Abstract
The quality of maize protein is poor due to the deficiencies of two essential amino acids, lysine and tryptophan, and excess of leucine. However, the discovery of association of high lysine and tryptophan with opaque-2 maize endosperm in 1964 opened up new vistas in improving maize protein quality. Consequently, many countries started developing maize varieties incorporating opaque-2 gene, and in the Indian Maize Programme, three opaque-2 composite varieties were developed and released for commercial production in the year 1970. Though the nutritional superiority of opaque-2 maize over normal maize was very well established, the newly developed high-lysine varieties could not become popular because of agronomic and acceptability problems associated with their soft chalky endosperm. To circumvent this problem, efforts were initiated in 1971 itself towards developing hard-endosperm opaque-2 maize lines/strains through the continuous process of vigorous selection for kernel vitreosity and monitoring tryptophan/lysine for maintaining protein quality. The modified opaque-2 maize with hard endosperm and having high lysine and tryptophan is known as quality protein maize (QPM). At present, in India we have one QPM composite and more than nine QPM hybrid varieties released, and many more are in pipelines. However, what is required is the popularization of these varieties with farmers as well as consumers highlighting their nutritional significance, especially to the vulnerable group, i.e. infants and preschool children and pregnant and lactating mothers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In India, maize is the third most important food crop after rice and wheat. Its production has reached 19.77 million tons, out of which about 25 % is used as human food, 12 % as animal feed and 49 % as poultry feed (Dass et al. 2008). Thus, maize plays an important role in food and nutritional security. Besides being the principal source of carbohydrates and energy, maize like other cereals is also the largest single source of protein in the diet of the people for whom it is a staple food. However, the nutritional quality of maize protein is poor because of imbalanced amino acid composition due to deficiencies of two main essential amino acids, lysine and tryptophan, and excess of leucine. Alcohol-soluble protein fraction zein (prolamine), which is extremely deficient in lysine and tryptophan, is the most abundant protein (40–60 %) in maize endosperm. In whole grain of maize varieties, the protein content ranges from 8.5 % to 13.6 %, whereas lysine from 2.5 % to 3.6 % and tryptophan from 0.37 % to 0.67% of protein. In mature maize kernel, endosperm accounts for 80–85 % and contributes as much as 80 % of total kernel protein. Embryo (germ) accounts for 8–10 % of total weight and contributes 15–20 % of total protein. The niacin/tryptophan deficiency disease, pellagra, caused by high concentration of leucine or high leucine–isoleucine ratio, is commonly associated with maize-eating people. The deficiency is recognized by 3 Ds: dermatitis, diarrhoea and dementia.
The discovery of association of high lysine and tryptophan with opaque-2 maize endosperm by Mertz et al. (1964) opened up new vistas in improving the protein quality of maize. The opaque-2 (o2) single recessive gene, which is located on chromosome 7, encodes a transcriptional activator that regulates the expression of alpha-zein genes (Schmidt et al. 1990). In the presence of opaque-2 gene, the synthesis of 22 kD alpha-zein, which is extremely deficient in lysine and tryptophan, is reduced and the synthesis of non-zein protein fractions, particularly glutelin, is increased in the endosperm resulting in overall increase in the concentrations of lysine and tryptophan in endosperm protein. In addition, there exists a highly positive correlation between lysine concentration and protein synthesis factor EF-1alpha, which is a lysine-rich abundant protein and can be used as a biochemical marker in the assay of lysine-containing proteins (Habben et al. 1995; Moro et al. 1996).
2 Essential Amino Acids and Protein Quality
Proteins are an important constituent of our diet and are required for (i) foetal development in pregnancy and milk output during lactation, (ii) growth in infants and children and (iii) maintenance (replacing the wear and tear in tissues) in adults. Amino acids are the building blocks of the proteins. Food and tissue proteins contain 20 amino acids, of which eight (nine for infants) are designated as ‘essential amino acids’, since they cannot be synthesized in the body. The rest of the amino acids are called ‘non-essential’ as they can be formed in the body. The proteins needed by our body are supplied through the diet we consume. The dietary proteins are broken down into amino acids, absorbed as such and used by the body to synthesize the proteins needed for various functions like tissue building, replacement of proteins depleted and synthesis of various functional molecules.
