Abstract
Before setting out to a conclusion of the critical analysis of structural transformation of kaolinite as described in Chaps. 19–25, it is important to recapitulate the past and present scenario on thermal decomposition of it. Kaolin at high temperature finally transforms to yield stable mullite phase and β-cristobalite as per the prediction of equilibrium phase diagram of the SiO2-Al2O3 system. Many investigations explained the fundamentals of the breakdown of kaolinite and the subsequent recrystallization of it to mullite. Although intermediate steps of reaction have been extensively studied the interpretations are full of controversies. It is reviewed that even with the application of many recent experimental techniques to resolve one of the most interesting crystallo–chemical problems of K-M reaction series, a few areas are yet to be solved or there is absence of general agreement on various issues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Before setting out to a conclusion of the critical analysis of structural transformation of kaolinite as described in Chaps. 19–25, it is important to recapitulate the past and present scenario on thermal decomposition of it. Kaolin at high temperature finally transforms to yield stable mullite phase and β-cristobalite as per the prediction of equilibrium phase diagram of the SiO2-Al2O3 system. Many investigations explained the fundamentals of the breakdown of and the subsequent recrystallization of it to mullite. Although intermediate steps of reaction have been extensively studied the interpretations are full of controversies. It is reviewed that even with the application of many recent experimental techniques to resolve one of the most interesting crystallo–chemical problems of K-M reaction series, a few areas are yet to be solved or there is absence of general agreement on various issues.
1. Earlier problems
-
(i)
Metakaolin, its nature and structural characteristics. It is amorphous to X-ray and electron diffraction. Standard methods are inapplicable to analysis of its structure. It is forced to rely upon indirect evidences for its elucidation.
-
(ii)
Crystal structure and chemistry of cubic spinel phase. There is a great disagreement over the nature of it and the temperature at which transformation takes place.
-
(iii)
The radial Patterson Function is suited for studying the structural aspects of weakly crystalline transitional spinel phase. Secondly, systematic determination of relative amount of AlO4 group throughout the thermal evolution of kaolinite by XRF will provide a complementary check of RED result. Unfortunately, the coefficients of 980°C exothermic reaction products of kaolinite are unavailable.
-
(iv)
Composition of primary mullite and whether the ratio of Al and Si in mullite changes with increasing temperature is a question. Determination of lattice parameter of primary mullite may be erroneous. Formation of mullite of tetragonal variety (t-mullite) at ~1000°–1050°C requires complementary proof.
-
(v)
Reason of 980°C exothermic event. Identification of reaction products that results in exothermic reaction is an essential step. Is it due to spinel phase? Whether a fraction of mullite phase also contributes to this exotherm. Or is nucleation of mullite solely responsible for the exotherm? Interpretation of it is still a matter of speculation. It is also believed that γ-Al2O3 is not a factor in the mechanism. It is even conjectured that evolution of energy with exhibition of characteristic exotherm, with concurrent and marked decrease in solubility, are indicative of stable crystallization phenomenon of mullite. Exothermic event is also demonstrated as of two-fold origin, i.e., both nucleation of mullite and formation of γ-Al2O3 is the reason behind exotherm. Moreover, there is absence of accurate and reproducible value of enthalpy data of 980°C exotherm.
-
(vi)
Endothermic dip (D) preceding first exotherm plays an important role in the transitional stage to exothermic reaction. This is generally ignored. General DTA and TGA curves do not provide convincing information in this matter.
-
(vii)
The second and third exothermic peak temperatures are sensitive to the presence of impurities available in the origin of kaolinite. The fluxing effect of interlayer cations, e.g., potassium, sodium present in mica and illite inhibit the crystallization of cristobaite. Thus, naturally occurring kaolinites contain chemical and mineralogical impurity oxides which influence the thermal behavior of clays and likely change the DTA traces. Due to this reason, a large variation of results are seen in the literature.
-
(viii)
Characteristics of natural kaolinites and their differences in their structural order play a role in their transformation behavior. Accordingly, variations are noted in the occurrence of phases in stability range of temperature.
-
(ix)
Based on topotatic relationship between kaolinite and its transformed phases, IR, and DTA curves, two mechanisms of dehydroxylation (either homogeneous or inhomogeneous) are suggested. There are no evidences in either of the mechanisms.
These problems hinder to arrive at a definite general conclusion in interpretations of K-M reaction processes. It is hoped that the pertinent problems in this reaction series are chronologically presented in the individual chapter of the book.
2. Predictions by scientific communities
There are a lot of predictions in the K-M literature. These are not always a matter of speculation. Some of the findings are experimentally based. Yet, one theory does not explain other established results of the methods applied in the characterization process.
