Abstract
Infertility specialists have been struggling to deal with poor responders, reported to range from 9 to 24 %. However, the incidence is abruptly increasing lately due to the increasing population of aged infertile women. The long gonadotropin-releasing hormone (GnRH) protocol is the first line of treatment if more than plural numbers of oocytes are expected, which is followed by the short protocol, and the GnRH antagonist stimulation is used as the last treatment option. Among the GnRH agonist protocols, the microdose agonist flare with oral contraceptive (OC) pretreatment appears more effective. Natural cycle or mild stimulation is used when the stimulation protocols fail. IVM is an alternative choice when none of the above-mentioned protocols succeed. Growth hormone (GH) appears to have a beneficial effect, but indications for using GH as well as growth hormone-releasing hormone (GH-RH) are not well defined. In addition, it is too early to determine if androgen pretreatment [dehydrepiandrosterone sulfate (DHEA), testosterone] is beneficial in the treatment of poor responders. It is most important for ART physicians to evaluate the patient response before starting the stimulation and choose the best protocol for the poor responder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- DOR (diminished ovarian reserve)
- AMH
- AFC
- Poor responder
- Aged patient
- IVM-IVF (in vitro maturation in vitro fertilization and embryo transfer)
Background
The incidence of poor responders to ovarian stimulation has been reported to range from 9 to 24 % [1–3]. The poor response to ovarian stimulation is attributed to patients with advanced age and iatrogenic reasons, such as ovarian surgery, pelvic adhesions, and obesity, indicated by body mass index (BMI) [4–8]. The incidence of sporadic poor response to stimulation and primary ovarian insufficiency has been well known for a long time [9, 10]. Recently, there has been increased interest in improving the reproductive capacity of older women because of the changing social structure and the worldwide trend of delaying marriage and childbirth. In the United States, the number of births in women aged between 40 and 44 years has nearly doubled between 1990 and 2002 [11]. The birth rate in women aged 45–49 years is 0.5 births per 1000 women, indicating that it has increased by more than two-folds. However, majority of these births can be attributed to the use of donor oocytes [12]. In the United States, 19 % of all women using assisted reproductive technologies (ART) are ≥40 years in age [12]. In Europe, during 2005, the percentage of women aged ≥40 years undergoing ART, such as conventional in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles, was 15.4 % and 13.0 %, respectively. The number of women delaying childbearing to the fifth decade of their life has markedly increased, and, consequently, 50 % of them will experience some difficulty in their attempt to have children [13]. The age-related decline in fecundity in spontaneous conception and ART success rates has long been known [14–17]. The decline in fertility is mainly due to a decrease in oocyte quality, which is linked to a single chromatid abnormality [18]. There is little evidence that uterine factors have a significant impact on age-related infertility [19]. Although ART with donor oocytes has helped woman in the fifth and sixth decades of their life to achieve a high pregnancy and childbirth rates [20], the procedure is associated with legal, ethical, religious, and cultural problems that have limited its universal accessibility in societies around the world [21, 22]. Extensive efforts to improve pregnancy rates in poor responders have been made using several stimulation protocols, but despite these efforts, pregnancy rates after IVF remain disappointingly low [23, 24]. Currently, the evidence available from both, retrospective and prospective studies is based on the variable definitions of poor ovarian response. The European Society for Human Reproduction and Embryology (ESHRE) consensus group has developed a new definition that may help in selecting a more uniform group of patients for future clinical trials [25]. A systematic appraisal of the available evidence, aiming to draw reliable conclusions is currently lacking. In this chapter, the current situation of stimulation protocols, interventions, and strategy at our facility is outlined.
Bologna Criteria as Defined by ESHRE
At least 2 of the following 3 features must be present to fulfill the Bologna criteria: advanced maternal age (≥40) or any other risk factor for poor ovarian response, previous poor response (≤3 oocytes with a conventional stimulation protocol), and abnormal ovarian reserve test (AFC < 5–7 follicles or AMH < 0.5–1.1 ng/mL). However, the definition of poor responder has not been standardized till date. Several groups have defined poor responders on the basis of variable numbers of mature follicles noted on ultrasound, ranging from <2 to <5. Others base their definition on elevated serum follicle-stimulating hormone (FSH) levels in the early follicular phase, with values ranging from 6.5 to 15 mIU/mL; the use of various maximal estradiol (E2) levels compared with the prior standard controlled ovarian hyperstimulation (COH); a minimal cumulative dose or the number of days of gonadotropin stimulation required in a prior cycle; or on the basis of differing numbers of mature oocytes obtained (≤4 or ≤6).