The quality of dietary proteins depends on the pattern of essential amino acids it supplies. The best quality protein is one which provides essential amino acid pattern very close to the pattern of the tissue proteins. The minimum amount of essential amino acids required by infants is also taken as reference pattern for defining the quality of proteins. Milk and egg proteins, which are of superior quality, also serve as reference protein. As per the recommendations of the Joint FAO/WHO Expert Consultation (1991), the essential amino acid composition (mg/g protein) of reference protein for 2–5-year-old children is lysine 58, threonine 34, tryptophan 11 and methionine + cysteine 25. As such, the first limiting amino acid in cereal proteins is lysine, while the second limiting amino acid is threonine/tryptophan. Pulse proteins are rich in lysine but deficient in sulphur-amino acids, mainly methionine. Cereal or pulse proteins individually are, therefore, incomplete proteins. However, when cereals and pulses are consumed together, they have a mutually supplementary effect; deficiency of an amino acid in one can be made good by an adequate level in another. A mixed cereal-based diet can meet the protein requirements of adults and older children provided they consume enough of the diet to meet their energy needs. However, it is the vulnerable group of infants and preschool children who need protein of superior quality, and cereal-based diet or a mixed diet of plant proteins cannot meet their protein requirements adequately. For this group, the requirement of protein per unit body weight is also higher, the safe level of reference high-quality protein for preschool children being 1.1 g/kg and for adults 0.75 g/kg body weight.
The nutritive value of food grain proteins, depends upon their essential amino acid make up as well as digestibility. For chemical evaluation of protein quality, first and/or second limiting amino acid in respective food grains is determined. For example, in maize protein, lysine is the first limiting amino acid and tryptophan is the second. The overall quality, i.e. the nutritive value of food grain proteins is determined in a nitrogen-balance study with laboratory animals like growing albino rats. From this study, biological value (BV) of proteins, which is a percentage of N-retained by the experimental animals out of N-absorbed from the diet, is obtained. This test also gives an estimate of true digestibility (TD) of proteins which indicates the per cent N-absorbed from the diet. Net protein utilization (NPU) value, a multiplication product of TD and BV, gives a measure of per cent N-retained out of total N-consumed. Utilizable protein (UP) value, a multiplication product of NPU and per cent protein in the test food grain, is a measure of both protein quality as well as quantity. Another simple measure of the nutritive value of proteins could be protein efficiency ratio (PER), which indicates the gain in weight of young animals per unit weight of protein consumed. For this type of study, usually weanling albino rats are used.
3 Development of Opaque-2 Maize Varieties and Improvement in Protein Quality
Soon after the discovery that opaque-2 gene improves the contents of lysine and tryptophan in maize endosperm, maize breeders all around the world started incorporating this gene into their elite maize varieties. In India also the work started in 1966, and three opaque-2 maize composite varieties, namely, Shakti, Rattan and Protina, were developed under the auspices of All India Coordinated Maize Improvement Project and released for commercial production during 1970. In the endosperm of these varieties, lysine and tryptophan contents increased by about 100 % and 160 %, respectively, while leucine content decreased by about 35 % as compared to normal maize hybrid Ganga-5 (Table 3.1).
The opaque-2 gene apparently acts as zein suppressant in the developing endosperm. In normal maize endosperm, zein accounts for more than 50 % and glutelin for about 35 % of the total protein. In opaque-2 endosperm, however, glutelin becomes the major fraction and zein content is reduced drastically. In addition, there are relative increases in albumin and globulin fractions (Table 3.2), which are rich in lysine and tryptophan.
The nutritional quality of normal and opaque-2 maize protein has been evaluated using albino rats, swine, children as well as adults.
4 Rat-Feeding Experiments
The nutritional superiority of opaque-2 maize over normal maize has been well established in feeding trials with albino rats as experimental animals. In a nitrogen-balance study, the BV of opaque-2 maize (Shakti) protein was found to be 90 % of milk protein casein, while that of normal maize (Vijay, Ganga-5, Bassi) protein was less than 70 % of casein (Lodha et al. 1976b). The NPU and UP values were also higher for opaque-2 maize (Table 3.3). The protein efficiency ratio (PER) value for opaque-2 maize has been found to be 62–110 % superior to that of ordinary maize, the PER for opaque-2 maize being 2.3–2.8 and that for normal maize 1.3–1.6. Further, 1 g of dietary opaque-2 maize resulted in 0.226 g weight gain in albino rats as against only 0.131 g with normal maize.
