Abstract
Acinetobacter baumannii is an opportunistic nosocomial pathogen that causes severe infections especially in patients with compromised immune systems. Many Gram-negative pathogens regulate the expression of their virulence factors through cell density dependent N-acyl homoserine lactone (AHL)-mediated quorum-sensing (QS) system. A. baumannii has one quorum-sensing system which involves AbaR receptor protein that forms complex with AbaI (autoinducer synthase)-generated N-(3-hydroxydodecanoyl)-L-homoserine lactone (3-OH-C12 HSL) that regulates virulence factors, namely, biofilm formation and surface motility. Survival of A. baumannii against oxidative stress is also facilitated by QS-mediated expression of antioxidant enzymes, catalase and SOD. Phylogenetic analysis showed that A. baumannii may have acquired its QS genes from Halothiobacillus neapolitanus despite being closely related to Burkholderia ambifaria. A. baumannii shares its sites of infections in patients with Pseudomonas aeruginosa, another Gram-negative pathogen exhibiting QS. Both these pathogens show two-way AHL-mediated interspecies interactions. Such interspecies interactions could bear serious implications on severity and treatment of disease. In the present era of increasing multidrug resistance, alternative therapies like inhibition of quorum sensing seem attractive to control infections caused by A. baumannii.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Quorum Sense
- Acinetobacter Baumannii
- Homoserine Lactone
- Acyl Homoserine Lactone
- Interspecies Interaction
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Despite all advances in antimicrobials and vaccines development, infectious agents rank among the most common causes of death worldwide (Perez et al. 2007). Microorganisms cover almost all the outer surfaces of the human body and normally do no harm to the host. They exist as commensals and play a vital role in maintaining human body homeostatis. At times these microbes may also result in severe infections in individuals with a compromised immune system. Such microorganisms are called opportunistic. Breach in the first line of defence, i.e., the skin, may allow bacteria to enter the host. Production of various virulence factors by the pathogen may cause host tissue damage, thus leading to establishment of infection in the host (Fig. 1). Factors which predispose such occurrences include increased number of invasive medical procedures, long tenure of hospitalization and capability of the microorganisms to evolve mechanisms of antimicrobial resistance. An exponential increase in drug resistance among pathogens is a major challenge for the management of infectious diseases.
Acinetobacter baumannii is a Gram-negative, non-fermentative coccobacillus that is widely distributed in nature and colonizes the human skin. It has recently gained importance in the health-care setup because of its ability to survive in the hospital environment for extended periods of time which makes it a frequent cause of health-care-associated infections. A. baumannii shows immunity to disinfectants, can survive desiccation conditions and also resists antibiotics. It is an opportunistic pathogen which has been implicated in a wide spectrum of infections, e.g., nosocomial pneumonia, urinary tract infections, secondary meningitis and superinfections in burn patients (Adams et al. 2011) with varying frequency of occurrence (Gaynes and Edwards 2005). A. baumannii has emerged as an important pathogen associated with the most common autosomal genetic disorder with high death toll called cystic fibrosis (Davies and Rubin 2007). The infection rate of A. baumannii in populations is alarming and accounts for 20 % of all the organisms isolated from intensive care units (ICUs). The increasing number of A. baumannii-related infection cases has led to its comparison with methicillin-resistant Staphylococcus aureus (MRSA) and accounts for 5 % mortality in general wards and 54 % mortality in ICUs (Fournier and Richet 2006).
Global surveillance programmes conducted over the last decade have shown an unparallel increase in resistance rates against different classes of antimicrobials among the clinical isolates of Acinetobacter spp. (Adams et al. 2011). This situation is variable in different countries but has gained worldwide importance, and multiple outbreaks are being reported. A. baumannii shows resistance against major antibiotic classes, namely, broad-spectrum β-lactams (Bonnin et al. 2011), third-generation cephalosporins (Meyer et al. 2010), carboxypenicillins (Mammeri et al. 2003) and carbapenems (Poirel and Nordmann 2006). They also produce a wide range of aminoglycoside-inactivating enzymes, and most strains are resistant to fluoroquinolones (Singh et al. 2013). Such resistance pattern is defined as multidrug resistance (MDR) and is a major cause of worry as it is rapidly nullifying the therapeutic armamentarium. The emergence of carbapenem-resistant New Delhi metallo-β-lactamase-producing A. baumannii strains has started a new wave of MDR worldwide (Bonnin et al. 2012). Drug resistance in A. baumannii is due to both acquired and intrinsic mechanisms. The main underlying mechanisms of resistance to multiple antibiotics in Acinetobacter spp. can be summarized as follows:
-
1.
Production of hydrolyzing enzymes, e.g., β-lactam hydrolysis by different kinds of β-lactamases (Classes A to D)
-
2.
