Abstract
In teleost embryos, the formation of the body axes is controlled by both maternal and zygotic factors. During oogenesis, the formation of the oocyte’s animal–vegetal polarity is maternally controlled. A mature oocyte contains a set of factors (dorsal determinants) involved in dorsal determination at the vegetal pole. After fertilization, a parallel array of microtubules forms briefly at the yolk’s vegetal pole to transport the dorsal determinants to the prospective dorsal side. The dorsal determinants activate Wnt/β-catenin signaling and induce expression of dorsal-specific genes required for forming the dorsal organizer. The molecules expressed in the dorsal organizer antagonize the signaling of ventralizing or posteriorizing factors such as Bmps and Wnts, thereby establishing the signaling gradients that are subsequently required to properly form the dorsoventral (DV) and anteroposterior (AP) axes. Genetic analyses of zebrafish mutants have identified the maternal and zygotic genes that control formation of the body axes. Comparative studies of zebrafish, the primitive ray-finned fish bichir, the basal vertebrate lamprey, and the amphibian Xenopus indicate that bichir embryogenesis is a good model for understanding the evolution of DV axis formation. This chapter focuses on the genetic control of DV and AP axis formation, and its evolution in ray-finned fish.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In vertebrate embryogenesis, the dorsoventral (DV), anteroposterior (AP), and other body axes are formed through an intricate developmental process involving signaling, transcriptional regulation, organelle transport, and cell differentiation and movement. The molecular mechanisms that form these axes have been relatively well studied in zebrafish and other model animals. A zebrafish oocyte has clear animal–vegetal (AV) polarity in which the blastodisc and the yolk are located at the animal (A) and vegetal (V) poles, respectively, but there is no apparent DV axis until the early gastrula stage, at around 6 hours postfertilization (hpf), when the embryo’s dorsal side is marked by the embryonic shield (the dorsal organizer structure). However, the program that forms the DV axis is thought to be initiated soon after fertilization. Recent studies have revealed that oocyte AV polarity is linked to the formation of the embryonic DV axis. Dorsal determinants, which are deposited at the vegetal pole of the egg during oogenesis, are thought to be transferred after fertilization to the dorsal blastomeres, where they induce the dorsal-specific genes that establish the dorsal organizer. The organizer factors interact with ventralizing and posteriorizing signals to establish the embryonic DV and AP axes. In this chapter, we first describe the molecular mechanisms in zebrafish by which (1) oocyte AV polarity is established and linked to the embryo’s DV axis; (2) the dorsal organizer is formed; and (3) interactions between the organizer factors and ventralizing factors determine the DV and AP body axes. Next, we compare the developmental processes of zebrafish with those of other vertebrate species—including bichir, lamprey, and Xenopus—and discuss the evolution of the germ layer and DV axis formation in ray-finned fish.
2 Embryonic Development of Zebrafish
Zebrafish sperm enters the oocyte at the animal pole. After fertilization, the chorion is detached from the egg surface, and cytoplasmic materials in the yolk are transported toward the animal pole to form the blastodisc. After the first blastodisc cleavage at 45 minutes postfertilization (mpf), synchronous cleavages occur every 15 min until the midblastula transition (MBT) at the 512-cell stage, at around 3 hpf (Kimmel et al. 1995). Zygotic gene expression is initiated at the MBT. Cell differentiation and morphogenetic processes start concomitantly, including (1) formation of the enveloping layer (EVL), which is an epithelial cell sheet covering the blastoderm; (2) formation of the yolk syncytial layer (YSL), which occurs when marginal blastomeres (connected to the yolk) collapse and release their nuclei into the yolk; and (3) epiboly, in which the blastomeres and EVL move to the vegetal pole to cover the yolk; this event starts slightly later than the MBT (Kimmel et al. 1995). Although the YSL is an extraembryonic structure, it is important in forming the germ layer. Transplanting the YSL to the animal pole of the blastoderm ectopically induces the endoderm and mesoderm (Mizuno et al. 1996; Ober and Schulte-Merker 1999; Rodaway et al. 1999), and depletion of RNAs from the yolk inhibits mesoderm and endoderm formation (Chen and Kimelman 2000), indicating that the YSL functions to induce the endoderm and mesoderm (Sakaguchi et al. 2002). The YSL attaches to the EVL, possibly through E-cadherin, and takes the EVL and the blastoderm to the vegetal pole during epiboly (Shimizu et al. 2005b; Solnica-Krezel and Driever 1994).
