Abstract
Premature rise of progesterone in controlled ovarian stimulation cycles influences IVF outcome. Several authors failed to demonstrate any negative impact while others reported the detrimental effect associated with progesterone rise (pre-ovulatory). It seems that P rise >1.5 ng/ml may have deleterious effect on endometrial receptivity, accelerating the endometrial maturation process that desynchronizes the crosstalk between the embryo and endometrium during implantation. This decreases the pregnancy rate. Progesterone elevations on the day of hCG in GnRH analogue cycles are the result of the ovarian stimulation itself, driven by high follicle-stimulating hormone dosages, high oestradiol levels, the increased number of follicles and oocytes, increased sensitivity of LH receptor of the granulosa cells to FSH or poor ovarian response with increased LH sensitivity. To prevent the premature rise of progesterone in COS, we should use milder stimulation protocols, earlier trigger of ovulation in high responders and single-blastocyst transfer on day 5. The optimal GnRH analogue protocols during the entire stimulation period appear to be the long agonist as well as ‘long’ and long GnRH antagonist regimens (oral contraceptive pre-treated fixed antagonist regime). The most appropriate choice to avoid the negative effects of follicular progesterone elevations is to cancel fresh embryo transfer and to transfer frozen-thawed embryos in natural cycles.
Premature luteinization (PL) refers to a rise in serum progesterone (P) levels on the day of hCG administration. Most studies used an absolute P level on the day of hCG administration as an indicator of PL, and the cut-off level differed from 0.8 to 2 ng/mL. Some authors defined PL as a P/E2 ratio of >1. There is a marked variation in the incidence (13–71 %) of PL due to discrepancies in definition, population characteristics and/or treatment protocols. The pathogenesis of PL in COH is still poorly understood. Several hypotheses may be considered to explain this phenomenon: elevation of follicular LH levels, serum accumulation of HCG from HMG, increased LH receptor sensitivity of the granulosa cells to FSH or poor ovarian response with increased LH sensitivity. The consequences of this premature elevation of serum P on IVF outcome remain controversial. Attempts to prevent COH include use of low-dose hCG alone in the late COH stages, flexible antagonist protocol, use of mifepristone, aspiration of a single leading follicle and hCG administration when the levels of serum P exceeded 1.0 ng/mL.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Premature rise of progesterone
- Ovulation induction
- Endometrium
- Pregnancy rate
- Premature luetinization
- Ovarian stimulation
- LH
1 Introduction
The incidence of premature luteinizing hormone (LH) surge has significantly decreased by the introduction of gonadotropin-releasing hormone (GnRH) analogues for pituitary suppression in in vitro fertilization (IVF) [1]. Despite pituitary down-regulation, however, several researchers have described a phenomenon reported as premature rise in serum progesterone levels on the day of human chorionic gonadotropin (hCG) administration or late follicular phase [2]. Decreased implantation and pregnancy rates have been reported with this phenomenon. Its pathogenesis is still poorly understood. One of the major reasons for the controversy has been the diverse definitions of P rise in literature.
2 Definition
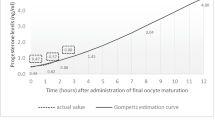
In past, an absolute progesterone concentration on the day of HCG administration was taken as an indicator of progesterone elevation with arbitrarily set cut-off concentrations ranging from 0.8 to 2 ng/ml [3–7]. In recently published studies, using new methods for serum progesterone assessment, this cut-off concentration is usually set at 1.5 ng/ml [8]. This cut-off is supported by the presence of a marked difference in endometrial gene expression profile between patients with a progesterone serum concentration above and below the threshold of 1.5 ng/ml on the day of HCG administration [8, 9].
More follicles produce more serum P. It would, therefore, be better to take into account the ovarian response, rather than the serum P levels only. Progesterone >1.5 ng/mL and P/E(2) >0.55 affect the clinical pregnancy rate. P/E(2) ratio is the only independent prognosticator for cycle outcome [10].
3 Incidence
There is a marked variation in the incidence of premature rise of progesterone due to discrepancies in definition, population characteristics and/or treatment protocols among the studies. Although the frequency of elevated serum progesterone concentrations varies, incidences as high as 35 % of stimulated cycles in women treated with GnRH agonists [3, 6] and 38 % of cycles in women treated with GnRH antagonists [7, 11] have been reported. However, in a large retrospective analysis of over 4,000 cycles, the incidence of progesterone rise (above 1.5 ng/ml) on the day of HCG administration was estimated to be 8.4 % in agonist and antagonist cycles [12].