5 Child-Feeding Experiments
Opaque-2 maize has been proved to be a good supplement to the home diet of weanling and young children in terms of their weight and height gain and gain in mid-arm and chest circumference. The experiments were carried out under the auspices of All India Coordinated Maize Improvement Project, IARI, for a period of 6 months (November 1975–May 1976) with 18–30-month-old children from low-income families. The children were fed on isocalorie (405 Kcal) and comparable protein (about 10 g) diets as a supplementary midday meal, to meet out one-third of the recommended daily allowances. The children fed on opaque-2 maize diet gained in weight 8.3 % and 25 % more as compared to those fed on skim milk and normal maize-based diets, respectively. As regards height, children fed on opaque-2 maize diet gained 29.4 % more compared to normal maize. Similarly, children of the milk group showed maximum gains in chest and arm circumference, followed by opaque-2 maize group and least gains were observed in normal maize group (Table 3.4).
6 Therapeutic Uses of Opaque-2 Maize
Studies carried out at Universidad del Valle Hospital in Cali, Colombia, and at the National Institute of Nutrition (NIN), Hyderabad, have shown that children severely ill with ‘kwashiorkor’ can be easily cured from the protein deficiency disease if fed regularly on opaque-2 maize diet. In addition, another study carried out at NIN with dogs suggested that if the population having maize as a staple food regularly consumes opaque-2 maize diet, then the incidence of pellagra could be prevented.
7 Protein Quality of Immature Kernels
In addition to their direct use after roasting or boiling, immature kernels from green ears are used in preparing many recipes. With the development of the kernels, the TD of the protein is improved; however, the BV decreases in both normal and opaque-2 maize. The BV values being equal (76.3 and 76.0), immature normal maize kernels harvested at the early-dough stage (25 days post-pollination) were found to be as good as mature opaque-2 maize kernels from their BV point of view (Table 3.5).
8 Problems Confronting Chalky Opaque-2 Maize and Development of Modified Opaque-2 Maize
Though the nutritional superiority of opaque-2 maize over normal maize has been very well established, the opaque-2 maize varieties could not become popular all over the world due to the following agronomic and acceptability problems associated with soft and chalky endosperm: 10–15 % lower grain yield, poor kernel appearance, high susceptibility to ear rot and stored grain pests (Singh et al. 1974; Vasal 2000).
Fortunately, during the process of converting normal maize populations to their opaque-2 versions, partially hard endosperm (i.e. vitreous) or ‘modified’ grains had been observed by many researchers including breeders at CIMMYT in Mexico. Separation of such grains began as early as in 1969 (Prasanna et al. 2001). At CIMMYT, under the dynamic leadership of Dr. S.K. Vasal, and at the University of Natal, various endosperm modifier genes were identified that could favourably alter the grain characteristics, thereby overcoming an important obstacle in the popularization of high-lysine opaque-2 maize (Sofi et al. 2009). The mechanism by which the endosperm modifiers change the grain structure from chalky to vitreous in modified opaque-2, is not clearly understood. The modified opaque-2 maize with hard endosperm is known as quality protein maize (QPM). Thus, the term QPM now refers to maize homozygous for the o2 allele, with increased lysine and tryptophan contents but without the negative secondary effects of a soft and chalky endosperm. The QPM essentially has about twice the levels of lysine and tryptophan than normal maize and also increased levels of histidine, arginine, aspartic acid and glycine, but reduced level of leucine.