During gastrulation, the embryonic structure is further established with a series of cell movements: involution/ingression of the mesendoderm, convergent extension, and migration of the axial mesoderm, in addition to epiboly (Solnica-Krezel and Sepich 2012). Mesoderm convergence and extension bring more cells to the dorsal side to form the dorsal thick (axial) mesendoderm. The axial mesendoderm elongates in the AP axis to become the prechordal plate and notochord. Various signaling pathways, including the Wnt/PCP (planar cell polarity) pathway, are involved in convergent extension [see review by Solnica-Krezel and Sepich (2012)]. Signaling molecules generated from the embryo’s dorsal and ventral regions act in coordination with gastrulation movements to establish the DV and AP axes.
3 Establishment of Oocyte Animal–Vegetal Polarity During Oogenesis in Zebrafish
Mature zebrafish oocytes contain dorsal determinants at the vegetal pole (see Sect. 32.4.2). Thus, the formation of AV polarity in oocytes is important for forming the embryonic DV and AP axes. A zebrafish oocyte develops through four stages until it is mature and competent for fertilization (Lessman 2009; Marlow 2010; Nagahama and Yamashita 2008). Oocyte AV polarity begins to form during stage I with the appearance of the Balbiani body (Fig. 32.1a), which is composed of a variety of messenger RNAs (mRNAs), proteins, and organelles, including mitochondria. The Balbiani body initially localizes adjacent to the nucleus on the future vegetal pole side and then moves to the vegetal pole and releases its contents (e.g., mRNAs) to the vegetal cortex (Bontems et al. 2009; Marlow and Mullins 2008). In Xenopus and zebrafish oocytes, some germline-specific transcripts are transported via the Balbiani body to the vegetal pole (Wilk et al. 2005; Kloc et al. 1996, 2001; Kloc and Etkin 1995; Kosaka et al. 2007).
Maternally controlled formation of the oocyte animal–vegetal (AV) axis and embryonic dorsoventral (DV) axis in zebrafish. (a) Formation of the oocyte AV axis. In the stage I oocyte, messenger RNAs (mRNAs) involved in dorsal determination and germ cell development are transcribed in the nucleus and deposited in the Balbiani body (Bb); these include glutamate receptor interacting protein 2a (grip2a), syntabulin (sybu), wnt8a, and daz-like (dazl) mRNAs. During oocyte maturation, mRNAs are transferred from the Balbiani body to the vegetal cortex. (b) Microtubule-dependent dorsal determination. Sperm entry activates Ca2+ signaling, which travels from the animal pole to the vegetal pole, where it induces microtubule formation. The microtubules are bundled to form an array at around 20 minutes postfertilization. (c) Dorsal determinants (DDs) or vesicles containing DDs are transported through the vegetal microtubule array. At the early cleavage stage, an unknown mechanism transports DDs (or vesicular DDs) to prospective dorsal blastomeres, where they activate canonical Wnt signaling, which causes β-catenin to accumulate at the blastula stage (Figure modified from Hibi et al. 2002; Langdon and Mullins 2011; Nojima et al. 2010)
Genetic studies with a maternal mutant have revealed that formation of the Balbiani body is controlled by Bucky ball, a protein that lacks obvious sequence similarities to other proteins (Bontems et al. 2009; Marlow and Mullins 2008). Oocytes with a bucky ball mutation fail to form the Balbiani body, to localize germline mRNAs to the vegetal pole, and to establish AV polarity (Bontems et al. 2009; Marlow and Mullins 2008). In these bucky ball–mutant oocytes, mRNAs that normally localize to the vegetal pole localize radially instead (Bontems et al. 2009; Dosch et al. 2004; Nojima et al. 2010). The vegetal pole localization of the transcripts for glutamate receptor interacting protein 2a (Grip2a) and Syntabulin, which are reported to be involved in dorsal determination, is dependent on Bucky ball (Nojima et al. 2010; Ge et al. 2014); wnt8a transcripts, which are probably involved in dorsal determination (Sect. 3.2), are also transferred from the Balbiani body to the vegetal pole (Lu et al. 2011). These data suggest that the localization of mRNAs to the vegetal pole, which depends on the Balbiani body, may be indispensable in dorsal determination. Study of another maternal mutant, magellan, has revealed that microtubule–actin crosslinking factor 1a (Macf1a) regulates oocyte AV polarity, possibly through microtubule-dependent transport of Balbiani body components (Gupta et al. 2010).
