Abstract
Maize, one of the three most popular cereal crops of the world, globally contributes 15 % of the protein and 20 % of the calories derived from food crops in the world’s diet. However, cereals do not provide a nutritionally balanced source of protein. For nutritional security, it is necessary to adopt a genetic enhancement strategy in which essential amino acids are either incorporated or increased in grain protein to alleviate hunger, increase income, and improve livelihood. Quality protein maize (QPM) is having high nutritive value of endosperm protein with opaque2 (o2) mutation leading to 60–100 % increased content of lysine and tryptophan. The lysine value of o2 maize is 2.5–4.0 g/100 g of endosperm protein, which is more than twice that of the normal maize (1.3 g lysine/100 g protein). International Maize and Wheat Improvement Center (CIMMYT), Mexico, played a significant role in the development of QPM maize. The breeding of QPM involves three genetic systems: (i) the recessive mutant allele of the o2 gene, (ii) the endosperm hardness modifier genes, and (iii) the amino acid modifier genes influencing free amino acid content in the endosperm. Due to recessive nature of the o2 gene, complex action of modifier genes, and presence of amino acid enhancer genes, the use of DNA marker-assisted selection (MAS) accelerated the selection efficiency and expedited the development of new QPM cultivars. Using a combination of MAS and phenotypic selection techniques, a single cross, short duration Vivek QPM 9 hybrid was developed and released in 2008 by Vivekananda Parvatiya Krishi Anusandhan Sansthan, Almora, India. Alternatively, manipulating the plant lysine metabolic pathway provides possible enzyme targets for genetic engineering to increase free lysine content in corn grain. Furthermore, RNA interference (RNAi) has been used to specifically suppress α-zein production in transgenic corn, resulting in a doubling of the lysine content of corn grain. QPM is likely to gain wider acceptance if QTLs for kernel modification, and enhancers for amino acids are fine mapped to develop markers to follow MAS for vitreous kernels and high levels of lysine. However, the major constraints in adoption of QPM hybrids are contamination with normal maize pollen in field, resulting in erosion of the trait in farmer-saved seed system. It is essential to give training on good seed production practices to the local communities and development of linkage between the seed producers, farmers, and the industry for sustainable higher nutritional benefits of QPM in the long term.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Maize (Zea mays L.) is one of the world’s three most popular cereal crops. It is a major cereal crop for both livestock feed and human nutrition worldwide and of great economic importance not only as a grain and fodder crop but also as an industrial raw material. Maize ranks next to rice and wheat with respect to area and grain production. India is the fifth largest producer of maize in the world contributing about 3 % of the global production. In India, maize was grown on an area of 8.33 million hectare with productivity of 2.4 tonnes/hectare in the year 2010–2011. The demand for cereal grains will continue to increase as a consequence of the expanding human population, which could add >1.5 billion people by the year 2025 (Lutz 2001). However, cereals do not provide a nutritionally balanced source of protein. Protein-containing foods are necessary for the rapid growth of children (Millward and Rivers 1989), and in some countries, maize is a primary weaning food for babies.
From nutritional perspective, the protein of normal maize is deficient in the essential amino acids, lysine and tryptophan (Bhatia and Rabson 1987). Protein deficiency, especially in children, causes kwashiorkor, a potentially fatal syndrome characterized by initial growth failure, irritability, skin lesions, edema, and fatty liver. Nutritional deficiency is of concern, particularly for people with high protein requirements, e.g., young children, pregnant and lactating women, and patients. For nutritional security, it is necessary to adopt a genetic enhancement strategy in which essential amino acids are either incorporated or increased in grain protein to alleviate hunger, increase incomes, and improve livelihoods.
2 Maize Protein
Maize kernel consists of endosperm, embryo, and the outer seed coat. Both the embryo and endosperm contain proteins but differ substantially in the content and the quality of the protein. The endosperm accounts for 80–85 % and the embryo 8–10 % of the total kernel dry weight. The endosperm of maize has two district regions. The aleurone layer, the outermost layers, is rich in hydrolytic enzymes. The starch-rich endosperm is present within the aleurone layer, which is vitreous (Fig. 4.1). The protein fraction constitutes only 8–10 % of the endosperm, while starch accounts for about 70 % of the kernel (Lawton and Wilson 1987; Prasanna et al. 2001). The various fractions of endosperm protein are albumin 3 %, globulin 3 %, zein (prolamine) 60 %, and glutelin 34 %, whereas the embryo protein largely consists of albumins (60 %).
Structure of maize kernel (Source:http://www.public.iastate.edu/~becraft/Endosperm.htm)
The zein proteins found in vitreous region form insoluble accretions called protein bodies in the lumen of rough endoplasmic reticulum and toward maturation are densely packed between starch grains (Gibbon and Larkins 2005). Based on their solubility, genetic properties, and the apparent molecular masses, zeins have been classified into α-zein (22 and 19 kDa), the most abundant; β-zein (14 kDa); γ-zein (27 and 16 kDa); and δ-zein (10 kDa) (Wilson et al. 1981). The zein fraction α is rich in cysteine, while β and γ fractions are rich in methionine. The zein fraction in normal maize generally contains a higher proportion of leucine (18.7 %), phenylalanine (5.2 %), isoleucine (3.8 %), valine (3.6 %), and tyrosine (3.5 %) but smaller amounts of other essential amino acids such as threonine (3 %), histidine and cysteine (1 %), methionine (0.9 %), and lysine (0.1 %) and is devoid of tryptophan. The non-zein protein fraction is balanced and rich in lysine and tryptophan. The high proportion of zeins in the endosperm is the primary reason for the poor protein quality of maize (Vasal 2000). Protein supplementation to correct such deficiencies is costly and wasteful of energy in animal nutrition and is not feasible for most developing countries, which rely on cereal for human consumption. Therefore, increasing the amounts of protein-bound essential amino acids depends on improving cereal storage proteins.
