Abstract
Co(1−x)MnyZnyFe2O4 (x = 0.9,0.5, 0.1 and y = 0.45, 0.25, 0.05.) nanoparticles of average crystalline size of 61 nm were prepared by chemical co-precipitation method. XRD, FTIR, SEM were utilized in order to study the effect of variation in the cobalt substitution and its impact on size and associated water content. The average crystalline size (DaveXR) of the particles was found to be decreased from 85 to 41 nm with the increase in cobalt concentration. Fourier transform infrared spectroscopy (FTIR) spectra of the Co(1−x)MnyZnyFe2O4 in the range 400–4000 cm−1 were reported. The spinel structure and the crystalline water adsorption of cobalt doped Mn-Zn nanoparticles were studied by using FTIR.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Magnetic nanoparticles are of great technological importance because of their use in magnetic fluid, information storage system, medical diagnostics, and so on. Various preparation techniques have been used for the synthesis of fine particles of ferrites, which exhibit novel properties when compared to their properties in the bulk. Non-conventional methods such as co-precipitation, thermal decomposition, sol-gel and hydrothermal methods have been widely used. Ultra fine ferrite particles can be prepared by chemical co-precipitation method. Auzans et al. [1, 2] have studied the preparation and properties of Mn-Zn ferrite nano particles, which were, used in ionic and surfacted ferrofluids with different degrees of Zn substitution prepared by co-precipitation method. Chandana Rath et al. [3] have reported the dependence on cation distribution of crystallite size, lattice parameter and magnetic properties in nano size Mn-Zn ferrite for different degrees of inversion of Zn substitution prepared by hydrothermal precipitation method.
The use of Mn-Zn ferrite for the preparation of temperature sensitive magnetic fluid by co-precipitation method has already been studied [4–6]. Co0.2Zn0.8Fe2O4 fine particles have been prepared by chemical co-precipitation method followed by sintering [7]. Control of crystalline size in the nanometer range by the variation of synthesis condition is always a difficult task. It becomes mandatory in the case of ferrofluid preparation using co-precipitation method. In order to prepare ferrofluid having such fine particles, specific size restriction is imposed considering the stability criteria. Co(1−x)MnyZnyFe2O4 (x = 0.9,0.5,0.1 and y = 0.45, 0.25, 0.05.) substituted ferrites were prepared by co-precipitation method have not yet been fully studied like Mn-Zn substituted ferrites. In this paper we report preparation of Me1−xMnyZnyFe2O4 fine particles, where Me = Co2+ with x = 0.9, 0.5, 0.1. Average crystalline size 61 nm by chemical co-precipitation method and the consequent change in their lattice parameter, crystalline size and associated water content due to increase in cobalt concentration were reported.
2 Synthesis and Characterization of Co(1−x)MnyZnyFe2O4 of Nanoparticles
2.1 Synthesis of Cobalt Doped Mn-Zn Ferrites
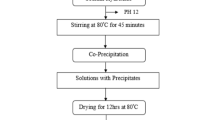
The cobalt doped ferrite nanoparticles synthesized by co-precipitation depends mostly on parameters such as reaction temperature, pH of the suspension, initial molar concentration etc. [4].Ultra fine particles of Co(1−x)MnyZnyFe2O4 (x = 0.9, 0.5, 0.1 and y = 0.45, 0.25, 0.05.) were prepared by co-precipitating aqueous solutions of CoCl2, MnCl2, ZnCl2 and FeCl3 mixtures respectively in alkaline medium. The mixed solution of CoCl2, MnCl2, ZnCl2 and FeCl3 in their respective stoichiometry (100 ml of 0.1 M CoCl2, 100 ml of 0.45 M ZnCl2, 100 ml of 0.45 M MnCl2 and 100 ml of. 2 M FeCl3 in the case of Co0.1Mn0.45Zn0.45Fe2O4 and similarly for the other values of x) was prepared and kept at 333 K (60 ℃).
This mixture was added to the boiling solution of NaOH (0.63 M dissolved in 1200 ml of distilled water) within 10 s under constant stirring. Nano ferrites are formed by conversion of metal salts into hydroxides, which take place immediately, followed by transformation of hydroxides into ferrites. The solutions were maintained at 358 K (85 ℃) for 1 h. This duration was sufficient for the transformation of hydroxides into spinel ferrite (dehydration and atomic rearrangement involved in the conversion of intermediate hydroxide phase into ferrite) [4]. Sufficient amount of fine particles were collected at this stage by using magnetic separation. These particles were washed several times with distilled water followed by acetone and dried at room temperature.