Changes in penicillin-binding proteins (PBPs) that prevent action of β-lactams
-
3.
Alterations in the structure and number of porin proteins that result in decreased permeability of antibiotics through the outer membrane of the bacterial cell
-
4.
The activity of efflux pumps that further decrease the concentration of antibiotics within the bacterial cell
MDR as well as the ability to stand environmental stresses makes eradication of A. baumannii infection difficult, particularly from hospital settings. A possible remedy to the emergence of A. baumannii as a superbug is to adopt anti-virulence strategy targeting its quorum-sensing system with compounds or enzymes holding quorum-quenching potential.
Quorum Sensing in A. baumannii
During infections, bacteria interact with each other (intraspecies), with other bacteria (interspecies) and with eukaryotic hosts. Gram-negative bacteria communicate with their neighbours through chemical signals, namely, N-acyl homoserine lactones (AHLs). The density of these signal molecules indicates the number of bacteria in a population. Single-celled bacteria at a minimum population unit, ‘quorum’, exhibit a complex pattern of multicellular behaviour. Such concerted population response is called quorum sensing (QS) (Diggle et al. 2007).
Quorum-sensing system in A. baumannii, like other Gram-negative bacteria, consists of:
-
Signal molecules: AHLs (autoinducers)
-
AbaI: AHL synthase
-
AbaR: AHL receptor (regulator)
Acyl Homoserine Lactones in A. baumannii
Crosstalking between Gram-negative bacteria belonging to the same or different species or genera is carried out through N-acyl homoserine lactones (AHLs) which are hormone like signal molecules. These molecules have a conserved homoserine lactone ring that is acylated with an acyl group at the α-position. AHL may contain a C4–C18 long acyl side chain with either oxo, hydroxy or no substitution at C3 position. Based on the length of the acyl chain, AHLs are classified as short- or long-chain molecules. A. baumannii synthesizes more than one long-chain (≥C10) AHLs (Table 1). Other species of Acinetobacter like A. calcoaceticus also produce one or more than one AHL molecules. However, none of the AHL signals could be specifically assigned to a particular species of this genus (Gonzalez et al. 2009). Since quorum signals are not homogenously distributed among the different strains of Acinetobacter, distinction between virulent and non-virulent strains on the basis of quorum-sensing signals is difficult. Transportation of AHLs is fundamental in the process of QS. Movement of AHLs from inside of the cell to the environment and vice versa is by diffusion where AHLs move freely across the bacterial membrane in a density-dependent manner. Since permeability of bacterial membrane is limited only to short-chain AHLs, transportation of long-chain AHLs (>C8) takes place through efflux pumps. However, very little is known about the pumps involved in transportation of AHLs in Acinetobacter.
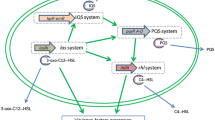
Quorum-sensing system in A. baumannii consists of one chromosomally encoded AHL-dependent signalling system, comprising of LuxI and LuxR homologues. abaI is responsible for the synthesis of 3-hydroxy-dodecanoyl-(L)-homoserine lactone (3-OH-C12 HSL), and the cognate receptor of this ligand is synthesized by abaR. When a threshold concentration is attained within the culture, the AHL molecules which are either present inside the cell or have been transported into it from the environment bind to its cognate receptor, AbaR (Fig. 2). This complex binds to the putative lux-box (CTGTAAATTCTTACAG) which is located 67 bp upstream the putative ATG of abaI and results in the synthesis of more AHL molecules. Since AHL molecules can induce production of more ‘like’ molecules themselves, they are referred to as autoinducers. It is suggested that all those virulence genes whose expression is controlled by AHL-mediated quorum-sensing system in Acinetobacter are likely to have the lux-box sequence in their respective promoter region. Since abaI is the only autoinducer synthase deciphered in A. baumannii which is responsible for synthesis of 3-OH-C12 HSL, other types of AHLs may probably be synthesized by the organism through the action of acyltransferases (Niu et al. 2008).