3 High-Lysine Mutants in Maize
Several mutations, both spontaneous and induced, have been recognized that affect the amino acid composition of maize endosperm. In the early 1920s, opaque2 (o2) mutation was first described by Jones and Singleton; but later on in 1963, researchers at Purdue University, USA, discovered that opaque2 (o2) increases grain proteins nearly twice as those of normal maize proteins (Mertz et al. 1964). In fact, maize homozygous for the recessive o2 allele has substantially higher lysine (>69 %) and tryptophan content compared to normal maize. Nelson et al. (1965) discovered another mutation, the floury2 gene, which in homozygous form also increases lysine and tryptophan levels in maize. Later on, a large number of mutants were discovered, which fall into three categories: the recessive mutants opaque1 through opaque17 (o1, o2, o5, o7, o9–o11, and o13–o17), the semidominant floury mutants (fl1, fl2, and fl3), the dominant mutants Mucronate (Mc), and Defective endosperm B30 (De-B30). The position of these mutations and zein genes on maize genetic map is shown in Fig. 4.2. The o2 and De-B30 mutants are located on chromosome 7, fl2 on chromosome 4, fl3 on chromosome 8, and o7 on chromosome 10. Chromosome 2 carries o4, o8, fl1, and Mc mutants.
It has been reported that recessive mutations affect regulatory genes, whereas semidominant and dominant mutations affect the storage proteins. All these mutations are associated with increase in non-zein protein content and decrease in the level of zein subunits. However, premature death of the mutant seedlings and the complex genetics behind lysine trait hinder utilization of these mutant genes except for o2 in corn breeding and production (McWhirter 1971; Nelson 1979, 1981). Epistatic interactions have also been reported among various regulatory mutants; o2 and o7 are epistatic over fl2, whereas o2 and Mc have synergistic effect (Prasanna and Sarkar 1991).
4 Beginning of Genetic Manipulation of Protein Quality
The development of quality maize begins initially with the manipulation of o2 and fl2 mutants singly or in combination. Later on, it was realized that fl2 conversion programs were difficult to handle as the kernel expression and quality depended on dosage effect. On the other hand, o2 mutants have simple recessive inheritance, no dosage effect, and easily scorable phenotypic expression as opaque kernels help in identification of the presence/absence of o2 gene. The intensive work for utilization of o2 mutation in breeding programs was continued in 1969 at CIMMYT, Mexico. All potentially important maize material was converted to opaque2 with varying degrees of backcrosses. India was among the first few countries in the world to focus on improvement of maize quality and a research program initiated in 1966 under the All India Coordinated Maize Improvement Project (AICMIP). Three o2 composites, namely, Shakti, Rattan, and Protina, were developed and commercially released in 1970 (Dhillon and Prasanna 2001; Prasanna et al. 2001). The o2 composites and hybrids were experimentally tested and grown commercially in Brazil, Colombia, India, the USA, South Africa, Yugoslavia, and Hungary during the late 1960s and early 1970s. The o2 maize had some pleiotropic effects such as reduced grain yield, chalky and dull kernel appearance, susceptibility to ear rots and stored grain pests, and slower drying of grain following physiological maturity. Due to these limitations, the o2 maize did not become popular with farmers as well as with consumers, despite its nutritional superiority. The kernel became soft and more prone to mechanical damage that decreased the yield by 8–15 %, and the plants were more susceptible to fungi and insects (Lambert et al. 1969; Salamini et al. 1970). The search then continued by exploiting other mutants in breeding programs such as o6, fl3, and Mc, but none of them showed any additional advantage over o2 and declined the efforts for enhancing the nutritional value of maize kernel. Double-mutant combination of o2su2 had vitreous and small kernels and protein quality at par to o2 but reduced grain yield by 15–25 %, whereas o2fl2 double-mutant combination resulted in vitreous kernels only in few genetic backgrounds. Despite the discouraging results, researchers at CIMMYT, Mexico; the University of Natal, South Africa; and Crow’s Hybrid seed company at Milford, IL, continued their efforts for improving the protein quality of maize. The only viable strategy that came out was to combine o2 with genetic modifiers of o2 for rewarding results.