4 Pathogenesis
The pathogenesis of P elevation in COS is still poorly understood. But it has become certain that it is multi-factorial. Several hypotheses may be considered to explain this phenomenon:
-
1.
In GnRH agonist cycles, P elevation is a magnitude response to FSH rather than LH [12, 13]. P elevation is positively correlated with (a) high FSH daily doses and total FSH doses, (b) prolongation of follicular phase, e.g. in rFSH/GnRH antagonist cycle delaying hCG administration 2 days after presence of >3 follicles (>17 mm) [15], (c) high oestradiol concentrations, (d) increased steroidogenic activity, (e) increased number of retrieved ocytes, (f) increased number of follicles. In a study [14], patients with P >1.5 ng/ML were found to have high concentration of oestradiol and increased number of follicles [2].
-
2.
Increased follicular steroidogenic activity: An excessive amount of progesterone is produced by granulosa cells as part of early luteinization. In COS cycles, there are excess number of follicles, each one producing a normal amount of progesterone consistent with the late follicular phase [2]. Early increase in progesterone levels that result from an initial intense FSH stimulation leads to increased granulosa cell steroidogenic activity [11] (mature granulosa cell response to high FSH exposure).
-
3.
Increased follicular phase LH activity: No relationship exists between LH and progesterone levels at the end of the follicular phase since the observed increases in progesterone were not accompanied by increases in LH [11].
-
4.
Serum accumulation of HCG from HMG [15]: A systematic review shows that providing LH activity supplementation in combination with FSH during ovarian stimulation does not have a consistent effect on serum progesterone concentrations at the time of hCG administration. However, these data also suggest that in accordance with physiological concepts, the timing of LH activity administration could influence the impact on serum progesterone level. Progesterone rise was even higher in recombinant FSH as compared with HMG ovarian stimulation [16, 17] supporting the fact that LH reduces progesterone level rather than contributing to progesterone rise. In a prospective study, LH rise was not found on the day of hCG stimulation in GnRH analogue cycles.
-
5.
Increased sensitivity of LH receptors of the granulosa cells to FSH: LH acts on granulosa cells when LH receptors have been induced by FSH at the later stage of follicular phase. In vitro experiments have clearly demonstrated that LH has a synergistic effect with FSH on granulosa cells to stimulate progesterone production [18, 19] and that LH is far more potent than FSH on granulosa cells to produce steroids as assessed by cAMP accumulation [19].
As the granulosa cells respond to FSH, proliferation and growth are associated with an increase in FSH receptors. The theca cells are characterized by steroidogenic activity in response to LH, converting pregnenolone into androgens. Aromatization of androgens to oestrogens is a distinct activity within the granulosa cell layer induced by FSH by activation of the P450 aromatase gene. Androgens produced in the theca layer diffuse into the granulosa layer, where they are converted to oestrogens that are released into the follicular fluid and from here into the peripheral circulation. Prior to ovulation, the granulosa cell layer is characterized by aromatization activity and conversion of theca androgens to oestrogens, an FSH-mediated response.
Factors that are associated with progesterone rise are the prolongation of the follicular phase (by delaying HCG administration) [20] and the oestradiol concentrations [14]. A study [20] reported that if the follicular phase is prolonged by 2 days after the presence of >3 follicles >17 mm is confirmed at ultrasound scan in recombinant FSH/GnRH antagonist stimulated cycles, a lower probability of ongoing pregnancy rate can be expected, probably through prolonged exposure of the endometrium to raised concentrations of progesterone. Hence, prolongation of stimulation is an important factor to be considered. Prolongation of follicular phase is related to the rise of oestradiol. Moreover, the rise in oestradiol concentration is associated with high risk of premature progesterone rise [21].
The adrenal is a secretory source of circulating progesterone during early follicular phase. This was demonstrated by the rapid rise of progesterone after administration of ACTH during suppression of endogenous gonadotropin secretion with triptorelin acetate. ACTH stimulates the conversion of cholesterol to pregnenolone in the adrenal cortex which is rapidly converted to progesterone. Moreover, it seems that the source of progesterone shifts towards the ovaries just prior to the ovulation [22].