4 Microtubule-Dependent Dorsal Determination in Zebrafish
4.1 Microtubule Array Formation
An array of parallel microtubules forms at the vegetal pole of the zebrafish embryo at around 20 mpf (Jesuthasan and Stahle 1997) (Fig. 32.1b). Disruption of the microtubules by nocodazole treatment, cold temperatures, or ultraviolet irradiation at this point causes a loss of the dorsal organizer and ventralization of the embryo (Jesuthasan and Stahle 1997). Removal of the vegetal yolk mass at the early one-cell stage also severely ventralizes the embryo (Mizuno et al. 1999; Ober and Schulte-Merker 1999). These data have established the hypothesis that dorsal determinants initially localize to the vegetal pole and are then transported along the vegetal microtubules to the prospective dorsal side, where they activate the genetic program(s) that induce dorsal tissue.
The relevance of the vegetal microtubule array in dorsal determination was initially proposed for Xenopus embryogenesis. After fertilization, the Xenopus egg cortex rotates relative to the sperm entry point during the first cell cycle (cortical rotation). Microtubules initially appear to be randomly oriented at the vegetal cortex. However, during cortical rotation, the cortical microtubules become aligned with the plus ends toward the prospective dorsal side (Olson et al. 2015). During this process, organelles are transported along the microtubule array. Inhibition of microtubule formation disrupts dorsal tissue formation (Elinson and Rowning 1988; Houliston and Elinson 1991; Rowning et al. 1997), supporting the microtubule array’s role in transporting dorsal determinants (or organelles containing the dorsal determinants) in Xenopus embryos. In Xenopus, sperm entry is proposed to control the orientation of vegetal microtubules by providing nascent microtubules originating from the sperm-derived centrosome (aster) (Houliston and Elinson 1991; Schroeder and Gard 1992).
In zebrafish, the micropyle is located at the animal pole region of the chorion, and the sperm can enter only into the animal pole of the egg. No microtubules are seen in the area between the pronucleus (its associated centrosome) and the vegetal cortex. Therefore, the sperm entry provides little, if any, information regarding the bias of vegetal microtubule orientation. Nevertheless, the plus ends of the vegetal microtubules that form at around 20–30 mpf are oriented to the prospective dorsal side (within 30° of the embryonic shield) (Tran et al. 2012) (Fig. 32.1b). Vegetal microtubule formation depends on Ca2+ signaling, which is potentially activated by sperm entry (Tran et al. 2012) (Fig. 32.1b). Vesicular structures (called cortical granules) are transported along the dorsal-oriented microtubules to the prospective dorsal side (Tran et al. 2012). Some mRNAs that localize to the vegetal pole, such as wnt8a mRNA, are translocated slightly to the prospective dorsal side. Although it is not clear how the vegetal microtubules are oriented to the prospective dorsal side, these data suggest that microtubule-dependent transport and a cortical rotation–like movement take place in zebrafish. These events are likely to be involved in dorsal determination in zebrafish, as in Xenopus (Fig. 32.1c). However, the vegetal microtubules do not reach the blastodisc (Tran et al. 2012), implying that the vegetal microtubule-dependent mechanism only provides a bias in positioning the dorsal determinants at the vegetal pole, and that other mechanism(s) translocate the dorsal determinants to the dorsal blastomeres.