Structure of AHL Synthase (AbaI)
Sequence analysis of A. baumannii AHL synthase (AbaI) shows that it contains a conserved consensus pattern, [LMFYA]-R-x(3)-F-x(2)-W-x-[LIVM]-x(6,9)-E-x-D-x-[FY]-D, which is characteristic of autoinducer synthase family. The protein contains a single domain and is predicted to perform the function of signal transduction and quorum sensing. LasI, autoinducer synthase in Pseudomonas aeruginosa, is the only protein closest (46 % similarity and 27.5 % identity) to AbaI whose X-ray crystal structure (1RO5) is known (Gould et al. 2004). Thus, similarity in the primary structure of LasI and AbaI could form the basis for making predictions about the tertiary structure of AbaI. LasI structure is a three-layered (αβα) sandwich containing eight α helices (31 %) surrounding a highly twisted platform of nine β strands (29 %) (Fig. 3). This platform is composed of antiparallel β-sheet with prominent V-shaped cleft between parallel β-strands, β4 and β5. A six-stranded β-sheet platform is buttressed by three α helices, forming a V-shaped substrate binding cleft that leads to a tunnel passing through the enzyme, which could accommodate the acyl chain of acyl-acyl carrier protein (acyl-ACP). Residues Arg23, Phe27 and Try33 in the N-terminal region of LasI form the substrate binding pocket. Several of these residues, including Met79, Phe105, Thr142 and Thr144, are well conserved among AHL synthases and appear to position the acyl chain in the proper orientation for catalysis. The conservation of the same residues in AbaI suggests the same role being played by these residues in catalysis of AHL molecule in Acinetobacter. Mutational studies reveal that Arg23, Asp42, Asp44, Asp47, Arg70, Glu101, Ser103 and Arg104 residues play a role either in stabilizing the interactions or in catalytic function (Gould et al. 2004). AbaI is predicted to interact with chemical ligands, sulphate and zinc ions and contains two cysteine molecules which may be involved in disulphide bond formation.
Structure of AHL Receptor/Regulator (AbaR)
AHL receptor protein in A. baumannii, AbaR, is 198 amino acids in length and is 29.2 % identical and 45 % similar to autoinducer receptor, LasR, of P. aeruginosa. Since the structures of autoinducer synthases of P. aeruginosa (LasI) and A. baumannii (AbaI) are similar, the structure of AbaR too was predicted based on the X-ray crystallographic structure of LasR (3IX3). LasR protein is 239 amino acids in length and is comprised of 50 % helical and 15 % β-sheet structure (Fig. 4). The protein has LuxR-type DNA-binding, helix-turn-helix (HTH) domain of about 65 amino acids, present in transcription regulators of the LuxR/FixJ family of response regulators. The domain is named after V. fischeri LuxR, a transcriptional activator for quorum-sensing-controlled luminescence. The DNA-binding HTH domain is usually located in the C-terminal, and the N-terminal contains an autoinducer binding domain or a response regulatory domain. Most LuxR-type regulators act as transcription activators, but some can be repressors or have a dual role for different sites. LuxR-type HTH regulators control a wide variety of activities in various biological processes. Several structures of LuxR-type HTH proteins have been resolved and show that the DNA-binding domain is formed by a four-helix bundle. The HTH is involved in DNA-binding into the major groove, where the N-terminal part of the recognition helix makes most DNA contacts. The regulators bind DNA as multimers (Ducros et al. 2001; Egland and Greenberg 2001; Vannini et al. 2002; Pristovsek et al. 2003).
LuxR-type HTH protein can be activated to form multimers by four different mechanisms:
-
(a)
Regulators belonging to two-component sensory transduction systems in bacteria are activated by phosphorylation, generally on an aspartate residue, by a transmembrane kinase.
-
(b)
Regulators involved in quorum-sensing systems get activated on binding with autoinducer molecule. AbaR falls under this category with consensus pattern of the LuxR-type HTH domain: [GDC] – x(2) – [NSTAVY] – x(2) – [IV] – [GSTA] – x(2) – [LIVMFYWCT] – x – [LIVMFYWCR] – x(3) – [NST] – [LIVM] – x(2) – {T} – x(2) – [NRHSA] – [LIVMSTA] – x(2) – [KR]. It gets activated and dimerizes on binding to its cognate lactone (3-OH-C12 HSL).
-
(c)
Spore germinating protein (GerE) in Bacillus subtilis is regulated by an autonomous effector but lacks the regulatory domain.
-
(d)
Regulators with multiple ligand binding, exemplified by MalT in E. coli, activate the maltose operon by binding to maltose and ATP.
Evolution of A. baumannii Quorum-Sensing System
A. baumannii was found to be closest to Bukholderia ambifaria on the basis of 16S rRNA sequence. Phylogenetic comparison of autoinducer synthase (AbaI) and autoinducer receptor protein (AbaR) sequences of pathogenic and nonpathogenic organisms exhibiting quorum sensing shows that these two proteins of A. baumannii ATCC 17978 were most closely related to those of the archaebacterium Halothiobacillus neapolitanus. A. baumannii and H. neapolitanus belong to the order Pseudomonadales and Chromatidales, respectively. Evolutionary studies on members of the class Gammaproteobacteria suggested that Pseudomonadales are more recent than Chromatidales. This suggested that abaI might have been acquired by A. baumannii from H. neapolitanus and not from B. ambifaria with which it shared ancestry (Bhargava et al. 2010).