5 Development of Quality Protein Maize (QPM)
Maize breeders began to identify modifier genes (mo2) that alter the soft, starchy texture of the endosperm, giving it a normal appearance while maintaining the increased essential amino acid content of o2 germplasm. During the process of converting normal maize to o2 version, partially hard endosperms were observed by Paez et al. (1969). The plant breeders at CIMMYT, Mexico (Vasal et al. 1980), and Pietermaritzburg, South Africa (Gevers and Lake 1992), developed hard endosperm o2 mutants, designated as quality protein maize (QPM). QPM looks and performs like normal maize, except that their nutritional value gets improved. Since the mid-1990s, QPM was tested at multiple research stations all over the world, with encouraging results. Data from 32 locations across Africa, Asia, and Latin America showed that QPM hybrids were capable of outperforming the normal in some of the poorest parts of countries such as China, Mexico, Ghana, and Peru. In India, it led to the development of a modified, nutritionally superior o2 composite in 1997, designated as “Shakti-I.” The QPM research gained further momentum by the launch of National Agricultural Technology Project (NATP) on QPM in 1998 by the Indian Council of Agricultural Research (ICAR). In this project, a multidisciplinary team of multi-institutes involving Directorate Maize Research (DMR), New Delhi; Punjab Agricultural University (PAU), Ludhiana; Chaudhary Charan Singh Haryana Agricultural University (CCSHAU), Karnal Center; Acharya NG Ranga Agricultural University (ANGRAU), Hyderabad; and Rajendra Agricultural University (RAU), Pusa, wherein the QPM germplasm received from CIMMYT was acclimatized to suit the local agroclimatic conditions in India. The lines were evaluated for their productivity and deployed in combination breeding, which led to release of first QPM three-way cross hybrid, Shaktiman-1, in 2001 followed by the release of the first QPM single-cross hybrid, Shaktiman-2, in 2004. Another QPM single-cross hybrid, HQPM-1, is the first yellow grain QPM single-cross hybrid released for its cultivation across the country in 2005. Later in the series of QPM, Shaktiman-3, Shaktiman-4, HQPM-5, HQPM-7, and Vivek QPM-9 were released. Vivek QPM-9 has a unique distinction of the first molecular-marker-assisted (MAS) converted product of normal hybrid Vivek-9. The cultivation of QPM hybrids will ensure higher income to farmers as well as nutritionally superior food to the consumers. Therefore, the QPM can be a strong support to the mission of food and nutritional security of the country particularly in underprivileged and tribal regions, where maize is consumed as a staple food. QPM will also ensure quality feed for poultry and animal sector, which are the largest consumers of maize in India.
6 Molecular Basis of o2 and Modifier Gene Action
The breeding of QPM involves three genetic systems: (i) the recessive mutant allele of the o2 gene, (ii) the endosperm hardness modifier genes, and (iii) the amino acid modifier genes influencing free amino acid content in the endosperm. Isolation and characterization of o2 gene revealed that it encodes a transcriptional factor that regulates the expression of zein gene and a gene encoding a ribosomal inactivating protein (Schmidt et al. 1990; Lohmer et al. 1991; Bass et al. 1992). The o2 mutation typically reduces the level of 22-kDa α-zein and enhances the synthesis of a number of non-zein proteins, particularly EF-1α, that contain relatively higher levels of lysine and tryptophan (Damerval and Devienee 1993; Habben et al. 1993; Gaziola et al. 1999). In higher eukaryotes, EF-1α is encoded by a multigene family. In maize, there are 10–15 genes, five of which are expressed in endosperm (Carneiro et al. 1999). Wu et al. (2002) identified two significant QTLs (on chromosomes 2S and 4S) and two suggestive QTLs (on chromosomes 5S and 6S) for EF-1α. The opaque2 gene is also known to be involved in the synthesis of the enzyme lysine-ketoglutarate reductase that is associated with free lysine degradation. As a consequence, in the grain with o2 mutation, a dramatic reduction in this enzyme leads to a corresponding increase in free lysine in the endosperm (Brochetto-Braga et al. 1992). The o2 allele is inherited in a simple recessive manner, and the presence of o2 allele in homozygous state (o2o2) is the prerequisite for the entire process of obtaining high-lysine/high-tryptophan maize.
The genetic modifiers are complex in their inheritance and they are additive in nature. The expression of modifiers varies with respect to kernel modification, density, and biochemical composition of the grain of the genetic background. Genetic analysis has shown that there are two major modifier genes: one modifier locus is tightly linked with γ-zein (27 KDa) encoding sequence near the centromere of chromosome 7, while the other is near the telomere of chromosome 7L (Lopes et al. 1995). Using two independently developed QPM lines, Holding et al. (2008) mapped several major o2 modifiers (Opm) QTLs to chromosomes 1, 7, and 9. In addition, five other QTLs are also present: one each on chromosomes 5S, 7L, and 10S and two on chromosome 1L. So far, 10 gene modifiers have been identified. A microarray hybridization and real-time PCR performed with RNA from true breeding o2 progeny with vitreous and opaque kernel phenotypes identified several candidate genes that were upregulated and mapped at or near the Opm QTLs, suggesting linkage of o2 endosperm modification with these differentially expressed genes. One of the QTLs is linked to the 27-kDa γ-zein locus on chromosome 7S. Moreover, QPM lines have 2–3-fold higher levels of the 27-kDa γ-zein, but the physiological significance of this increase is not known. Because the genes encoding 27- and 16-kDa γ-zein are highly conserved in DNA sequence, Wu et al. (2010) introduced a dominant RNAi transgene into a QPM line (CM105Mo2) to get rid of their expression. Elimination of γ-zeins disrupts endosperm modification by o2 modifiers, signifying their hypostatic action to γ-zeins. Abnormalities in protein body structure and their interaction with starch granules in the F1 with Mo2/+; o2/o2; γRNAi/+ genotype suggest that γ-zeins are essential for restoring protein body density and starch grain interaction in QPM. RNAi is to eliminate pleiotropic effects caused by o2, resulting in protein bodies formation such as honeycomb-like structures that clearly demonstrate that γ-zeins play a mechanistic role in QPM molecular breeding. Proteomic analysis revealed that altered starch structure was associated with endosperm modification in QPM as granule-bound starch synthase I (GBSS I) level was increased in modified kernels, which alters the amylopectin branching in QPM. Compared with normal and soft o2 genotypes, amylopectin in QPM had reduced levels of intermediate length α-1, 4-linked glucose chains. As a consequence, the QPM starch swelled more than the normal and formed tight contacts between starch granules in mature endosperm, resulting in vitreous kernel phenotype. However, the mechanism by which modifier genes create a vitreous phenotype is yet to be understood. The beneficial alleles of the modifying loci that control γ-zein production can be selected using a rapid, low-cost, light table method. Due to segregation of genes for endosperm hardness (or softness), varying degrees of softness/hardness are expressed in the endosperm of segregating generations (i.e., varying levels of opaqueness are observed on a light table (Fig. 4.3). Gradation in the opaqueness is scored on a 1–5 scale for easy description of the various classes. Score 1 is given for fully modified kernels (0 % opaqueness), scores 2, 3, and 4 for 25 %, 50 %, and 75 % opaqueness, respectively, whereas score 5 for soft, chalky, and 100 % opaque kernels.