4.2 Mechanisms That Control Microtubule Array Formation and Transport of Dorsal Determinants
Studies of maternal effect mutations in zebrafish that affect the initial dorsal determination have identified molecules that function in microtubule-dependent dorsal determination (Table 32.1, Fig. 32.1b). The hecate mutants are deficient in genes that encode Grip2a, an adaptor protein that contains multiple PDZ domains (Ge et al. 2014), and whose mRNA localizes to the vegetal pole. The hecate mutant embryos fail to bundle microtubules at the vegetal pole and do not form a parallel microtubule array, implying that Grip2a is involved in bundling the vegetal microtubules (Ge et al. 2014). Embryos with the maternal effect mutant brom bones, which are deficient in the gene encoding polypyrimidine tract binding protein 1a (Ptbp1a, also known as hnRNPI), are also severely ventralized. Embryos with a brom bones mutation do not activate inositol 1,4,5-triphosphate (IP3)-mediated Ca2+ release, do not undergo exocytosis of the cortical granules, and do not form a vegetal microtubule array (Mei et al. 2009), suggesting that Ptbp1a is involved in processing the pre-RNA for IP3–Ca2+ signaling components. It has been suggested that the persistent cortical granules in the vegetal cortex of brom bones mutants secondarily affect the formation of the vegetal microtubule array (Mei et al. 2009). However, it is also possible that the IP3-dependent activation of Ca2+ signaling plays a role in formation of the vegetal microtubule array. In any case, these data provide genetic evidence that the parallel microtubule array plays a pivotal role in dorsal determination. Embryos of the maternal effect mutant tokkaebi also show ventralization but do not display abnormalities in formation of the vegetal microtubule array (Nojima et al. 2004). The tokkaebi locus encodes Syntabulin, which is a linker for the kinesin motor protein and is involved in microtubule-dependent transport of organelles in neurons (Cai et al. 2005; Su et al. 2004). Since syntabulin mRNA is localized to the vegetal pole, Syntabulin protein is also localized to the vegetal pole until the vegetal microtubules form, after which Syntabulin is translocated from the vegetal pole in a microtubule-dependent manner and is degraded at the two-cell stage (Nojima et al. 2010). These data suggest that Syntabulin assists in transporting the dorsal determinants—or the organelles (e.g., vesicles) containing the dorsal determinants—along the vegetal microtubules and in releasing them from the vegetal microtubules after the two-cell stage. It was recently reported that maternal effect mutants of kif5Ba, which encodes a heavy chain of Kinesin-1, showed abnormal formation of the vegetal microtubules (random orientation or nonbundled), aberrant localization of Syntabulin and wnt8a mRNA, and ventralized phenotypes (Campbell et al. 2015), suggesting that Kinesin-1 plays an important role in the vegetal microtubule formation and the subsequent microtubule-dependent dorsal determination.
4.3 Dorsal Determinants
The canonical Wnt pathway, which results in β-catenin accumulation, is believed to play an essential role in dorsal determination in Xenopus and zebrafish embryos. In these animals, activating the canonical Wnt pathway elicits ectopic formation or expansion of the dorsal organizer, whereas its inhibition impairs dorsal axis formation (Hibi et al. 2002). Furthermore, the maternal effect mutant ichabod, which lacks the expression of maternal β-catenin 2, fails to establish dorsal tissues (Kelly et al. 2000). In zebrafish, β-catenin accumulation is detected in the nuclei of dorsal blastomeres by the 128-cell stage, and in the dorsal blastoderm and dorsal YSL of midblastula-stage embryos (Dougan et al. 2003; Schneider et al. 1996). These data suggest that dorsal determinants activate the canonical Wnt pathway to induce dorsal-specific genes. It was initially thought that Wnt molecules may not be directly involved in dorsal determination, since inhibition of Wnt molecules by either dominant negative Wnt1/8 or a secreted Frizzled-related protein, Frzb1, did not suppress dorsal axis formation in Xenopus embryos (Tao et al. 2005; Hoppler et al. 1996; Leyns et al. 1997; Wang et al. 1997). However, maternal Wnt11 was shown to activate the canonical Wnt pathway, and the Wnt11/5a complex has been suggested to be a dorsal determinant in Xenopus (Cha et al. 2008; Cha et al. 2009). Neither wnt11 nor wnt5a can activate the canonical Wnt pathway and induce dorsal-specific genes in zebrafish (Lu et al. 2011; Nojima et al. 2010). In zebrafish, wnt8a mRNA localizes to the egg’s vegetal pole (Lu et al. 2011) and then is translocated from the vegetal pole in a microtubule-dependent manner. Expression of a dominant negative Wnt8a abolishes the expression of the dorsal-specific gene chordin (Lu et al. 2011; Ge et al. 2014; Tran et al. 2012), suggesting that wnt8a mRNA is a dorsal determinant. Many vegetal mRNAs and proteins are translocated by the movement accompanying cortical rotation. It is not clear whether it is the wnt8a mRNA or Wnt8a protein that acts as a dorsal determinant. It also remains to be elucidated how Wnt8a is transported to the dorsal blastoderm. Before the 128-cell stage, wnt8a mRNA is not detected in dorsal blastomeres, yet Wnt8a protein may be translated and transported to the dorsal blastomeres before that stage. Genetic analyses of maternal wnt8a mutants and detailed localization analyses of wnt8a mRNA or Wnt8a protein should provide compelling evidence for the role of Wnt8a in dorsal determination. In any case, Wnt8a may play a major role in activating the canonical Wnt pathway (Fig. 32.1c).