Pathogenicity and Virulence Factors of A. baumannii
Pathogenicity is the ability of microorganisms to cause disease. Virulence is the degree of pathogenicity of a microorganism that can vary among the members of the same species. Virulence depends on various parameters of the microorganism, the host and the interaction between both (Winn et al. 2005). An infection begins when the balance between the host resistance and bacterial pathogenicity is not stable. Although virulence factors responsible for pathogenicity of Acinetobacter are still not well defined, a few of them are described below:
Quorum-Sensing-Regulated Production of Virulence Factors in A. baumannii
Quorum sensing in A. baumannii is a central mechanism known for the autoinduction of two virulence factors: biofilm formation and surface motility (Fig. 5).
-
Biofilm: It is a sedentary mode of life exhibited by bacteria. Cells in biofilm, unlike their planktonic counterparts, attach themselves to the substratum irreversibly and exist as a sessile community. The biofilm cells show an altered phenotype with respect to growth rate and gene transcription as compared to the planktonic cells. The biofilm cells are embedded in a matrix of extracellular polymeric substances comprising of polysaccharides, proteins and DNA which makes it 1,000–1,500-fold more resistant to antibiotics as compared to the free living cells.
Formation of biofilm is a complex process which can be described in four stages: (1) attachment, (2) growth, (3) maturity and (4) dispersal. A. baumannii, like many other Gram-negative pathogens, also forms biofilm. Biofilm formation is a community phenotype and is associated with quorum sensing. An autoinducer synthase mutant (∆abaI) of A. baumannii strain M2, exhibiting a disrupted QS system, formed biofilm which was impaired in the later stages of development (Niu et al. 2008). Exogenous supplementation of its quorum-sensing molecule (3-OH-C12 HSL) compensated for the deficiency which occurred due to mutation and rescued the phenotype similar to the wild type. AbaI deficiency did not affect the survival of the pathogen but was vital for the formation of mature biofilm.
-
Surface motility: Bacteria use surface appendages such as flagella and pili to show motility of different types, namely, swimming, swarming, twitching, gliding and sliding. Motility is an important virulence factor as it allows bacteria to penetrate into the host followed by its colonization. Genetically, A. baumannii is regarded as nonmotile as genes coding for flagellar assembly responsible for motility are absent in it. However, it demonstrated motility on soft agar (agar concentration ranging from 0.2 to 0.4 %) which was defined as surface motility. In A. baumannii, surface motility was found to be quorum sensing dependent as decreased motility of autoinducer synthase mutant (∆abaI) of A. baumannii strain M2 was restored on exogenous addition of 3-OH-C12 HSL (Clemmer et al. 2011). Further, RNA sequencing and transcriptome analysis of A. baumannii M2 revealed a subset of the genes (A1S_0112 to A1S_0118) which were activated by quorum sensing. One of the genes, A1S_0119, encoded phosphopantetheine protein transferase which has a function in the production of a lipopeptide, but its role in virulence is not yet determined.
Other Virulence Factors
-
Hydrolytic enzymes: Many bacteria synthesize enzymes that play an important role in resisting the host immune system. Enzymatic activities, namely, esterases, certain aminopeptidases, ureases and acid phosphatases, have been reported in A. baumannii and may also be associated with virulence (Bergogne-Berezin et al. 2008). Esterases might help the pathogen to successfully enter the host and damage host lipid tissues by hydrolyzing short-chain fatty acids at ester linkages, while urease production might help them to colonize the hypochlorhydric or achlorhydric stomach causing inflammation (Rathinavelu et al. 2003).
-
Lipopolysaccharide (LPS): The outer membrane of A. baumannii contains LPS which renders the organism resistant to bactericidal action of host serum (Panilaitis et al. 2002). As a result, bacteria survive in the blood and cause infection. Diamine tetraacetic acid treatment made the pathogen susceptible to antibacterial action of serum as it reduced the LPS content.
-
Hydrophobicity: Surface hydrophobicity of bacteria protects it from being phagocytozed and plays an important role in attachment to various surfaces such as that of catheters. Surface hydrophobicity of strains obtained from catheter surfaces was higher than that of strains obtained from healthy carrier skin (Boujaafar et al. 1990). Thus, virulent and non-virulent bacteria could be differentiated on the basis of their hydrophobicity.
-
Siderophore – the iron regulator: A. baumannii isolates survive iron-deficient conditions by producing siderophores which solubilize the polymeric ferric oxy-hydroxides into soluble iron (Actis et al. 1993). The ability of bacteria to assimilate iron is known to be related to invasiveness and thus virulence. Other genes responsible for iron uptake in A. baumannii are regulated by the Fur protein of A. baumannii. Since Fur and Fur-like repressors are known to regulate some virulence-determinant genes in other bacteria (Wooldridge and Williams 1993), similar genes in A. baumannii may also regulate a subset of genes with vital role in pathogenesis.