In the Indian Maize Programme, though the initial emphasis was on the development of soft-endosperm opaque-2 maize varieties, gradually the emphasis shifted to developing modified opaque-2 maize strains with hard endosperm. The work was initiated during kharif (rainy season) of 1971, and a large number of inbred lines of varying vitreosity were selected from the three opaque-2 composites, Shakti, Rattan and Protina. These lines were subjected to vigorous selection for kernel vitreosity by growing them in Delhi during kharif (rainy season) and during rabi (winter season) in Hyderabad for 3 years (1972–1974). After each harvest, the selection was made based on kernel vitreosity and tryptophan content. In each cycle of selection, semi-opaque and semi-normal kernels were sown separately. Based on light transparency, the kernels were classified as opaque (O), 100 % opaque with no light transmission; semi-opaque (SO), partial light transmission; semi-normal (SN), over two-third of the kernel from the crown is vitreous; and normal (N), nearly vitreous kernels with slight spotting or cloudiness. The development of hard-endosperm inbred lines of opaque-2 maize over five cycles of selection and their analyses showed that protein content, 100-kernel weight and kernel density increased with the increase in kernel vitreosity; however, tryptophan (as % of endosperm protein) decreased slightly. There was variability in tryptophan content suggesting a possibility of selecting hard-endosperm opaque-2 strains with high tryptophan (more than 0.7 % in endosperm protein) and nearly normal-looking vitreous type of kernels (Lodha et al. 1976a; Singh et al. 1974, 1985). Utilizing the selected hard-endosperm opaque-2 inbred lines, a SO/SN composite was developed which was subjected to extensive biochemical and nutritional studies. Later on, some of these lines were used by the Directorate of Maize Research (DMR) in developing a QPM composite, Shakti-1 (0.92 % tryptophan), which was released in 1997.
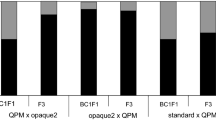
The major emphasis was, however, given on the development of QPM hybrids by practically all the breeders. CIMMYT-developed QPM lines along with their own lines were mostly involved in two-parent QPM hybrids. At present, in India we have one QPM composite (Shakti-1) and more than nine QPM hybrid varieties, including Shaktiman and HQPM series and Vivek QPM 9. The QPM version of extra-early Vivek Hybrid 9, Vivek QPM 9, was developed by following marker-assisted selection (MAS) and had been released for commercial cultivation in 2008. Phenotypically, the kernels of the QPM hybrid are as vitreous as those of its normal maize counterpart (Fig. 3.1). This QPM hybrid contains 4.19 % lysine and 0.83 % tryptophan in its endosperm protein as against 3.25 % lysine and 0.59 % tryptophan in its normal counterpart (Gupta et al. 2009).
The QPM hybrids, Shaktiman-1, Shaktiman-2, Shaktiman-3 and Shaktiman-4, respectively, contain 1.01 %, 1.04 %, 0.73 % and 0.93 % tryptophan in their endosperm proteins. The protein in the endosperms of these QPM hybrids ranges from 9.3 % to 9.9 %. Similarly, in the QPM hybrids, HQPM-1, HQPM-5 and HQPM-7, which respectively contain 0.94 %, 0.76 % and 0.72 % tryptophan, protein in the endosperms ranges from 9.36 % to 9.80 %.
9 Chemical and Biological Evaluation of Modified Opaque-2 Maize Protein Quality
As a result of selection for modifiers, several changes occur in physical and biochemical characteristics of the modified opaque-2 maize kernels. Based on light transparency, modified opaque-2 maize kernels are classified into the following categories: vitreous (nearly normal), 25 % opaque–75 % vitreous, 50 % opaque–50 % vitreous, 75 % opaque–25 % vitreous and completely opaque. The vitreous fraction of endosperm contains more protein but less lysine and tryptophan than the opaque fraction (Lodha et al. 1976a). With the increase in kernel vitreosity, protein and leucine contents increase, while lysine and tryptophan contents decrease in the endosperm. This is because albumin, globulin and glutelin fractions decrease, while the zein fraction increases in the endosperm with the increase in kernel vitreosity (Gupta et al. 1979a). Although chemical analysis showed differences in endosperm protein quality of various categories of modified opaque-2, rat-feeding experiments showed more or less similar nutritional quality of the grain protein based on UP values, as described below.
Nitrogen-balance study carried out with albino rats utilizing three kernel categories of modified opaque-2 with hard endosperm (SO/SN composite), viz. 25 % opaque, 50 % opaque and 75 % opaque; normal composite Vijay and Shakti opaque-2 showed about 20 % higher BV for different categories of modified opaque-2 as compared with normal maize (Table 3.6). Although the BV of Shakti opaque-2 was slightly better (6.6–8.2 %) than that of various categories of modified opaque-2, the UP values of Shakti and three categories of modified opaque-2 were not significantly different from one another. The change was mainly because of higher protein in different kernel categories of modified opaque-2 composite in comparison to Shakti composite, which has got chalky endosperm (Table 3.6). Chalky opaque-2 and modified opaque-2 maize also supported better liver growth compared to normal maize in albino rats. A similar trend was also observed for protein per cent in liver.