Poor ovarian response with increased LH sensitivity. In poor ovarian responders, premature rise as defined by the P/E2 ratio was more prevalent. It was associated with poor ovarian response with increased LH sensitivity, similar to the report by Younis et al., who concluded that neither the LH nor the hCG content of the recombinant preparations is responsible for this elevation of P/E2 ratio level and suggested that P elevation is not necessarily an LH-dependent event and may be primarily related to an adversely affected cumulus–oocyte complex [23]. When considering P rise, ovarian response or reserve may be of critical importance [24]. The main factors associated with increased risk of progesterone rise during COS cycles are ovarian parameters, including the total FSH dose, the intensity of the ovarian response, and excess number of follicles or oocytes [15].
Recently emerging evidence points to the existence of an oocyte granulosa cell regulatory loop by which complementary signalling and metabolic pathways drive the development and function of both the oocytes and follicular somatic compartments [25, 26]. Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) are two well-characterized oocyte-derived growth factors that play crucial roles in follicle growth and ovulation in all mammalian species including humans [25–29]. Spontaneous mutations or genetic targeting of either Gdf9 or Bmp15 in mammals affect fertility in females [30]. Disruption of signalling in the ovarian granulosa cells leads to their premature luteinization [31].
5 Impact
The impact of premature serum progesterone elevation at the end of the follicular phase under controlled ovarian stimulation (COS) cycle for in vitro fertilization (IVF) is still debated. While several studies reported lower pregnancy rates in patients with high progesterone concentration on the day of human chorionic gonadotropin (hCG) administration [6, 11, 12, 29–32], one found a favourable effect on pregnancy outcome [33], and others failed to demonstrate any association [3, 4, 7].
No significant difference in pregnancy rate was observed by Hofman et al. [33] in patients undergoing IVF/embryo transfer with high or low progesterone concentrations on the day of HCG administration and in patients who received oocytes donated from women with high or low progesterone concentrations. On the contrary, other authors reported that pregnancy rate has been inversely related to serum progesterone levels on the day of HCG administration [3, 4, 6, 11]. The involved endocrinologic mechanism of such an observation, however, is unclear.
Adverse effects of elevated P levels on oocyte maturation, fertilization or early cleavage have been described [6, 11]. On the other hand, no negative impact of progesterone rise on oocyte/embryo quality could be found in several studies [2, 6, 34, 35]. Systematic review and meta-analysis conducted by Venetis C et al. showed that E2 levels (pg/mL) on the day of hCG administration were significantly higher in the group of patients that exhibited progesterone elevation on the day of hCG. No significant difference in the number of COCs retrieved was detected between the patients with and those without progesterone elevation on the day of hCG administration [2]. These findings suggest that P elevation may influence the endometrium, adversely affecting implantation and subsequent embryo development. Elevated progesterone levels might induce premature endometrial maturation and, as a consequence, earlier opening of the implantation window that leads to asynchronization of the crosstalk between embryo and endometrium. Accelerated endometrial maturation following COS has been clearly demonstrated by histological dating on the day of oocyte retrieval [8], but this is not the case during the implantation window [9]. When the endometrial receptivity was studied, findings pointed to an abnormally accelerated endometrial maturation but only during the pre-receptive secretory phase and not during the implantation window. Consequently, transfer of a day-3 embryo in such too precociously mature endometrium would not allow the proper establishment of the embryo-endometrium crosstalk; this might explain why the pregnancy outcome was impaired when embryo transfer was performed on day 3 (hCG + 5) in patients with high serum [P] on the day of hCG administration [36]. On the other hand, when embryo transfer was performed on day 5 (hCG + 7), no detrimental effect on the pregnancy outcome was observed. The deleterious effect of premature progesterone rise is probably not due to an impact on endometrial receptivity or ovarian parameters but rather to a desynchronized dialogue between embryo and endometrium. [37].