Which Types of Gonadotropins Are Effective?
Ovarian stimulation using recombinant FSH (r-FSH) is possibly associated with the retrieval of significantly higher numbers of oocytes, greater numbers of embryos, and higher pregnancy rates compared with ovarian stimulation using urinary FSH (u-FSH) [26]. However, the potential benefit of ovarian stimulation using r-FSH with respect to pregnancy rates in poor responders is unclear [27]. Perhaps, the most logical approach to the management of patients, who fail to respond to a standard gonadotropin stimulation protocol, is to consider increasing the dose of gonadotropins. Although no single maximally effective gonadotropin dose has been defined, there would be little benefit in raising the initial daily dose of FSH to >450 IU/day.
Short or Long GnRH Agonist Protocol
Preference, not protocol efficiency, dictates the selection of a short- or long-agonist protocol for the suppression of a premature luteinizing hormone (LH) surge in women undergoing IVF treatment. The flare-up effect of gonadotropin-releasing hormone (GnRH) agonist on pituitary gonadotropin release is used in the short protocol to enhance initial follicular growth. In contrast, the long protocol results in a more co-ordinated follicular growth. The improvement in clinical pregnancy rate does not appear to be dependent on the type of GnRH agonist protocol applied [28].
GnRH Antagonist or GnRH Agonist Protocol
The use of GnRH antagonists to improve pregnancy rate in poor responders is based on the fact that endogenous gonadotropin secretion is not suppressed during follicular recruitment [29]. A meta-analysis suggests that the type of GnRH analog used to inhibit the LH surge does not appear to be associated with ongoing pregnancy [30]. In contrast, significantly better results were demonstrated with the use of GnRH antagonists with regard to the duration of stimulation, the total dose of gonadotropins required, and the number of cumulus-oocyte complexes retrieved, but further comparative studies may be required to substantiate these results.
Microdose GnRH Agonist Flare Regimen
Several studies have supported the use of a microdose GnRH agonist flare protocol in poor responders, which has demonstrated an improvement in the ovarian responses and clinical outcomes in these cases [31]. This approach takes advantage of the initial release of endogenous gonadotropins, induced by a low-dose GnRH agonist administration during the early follicular phase, and is aimed at enhancing the response to the subsequent administration of exogenous gonadotropins. Moreover, the blastocysts generated from a microdose GnRH agonist flare regimen showed a significantly lower incidence of blastocysts with chromosome aneuploidy [32].
Stimulation Protocols or Natural/Mild Stimulation Cycles
Natural cycle IVF in poor responders has been proposed as an alternative to standard stimulation protocols. This approach appears to be less invasive and less expensive for poor responders who do not show an increase in oocyte production, with standard ovarian stimulation. However, there is a study suggesting that such a strategy is not beneficial for clinical pregnancy rates [33]. In contrast, Clomiphene citrate (CC) administration in the early follicular phase with r-FSH may improve the outcome of stimulation in poor responders [34]. The use of Letrozole with FSH does not appear to improve the pregnancy rates [35]. Moreover, safety concerns regarding Letrozole administration in assisted reproduction have been noted [36].
Transdermal Testosterone Priming
The addition of androgens during the early follicular phase may have a beneficial effect on the increase in number of small antral follicles and improve the ovarian sensitivity to FSH. Pretreatment with transdermal testosterone (TT) may improve ovarian sensitivity to FSH and follicular response to gonadotropin treatment in previous IVF patients who were poor responders. This approach leads to an increased follicular response compared with a high-dose gonadotropin and minidose GnRH agonist protocols [37]. Moreover, the numbers of oocytes retrieved, mature oocytes, fertilized oocytes, and good quality embryos were significantly higher in the TT pretreatment group. Embryo implantation rate and clinical pregnancy rate per cycle initiated were also significantly higher in the TT group [38].