The modified o2 version maize lines have varying levels of lysine and tryptophan content. This indicates presence of amino acid enhancer genes (minor modifying genes), which necessitate systematic biochemical evaluation of lysine and tryptophan levels in each segregating generation. At least three genes associated with lysine level have been mapped to locations on chromosomes 2, 4, and 7 (Wang et al. 2001). Ten significant and one suggestive QTL for free amino acid (FAA) content were identified on all 10 chromosomes (Wu et al. 2002). The QTL on chromosome 2L is coincident with genes encoding two aspartate kinase enzymes, which control important steps in amino acid biosynthesis and lysine degradation pathways. A negative correlation was observed between endosperm texture trait and amino acid content (Gutierrez-Rojas et al. 2008). Therefore, their regular monitoring at each step is essential as one could end up with a maize cultivar having the o2o2 genotype with lysine and tryptophan levels equivalent to those in normal maize (Krivanek et al. 2007).
7 Molecular Interventions for Enhancing the Protein Quality
The expression of the o2 allele is specific to seeds, and recessive, conventional introgression approaches require the inclusion of a selfing progeny test to monitor the introgression of the o2 allele within each backcross (BC) population, and also higher plant population, enormous time duration, labor, and spatial resources are required. Numerous advantages such as reduced time and population size are known to accrue to the breeder by use of marker-facilitated genotype selection rather than classical phenotypic selection or by genetic engineering for endosperm-specific expression of high lysine and tryptophan content.
7.1 Molecular Breeding for QPM
Mapping and tagging of agriculturally important genes have been greatly accelerated by an array of molecular markers in crop plants. The use of DNA markers in backcrossing has greatly increased the efficiency of selection. The practical application of molecular markers in crop improvement is in marker-assisted selection (MAS), which is of great importance as it helps in improving the efficiency of plant breeding through specific transfer of genomic regions of interest (foreground selection) and accelerating the recovery of the recurrent parent genome (background selection). MAS have been more extensively employed for simply inherited traits. In 2001, the primer sequence of three SSR markers (phi112, umc1066, and phi057), all at the o2 locus, were released at the website www.agron.missouri.edu, which facilitated the study and application of the o2 gene. Phi057 is located between G box and 3 upstream ORF in the leader sequence of the o2 gene, and its mutation can affect transcription of the o2 gene. The umc1066 and phi057 are located in exon 1 and exon 6, respectively; these are the two largest exons among the six exons within the o2 gene. To follow MAS, there are three main steps:
-
1.
Validation of markers, i.e., the markers for the target gene should give polymorphism between recipient and the donor parent.
-
2.
Foreground selection of the target gene with linked molecular markers and recombinant selection for recurrent parent allele at markers flanking the target allele. Flanking markers are used to select rare individuals that are the result of recombination near the target gene, thus minimizing the effects of linkage drag (Ibitoye et al. 2010). Using very close markers is the only way to reduce linkage drag, substantially.
-
3.
Marker-assisted background selection for noncarrier chromosomes. Using markers, it can be achieved by BC2 generation (Visscher et al. 1996; Hospital and Charcosset 1997, Frisch et al. 1999a, b), thus saving four BC generations accelerating the rate of recurrent parent genome recovery.
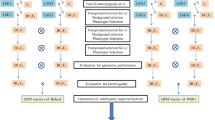
The utility of three maize SSR markers present within the o2 gene in molecular-marker-assisted selection for o2 has been successfully used for conversion of non-QPM to QPM lines in many parts of the world including CIMMYT, Mexico; VPKAS, Almora; and PAU, Ludhiana, in India and elsewhere in Asia and Africa (Babu et al. 2005; Manna et al. 2005; Jompuk et al. 2006; Gupta et al. 2009; Agrawal and Gupta 2010; Zhang et al. 2010). At PAU, Ludhiana, three microsatellite primers, viz., phi112, umc1066, and phi057, within the gene o2 have been surveyed among non-QPM lines (LM5, LM6, LM11, LM12, LM13, and LM14), CML lines (161, 163, 170, 189, 193, and 165), DMR lines (7, 11, 56–1, 56–4, 56–8, 72, and 74), and derived QPM lines (in different generations, S1–S4) for their validation. With phi057, three alleles were detected, and this primer clearly distinguished between QPM and normal lines. With primer phi112, null allele was observed in most of the QPM lines except CML 189, DMR 7, DMR 56–8, and few derived QPM lines. With primer umc1066, only two of the non-QPM lines, viz., LM11 and LM13, were distinguished from QPM lines (unpublished data). Xu and Crouch (2008) also reported that umc1066 is easily visualized on agarose gels but is commonly not polymorphic in CIMMYT breeding populations, while phi112 is a dominant marker and hence cannot be used in the identification of heterozygotes in F2/BC populations. It is best to use three markers together in MAS for high-lysine maize materials (Babu et al. 2005; Danson et al. 2006; Mboogoi et al. 2006). A rapid line conversion strategy using MAS program is given in Fig. 4.4. Using combination of MAS and phenotypic selection techniques, linkage drag can be reduced by selecting for flanking markers of recipient allele type (selection of either single or double recombinants) and recovery of the maximum amount of recurrent parent genome (90–95 %) coupled with higher lysine and tryptophan content within a short span of 3 years, which would otherwise require 10 generations by conventional backcrossing method.
