Induction of plant defense enzymes and phenolics by treatments with plant growth promoting rhizobacteria Serratia marcescens NBRI 1213. Curr Microbiol 52:363–368
Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41:455–496
Liu A, Hamel C, Hamilton RI, Ma BL, Smith DL (2000) Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) growth in soil at different P and micronutrient levels. Mycorrhiza 9:331–336
Lugtenberg BJJ, Chin-A-Woeng TFC, Bloemberg GV (2002) Microbe– plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek 81:373–383
Luna E, Ton J (2012) The epigenetic machinery controlling transgenerational systemic acquired resistance. Plant Signal Behav 7:615–618
Luna E, Bruce TJA, Roberts MR, Flors V, Ton J (2012) Next-generation systemic acquired resistance. Plant Physiol 158:844–853
Mäe A, Montesano M, Koiv V, Palva ET (2001) Transgenic plants producing the bacterial pheromone N-acyl-homoserine lactone exhibit enhanced resistance to the bacterial phytopathogen Erwinia carotovora. Mol Plant-Microbe Interact 14:1035–1042
Mandal S, Mitra A (2007) Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol Mol Plant Pathol 71:201–209
Meziane H, Van der Sluis I, Van Loon LC, Ho¨fte M, Bakker PAHM (2005) Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol Plant Pathol 6:177–185
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Molina L, Constantinescu F, Michel L, Reimmann C, Duffy B, Defago G (2003) Degradation of pathogen quorum-sensing molecules by soil bacteria: a preventive and curative biological control mechanism. FEMS Microbiol Ecol 45:71–81
Morrissey JP, Dow JM, Mark L, O’Gara F (2004) Are microbes at the root of a solution to world food production? EMBO Rep 5:922–926
Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30:369–389
Ongena M, Jourdan E, Scha¨ fer M, Kech C, Budzikiewicz H, Luxen A, Thonart P (2005) Isolation of an N-alkylated benzylamine derivative from Pseudomonas putida BTP1 as elicitor of induced systemic resistance in bean. Mol Plant Microbe Interact 18:562–569
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny JL, Thonart P (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9:1084–1090
Park KS, Kloepper JW (2000) Activation of PR-1a promoter by rhizobacteria which induce systemic resistance in tobacco against Pseudomonas syringae pv. tabaci. Biol Control 18:2–9
Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406:731–734
Pierson EA, Wood D, Cannon JAW, Blachere FM, Pierson LS (1998a) Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol Plant-Microbe Interact 11:1078–1084
Pierson LS, Wood DW, Pierson EA (1998b) Homoserine lactone-mediated gene regulation in plant-associated bacteria. Annu Rev Phytopathol 36:207–225
Pieterse CMJ (2012) Prime time for transgenerational defense. Plant Physiol 158:545
Pieterse CM, Dicke M (2007) Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci 12:564–569
Pieterse CMJ, van der Does D, Zamioudis C, Leon-Reyes A, van Wees SCM (2012) Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol 28:489–521
Pineda A, Zheng SJ, van Loon JJA, Pieterse CMJ, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15:507–514
Pozo MJ, Azcon-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Ran LX, Li ZN, Wu GJ, Van Loon LC, Bakker PAHM (2005) Induction of systemic resistance against bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. Eur J Plant Pathol 113:59–70
Ride JP (1978) The role of cell wall alterations in resistance to fungi. Ann Appl Biol 89:302–306
Ryan RP, Monchy S, Cardinale M, Taghavi S, Crossman L, Avison MB, Berg G, van der Lelie D, Dow JM (2009) Versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Microbiol Rev 7:514–525
Ryu CM, Farag MA, Hu CH, Reddy MS, Wie HX, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth of Arabidopsis. Proc Natl Acad Sci 100:4927–4932
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare’ PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Schrey SD, Tarkka MT (2008) Friends and foes: Streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek 94:11–19
Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Hartmann A, Langebartels C (2006) Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ 29:909–918
Sederoff RR, MacKay JJ, Ralph J, Hatfield RD (1999) Unexpected variation in lignin. Curr Opin Plant Biol 2:145–152
Serfling A, Wirsel SGR, Lind V, Deising HB (2007) Performance of the biocontrol fungus Piriformospora indica on wheat under greenhouse and field conditions. Phytopathology 97:523–531
Shoresh M, Harman GE, Mastouri F (2010) Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 48:21–43
Silva HSA, Romeiro RDS, Macagnan D, Halfeld-Vieira BDA, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants non-specific protection and increase in enzyme activities. Biol Control 29:288–295
Singh A, Sarma BK, Upadhyay RS, Singh HB (2013) Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol Res 168:33–40
Singhai PK, Sarma BK, Srivastava JS (2011) Biological management of common scab of potato through Pseudomonas species and vermicompost. Biol Control 57:150–157
Slaughter A, Daniel X, Flors V, Luna E, Hohn B, Mauch-Mani B (2012) Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol 158:835–843
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057
Spaink HP (2000) Root nodulation and infection factors produced by rhizobial bacteria. Annu Rev Microbiol 54:257–288
Stein E, Molitor A, Kogel KH, Waller F (2008) Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol 49:1747–1751
Tran H, Ficke A, Asiimwe T, Ho¨fte M, Raaijmakers JM (2007) Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol 175:731–742
Truchado P, Tomás-Barberán F, Larrosa M, Allende A (2012) Food phytochemicals act as quorum sensing inhibitors reducing production and/or degrading autoinducers of Yersinia enterocolítica and Erwinia carotovora. Food Control 24:78–85
Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M (2005) Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of lupin analysed by phytate utilization ability. Environ Microbiol 7:396–404
Van der Putten WH, Vet LM, Harvey JA, Wäckers FL (2001) Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends Ecol Evol 16:547–554
Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Van Oosten VR, Bodenhausen N, Reymond P, Van Pelt JA, Van Loon LC, Dicke M, Pieterse CMJ (2008) Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol Plant-Microbe Interact 21:919–930
van Rhijn P, Vanderleyden J (1995) The Rhizobium-plant symbiosis. Microbiol Rev 59:124–142
van Wees SCM, de Swart EAM, van Pelt JA, van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate – and jasmonate-dependent defense pathways in Arabidopsis thaliana. Proc Natl Acad Sci U S A 97:8711–8716
Vance CP, Anderson JO, Sherwood RT (1976) Soluble and cell wall peroxidases in reed canary grass in relation to disease resistance and localized lignin formation. Plant Physiol 57:920–922
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Barbetti MJ, Li H, Woo SL, Lorito M (2008) A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol 72:80–86
Whitmore FW (1978) Lignin-carbohydrate complex formed in isolated cell walls of callus. Phytochemistry 17:421–425
Woo SL, Scala F, Ruocco M, Lorito M (2006) The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi and plants. Phytopathology 96:181–185
Yates IE, Bacon CW, Hinton DM (1997) Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis 81:723–728
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Par’e PW (2008) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56:264–273
Acknowledgments
HBS and BKS are grateful to the Department of Biotechnology, Govt. of India, for providing financial support (BT/PR5990/AGR/5/587/2012). SM is thankful to UGC for awarding Dr. D.S. Kothari Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Mishra, S., Singh, A., Keswani, C., Saxena, A., Sarma, B.K., Singh, H.B. (2015). Harnessing Plant-Microbe Interactions for Enhanced Protection Against Phytopathogens. In: Arora, N. (eds) Plant Microbes Symbiosis: Applied Facets. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2068-8_5
Download citation
DOI: https://doi.org/10.1007/978-81-322-2068-8_5
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2067-1
Online ISBN: 978-81-322-2068-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)