Dehydroepiandrosterone (DHEA) Supplementation
Supplementing poor responders with 75 mg of micronized DHEA daily for up to 4 months before the initiation of IVF resulted in significantly higher pregnancy rates. The beneficial effect of DHEA supplementation was suggested [39]; however, the definitive effect of this supplementation is still under discussion.
Growth Hormone (GH) and GH-Releasing Hormone (GH-RH)
The concept of potentiating the effect of exogenous gonadotropins with GH or GH-RH can be used as an alternative approach to improve pregnancy rates instead of changing the type or dose of gonadotropin administration. GH plays an important role in ovarian steroidogenesis and follicular development. Treatment with GH appears to modulate the action of FSH on granulosa cells by upregulating the local synthesis of insulin-like growth factor-I (IGF-I) [40, 41]. Live birth rates are improved when GH is co-administered to poor responders during ovarian stimulation for IVF; however, the clinical significance of this difference may be small. Interestingly, the inclusion of GH resulted in a significant decrease in the total dose of gonadotropins required for ovarian stimulation [42]. GH co-treatment and the optimal dose required in the ovarian stimulation of poor responders need to be evaluated further. An enhanced ovarian response, however, with little impact on conception rates was reported following treatment of poor responders with adjunctive GnRH [43].
Our Strategy for Poor Responders Based on the Treatment Protocols Used in Patients with Advanced Age
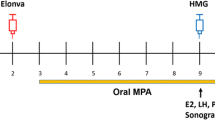
We established a new protocol for poor responders on the basis of the concept followed for treating patients >40 years old. Fecundity in female patients declines with age because of a decrease in the number of oocytes in the ovarian reserve and also because of impaired oocyte quality. These findings are similar to those observed in a poor responder. The pregnancy rate following ART is closely related to the number of oocytes/embryos obtained and the quality of the embryos prior to transfer. It is not easy to retrieve multiple oocytes from poor responders. At the same time, to achieve pregnancy, it is imperative to transfer >2 embryos. Therefore, our new strategy for the poor responder is to transfer ≥2 good frozen-thawed embryos, which are accumulated and chosen from several oocyte retrievals (Fig. 9.1). In addition to various stimulation protocols or natural/mild stimulation cycles, we also use in vitro maturation (IVM) to produce embryos [44].
Conclusions
Numerous papers have discussed the strategy for the treatment of poor responders during IVF, but the lack of a uniform definition of the poor responder makes an accurate comparison among results difficult. Conventional stimulation protocols should be applied if >2 follicles are present in each ovary on day 3 of ultrasound assessment. Recombinant FSH is usually more effective than u-FSH; however, in some instances, the addition of u-FSH to r-FSH can encourage follicular development in poor responders. The long protocol is the first line of treatment, which is followed by the short protocol, and the GnRH antagonist stimulation is used as the last treatment option. Among GnRH agonist protocols, the microdose agonist flare with OC pretreatment appears more effective. However, whether microdose flare protocols are more effective than GnRH antagonist protocols has not been determined till date. Natural cycle or mild stimulation is used when the stimulation protocols fail. IVM is an alternative choice when none of the above-mentioned protocols succeed. GH appears to have a beneficial effect, but indications for using GH as well as GH-RH are not well defined. In addition, it is too early to determine if androgen pretreatment (DHEA, testosterone) is beneficial in the treatment of poor responder cases in ART.
The available number of good quality embryos is the most important factor in achieving a pregnancy using ART. It is very difficult for poor responders to produce more than a few embryos in a single retrieval. Therefore, the accumulation of multiple cryopreserved embryos is key to achieve pregnancy in these patients. A Clomiphene regimen can be considered as an alternative choice for mild ovarian stimulation, as the endometrial condition is not considered if all embryos are frozen. Ovarian stimulation for poor responders is challenging; however, increasing the number of protocol choices may increase the prospects of achieving a pregnancy.
References
Surrey ES, Schoolcraft WB. Evaluating strategies for improving ovarian response of the poor responder undergoing assisted reproductive techniques. Fertil Steril. 2000;73:667–76.
Jenkins JM, Davies DW, Devonport H, Anthony FW, Gadd SC, Watson RH, et al. Comparison of “poor” responders with “good” responders using a standard buserelin/human menopausal gonadotrophin regime for in-vitro fertilization. Hum Reprod. 1991;6:918–21.