5 Program for Forming the Dorsal Organizer in Zebrafish
5.1 Wnt Signaling and Dharma
The canonical Wnt pathway induces dorsal-specific genes by binding a complex of Tcf/Lef family protein and β-catenin to their promoter/enhancer regions at the MBT; this process is controlled by both positive and negative regulators. Caveolin-1 is reported to inhibit the nuclear translocation of β-catenin, and Tob1a inhibits the formation of the Tcf/Lef and β-catenin complex (Mo et al. 2010; Xiong et al. 2006).
The dorsal-specific gene dharma (also known as nieuwkoid) encodes a homeodomain-containing transcriptional repressor, which harbors an Engrailed homology 1 (Eh1) repressor motif (Koos and Ho 1998; Yamanaka et al. 1998), The dharma-defective mutant bozozok exhibits various degrees of defects in the dorsoanterior tissues, including defects in organizer formation (Fekany et al. 1999; Koos and Ho 1999). At the MBT, dharma is expressed in the dorsal blastoderm; thereafter, its expression is confined to the dorsal YSL at the early gastrula stage (Koos and Ho 1998; Yamanaka et al. 1998). The canonical Wnt pathway induces dharma expression. The dharma promoter/enhancer contains many Tcf/Lef-binding sites involved in dorsal-specific dharma expression (Leung et al. 2003b; Ryu et al. 2001; Shimizu et al. 2000), indicating that dharma is a direct target of the maternal canonical Wnt pathway (Fig. 32.2a). Dharma represses the ventral expression of the homeobox genes vox, vent, and ved (Kawahara et al. 2000a, b; Imai et al. 2001; Shimizu et al. 2002). The expression of vox, vent, and ved is positively regulated by the maternal factor Runx2b type2 (Runx2bt2) (Flores et al. 2008). Vox, Vent, and Ved are also transcriptional repressors, which repress dorsal organizer genes such as goosecoid and chordin (Shimizu et al. 2002; Imai et al. 2001; Melby et al. 2000). Hence, the Dharma-mediated repression of vox, vent, and ved releases the expression of dorsal organizer genes in the dorsal blastomeres. Vox, Vend, and Ved also repress dharma expression. Thus, the mutual repression of vox/vent/ved and dharma refines the dorsal organizer domain (Kawahara et al. 2000a, b; Imai et al. 2001; Shimizu et al. 2002). The SoxB1 transcription factors Sox3 and Sox19b also restrict dharma expression (Shih et al. 2010). Dharma not only represses vox/vent/ved expression but also directly represses the gene expression of the ventralizing factors Bmp2b and Wnt8a (Erter et al. 2001; Leung et al. 2003a). The Dharma-mediated inhibition of Bmp and Wnt signals may contribute to Dharma’s non-cell-autonomous role in forming dorsal and anterior tissues (Koos and Ho 1998; Yamanaka et al. 1998). The stability of the Dharma protein is regulated by protein degradation mediated by the E3 ubiquitin ligase Lnx2b (Ro and Dawid 2009). The regulation of dharma expression and its role in DV axis formation are summarized in Fig. 32.2a.