6 Prevention
If a negative association between progesterone elevation on the day of hCG administration and the probability of pregnancy exists, it might be worth examining the progesterone level at the beginning of a cycle and on the day of hCG administration, modification of the protocol and timing of triggering of final oocyte maturity, cryopreserving the resulting embryos and their transfer in a subsequent frozen-thawed cycle [6, 34]. A literature search identified several regimens for prevention of P elevation:
6.1 Milder Stimulation Protocols
To prevent follicular phase elevations, it might be preferable to use milder stimulation protocols. When comparing the optimal GnRH agonist with antagonist, it was found that with GnRH agonist cycle an early and stable suppression of endogenous FSH led to more synchronized development of follicles compared to fewer follicles and oocyte with fixed GnRH antagonist regime. Several RCTs comparing OC-pre-treated GnRH antagonist with long agonist protocols could not observe significant differences with respect to the number of oocytes retrieved and pregnancy rates [38–40].
6.2 Flexible Antagonist Protocol [41]
In IVF-ICSI patients undergoing COS with the antagonist protocol, the antagonist administration was initiated according to at least one of the following patient-specific criteria: (i) at least one follicle measuring >14 mm; (ii) oestradiol levels >600 pg/ml; and (iii) ET > 6 mm. Rapid response, causing earlier antagonist administration initiation, according to the proposed criteria for the prevention of premature LH surges, and the absence of P rise, as evidenced by normal progesterone levels on HCG day, were found to be independent positive predictive factors for favourable IVF outcome. The employment of an algorithm of criteria, aimed at the prevention of premature LH surges in a flexible antagonist protocol, resulted in antagonist initiation earlier than on stimulation day 6 in a significant proportion of patients. A higher pregnancy rate was observed in these patients.
6.3 Earlier Trigger for Ovulation [42]
Altering the timing of hCG injection according to serum progesterone concentrations improves embryo quality in cycles with subtle P rise. Serum was obtained daily or every 12 h from day 7 until the administration of hCG. hCG injection was given when the levels of serum P exceeded 1.0 ng/mL (‘rescued’ subtle P rise). The mean day of hCG administration in the rescued cycles was 1 day earlier than those of the subtle P rise and no P rise cycles. The rate of embryonic development beyond four-cell stage was increased significantly in the rescued cycles and no P rise cycles versus the subtle P rise cycles. Embryos obtained in the no P rise and rescued cycles were of better morphological quality than those obtained in the P rise cycles. The implantation rate was significantly higher in the rescued cycles than in the P rise cycles. The data suggest that if hCG is administered when a subtle P rise is detected, embryo quality and subsequent implantation rate can be improved.
An earlier hCG trigger in patients stimulated with rFSH/GnRH antagonists for IVF resulted in significant differences between the early-hCG and the late-hCG groups regarding oestradiol (1,388 versus 2,040 pg/mL, respectively) and P (0.8 versus 1.1 ng/mL, respectively) levels on the day of hCG. However, no significant differences were observed between the early-hCG and the late-hCG groups regarding positive hCG (46.2 versus 50 %, respectively) or ongoing pregnancy rates (34.6 versus 40.7 %, respectively). In cycles stimulated with rFSH and GnRH antagonists, an earlier administration of hCG was not associated with an increased probability of pregnancy, but investigators found significant difference in oestradiol and P level in early-hCG and late-hCG groups [43]. An earlier trigger in high responders in order to avoid premature progesterone elevation is feasible.
6.4 Cryopreservation and Subsequent Frozen-Embryo Transfer
The most appropriate choice to avoid the negative effects of follicular progesterone elevations is to cancel fresh embryo transfer and to transfer frozen-thawed embryos in natural cycles. Next cycle would have a more synchronized endometrium, thus improving implantation rates.
7 Conclusion
To prevent follicular phase elevations, it might be preferable to use milder stimulation protocols, earlier trigger of ovulation in high responders and single-blastocyst transfer on day 5. The optimal GnRH analogue protocols during the entire stimulation period appear to be the long agonist as well as ‘long’ and long GnRH antagonist regimens (oral contraceptive pre-treated fixed antagonist).
References
Smitz J, Ron-El R, Tarlatzis BC. The use of gonadotrophin releasing hormone agonists for in vitro fertilization and other assisted procreation techniques: experience from three centres. Hum Reprod. 1992;7 Suppl 1:49–66.
Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007;13(4):343–55.
Edelstein MC, Seltman HJ, Cox BJ, Robinson SM, Shaw RA, Muasher SJ. Progesterone levels on the day of human chorionic gonadotropin administration in cycles with gonadotropin-releasing hormone agonist suppression are not predictive of pregnancy outcome. Fertil Steril. 1990;54(5):853–7.