Ben-Rafael Z, Bider D, Dan U, Zolti M, Levran D, Mashiach S. Combined gonadotropin releasing hormone agonist/human menopausal gonadotropin therapy (GnRH-a/hMG) in normal, high, and poor responders to hMG. J In Vitro Fert Embryo Transf. 1991;8:33–6.
Akande VA, Fleming CF, Hunt LP, Keay SD, Jenkins JM. Biological versus chronological ageing of oocytes, distinguishable by raised FSH levels in relation to the success of IVF treatment. Hum Reprod. 2002;17:2003–8.
Nargund G, Bromhan D. Comparison of endocrinological and clinical profiles and outcome of IVF cycles in patients with one ovary and two ovaries. J Assist Reprod Genet. 1995;12:458–60.
Keay SD, Liversedge NH, Jenkins JM. Could ovarian infection impair ovarian response to gonadotrophin stimulation? Br J Obstet Gynaecol. 1998;105:252–3.
Ragni G, De Lauretis Yankowski L, Piloni S, Vegetti W, Guermandi E, Colombo M, et al. In vitro fertilization for patients with poor response and occult ovarian failure: a randomized trial. Reprod Technol. 2000;10:98–102.
Loh S, Wang JX, Matthews CD. The influence of body mass index, basal FSH and age on the response to gonadotrophin stimulation in non-polycystic ovarian syndrome patients. Hum Reprod. 2002;17:1207–11.
Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol. 1997;104:521–7.
Nikolaou D, Templeton A. Early ovarian aging: a hypothesis. Detection and clinical relevance. Hum Reprod. 2003;18:1137–9.
U.S. National Health Center for Health National Statistics. Health and injury chartbook. In U.S. National Health Center for Health Statistics, National vital statistics report, Vol. 50(5). Hyattsville: Centers for Disease Control and Prevention; 2002
Speroff L, Fritz MA. Clinical gynecologic and endocrinology and Infertility. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2004.
Bopp BL, Alper MM, Thompson IE, Mortola J. Success rates with gamete intrafallopian transfer and in vitro fertilization in women of advanced maternal age. Fertil Steril. 1995;63:1278–83.
Hull MG, Flemming CF, Hughes AO, McDermont A. The age-related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril. 1996;65:783–90.
Lass A, Croucher C, Duffy S, Dawson K, Margara R, Winston RM. One thousand initiated cycles of in vitro fertilization in women ≧ 40 years of age. Fertil Steril. 1998;70:1030–4.
Baired DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, et al. Fertility and aging. ESHRE Capri Workshop Group. Hum Reprod Update. 2005;11:261–76.
Vialard F, Lombroso R, Bergere M, Molina Gomes D, Hammonud I, Bailly M, et al. Oocyte aneuploidy mechanisms are different in two situations of increased chromosomal risk: older patients and patients with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2007;87:1333–9.
Practice Committee of the American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86 Suppl 4:S248–52.
Paulson RJ, Boostanfer R, Saadar P, Mor E, Tourgeman D, Slater CC, et al. Pregnancy in the sixth decade of life obstetric outcomes in women of advanced age. JAMA. 2002;288:2320–3.
Serour GI. Islamic perspectives in human reproduction; ethical issues in ART. Reprod Biomed Online. 2008;17 Suppl 3:34–8.
Serour GI. Medical and socio-cultural aspects of infertility in the middle east. ESHRE Monogr. 2008;1:34–41.
Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update. 2003;9:61–76.
Ubaldi FM, Rienzi L, Ferrero S, Baroni E, Sapienza F, Cobellis L, et al. Management of poor responders in IVF. Reprod Biomed Online. 2005;10:235–46.
Ferraretti AP, Ia Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: Bologna criteria. Hum Reprod. 2011;26:1616–24.
Out HJ, Mannaerts BM, Driessen SG, Coelingh Bennink HJ. Recombinant follicle stimulating hormone (rFSH; Puregon) in assisted reproduction: more oocytes, more pregnancies. Results from five comparative studies. Hum Reprod Update. 1996;2:162–71.