-
Phospholipases: Phospholipases are group of enzymes produced by A. baumannii which cleave phospholipids present in the cell membrane and are linked to virulence.
Phospholipase-C (PLC) and phospholipase-D (PLD) remove glycerophosphate bond and the head group, respectively, from the phospholipid molecule. PLC production increases toxicity of epithelial cells (Camarena et al. 2010), whereas PLD production is linked to serum resistance and epithelial cell invasion (Jacobs et al. 2010).
Quorum-Sensing-Controlled Production of Superoxide Dismutase (SOD) and Catalase Enzymes
A. baumannii produces SOD and catalase to protect itself against toxic products of oxygen reduction, namely, hydrogen peroxide (H2O2), superoxide (O2 *) and hydroxyl (OH*) radicals. Pathogens are exposed to killing mechanisms by the host including various reactive oxygen species (ROS) generated by respiratory burst response. It is proposed that by the production of SOD and catalase enzymes, the pathogen is able to resist free radicals and persist under oxidative stress.
Autoinducer synthase mutant (∆abaI) of A. baumannii strain M2 produced lower levels of antioxidant enzymes, namely, SOD and catalase, as compared to the wild type (unpublished data). Production of these enzymes in the mutant increased on exogenous addition of the quorum-sensing molecule of A. baumannii (3-OH-C12 HSL). Treatment of the wild type with a known quorum-quencher salicylic acid interfered with the quorum-sensing system and decreased the SOD and catalase enzyme production. This suggested that production of antioxidant enzymes was controlled by AbaI-mediated quorum-sensing system in A. baumannii (Bhargava et al. 2014).
Crosstalking of A. baumannii with Other Pathogens
A. baumannii and P. aeruginosa have overlapping sites of infection and exist as coinfecting organisms in patients (Dent et al. 2011). None of the two pathogens showed any inhibitory effect on the other, and both could coexist stably in vitro (Bhargava et al. 2012). Comparative genomics analysis of A. baumannii with P. aeruginosa reveals that they share 65 % orthologs (Gospodarek et al. 2009). Both the organisms exhibit quorum sensing which involves acyl homoserine lactone (AHLs)-mediated regulation.
P. aeruginosa has two AHL-dependent quorum-sensing systems, lasI-R and rhlI-R, and one 2-heptyl-3-hydroxy-4-quinolone (PQS)-dependent quinolone system (Dekimpe and Deziel 2009). A. baumannii has only one AHL-dependent AbaI-R-mediated quorum-sensing system (Niu et al. 2008). LasR in P. aeruginosa is a transcriptional regulator protein which responds to N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12 HSL) generated by lasI, and AbaR in A. baumannii is a transcriptional regulator that responds best to abaI-generated N-(3-hydroxydodecanoyl)-l-homoserine lactone (3-OH-C12 HSL). Due to high similarity between the receptor proteins of the two organisms, they were probably able to interact with each other’s AHL molecules. Such flexibility of AbaR and LasR to C-12 HSL, irrespective of the modification at C3, suggested that A. baumannii and P. aeruginosa can show two-way communication with each other (Bhargava et al. 2012). It is suggested that interspecies interactions between A. baumannii and P. aeruginosa could have serious medical implications as both the nosocomial pathogens are associated with overlapping sites of infection and can influence disease courses and therapeutic control measures during infection.
Managing Acinetobacter Infections by Inhibiting Quorum Sensing
Quorum sensing has drawn the interest of medical community due to the role it plays in controlling the virulence of A. baumannii as it does in other Gram-negative pathogens such as Yersinia enterocolitica, P. aeruginosa and Vibrio cholera. Not much work has been reported on quorum-sensing inhibition of A. baumannii. The continued evolution of resistance mechanisms and alarming increase in the prevalence of multiple drug resistance among the emerging bacterial pathogens like A. baumannii necessitates the search for new antibacterial agents aimed at new targets. Quorum-sensing inhibition can provide an alternative treatment option when the ‘last- resort’ drugs such as colistin and tigecycline lose their efficacy. Since QS is not directly involved in essential processes, such as growth of the bacteria, one can reason that unlike antibiotics, the use of quorum-sensing inhibitors (quorum quenchers) will not lead to selective pressure on the bacteria for the development of resistance (Sperandio 2007). Misregulation or inhibition of quorum sensing can be achieved through three mechanisms:
-
1.
Inhibition of AHL synthesis
-
2.
Inhibition of AHL binding to AbaR receptor
-
3.