Marker-assisted foreground and background selection scheme for quality protein maize (Source: Babu et al. 2005)
Danson et al. (2006) used a modifier marker, umc 1216, to select modifiers for the o2 phenotype showing two peaks for the non-QPM at 112 bp and 115 bp, while only one peak for the QPM donor at 115 bp. However, Micic-Ignjatovic et al. (2008) reported this marker as monomorphic. So, this marker could not be employed in MAS for selection of modified endosperm. Effective markers associated with modifying loci for both endosperm hardness and amino acid levels need to be identified. Besides, phenotypic selection is applied for grain hardness and amino acid content; the rapid line conversion strategy using MAS was followed to develop QPM hybrid Vivek QPM 9 in India at VPKAS, Almora (Gupta et al. 2009), which was released in 2008 At PAU, Ludhiana, non-QPM lines, viz., LM11, LM12, LM13, and LM14, have been converted into QPM versions, which are parental lines of two important hybrids, Buland (LM11 × LM12) and PMH1 (LM13 × LM14).
Another high-lysine mutant opaque16 (o16) has been isolated from the Robertson Corn Mutant library in Guizhou Institute of Upland Food Crops, China. It has been mapped on long arm of chromosome 8 and umc1141 marker is linked to this gene. Double-recessive mutant effect of the o16 with o2 was evaluated on lysine content and the double-recessive hybrid showed 6 % higher lysine content than both the parents (Yang et al. 2005; Zhang et al. 2010). This information may be useful for marker-assisted selection and gene pyramiding in high-lysine maize breeding programs.
7.2 Transgenics for QPM
Efforts in genetic transformation are focused on developing a dominant opaque2 trait in maize. RNA interference (RNAi) has been used to specifically suppress α-zein production in transgenic corn, resulting in doubling of the lysine content of corn grain. Segal et al. (2003) used RNAi technology to engineer transgenic maize for 22-KD α-zein gene, producing a dominant opaque phenotype. The phenotype segregated in a normal Mendelian fashion and eliminated 22-kD zein without affecting the accumulation of other zein proteins. It was also found that the dominant phenotypes generated were correlated with increased lysine content. Similarly in another experiment, 19-kD alpha-zeins got reduced by using antisense transformation constructs (Huang et al. 2004, 2005). In both the studies, the increase of lysine was far below than is reported in o2o2 maize. Huang et al. (2006) changed the gene construct and used a double-stranded RNA (ds RNA) to suppress the 19-kD and 22-kD α-zein gene families. Thus they could achieve the lysine 5.62 % and tryptophan 1.22 %, which is higher that is achievable with opaque2. This approach of using ds RNA looks promising. While the dominant nature of the antisense transgene is a definite advantage as compared to recessive allele of o2, the opaque endosperm still needs to be modified by endosperm modifier genes whose epistasis with the transgene has not yet been tested.
Alternatively, the plant lysine metabolic pathway provides possible enzyme targets for genetic engineering to increase free lysine content in corn grain. As shown in Fig. 4.5, lysine, along with methionine, threonine, and isoleucine, is derived from aspartate; dihydrodipicolinate synthase (DHDPS) catalyzes the first committed step of lysine biosynthesis. A bifunctional enzyme, lysine-ketoglutarate reductase/saccharopine dehydrogenase (LKR/SDH), is responsible for lysine catabolism (Azevedo et al. 2006; Stepansky et al. 2006).
(a) The lysine metabolic pathway in plants. In the biosynthesis of aspartate (Asp)-derived amino acids, dihydrodipicolinate synthase (DHDPS) catalyzes the committed step to (Lys) production and is sensitive to feedback inhibition by Lys (red line). The bifunctional lysine catabolic enzyme lysine-ketoglutarate reductase/saccharopine dehydrogenase (LKRSDH) is activated by excess lysine (green line). Other amino acids (shaded box) and metabolites (open box) shown in the pathway are threonine (Thr), methionine (Met), isoleucine (Ile), glutamate (Glu), 3 aspartate semialdehyde (ASA), and α-aminoadipic-8-semialdehyde (AASA). (b) The design of a bifunctional transgene cassette that simultaneously deregulates lysine biosynthesis and reduces lysine degradation (Source: Huang et al. 2008)
The free lysine level in plant cells is thought to be regulated by lysine feedback inhibition of DHDPS and feed-forward activation of LKR/SDH. Indeed, the expression of a lysine feedback-insensitive DHDPS from Corynebacterium glutamicum, CordapA, as well as the suppression of LKR/SDH have resulted in transgenic corn with higher levels of free lysine (Huang et al. 2005; Houmard et al. 2007). To overcome lysine catabolism in the endosperm, maize plants were transformed with a single endosperm-specific bifunctional expression/silencing transgene, which encodes a bacterial feedback-insensitive DHDPS with a LKR/SDH RNAi sequence in an intron (Frizzi et al. 2008). The suppression of LKR/SDH in the endosperm tissue increases free lysine to 1,324 ppm (~30-fold increase). The combination of CordapA expression and LKR/SDH suppression in a single transgene produces over 4,000 ppm free lysine (~100-fold increase), the highest ever reported in corn kernels. This construct resulted in a significant elevation in the seed lysine level, proving that maize endosperm possesses enzymatic activity responsible for both lysine biosynthesis and catabolism. GM crops with higher levels of other important amino acids, namely, methionine, tryptophan, and threonine, are also expected. The opportunities for and the impacts of GM crops with enhanced nutritional quality depend on public acceptance.