Molecular mechanisms controlling dorsoventral (DV) axis formation in zebrafish. (a) Control of DV axis formation at the midblastula transition (left panel) and at the late blastula to early gastrula stages (right panel). Expression of the ventralizing factors Bmp2b/4/7, Vox/Vent/Ved, and Wnt3a/8a is regulated by the maternal factors Gdf6a/Pou5f3, Runx2b, and Kzp, respectively. Expression of the dorsalizing factors Dharma and Ndr1 is regulated by maternal Wnt signaling. The mutual repressive interaction between Dharma and Vox/Vent/Ved defines the DV axis. Dharma releases the expression of dorsal organizer genes by suppressing Vox/Vent/Ved-mediated repression of these genes. (b) Regulation of DV axis formation at the gastrula stage. Dorsalizing and ventralizing signals are marked by blue lines and red lines, respectively; maternal factors are indicated by gray letters. (c) Regulation of Chordin and Bmps. Chordin has four cysteine-rich (CR) domains. Tolloid and Bmp1—which have proteinase, CUB, and epidermal growth factor–like domains (E)—cleave Chordin protein. Sizzled, which has a CR domain similar to that of Frizzled, binds Bmp1 and inhibits Bmp1-dependent (and possibly Tolloid-dependent) Chordin degradation. Crossveinless 2 (CV2) contains CR domains and a von Willebrand factor domain (vWFd). The cleaved form of CV2 suppresses Chordin-mediated Bmp inhibition and thus displays pro-Bmp activity. Twisted gastrulation (Tsg) binds Chordin and Bmps, and promotes Bmp signaling. Tsg enhances Tolloid-dependent Chordin degradation (Langdon and Mullins 2011; Muraoka et al. 2006; Shimizu et al. 2000)
5.2 Nodal-Related Genes
The Nodal-related gene ndr1 (also known as squint) is also thought to be a target of the maternal canonical Wnt pathway (Shimizu et al. 2000; Kelly et al. 2000). The dorsal expression of dharma and ndr1 is lost in ichabod mutants, which are defective in β-catenin 2; β-catenin rescues the expression of these two genes (Kelly et al. 2000), supporting the idea that dharma and ndr1 function downstream from the maternal Wnt pathway. Ndr1 functions with another Nodal-related gene, ndr2 (known as cyclops), to form the endoderm and the dorsal mesoderm (Dougan et al. 2003; Erter et al. 1998; Rebagliati et al. 1998a, b; Sampath et al. 1998; Feldman et al. 1998; Schier and Talbot 2005). A combined deficiency of dharma and ndr1 (zygotic combined mutations) severely reduces the dorsoanterior tissues (Shimizu et al. 2000; Sirotkin et al. 2000), revealing that dharma and ndr1 play major roles in forming the dorsal organizer and the dorsoanterior tissues (Fig. 32.2a). In addition to this zygotic Ndr1 function, a role has also been proposed for maternal ndr1 transcripts in dorsal determination. Maternally deposited ndr1 mRNA is distributed to the two prospective dorsal blastomeres at the four-cell stage; morpholino-mediated ndr1 knockdown causes severe ventralization (Gore et al. 2005), suggesting that maternal Ndr1 functions in dorsal determination. However, arguing against this role of Ndr1, dorsal axis formation is not severely defective in maternal effect ndr1 mutants that cannot generate the Ndr1 protein (Erter et al. 2001; Feldman et al. 1998; Heisenberg and Nusslein-Volhard 1997; Amsterdam et al. 2004). It was recently suggested that a noncoding function of ndr1 RNA might activate the maternal canonical Wnt pathway, although the mechanism remains elusive (Lim et al. 2012).