2.2 XRD
The X-ray diffraction (XRD) patterns of the samples were recorded on a BRUKER-binary V2 (.RAW) powder diffractometer using Cu K∝(λ = 1.54060 A°) radiation. Slow scans of the selected diffraction peaks were carried out in step mode (step size 0.02°, measurement time 5 s, measurement temperature 323 K (25 °C), standard: Si powder). The crystalline size of the nanocrystalline samples was measured using Debye- Scherrer formula,
2.3 FTIR
FTIR spectra were recorded for the dried samples of Co(1−x)MnyZnyFe2O4 (x = 0.9, 0.5, 0.1 and y = 0.45, 0.25, 0.05.) with Nexus 670 (range 400–4000 cm−1) spectrometer. The dried samples were mixed with KBr and spectra were measured according to transmittance method.
3 Results and Discussions
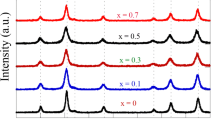
Generally, XRD can be used to characterize the crystallinity of nanoparticles, and it gives average diameters of all the nanoparticles. The precipitated fine particles were characterized by XRD for structural determination and estimation of crystallite size. XRD patterns were analyzed and indexed using JCPDS. All experimental peaks were matched with (JCPDS #653111) the theoretically generated one and indexed. Analysis of the diffraction pattern using powder-X software [8] confirms the formation of cubic spinel structure for all the samples. All the compositions had a spinel structure. The XRD pattern for Co(1−x)MnyZnyFe2O4 (x = 0.9, 0.5, 0.1 and y = 0.45, 0.25, 0.05.) shown Fig. 1.
The broad XRD lines indicate that the particles are of nanosize range. The particle size was found to decrease from 85 to 41 nm with the increase in cobalt concentration. The crystallite size (DXRD) was estimated by the Debye-Scherrer formula [9] using the full width at half maximum value of the respective indexed peaks. Though all the samples were prepared under identical condition, the crystallite size was not the same for all concentrations (Table 1). This was probably due to the preparation condition followed here, which gave rise to different rate of ferrite formation for different concentrations of cobalt, favoring the variation of crystalline size.
The variation of average crystalline size with the cobalt concentration is given in Fig. 2. Ferrofluids can be conveniently prepared by making use of particles in this size range. The average crystallite size (DaveXR) for Co(1−x)MnyZnyFe2O4 (x = 0.9,0.5,0.1 and
y = 0.45,0.25,0.05.) is shown in Table 1. The particle size was confirmed by SEM data (Fig. 3).
3.1 SEM
3.2 FTIR
From the FTIR spectra for Fe3O4 and for Co(1−x)MnyZnyFe2O4 (x = 0.9,0.5,0.1 and y = 0.45, 0.25, 0.05.), the spectral similarities were observed. The main transmittance frequencies observed in the region 400–4,000 cm−1 of the FTIR spectra for Co(1−x)MnyZnyFe2O4 (x = 0.9, 0.5, 0.1 and y = 0.45, 0.25, 0.05.) are summarized in Table 2.
The broad feature between 3460.66 and 3031.56 cm−1 is due to O–H stretch (\( \nu_{1} \)), which corresponds to the hydroxyl groups attached by the hydrogen bonds to the iron oxide surface and the water molecules chemically adsorbed to the magnetic particle surface (associated water content) [8]. From these results, it appears that the hydroxyl groups are retained in the samples during the preparation of the uncoated Co(1−x)MnyZnyFe2O 4 . Ghose et al. [7] have reported that the presence of some hydroxyl ions are completely removed when the sample is sintered at temperatures \( \ge \)973 K. The O–H in-plane (\( \nu_{2} \)) and out-of-plane (\( \nu_{3} \)) bonds appear at 1640.02–1509.94 cm−1 and 988.21–986.49 cm1, respectively. The spectrum of the uncoated sample Co(1−x)MnyZnyFe2O4 shows a strong band at \( \nu_{4} \) (635.57–573.51 cm−1) due to Fe3O4 [10]. The transmittance waveband at \( \nu_{4} \)(584.74–583.66 cm−1), which corresponds to the metal-oxygen bonds are considered as the confirmation for the ferrite formation. This is in good agreement with Zins et al. [1, 11–14] (Fig. 4).