8 Nutritional Superiority and Biological Value of QPM
Maize, being a potential crop in India, occupies an important place as a source of human food (25 %), animal feed (12 %), poultry feed (49 %), industrial products mainly as starch (12 %), and 1 % each in brewery and seed. The maize grain accounts for about 15–56 % of the total daily calories in diets of people in about 25 developing countries, particularly in Africa and Latin America, where animal protein is scarce and expensive and consequently unavailable to a vast sector of the population (Prasanna et al. 2001). Research suggests that QPM can help reduce protein deficiencies, particularly in young children, where maize dominates in the diets (Vasal 2000). In QPM varieties, leucine/isoleucine ratio was improved and became better balanced which in turn is considered beneficial as it helps to liberate more tryptophan for more niacin biosynthesis, thus helping to combat pellagra (Prasanna et al. 2001). The other nutritional benefits of QPM include higher calcium and carbohydrate (Bressani 1995) and carotene utilization (De Bosque et al. 1988).
Because of the 60–100 % increase in these two essential amino acids, increased digestibility, and increased nitrogen uptake relative to normal-endosperm maize, the biological value of QPM is about 80 %, whereas that of normal maize is 40–57 % (Bressani 1992). The lysine content of o2 maize is 2.5–4.0 g/100 g of protein, which is more than twice that of an endosperm from the normal maize (1.3 g lysine/100 g protein). Only 37 % of common maize protein intake is utilized compared to 74 % of the same amount of o2 maize protein. A minimum daily intake of approximately 125 g of o2 maize might guarantee nitrogen equilibrium. This could not be obtained by using even twice the amount of normal maize. The nitrogen balance index for skim milk and o2 maize protein is 0.80 and 0.72, respectively, which indicates that the protein quality of QPM is 90 % of that of milk. Besides, around 24 g of normal maize per kg of body weight is required for nitrogen equilibrium, compared to only around 8 g for QPM (Bressani 1995; Graham et al. 1980). This nutritional value of the QPM genotypes meets the requirements of preschool children for their protein needs (Mertz et al. 1964; Vasal 2000).
The decreased level of zein (5–27 %) in o2 maize in contrast to normal endosperm (54–59 %) considerably improves its nutritional quality. Another important application of QPM is as animal feed, especially for monogastric animals such as pigs and poultry, as it results in improved growth. Small-holding farmers (who typically cannot afford balanced feeds) and commercial farmers find this extremely remunerative. Therefore, the development and adoption of improved QPM cultivars have significant potential to alleviate hunger, increase incomes, and improve livelihoods.
9 Conclusion
Malnutrition is one of the important issues in India and the world. Therefore, development of QPM hybrids, which are widely grown in specific locations will provide food and nutritional security and thereby help in reducing poverty. QPM is likely to gain wider acceptance if QTLs for kernel modification and enhancers for amino acids are fine mapped to develop markers to follow MAS for vitreous kernels and high levels of lysine. It also provides an ideal platform for stacking of other nutritional genes for enhanced Fe and Zn content and low phytate content for multiple benefits. However, the major constraint in adoption of QPM hybrids is contamination with normal maize pollen in field, resulting in erosion of the trait in farmer-saved seed system. It is essential to give training on good seed production practices to the local communities and development of linkage between the seed producers, farmers, and the industry for sustainable higher nutritional benefits of QPM in the long term.