So far as distribution of protein fractions in the endosperm is concerned, albumin content was more or less equal in chalky opaque-2 composite (Shakti) and modified opaque-2 with hard endosperm (SO/SN composite), and it was fourfold higher than in normal maize Vijay. The content of globulin was also twofold higher in chalky and 1.5-fold higher in modified opaque-2 compared to normal maize. However, the content of zein was drastically reduced in opaque-2 types, being 76 % lower in chalky opaque-2 and 58 % lower in modified opaque-2. The content of glutelin was about 20 % higher in opaque types compared to normal maize (Table 3.7).
10 Child-Feeding Experiments
Nutritional superiority of QPM over normal maize was also demonstrated in a child-feeding experiment. In a 6-month study carried out with preschool children at Rajendra Agricultural University, Pusa, gains in weight and arm circumference were, respectively, about 30 % and 100 % higher for Shakti-1 (QPM composite)-fed group compared to the group fed with normal maize (DMR 2001).
11 Genetic Systems and Need for Monitoring Protein Quality of QPM
There are three distinct genetic systems which are involved in the development of QPM: (i) recessive homozygous allele of the opaque-2 gene, (ii) modifiers for endosperm hardness and (iii) amino acid modifiers which affect the relative levels of lysine and tryptophan in the endosperm (Gupta et al. 2009).
The recessive mutant allele of the opaque-2 gene as described earlier is the first and most important component. The second distinct genetic system is comprised of the alleles of endosperm hardness modifier genes which convert the soft/opaque mutant endosperm to a hard/vitreous endosperm with little loss of protein quality. It has been shown that increased levels of gamma-zein contribute to the recovery of a hard-endosperm phenotype as the modified opaque-2 (QPM) grains have approximately double the amount of gamma-zein in the endosperm relative to the o2-only mutants. These modifiers along with the o2 mutant allele can be initially selected by using a rapid and low-cost method of selection, whereby light is projected through the vitreous grains or blocked by the opaque grains, respectively. Thus, when the kernels are screened against light, varying degrees of modification, ranging from completely vitreous to fully opaque, may be observed. As shown in Fig. 3.2, varying degrees of kernel modification may also be observed in QPM ears.
Two genetic loci which affect the modification of the endosperm hardness in o2o2 backgrounds have been mapped to the long arm of chromosome 7, and interestingly one endosperm modifier locus maps near a gamma-zein gene ‘gzr1’. The third genetic system critical to a QPM breeding programme is comprised of a distinct set of amino acid modifier genes which affect the relative levels of lysine and tryptophan content in the grain endosperm. The lysine level in normal and QPM maize average 2.0 % and 4.0 % of total protein in whole grain flour, respectively, but range across genetic backgrounds from 1.6 to 2.6 in normal maize and 2.7–4.5 % in their o2-converted counterparts (Moro et al. 1996). Since QPM looks like normal maize (Fig. 3.1), there is a need to monitor lysine or tryptophan levels in its endosperm continuously during the breeding process.
After initial screening of QPM materials against light, the endosperm samples of selected materials having vitreous kernels are subjected to chemical analysis for lysine/tryptophan and protein. Because of the existence of a relationship of approximately 1–4 in tryptophan and lysine in maize endosperm protein and also because of simplicity of its estimation, initially the tryptophan content may be used as a single parameter for protein quality evaluation. Because of its simplicity and rapidity, a colorimetric method in which a single-step papain hydrolysis is utilized for protein solubilization is used more extensively for screening maize germplasm for tryptophan content (Villegas and Mertz 1971). With this method, it is possible to analyse up to 75 samples each day. Subsequently, the lysine determination is performed only on those samples selected as having high tryptophan values or when lysine value in addition to tryptophan is desired. Because of its simplicity and rapidity, a colorimetric method modified by Villegas (CIMMYT), which also utilizes a single-step papain hydrolysis for protein solubilization, is followed extensively for screening maize germplasm for lysine content (Villegas and Mertz 1971). By this method up to eight samples can be processed simultaneously, and it gives an estimate of available lysine. In addition, since the concentration of lysine in the maize endosperm is highly correlated with the content of a single non-zein, protein synthesis factor EF-1 alpha, assay of its content by ELISA also provides a reliable index of lysine in endosperm (Habben et al. 1995; Prasanna et al. 2001). Once a QPM variety reaches a final stage of its release for commercial production, it is advisable to analyse it for complete amino acid profile and subject it to animal-feeding experiments for biological evaluation of its nutritive value.