4 Conclusion
The preparation technique of nano particles has a definite impact on the control of particle size and alteration of magnetic properties. The estimated cations from the product are in comparison with the initial substitution degree, indicating that the preparation procedure favors the formation of only ferrites. The formation of Co (1−x)MnyZnyFe2O4 (x = 0.9, 0.5, 0.1 and y = 0.45, 0.25, 0.05.) was confirmed by the X-ray diffraction. The average crystallite size (DaveXR) decreased when the partial substitution of cobalt increased. The Cobalt doped Mn-Zn ferrite particles can be used to prepare ferro fluids with higher magnetization. FTIR was used to confirm the formation of Fe–O bonds and presence of the associated water content in the samples.
References
Auzans E, Zins D, Blums E, Massart R (1999) Synthesis and properties of Mn-Zn Ferrite ferrofluids. J Mater Sci 34:1253
Auzans E, Zins D, Blums E, Massart R (1999) Magn. Gidrodinamika, Mn-Zn nanoparticles for ferrofluid preparation, Science direct 36:78
Chandana Rath S, Anand RP, Das KK, Sahu SD, Kulkarni SD, Date SK, Mishra NC (2002) Dependence on cation distribution of particle size, lattice parameter and magnetic properties in nanosized Mn-Zn ferrite. J Appl Phys 91(4):2211
Jeyadevan B, Chinnasamy CN, Shinoda K, Tohji K (2003) Mn-Zn ferrite with magnetization for temperature sensitivity magnetic fluid. J Appl Phys 93(10):8450
Parekh K, Upadhyay RV, Mehta RV (2000) Electron spin resonance study of temperature sensitive magnetic fluid. J Appl Phys 88(5):2799
Upadhyay T, Upadhyay RV, Mehta RV (1997) Characterization of a temperature sensitive magnetic fluid. Phys Rev B 55(9):5585
Dey S, Ghose J (2003) Synthesis, characterization and magnetic studies on nanocrystalline Co0.2Zn0.8Fe2o4. Mater Res Bull 38:1653
Creanga D, Calugaru Gh (2005) Physical investigations of a ferro fluid based on hydrocarbons. J Magn Magn Mater 289:81
Dong C (1999) PowderX: Windows -95 based program for powder X-ray diffraction data processing. J Appl Crystallogr 32:838
Ahn Y, Choi EJ, Kim EH (2003) Superparamagnetic relaxation in cobalt ferrite nanoparticles synthesized from hydroxide carbonate precursors. Rev Adv Mater Sci 5:477
Ma M, Zhang Y, Yu W, Shen H-Y, Zhang H-Q, Gu N (2003) Preparation and characterization of magnetic nano particles coated by aminosilane. J Colloid and Surface A: Physicochem Eng Aspects 212:219
Ahmed SR, Kofinas P (2005) Magnetic properties and morphologyof block co-polymer-cobalt oxide nanoparticles. J Magn Magn Mater 288:219
Ishikawa T, Nakazaki H, Yasukawa A, Kandori K, Seto M (1999) Influences of Co2 +, Cu2 + and Cr3 + ions on the formation of magnetite. Corros Sci 41:1665
Wu N, Fu L, Su M, Aslam M, Wong KC, Dravid VP (2004) Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Letters 4:383
Acknowledgments
One of the authors Dr. S. Sendhilnathan gratefully acknowledges DST (Ref No.SR/FTP/PS -59/2008) for the financial assistance received through the project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer India
About this paper
Cite this paper
Bhuvaneswari, M., Sendhilnathan, S. (2012). Preparation and Characterization of Cobalt Doped Mn-Zn Ferrites. In: Sathiyamoorthy, S., Caroline, B., Jayanthi, J. (eds) Emerging Trends in Science, Engineering and Technology. Lecture Notes in Mechanical Engineering. Springer, India. https://doi.org/10.1007/978-81-322-1007-8_55
Download citation
DOI: https://doi.org/10.1007/978-81-322-1007-8_55
Published:
Publisher Name: Springer, India
Print ISBN: 978-81-322-1006-1
Online ISBN: 978-81-322-1007-8
eBook Packages: EngineeringEngineering (R0)