Givens CR, Schriock ED, Dandekar PV, Martin MC. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration do not predict outcome in assisted reproduction cycles. Fertil Steril. 1994;62(5):1011–7.
Hofmann GE, Bentzien F, Bergh PA, Garrisi GJ, Williams MC, Guzman I, Navot D. Premature luteinization in controlled ovarian hyperstimulation has no adverse effect on oocyte and embryo quality. Fertil Steril. 1993;60(4):675–9.
Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration in in vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril. 1994;61(3):508–13.
Ubaldi F, Albano C, Peukert M, Riethmuller-Winzen H, Camus M, Smitz J, et al. Subtle progesterone rise after the administration of the gonadotrophin-releasing hormone antagonist cetrorelix in intracytoplasmic sperm injection cycles. Hum Reprod. 1996;11(7):1405–7.
Van Vaerenbergh I, Fatemi HM, Blockeel C, Van Lommel L, In’t Veld P, Schult F, et al. Progesterone rise on HCG day in GnRH antagonist/rFSH stimu- lated cycles affects endometrial gene expression. Reprod BioMed Online. 2011;22(3):263–71.
Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, Bosch E. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26(7):1813–25.
Elgindy EA. Progesterone level and progesterone/estradiol ratio on the day of hCG administration: detrimental cutoff levels and new treatment strategy. Fertil Steril. 2011;95(5):1639–44.
Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, Pellicer A. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80(6):1444–9.
Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25(8):2092–100.
Fleming R, Jenkins J. The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod Biomed Online. 2010;21(4):446–9.
Kyrou D, Al-Azemi M, Papanikolaou EG, Donoso P, Tziomalos K, Devroey P, Fatemi HM. The relationship of premature progesterone rise with serum estradiol levels and number of follicles in GnRH antagonist/recombinant FSH-stimulated cycles. Eur J Obstet Gynecol Reprod Biol. 2012;162(2):165–8.
Kolibianakis EM, Albano C, Camus M, Tournaye H, Van Stierteghem AC, Devroey P. Prolongation of the follicular phase in in vitro fertilization results in a lower ongoing pregnancy rate in cycles stimulated with recombinant follicle- stimulating hormone and gonadotropin-releasing hormone antagonists. Fertil Steril. 2004;82(1):102–7.
Hugues JN, Masse-Laroche E, Reboul-Marty J. Impact of endogenous luteinizing hormone serum levels on progesterone elevation on the day of human chorionic gonadotropin administration. Fertil Steril. 2011;96(3):600–4.
Smitz J, Andersen AN, Devroey P, Arce JC, MERIT Group. Endocrine profile in serum and follicular fluid differs after ovarian stimulation with HP-hMG or recombinant FSH in IVF patients. Hum Reprod. 2007;22(3):676–87.
Lindeberg M, Carlstrom K, Ritvos O, Hovatta O. Gonadotrophin stimulation of non luteinized granulosa cells increases steroid production and the expression of enzymes involved in estrogen and progesterone synthesis. Hum Reprod. 2007;22(2):401–6.
Young EL, Hillier SG, Turner M, Baird DT, Ng SC, Bongso A, Ratnam SS. Differential regulation of cholesterol side-chain cleavage (P450scc) and aromatase (P450arom) enzyme mRNA expression by gonadotrophins and cyclic AMP in human granulose cells. J Mol Endocrinol. 1994;12(2):239–49.
Kolibianakis EM, Bourgain C, Papanikolaou EG, Camus M, Tournaye H, Van Steirteghem AC, Devroey P. Prolongation of follicular phase by delaying hCG administration results in a higher incidence of endometrial advancement on the day of oocyte retrieval in GnRH antagonist cycles. Hum Reprod. 2005;20(9):2453–6.
Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjiens P, Van Landuyt L, Devroey P. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/ GnRH antagonist? Hum Reprod. 2009;24(11):2902–9.
De Geyter C, De Geyter M, Huber PR, Nieschlag E, Holzgreve W. Progesterone serum levels during the follicular phase of the menstrual cycle originate from the crosstalk between the ovaries and the adrenal cortex. Hum Reprod. 2002;17(4):933–9.
Younis JS, Matilsky M, Radin O, Ben-Ami M. Increased progesterone/estradiol ratio in the late follicular phase could be related to low ovarian reserve in in vitro fertilization-embryo transfer cycles with a long gonadotropin releasing hormone agonist. Fertil Steril. 2001;76(2):294–9.