12 Improving Nutritive Value of Normal Maize and QPM by Amino Acids and Pulse Supplementation
The protein quality of ordinary maize and even of hard-endosperm modified opaque-2 maize can be improved by supplementation with limiting essential amino acids or pulses because of the complementary nature of essential acids present in pulses and cereals. The UP value, which takes into account both protein quality as well as quantity, showed substantial improvement with amino acid supplementation as well as by addition of pulse to normal and modified opaque-2 diet. Studies carried out by Gupta et al. (1979b) with albino rats showed that supplementation with lysine alone in case of modified opaque-2 and with lysine + tryptophan in ordinary maize improved substantially the nutritive value. When modified opaque-2 was supplemented with 0.22 % L-lysine HCl, its UP value increased from 6.6 % to 8.3 %, showing thereby about 25 % improvement in nutritive value of protein. In the case of Vijay normal maize, the UP value increased from 6.1 % to 8.2 % (about 34 % improvement) when it was supplemented with 0.45 % L-lysine HCl + 0.06 % DL-tryptophan. At the same time, synergistic effect to the order of 25.0 % and 22.0 % has been observed in the case of normal maize and modified opaque-2 maize, respectively, when these were supplemented with chickpea pulse (7:3 on protein basis).
13 Future Strategies
The nutritional superiority of Quality Protein Maize (QPM) over normal maize is well established. QPM can become a boon to young children, especially to those suffering from protein malnutrition. More so, because its consumption does not require any change in traditional food habits. However, there is a need to popularize QPM varieties with farmers as well as consumers, highlighting its nutritional importance so that its cultivation gains the required momentum. To achieve this objective, it is also important that farmers get remunerative price for their produce. There is also a need to highlight that QPM is a product of conventional breeding, and no genetic engineering is involved in its development. The produce may be utilized in the midday meal programme of school children. In addition, value addition is also required and efforts are needed to popularize already developed QPM products, for example, infant food, health food, snacks and savoury items, convenience food and specialty food (Singh 2001). This is going to benefit specially the vulnerable groups, i.e. infants, preschool children, pregnant and lactating mothers and elderly people. From the poultry industry point of view, concerted efforts are required towards developing maize rich in amino acid methionine.
The lysine content of presently available QPM varieties is below 5 mg/100 mg protein, the FAO/WHO recommended minimum level for human diets. Therefore, by exploiting variability for lysine content in QPM germplasm, it may be possible to select economically viable genotypes with lysine content exceeding the recommended level. Moreover, it is also important to establish as to how the modification of opaque-2 locus by modifier genes is affected at biochemical and molecular level. This should help understand the mechanism of action of modifier genes, the expression of which is affected by genetic and environmental factors.
14 Conclusion
An important goal of plant breeding programmes has been improvement in nutritive value, in particular amino acid profile, of food grains. The biggest success was achieved when high-lysine opaque-2 mutant of maize was identified, and subsequently QPM developed by exploiting genetic modifiers of opaque-2 locus. QPMs are the mutants that have a hard, vitreous endosperm; a normal yield; and a high nutritional quality. Since, there exist a lot of variability for lysine/tryptophan content in the germplasm, and breeding and development of QPM varieties by accumulating the modifiers being a multistep process, monitoring of tryptophan/lysine level at each step is required as QPM kernels cannot be easily distinguished visually from normal maize kernels, and chemical analysis is the only way. Thus, establishment of a few well-equipped biochemical laboratories to provide rapid and reliable analyses would be able to give a desired boost to the ongoing QPM programme.