Quorum quenching enzymes
Inhibition of AHL Synthesis
Methylthioadenosine nucleosidase (MTAN) is a hydrolase found only in bacteria and not in humans which is involved in the synthesis of AHL. It salvages methionine for S-adenosyl methionine (SAM) regeneration and thus is linked to quorum sensing in Gram-negative bacteria. BuT-DADMe-ImmA is an MTAN inhibitor which could suppress AHL synthesis by disruption of SAM supply with high efficacy even at picomolar concentration (Schramm et al. 2008). Inhibition of MTAN could block the synthesis of quorum-sensing molecule without challenging the bacterial survival. Another protein with a vital role in AHL acyl chain formation is enoyl-acyl carrier protein reductase (FabI) and is a potential candidate for quorum-sensing inhibition of pathogenic bacteria. Triclosan at low concentration inhibited FabI to almost 50 % (Hoang and Schweizer 1999). Since enzymes like MTAN and FabI are involved in AHL synthesis, a process conserved in all Gram-negative bacteria exhibiting QS, it is suggested that inhibitors of these enzymes can be used to disrupt QS in A. baumannii as well.
Inhibition of AHL Binding to AbaR Receptor
Antagonists are structural analogues or chemicals which inhibit the binding of natural AHLs to AbaR receptor (Fig. 6). AHLs with non-native stereochemistry and chemicals containing aromatic acyl group act as antagonists and can disrupt the AbaR receptor’s binding activity for native AHLs in A. baumannii. L-Conformation of AHLs is biologically active while the inactive D-conformation is regarded as non-native. D-AHLs were successful in inhibiting quorum-sensing-regulated surface motility and biofilm formation in A. baumannii. Recently, it has been found that streptomycin (an aminoglycoside) at subinhibitory concentration acts as antagonist of AHL and prevents the formation of functional 3-OH-C12 HSL-AbaR complexes, thereby obstructing QS in A. baumannii (Saroj and Rather 2013). A macrolide like azithromycin, usually used for the treatment of cystic fibrosis patients infected with P. aeruginosa, besides showing antibacterial activity also decreases QS which reduces its pathogenicity and could show better clinical outcome (Skindersoe et al. 2008). Thus, it is suggested that these antibiotics could act as signalling agents in a concentration-dependent manner, thereby affecting quorum-sensing-mediated expression of virulence genes in pathogenic bacteria.
Compounds with not only structural but also functional similarity to AHLs are known as agonists. These can bind and activate AbaR to cause QS inhibition in A. baumannii. AHLs closely resembling OH-dDHLs (Stacy et al. 2012) and triazole HL (IV-AE) exhibited agonist activity (Stacy et al. 2013).
Quorum-Quenching Enzymes
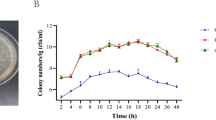
Gram-negative bacteria share common AHL types. Hence, enzymatic cleavage of these signalling moieties, also known as enzymatic quorum quenching, might be useful in broad-spectrum attenuation of virulence of various pathogens (Czajkowski and Jafra 2009). There are two kinds of quorum-quenching enzymes: (a) those that hydrolyze the lactone ring of AHL and (b) those that break the amide linkages of the AHLs. The former are called lactonases, while the latter are acylases (Fig. 7). Since the substitution groups on acyl chain do not affect the enzymatic activities, it is suggested that enzymes could be more effective in the disruption of a broad range of AHL signals. Lactonase from Geobacillus kaustophilus was engineered to enhance its activity against different AHLs (chain length range C6–C12) (Chow et al. 2014). The engineered lactonase was able to disrupt biofilm formation in A. baumannii (Fig. 8) due to its broad AHL specificity and could be used to treat infectious diseases caused by various Gram-negative bacteria.
Lactonase-mediated quorum quenching controls biofilm formation by Acinetobacter baumannii (Adapted from Tay and Yew 2013)
Conclusion
Bacteria have a tendency to adopt more economic processes such as quorum-sensing-controlled virulence which help the organism to conserve resources and avoid wasteful expression of virulence genes which are otherwise ineffective at lower cell densities (Czárán and Hoekstra 2009). The QS system, including autoinducer synthase and its cognate receptor, is regarded as a new target for antimicrobial strategies since, unlike antibiotics, quorum-sensing inhibitors control pathogenesis without placing immediate life-or-death pressure on the targeted pathogen. Currently there is a need to develop such alternate strategies to combat rapidly emerging multidrug resistance. Understanding the role of quorum-sensing-controlled network of genes in A. baumannii will suggest new targets and strategies to control infections.