-
(i)
It may be simple γ-Al2O3 by XRD but pioneering single crystal study predicts Al-Si spinel.
-
(ii)
It is also predicted that the composition of spinel may be analogous to composition of 3:2 orthorhombic mullite (o-mullite) and it is designated as cubic mullite (c-mullite), i.e., it contains as high as 28 wt % SiO2.
-
(iii)
Contrarily, spinel phase was found to contain SiO2 to the extent of 8–11 wt % only by analytical TEM-EDS analysis and HRTEM study respectively. Therefore, the question is how much silicon is present in the spinel phase?
-
(iv)
In most of the repetitive alkali leaching techniques, the end point of SiO2(A) – alkali reaction is not maintained properly, which may lead to erroneous prediction in determination of silica content in spinel phase by energy dispersive X- ray spectroscopy.
-
(v)
MAS NMR was applied to characterize the disordered solids like metakaolinite and spinel phase to study the local atomic environment of Al and Si and it is predicted that 980°C exotherm is not associated with formation of a particular phase.
-
(vi)
To explain the reasons behind the cause for exothermic reaction in DTA, which is purely a dynamic heating process, some researchers analyzed heat treated clay samples of different thermal history which misleads the actual reason behind exotherm.
-
(vii)
Mullitization process may occur through a degree of segregation of silica and alumina by way of decomposition of metakaolinite and then a more extensive diffusional process of Al and Si elements. Author theory predicts simple structural transformation of orderly mixed spinel phase. Thus, contrast between segregation-recombination path versus. continuous structural transitional path is still to be a subject of study.
3. Difficulties encountered by distinguished authors
The main reasons and difficulties in characterization of phases are the following.
-
(i)
Important and landmark studies in phase transformation claim that powder X-ray pattern of metakaolin is amorphous and first crystalline product of it is also a poorly crystalline γ-Al2O3 followed by mullite.
-
(ii)
Nature of spinel phase, whether it is Al-Si spinel or Al spinel ( γ- Al2O3), is rather difficult to determine, because it is poorly crystalline, small crystal size and showing 2-3 number XRD peaks, are very broad and diffuse, and lastly even on heating for as long as 28 hr at ~950°C doesn’t develop into a well crystallized state. Now the reader will realize the tough problem of its identification. Thus, the grain size of spinel phase in the nanometer range makes the structural analysis most challenging. Not only this, the problem is most critical considering its composition of Al- Si spinel, i.e., the content of Si in spinel phase.
Other fundamental difficult problems are the following.
-
(i)
Crystallographic relationship between kaolinite to metakaoliite to spinel phase is evaluated . But there exist uncertainties on the clear axial relationship between spinel to mullite, i.e., between kaolinite to mullite as a whole.
-
(ii)
IR studies suggest three stages of dehydroxylation of kaolinite. The large principal and small final dehydroxylation peaks are relatively sharp. Those OH groups which stabilize the metakaolin structure release gradually over a range of temperature between ~700°C to temperature just below first exotherm. This result indicates the possibility of varying hydroxyl groups in structural units of metakaolin.
-
(iii)
During phase separation process at final stage of dehydroxylation of metakaolinite, a large quantity of siliceous phase SiO2(A) to the extent of ~35–37 wt % is liberated. This SiO2(A) part poses a great thrust to characterization of Al-Si spinel phase either by XRD or in TEM.
-
(iv)
Besides liberation of SiO2(A), a large quantity of mullite in amorphous state also remains as a coexisting phase in 980°C decomposition product of kaolinite. Now the reader must realize a very difficult task of identification of spinel phase whether it is γ-Al2O3 or Si bearing spinel due to its low content (~20–30 wt %), low crystallinity, and it is admixed with an average 70–80 wt % amorphous phase mixtures. Due to these reasons, composition analysis of spinel phase even by EF-TEM study is found inconclusive.
-
(v)
Interpretations on the kinetics and mechanism of phase transformation of kaolinite studied either by isothermal or by non-isothermal process are varying. Thermal transformation of kaolinite is governed by both thermodynamic and kinetic laws. However, most of the studies in this process are done from a kinetic point of view. It is necessary to present thermodynamic and kinetic contributions separately.
-
(vi)
The growth of mulite needles sometimes occurs in preferred orientation to hexagonal orientation to kaolinite flakes and supports the idea of epitaxial type of transformation. But it is not always noted in electron microscopic studies.