Hugues JN. Impact of ‘LH activity’ supplementation on serum progesterone levels during controlled ovarian stimula-tion: a systematic review. Hum Reprod. 2012;27(1):232–43.
Chand L, Ponnampalam P, Harris E, Winship M, Shelling N. Mutational analysis of BMP15 and GDF9 as candidate genes for premature ovarian failure. Fertil Steril. 2006;86(4):1009–12.
Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 2006;91(5):1976–9.
Juengel L, McNatty P. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11(2):143–60.
Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20(6):1406–22.
Kolibianakis EM, Venetis CA, Bontis J, Tarlatzis BC. Significantly lower pregnancy rates in the presence of pro- gesterone elevation in patients treated with GnRH antagonists and gonadotrophins: a systematic review and meta-analysis. Curr Pharma Biotechnol. 2012;13(3):464–70.
Andersen AN, Devroey P, Arce JC. Clinical outcome following stimulation with highly purified hMGorre combinant FSH in patients undergoing IVF: a randomized assessor-blind controlled trial. Hum Reprod. 2005;21(12):3217–27.
Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, Zhu G. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97(6):1321–7.
Doldi N, Marsiglio E, Destefani A, Gessi A, Merati G, Ferrari A. Elevated serum progesterone on the day of HCG administration in IVF is associated with a higher pregnancy rate in polycystic ovary syndrome. Hum Reprod. 1999;14(3):601–5.
Melo M, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohí J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod. 2006;21(6):1503–7.
Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte in-vitro fertilization. Hum Reprod. 1993;8(9):1506–11.
Fanchin R, Righini C, Olivennes F, de Ziegler D, Selva J, Frydman R. Premature progesterone elevation does not alter oocyte quality in in vitro fertilization. Fertil Steril. 1996;65(6):1178–83.
Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C, et al. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril. 2009;91(3):949–52.
Haouzi D, Bissonnette L, Gala A, Assou S, Entezami F, Perrochia H, et al. Endometrial receptivity profile in patients with premature progesterone elevation on the day of HCG administration. Biomed Res Int. 2014;2014:951937.
Huirne JA, Hugues JN, Pirard C, Fischl F, Sage JC, Pouly JL, et al. Cetrorelix in an oral contraceptive-pretreated stimulation cycle compared with buserelin in IVF/ICSI patients treated with r-hFSH: a randomized, multicentre, phase IIIb study. Hum Reprod. 2006;21(6):1408–15.
Rombauts L, Healy D, Norman RJ, Orgalutran Scheduling Study Group. A comparative randomized trial to assess the impact of oral contraceptive pre treatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. Hum Reprod. 2006;21(1):95–103.
Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to GnRH agonists for use in IVF? Hum Reprod. 2007;22(11):2805–13.
Lainas T, Zorzovilis J, Petsas G, Stavropoulou G, Cazlaris H, Daskalaki V, et al. In a flexible antagonist protocol, earlier, criteria-based initiation of GnRH antagonist is associated with increased pregnancy rates in IVF. Hum Reprod. 2005;20(9):2426–33.
Harada T, Katagiri C, Takao N, Toda T, Mio Y, Terakawa N. Altering the timing of human chorionic gonadotropin injection according to serum progesterone concentrations improves embryo quality in cycles with subtle P rise. Fertil Steril. 1996;65(3):594–7.
Kyrou D, Kolibianakis EM, Fatemi HM, Tarlatzis BC, Tournaye H, Devroey P. Is earlier administration of human chorionic gonadotropin (hCG) associated with the probability of pregnancy in cycles stimulated with recombinant follicle-stimulating hormone and gonadotropin-releasing hormone (GnRH) antagonists? A prospective randomized trial. Fertil Steril. 2011;96(5):1112–5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Sharma, R.K., Kapoor, A. (2015). Premature Rise of Progesterone During Ovarian Stimulation. In: Ghumman, S. (eds) Principles and Practice of Controlled Ovarian Stimulation in ART. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1686-5_25
Download citation
DOI: https://doi.org/10.1007/978-81-322-1686-5_25
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1685-8
Online ISBN: 978-81-322-1686-5
eBook Packages: MedicineMedicine (R0)