References
Agrawal PK, Gupta HS (2010) Enhancement of protein quality of maize using biotechnological options. Anim Nutr Feed Tech 10S:79–91
Azevedo RA, Lancien M, Lea PJ (2006) The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 30:143–162
Babu R, Nair SK, Kumar A, Venkatesh S, Sekhar JC, Singh NN, Srinivasan G, Gupta HS (2005) Two-generation marker-aided backcrossing for rapid conversion of normal maize lines to quality protein maize. Theor Appl Genet 111:888–897
Bass HW, Webster C, O’Brien GR, Roberts JKM, Bostou RS (1992) A maize ribosome inactivating protein is controlled by the transcriptional activator opaque 2. Plant cell 4:225–234
Bhatia CR, Rabson R (1987) Relationship of grain yield and nutritional quality. In: Olson RA, Frey KJ (eds) Nutritional quality of cereal grains: genetic and agronomic improvement, vol 28, Agronomy monograph. ASA, CSSA and SSSA, Madison, pp 11–43
Bressani R (1992) Nutritional value of high-lysine maize in humans. In: Mertz ET (ed) Quality protein maize. American Association of Cereal Chemists, St. Paul
Bressani R (1995) Proceedings of the international symposium on quality protein maize EMBRAPA/CNPMS, Sete Lagaos, Brazil, pp 41–63
Brochetto-Braga MR, Leite A, Arruda P (1992) Partial purification and characterization of lysine-ketoglutarate reductase in normal and opaque-2 maize endosperms. Plant Physiol 98:1139–1147
Carneiro NP, Hughes PA, Larkins BA (1999) The eEF1A gene family is differentially expressed in maize endosperm. Plant Mol Biol 41:801–813
Damerval C, Devienee D (1993) Quantification of dominance for proteins, pleiotropically affected by opaque-2 in maize. Heredity 70:38–51
Danson JW, Mercy M, Michael K, Martin L, Alex K, Alpha D (2006) Marker assisted introgression of opaque2 gene into herbicide resistant elite maize inbred lines. Afr J Biotech 5:2417–2422
De Bosque C, Castellanos EJ, Bressani R (1988) Reporte annual. INCAP, Guatemala
Dhillon BS, Prasanna BM (2001) In: Chopra VL (ed) Breeding field crops, Oxford & IBH, New Delhi, pp 149–185
Frisch M, Bohn M, Melchinger RAE (1999a) Comparison of selection strategies for marker assisted back crossing of a gene. Crop Sci 39:1295–1301
Frisch M, Bohn M, Melchinger RAE (1999b) Minimum sample size and optimum positioning of flanking markers in assisted back crossing for transfer of target gene. Crop Sci 39:967–975
Frizzi A, Huang S, Gilbertson LA, Armstrong TA, Luethy MH, Malvar TM (2008) Modifying lysine biosynthesis and catabolism in corn with a single bifunctional expression/silencing transgene cassette. Plant Biotech J 6:13–21
Gaziola SA, Alessi ES, Guimaraes PEO, Damerval C, Azevedo RA (1999) Quality protein maize: a biochemical study of enzymes involved in metabolism. J Agri Food Chem 47:1268–1275
Gevers HO, Lake JK (1992) Development of modified opaque-2 maize in South Africa. In: Mertz ET (ed) Quality protein maize. American Association of Cereal Chemists, St. Paul, pp 111–121
Gibbon BC, Larkins BA (2005) Molecular genetics approaches to developing quality protein maize. Trends Genet 21:227–233
Graham GG, Placko RP, Maclean WC (1980) Nutritional value of normal, opaque2 and, sugary 2 and opaque 2 maize hybrids for children and infants 2. Plasma free amino acids. J Nutr 110:1070–1074
Gupta HS, Aggarwal PK, Mahajan V, Mani VP, Bisht GS, Kumar A, Verma P, Babu R (2009) Quality protein maize for nutritional security : rapid development of short duration hybrids through molecular marker assisted breeding. Curr Sci 96:230–237
Gutierrez-Rojas A, Scott MP, Leyva OR, Menz M, Betran J (2008) Phenotypic characterization of quality protein maize endosperm modification and amino acid contents in a segregating recombinant inbred population. Crop Sci 48:1714–1722
Habben JE, Kirlies AW, Larkin BA (1993) The origin of lysine containing proteins in opaque-2 maize endosperm. Plant Mol Biol 23:825–838
Holding DR, Hunter BG, Chung T, Gibbon BC, Ford CF, Bharti AK, Messing J, Hamaker BR, Larkins BA (2008) Genetic analysis of opaque-2 modifier loci in quality protein maize. Theo App Genet 117:157–170
Hospital F, Charcosset A (1997) Marker-assisted introgression of quantitative trait loci. Genetics 147:1469–1485
Houmard NM, Mainville JL, Bonin CP, Huang S, Luethy MH, Malvar TM (2007) High-lysine corn generated by endosperm-specific suppression of lysine catabolism using RNAi. Plant Biotech J 5:605–614
Huang SS, Adams WR, Zhou Q, Malloy KP, Voyles DA, Anthony J, Kriz AL, Luethy MH (2004) Improving nutritional quality of maize proteins by expressing sense and antisense zein genes. J Agri Food Chem 52:1958–1964
Huang S, Kruger DE, Frizzi A, D'Ordine RL, Florida CA, Adams WR, Brown WE, Luethy MH (2005) High-lysine corn produced by the combination of enhanced lysine biosynthesis and reduced zein accumulation. Plant Biotech J 3:555–569
Huang S, Frizzi A, Florida CA, Kruger DE, Luethy MH (2006) High lysine and high tryptophan transgenic maize resulting from the reduction of both 19- and 22-kDα-zeins. Plant Mol Biol 61:525–535
Huang S, Frizzi A, Malvar T (2008) Genetically engineered high lysine corn. ISB News Report. pp 1–3
Ibitoye DO, Akin-Idowu PE (2010) Marker – assisted – selection (MAS): a fast track to increase genetic gain in horticultural crop breeding. Afr J of Biotech 52:8889–8895