References
Actis LA, Tolmasky ME, Crosa LM, Corsa JH (1993) Effect of iron-limiting conditions on growth of clinical isolates of Acinetobacter baumannii. J Clin Microbiol 31:2812–2815
Adams D, Yee L, Rimmer JA, Williams R, Martin H, Ovington C (2011) Investigation and management of an A. baumannii outbreak in ICU. Br J Nurs 20:140–147
Bergogne-Berezin E, Friedman H, Bendinelli M (2008) Acinetobacter biology and pathogenesis. Springer Science + Business Media, New York
Bhargava N, Sharma P, Capalash N (2010) Quorum sensing in Acinetobacter: an emerging pathogen. Crit Rev Microbiol 36:349–360. doi:10.3109/1040841X.2010.512269
Bhargava N, Sharma P, Capalash N (2012) N-acyl homoserine lactone mediated interspecies interactions between A. baumannii and P. aeruginosa. Biofouling 28:813–822. doi:10.1080/08927014.2012.714372
Bhargava N, Sharma P, Capalash N (2014) Pyocyanin stimulates quorum sensing-mediate tolerance to oxidative stress and increases persister cells population in Acinetobacter baumannii. Infect Immun pii:IAI.01600-14 [Epub ahead of print]
Bonnin RA, Nordmann P, Pottron A, Lecuyer H, Zahar JR, Piorel L (2011) Carbapenem hydrolyzing GES-type extended spectrum β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 55:349–354. doi:10.1128/AAC.00773-10
Bonnin RA, Poirel L, Naas T, Pirs M, Seme K, Schrenzel J, Nordmann P (2012) Dissemination of New Delhi metallo-β-lactamase-1-producing Acinetobacter baumannii in Europe. Clin Microbiol Infect 18:E362–E365. doi:10.1111/j.1469-0691.2012.03928.x
Boujaafar N, Freney J, Bouvet PJM, Jeddi M (1990) Cell surface hydrophobicity of 88 clinical strains of Acinetobacter baumannii. Res Microbiol 141:477–482. doi:10.1016/0923-2508(90)90073-Y
Camarena L, Bruno V, Euskirchen G, Poggio S, Synder M (2010) Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog 6:e1000834. doi:10.1371/journal.ppat.1000834
Chow JY, Yang Y, Tay SB, Chua KL, Yew SW (2014) Disruption of biofilm formation by the human pathogen Acinetobacter baumannii using engineered quorum-quenching lactonases. Antimicrob Agents Chemother 58:1802–1805. doi:10.1128/AAC.02410-13
Clemmer KM, Bonomo RA, Rather PN (2011) Analysis of surface motility in Acinetobacter baumannii. Microbiology 157:2534–2544. doi:10.1099/mic.0.049791-0
Czajkowski R, Jafra S (2009) Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol 56:1–16
Czárán T, Hoekstra RF (2009) Microbial communication, cooperation and chatting: quorum sensing drives the evolution of cooperation in bacteria. PLoS One 4(8):e6655. doi:10.1371/journal.pone.0006655
Davies JC, Rubin BK (2007) Emerging and unusual Gram-negative infections in cystic fibrosis. Semin Respir Crit Care Med 28:312–321. doi:10.1055/s-2007-981652
Dekimpe V, Deziel E (2009) Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155:712–723. doi:10.1099/mic.0.022764-0
Dent LL, Marshall DR, Pratap S, Hulette RB (2011) Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect Dis 10:196. doi:10.1186/1471-2334-10-196
Diggle SP, Crusz SA, Camara M (2007) Quorum sensing. Curr Biol 17:R907–R910
Ducros VM, Lewis RJ, Verma CS, Dodson EJ, Leonard G, Turkenburg JP, Murshudov GN, Wilkinson AJ, Brannigan JA (2001) Crystal structure of GerE, the ultimate transcriptional regulator of spore formation in Bacillus subtilis. J Mol Biol 306:759–771. doi:10.1006/jmbi.2001.4443
Egland KA, Greenberg EP (2001) Quorum sensing in Vibrio fischeri: analysis of the LuxR DNA binding region by alanine-scanning mutagenesis. J Bacteriol 183:382–386. doi:10.1128/JB.183.1.382-386.2001
Fournier PE, Richet H (2006) The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 42:692–699. doi:10.1086/500202
Gaynes R, Edwards JR (2005) Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41:848–854. doi:10.1086/432803
Gonzalez RH, Dijkshoorn L, Van den Barselaar M, Nudel C (2009) Quorum sensing signal profile of Acinetobacter and environmental sources. Rev Argent Microbiol 41:73–78
Gospodarek E, Bogiel T, Zalas-Wiecek P (2009) Communication between microorganisms as a basis for production of virulence factors. Pol J Microbiol 58:191–198
Gould TA, Schwelaer HP, Churchill MEA (2004) Structure of the Pseudomonas aeruginosa acyl-homoserine lactone synthase LasI. Mol Microbiol 53:1135–1146. doi:10.1111/j.1365-2958.2004.04211.x
Hoang TT, Schweizer HP (1999) Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J Bacteriol 181:5489–5497
Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison JM, Carson S, Sayood K, Iwen PC, Skaar EP, Dunman PM (2010) Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun 78:1952–1962. doi:10.1128/IAI.00889-09
Mammeri H, Poirel L, Mangeney N, Nordmann P (2003) Chromosomal integration of a cephalosporinase gene from Acinetobacter baumannii into Oligella urethralis as a source of acquired resistance to β-Lactams. Antimicrob Agents Chemother 47:1536–1542. doi:10.1128/AAC.47.5.1536-1542.2003
Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P (2010) Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit Care 14:R113
Niu C, Clemmer KM, Bonomo RA, Rather PN (2008) Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol 190:3386–3392. doi:10.1128/JB.01929-07
Panilaitis B, Johri A, Blank W, Kaplan D, Fuhrman J (2002) Adjuvant activity of emulsan, a secreted lipopolysaccharide from Acinetobacter calcoaceticus. Clin Diagn Lab Immunol 9:1240–1247. doi:10.1128/CDLI.9.6.1240-1247.2002
Perez F, Hujer AM, Hujer KM, Decker BK, Rather NP, Bonomo RA (2007) Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51:3471–3484. doi:10.1128/AAC.01464-06
Poirel L, Nordmann P (2006) Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836. doi:10.1111/j.1469-0691.2006.01456.x
Pristovsek P, Sengupta K, Löhr F, Schäfer B, von Trebra MW, Rüterjans H, Bernhard F (2003) Structural analysis of the DNA-binding domain of the Erwinia amylovora RcsB protein and its interaction with the RcsAB box. J Biol Chem 278:17752–17759. doi:10.1074/jbc.M301328200
Rathinavelu S, Zavros Y, Merchant JL (2003) Acinetobacter lwoffii infection and gastritis. Microbes Infect 5:651–657. doi:10.1016/S1286-4579(03)00099-6
Saroj SD, Rather PN (2013) Streptomycin inhibits quorum sensing in Acinetobacter baumannii. Antimicrob Agents Chemother 57:1926–1929. doi:10.1128/AAC.02161-12
Schramm VL, Gutierrez JA, Cordovano G, Basu I, Guha C, Belbin TJ, Evans GB, Tyler PC, Furneaux RH (2008) Transition state analogues in quorum sensing and sam recycling. Nucleic Acids Symp Ser 52:75–76. doi:10.1093/nass/nrn038
Singh H, Thangaraj P, Chakrabarti A (2013) Acinetobacter baumannii: a brief account of mechanisms of multidrug resistance and current and future therapeutic management. J Clin Diagn Res 7:2602–2605. doi:10.7860/JCDR/2013/6337.3626
Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, Bjarnsholt T, Nielsen TT, Hoiby N, Givskov M (2008) Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:3648–3663. doi:10.1128/AAC.01230-07
Sperandio V (2007) Novel approaches to bacterial infection therapy by interfering with bacteria-to-bacteria signalling. Expert Rev Anti Infect Ther 5:271–276. doi:10.1586/14787210.5.2.271
Stacy DM, Welsh MA, Rather PN, Blackwell HE (2012) Attenuation of quorum sensing in pathogen Acinetobacter baumannii using non native N-acyl homoserine lactones. ACS Chem Biol 7:1719–1728. doi:10.1021/cb300351x
Stacy DM, Le Quement ST, Hansen CL, Clausen JW, Tolker-Nielsen T, Brummond JW, Givskov M, Nielsen TE, Blackwell HE (2013) Synthesis and biological evaluation of Triazole-containing N-acyl homoserine lactones as quorum sensing modulators. Org Biomol Chem 11:938–954. doi:10.1039/c2ob27155a
Tay SB, Yew WS (2013) Development of quorum-based anti-virulence therapeutics targeting Gram-negative bacterial pathogens. Int J Mol Sci 14:16570–16599. doi:10.3390/ijms140816570
Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, De Francesco R, Neddermann P, Marco SD (2002) The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J 21:4393–4401. doi:10.1093/emboj/cdf459
Winn WC Jr, Allen SD, Janda WM, Koneman EW, Schreckenberger PC, Procop GW, Woods GL (2005) Koneman’s color atlas and textbook of diagnostic microbiology. Lippincott Williams & Wilkins, Philadelphia
Wooldridge KG, Williams PH (1993) Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev 12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Bhargava, N., Sharma, P., Capalash, N. (2015). Quorum Sensing in Acinetobacter baumannii . In: Kalia, V. (eds) Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1982-8_10
Download citation
DOI: https://doi.org/10.1007/978-81-322-1982-8_10
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1981-1
Online ISBN: 978-81-322-1982-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)