4. Investigation methods chosen by various researchers
Use of thermal analysis method for phase transformation of kaolinite has shown much and widespread interest among ceramists and mineralogists. Numeros efforts by them on the investigation on the heat effects of clays of various sources are obviously desirable. A short summary of the various investigation approaches are shown in Chap. 2 vide its Table 2.1. Detail investigations out of a large number of researchers over a period of more than 100 years are serially mentioned. Experimentally noted figures of some researchers are presented after obtaining their kind permission for reproduction along with the inclusion of author’s own newer data of several studies. These are chronologically and elaborately presented in Chaps. 3–18. Experimental results of various researchers and scientific personnel are comparable and imagined to different kinds of flowers blooming in their individual investigative fields of study. The present author just tried to pluck various types of colorful flowers from their clay –mullite research area and thereafter sorted out these flowers in different gradations considering their shape, size, and colors for future use. This way of presentation of the earlier literature along with the incorporation of new conceptions of present author are done in chapter wise mode with a view to develop lucid pictures of K-M reaction processes which are likely analogous to conglomeration of flowers kept in different vases such that these most adorable and would be acceptable globally by students, professors, researchers, and technocrats.
5. Visualization and envisagement
Based on the pertinent observations and experimental results of eminent scientists, the present author elaborately addresses the various issues e.g., (i) on the various earlier problems, (ii) difficulties encountered by distinguished authors, and (iii) predictions by scientific communities on the decomposition of kaolinite as stated above. These critical reviews are presented in Chaps. 19–25 with proper cross references from previous chapters. This service is considered as an equivalent to formulation of flower bouquet or fabrication of flowers garland, etc., with some insertion of his own conceptions and visualization regarding the problems and mechanisms of K-M reaction series out of sorted flowers that are kept in vases. It is largely expected that these sorts of critical exercises as submitted here may evoke a melody in the mind of a reader when they attentively go through this book. The present author with his bowed head dedicates these newly made garland and two bouquets to the feet of his Revered Sadhan-Siddha Sadguru Swami Nigamananda Pramhansa and ends the book.
6. Conclusion drawn by the present author
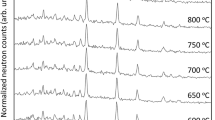
Reaction sequences of decomposition of kaolinite are chronologically enumerated and is shown in Fig. 26.1.
Finally, thermal events of kaolinite are explained and summarized below.
-
1.
At 100–150 °C. Physically adsorbed moisture is removed.
-
2.
At the 1st endothermic region at ~500–650 °C.
First step is kaolinite to metakaolinite transformation with loss of crystalline structure. Loss of structural OH groups amounting to ~87 % and forms metakaolinite with residual OH groups ~13 %.
-
3.
At the endo–exo peak region at the temperature 950–1,000 °C.
Second step is the rapid decomposition of metakaolinite at ~1,000 °C Loss of last traces of OH groups at the endothermic dip followed by phase separation into siliceous and aluminous regions. A part of the aluminous region crystallizes to weakly crystalline primary mullite (minor) with exhibition of a small exotherm just before 980° and other part crystallizes to Al–Si spinel phase (major) at the first exothermic peak. The significant amount of residual, two separate, amorphous phases e.g., siliceous region (SiO2(A)) and residual aluminous region (Mullite(A)) resulting to depict globally a very weak X-ray pattern and a band.
-
4.
Two exothermic regions of mullite formation in the temperature range 1,100–1,400 °C.
Third step is the mullitization out of Al–Si spinel and amorphous aluminosilicate phases (mullite (A)) over a long range of temperature from ~1,050 °C to ~1,350 °C. During the entire region, two growth steps of mullite formation occur (one starts from 1,050° to ~1,150 °C and other sharply at ~1,250 °C with continuous increase up to 1,350 °C). A broad exotherm is due mullitization out of residual aluminous phase (Mullite(A)) by nucleation–crystallization mechanism. A small exotherm is always noticed by various researchers may be due to phase transformation of Al–Si spinel phase to 3:2 mullite. Both sharpness and resolution of mullite peaks increase with continued heating up to 1,550 °C.
-
5.
Final exothermic region of cristobalite formation at 1,400–1,430 °C.
Concurrent to mullite formation, the remaining silica-rich alumina phase crystallizes at above 1,200 °C to β-cristobalite. The lattice parameter study shows a axis data of cristobalite is much more than that of the value noted in cristobalite samples obtained from pure silica gel this data indicates the presence of Al+3 in cristobalite structure. Thus, by lattice parameter data one can predict the formation of silica-rich aluminous phase during the decomposition of metakaolinite. Crystallization of cristobalite is very sharp at ~1,400 °C and cause a small exotherm in DTA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Chakraborty, A.K. (2014). Final Conclusion on the Thermal Effects of Kaolinite . In: Phase Transformation of Kaolinite Clay. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1154-9_26
Download citation
DOI: https://doi.org/10.1007/978-81-322-1154-9_26
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1153-2
Online ISBN: 978-81-322-1154-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)