Raga F, Bonilla-Musoles F, Casan EM, Bonilla F. Recombinant follicle stimulating hormone stimulation in poor responders with normal basal concentrations of follicle stimulating hormone and oestradiol: improved reproductive outcome. Hum Reprod. 1999;14:1431–4.
Weissman A, Farhi J, Royburt M, Nahum H, Glezerman M, Levran D. Prospective evaluation of two stimulation protocols for low responders who were undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2003;79:886–92.
Craft I, Gorgy A, Hill J, Menon D, Podsiadly B. Will GnRH antagonists provide new hope for patients considered “difficult responders” to GnRH agonist protocols? Hum Reprod. 1999;14:2959–62.
Marci R, Caserta D, Dolo V, Tatone C, Pavan A, Moscarini M. GnRH antagonist in IVF poor-responder patients: results of a randomized trial. Reprod Biomed Online. 2005;11:189–93.
Detti L, Williams D, Robins J, Maxwell R, Thomas M. A comparison of three down regulation approaches for poor responders undergoing in vitro fertilization. Fertil Steril. 2005;84:1401–5.
Schoolcraft WB, Surrey ES, Minjarez DA, Gustofson RL. Microdose GnRH agonist flare protocol results in lower incidence of chromosome aneuploid blastocysts. Fertil Steril. 2011;96:S255.
Morgia F, Sbracia M, Schimberni M, Giallonardo A, Piscitelli C, Giannini P, et al. A controlled trial of natural cycle versus microdose gonadotropin-releasing hormone analog flare cycles in poor responders undergoing in vitro fertilization. Fertil Steril. 2004;81:1542–7.
D’Amato G, Caroppo E, Pasquadibisceglie A, Carone D, Vitti A, Vizziello GM. A novel protocol of ovulation induction with delayed gonadotropin-releasing hormone antagonist administration combined with high-dose recombinant follicle-stimulating hormone and clomiphene citrate for poor responders and women over 35 years. Fertil Steril. 2004;81:1572–7.
Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19:2031–5.
Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole and gonadotrophins. Fertil Steril. 2005;84:S95.
Fábregues F, Penarrubia J, Creus M, Manau D, Casals G, Carmona F, et al. Transdermal testosterone may improve ovarian response to gonadotrophins in low-responder IVF patients: a randomized, clinical trial. Hum Reprod. 2009;24:349–59.
Kim C-H, Howles CM, Lee H-A. The effect of transdermal testosterone gel pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertil Steril. 2011;95:679–83.
Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007;24:629–34.
Baricca A, Artini P, Del Monte P, Ponzani P, Pasquini P, Cariola G, et al. In vivo and in vitro effect of growth hormone on estradiol secretion by granulosa cells. J Clin Endocrinol Metab. 1993;77:61–7.
Adashi E, Resnick C, D’Erole J, Svoboda M, Van Nyk J. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985;6:400–20.
Owen EJ, Shoham Z, Mason BA, Ostergaard H, Jacobs HS. Cotreatment with growth hormone, after pituitary suppression, for ovarian stimulation in in vitro fertilization: a randomized, double-blind, placebo-control trial. Fertil Steril. 1991;56:1104–10.
Busacca M, Fusi F, Brigante C, Bonzi V, Gonfiantini C, Vignali M, et al. Use of growth hormone-releasing factor in ovulation induction in poor responders. J Reprod Med. 1996;41:699–703.
Fukuda A, Kawata A, Tohnaka M, Yamazaki M, Iwamoto H, Nakaoka Y, et al. Successful pregnancy by intracytoplasmic sperm injection of in vitro matured oocytes from non-stimulated women. J Fertil Implant. 2001;18:1–4.
Hashimoto S, Murata Y, Kikkawa M, Sonoda M, Oku H, Murata T, et al. Successful delivery after the transfer of twice-vitrified embryos derived from in vitro matured oocytes: a case report. Hum Reprod. 2007;22(1):221–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Fukuda, A. (2016). Ovarian Stimulation for Poor Responders. In: Allahbadia, G., Morimoto, Y. (eds) Ovarian Stimulation Protocols. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1121-1_9
Download citation
DOI: https://doi.org/10.1007/978-81-322-1121-1_9
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1120-4
Online ISBN: 978-81-322-1121-1
eBook Packages: MedicineMedicine (R0)