Jompuk P, Wongyai W, Jamptong C, Apisitvanich S (2006) Detection of quality protein maize (QPM) using simple sequence repeat (SSR) markers and analysis of tryptophan content in endosperm. Nat Sci 40:768–774
Krivanek AF, Groote DE, Gunaratna H, Diallo NS, Friesen D (2007) Breeding and disseminating quality protein maize for Africa. Afr J Biotech 6:312–324
Lambert RJ, Alexander DE, Dudley JW (1969) Relative performance of normal and modified protein (opaque-2) maize hybrid. Crop Sci 9:242–243
Lawton JW, Wilson CM (1987) Proteins of the kernel. In: White PJ, Johnson LA (eds) Corn chemistry and technology. American Association of Cereal Chemists, St. Paul, pp 313–354
Lohmer S, Maddaloni M, Motto M, Dilonzo N, Hartings A, Salamini F, Thomson RD (1991) The maize regulatory locus opaque-2 encodes a DNA binding protein which activates the transcription of the B-32 gene. Embo J 10:617–624
Lopes MA, Takasaki K, Bostwick DE, Helentjaris T, Larkins BA (1995) Identification of opaque-2 modifier loci in quality-protein-maize. Mol Gen Genet 247:603–613
Lutz W (2001) The end of world population growth. Nature 412:543–545
Manna R, Okello DK, Imanywoha J, Pixley K, Edema R (2005) Enhancing introgression of the opaque-2 trait into elite maize lines using simple sequence repeats. Afr Crop Sci J 13:215–226
Mboogoi MN, Danson JW, Kimani M (2006) Using biotechnology to develop high lysine maize. Afr J of Biotech 5:693–696
McWhirter KS (1971) A floury endosperm, high lysine locus on chromosome 10. Corn Genet Coop Newslett 45:184
Mertz ET, Bates LS, Nelson OE (1964) Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 145:279–280
Micic-Ignjatovic D, Ristic D, Markovic K, Lazic-Jancic V, Denic M (2008) Quality protein maize – QPM. Genetika 40:205–214
Millward DJ, Rivers JP (1989) The need for indispensable amino acids: the concepts of the anabolic drive. Diabetes-Metab Rev 5:191–211
Nelson OE (1979) More precise linkage data on fl3. Maize Genet Coop Newslett 53:56
Nelson OE (1981) The mutations opaque-9 through opaque-13. Corn Genet Coop Newslett 55:68
Nelson OE, Mertz ET, Bates LS (1965) Second mutant gene affecting the amino acid pattern of maize endosperm proteins. Science 150:1469–1472
Paez AV, Helm JL, Zuber MS (1969) Lysine content of opaque-2 maize kernels having different phenotypes. Crop Sci 9:251–252
Prasanna B, Sarkar K (1991) Coordinate genetic regulation of maize endosperm. Maize Genetics Perspectives ICAR, pp 74–86
Prasanna BM, Vasal SK, Kassahun B, Singh NN (2001) Quality protein maize. Curr Sci 81:1308–1319
Salamini F, Borghi B, Lorenzon C (1970) Effect of opaque-2 gene on yield in maize. Euphytica 19:531–538
Schmidt RJ, Burr FA, Aukerman MJ, Burr B (1990) Maize regulatory gene opaque2 encodes protein with a “leucine zipper” motif that binds to zein DNA. Proc Natl Acad Sci U S A 87:46–50
Segal G, Song R, Messing J (2003) A new opaque variant of maize by a single dominant RNA interference-inducing transgene. Genetics 165:387–397
Stepansky A, Less H, Angelovici R, Aharon R, Zhu X, Galili G (2006) Lysine catabolism, an effective versatile regulator of lysine level in plants. Amino Acids 30:121–125
Vasal SK (2000) The quality protein maize story. Food Nutr Bull 21:445–450
Vasal SK, Villegas E, Bjarnason M, Gelow B, Goertz P (1980) Genetic modifiers and breeding strategies in developing hard endosperm opaque material. In: Pollmer WG, Phillips RH (eds) Improvement of quality traits of maize for grain and silage used. Mortinus Nijhoff Press, London, pp 37–73
Visscher PM, Haley CS, Thompson R (1996) Marker assisted introgression in backcross breeding programs. Genetics 144:1923–1932
Wang XL, Woo YN, Kim CS, Larkins BA (2001) Quantitative trait locus mapping of loci influencing elongation factor 1 alpha content in maize endosperm. Plant Physiol 125:1271–1282
Wilson CM, Shewry PR, Miflin BJ (1981) Maize endosperm proteins compared by sodium dodecyl gel electrophoresis and isoelectric focussing. Cereal Chem 58:275–281
Wu RL, Lou XY, Ma CX, Wang XL, Larkins BA, Casella G (2002) An improved genetic model generates high-resolution mapping of QTL for protein quality in maize endosperm. Proc Natl Acad Sci U S A 99:11281–11286
Wu YR, Holding DR, Messing J (2010) γ-zein are essential for endosperm modification in quality protein maize. Proc Natl Acad Sci USA 29:12810–12815
Xu Y, Crouch JH (2008) Marker assisted selection in plant breeding: from publication to practice. Crop Sci 48:391–407
Yang W, Zheng Y, Zheng W, Feng R (2005) Molecular genetic mapping of high lysine mutant gene (Opaque-16) and the double recessive effect with opaque-2 in maize. Mol Breed 15:257–269
Zhang WL, Yang WP, Chen ZW, Wang MC, Yang LQ, Cai YL (2010) Molecular marker assisted selection for o2 introgression lines with o16 gene in corn. Acta Agronomica Sinica 36:1302–1309
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Vikal, Y., Chawla, J.S. (2014). Molecular Interventions for Enhancing the Protein Quality of Maize. In: Chaudhary, D., Kumar, S., Langyan, S. (eds) Maize: Nutrition Dynamics and Novel Uses. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1623-0_4
Download citation
DOI: https://doi.org/10.1007/978-81-322-1623-0_4
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1622-3
Online ISBN: 978-81-322-1623-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)