References
Dass S, Jat ML, Singh KP, Rai HK (2008) Agro-economic analysis of maize-based cropping systems in India. Ind J Fert 4:53–62
DMR (2001) Production technology of quality protein maize. Technical Bulletin, Directorate of Maize Research, Pusa Campus, New Delhi
Gupta HO, Lodha ML, Singh J, Mehta SL (1978) Protein quality of normal and opaque-2 maize at different stages of ripening. J Food Sci Tech 15:148–149
Gupta HO, Lodha ML, Rastogi DK, Singh J, Mehta SL (1979a) Nutritional evaluation of hard endosperm opaque-2 maize (Zea mays L.). J Agri Food Chem 27:390–392
Gupta HO, Lodha ML, Mehta SL, Rastogi DK, Singh J (1979b) Effect if amino acid(s) and pulse supplementation on nutritional quality of normal and modified opaque-2 maize (Zea mays L.). J Agri Food Chem 27:787–790
Gupta HO, Lodha ML, Mehta SL, Rastogi DK, Singh J (1980) Changes in minerals, proteins and amino acids in hard endosperm opaque-2 Zea mays during development. Ind J Expt Biol 18:1419–1422
Gupta HS, Agrawal PK, Mahajan V, Bisht GS, Kumar A, Verma P, Srivastava A, Saha S, Babu R, Pant MC, Mani VP (2009) Quality protein maize for nutritional security: rapid development of short duration hybrids through molecular marker assisted breeding. Curr Sci 96:230–237
Habben JE, Moro GL, Hunter BG, Hamaker BR, Larkins BA (1995) Elongation factor-1 alpha concentration is highly correlated with the lysine content of maize endosperm. Proc Natl Acad Sci USA 92:8640–8644
Lodha ML, Gupta HO, Ram PC, Singh J (1976a) Some biochemical characteristics of modified phenotype strains of opaque-2. Curr Sci 45:285–286
Lodha ML, Srivastava KN, Gupta HO, Eggum BO, Mehta SL, Singh J (1976b) Nutritive value of normal and opaque-2 maize. Curr Sci 45:287–289
Mertz ET, Bates LS, Nelson OE (1964) Mutant gene that changes protein compositions and increases lysine content of maize endosperm. Science 145:279–280
Moro GL, Habben JE, Hamaker BR, Larkins BA (1996) Characterization of the variability in lysine content for normal and opaque-2 maize endosperm. Crop Sci 36:1651–1659
Prasanna BM, Vasal SK, Kasahun B, Singh NN (2001) Quality protein maize. Curr Sci 81:308–1319
Schmidt RJ, Burr FA, Aukeman MJ, Burr B (1990) Maize regulatory gene opaque-2 encodes a protein with a ‘leucine zipper’ motif that binds to zein DNA. Proc Natl Acad Sci USA 87:46–50
Singh U (2001) Quality protein maize products for human nutrition. Technical Bulletin, Directorate of Maize Research, Pusa Campus, New Delhi
Singh J, Koshy S (1974) Role of opaque-2 in child nutrition. Ind J Genet 34A:1183
Singh J, Lodha ML, Gupta HO (1974) Problems and prospects of breeding for better protein quality through the use of opaque-2. Ind J Genet 34A:651–656
Singh J, Koshy S, Agarwal KN, Singh NN, Lodha ML, Sethi AS (1980) Relative efficacy of opaque-2 maize in growth of preschool children. The Ind J Nutr Dietet 17:326–334
Singh J, Lodha ML, Gupta HO, Ram PC (1985) Development of hard endosperm opaque-2 strains of maize (Zea mays L.). Ann Agric Res 6:104–110
Sofi PA, Wani SA, Rather AG, Wani SH (2009) Review article- quality protein maize (QPM): genetic manipulation for the nutritional fortification of maize. J Plant Breed Crop Sci 1:244–253
Vasal SK (1994) High quality protein corn. In: Hallauer AR (ed) Specialty corns. CRC Press, Boca Raton, pp 80–121
Vasal SK (2000) The quality protein maize story. Food Nutr Bull 21:445
Villegas E, Mertz ET (1971) Chemical screening methods for maize protein quality at CIMMYT. Res. Bull. No. 20, International Maize and Wheat Improvement Centre, Mexico
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Lodha, M.L. (2014). Maize Protein Quality and Its Improvement: Development of Quality Protein Maize in India. In: Chaudhary, D., Kumar, S., Langyan, S. (eds) Maize: Nutrition Dynamics and Novel Uses. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1623-0_3
Download citation
DOI: https://doi.org/10.1007/978-81-322-1623-0_3
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1622-3
Online ISBN: 978-81-322-1623-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)