5.3 Fgf Signaling
The fibroblast growth factors (Fgf) fgf3, fgf8, and fgf24—which are expressed in the dorsal marginal blastomeres in the blastula period—control dorsal axis formation by repressing bmp gene expression (Furthauer et al. 1997, 2004). In ichabod mutants, β-catenin-dependent expression of the organizer genes depends on Fgf signaling. Fgf signaling is also involved in Ndr1-dependent chordin expression and in maintaining dharma expression (Maegawa et al. 2006) (Fig. 32.2a). Therefore, the fgf genes function in dorsal axis formation downstream from the maternal canonical Wnt pathway. The Fgf signaling in dorsal axis formation is negatively regulated by Sef, Sprouty2, and Dusp6 (Mkp3, a mitogen-activated protein kinase [MAPK] phosphatase), which function as feedback regulators (Furthauer et al. 2002, 2004; Tsang et al. 2002, 2004). Precise control of the Fgf signaling gradient should contribute to the regulation of Bmp signaling along the DV axis. Fgf signaling, together with Wnt and retinoic acid signaling, also controls the posteriorization of embryos (Koshida et al. 1998, 2002; Shimizu et al. 2005a, 2006; Kudoh et al. 2002, 2004) (Fig. 32.3c, d).
Fate determination according to dorsoventral (DV) and anteroposterior (AP) positional information in zebrafish. (a) Wnt signaling controls the fates of the ventrolateral versus axial mesendoderm. Wnts and Vox/Vent/Ved are involved in forming the ventrolateral mesoderm. Dkk1, Dharma, and Goosecoid control the formation of axial mesendoderm tissue by inhibiting Wnts and Vox/Vent/Ved. (b) A Bmp signal gradient controls cell fates on the DV axis. Strong Bmp activity is required for formation of the ventral mesoderm and epidermis. Bmp inhibition is required for neuroectoderm formation. (c) Control of AP axis formation in the neuroectoderm. The posterior region of the neuroectoderm, which receives Wnt and fibroblast growth factor (Fgf) signaling from the nonaxial mesoderm, is fated to become posterior neural tissue such as the spinal cord or hindbrain. The anterior (animal pole) region of the neuroectoderm, which does not receive posterior- Fig. 32.3 (continued) izing signals, becomes anterior neural tissues such as the forebrain and midbrain. (d) Molecular mechanisms controlling AP axis formation during early neural development. Wnts and Fgfs from the nonaxial mesoderm regulate the expression of the caudal-related homeodomain proteins Cdx1a and Cdx4, which induce the posterior hox genes that determine the fate of the posterior spinal cord. Retinoic acid (RA), generated by the anterior paraxial mesoderm, controls expression of the anterior hox genes required for fate determination of the hindbrain (posterior from rhombomere 2) and the anterior spinal cord. Posteriorizing signals (Wnt, Fgf, and RA) must be inhibited to induce anterior neural tissues
6 Interactions Between the Dorsal Organizer and Ventralizing/Caudalizing Factors in Zebrafish
6.1 Goosecoid
The Wnt target genes (e.g., dharma, ndr1, and fgfs) cooperatively regulate the expression of dorsal organizer genes such as goosecoid, chordin, and dickkopf1 (dkk1) (Hashimoto et al. 2000; Maegawa et al. 2006; Shimizu et al. 2000; Sirotkin et al. 2000) (Fig. 32.2a). The expression of goosecoid and dkk1 depends on both Dharma and Nodal signaling (Hashimoto et al. 2000; Shimizu et al. 2000). However, chordin expression depends more on Dharma than on Nodal signaling, as chordin expression is relatively well maintained in Nodal signal–deficient embryos, such as the maternal zygotic one-eyed pinhead (oep) mutant (Gritsman et al. 1999; Shimizu et al. 2000). Goosecoid is a homeodomain-containing transcription factor, which harbors an Eh1 repressor motif and exhibits sequence similarity to Dharma (Cho et al. 1991; Stachel et al. 1993). Goosecoid can inhibit Bmp signaling even in the absence of the secreted Bmp inhibitors Chordin, Noggin1, and Follistatin-like 1b (Dixon Fox and Bruce 2009) (Fig. 32.2a). Thus, it is possible that Goosecoid directly binds bmp gene promoters/enhancers to negatively regulate their expression in dorsal blastomeres, like Dharma. Although the goosecoid gene exists in most, if not all, vertebrate genomes, dharma is found only in teleost genomes. It is conceivable that goosecoid was duplicated during teleost-specific whole-genome duplication (WGD), and that the expression and function of the two genes were diversified during teleost evolution: dharma came to be expressed earlier, goosecoid came to function at a later stage, and the expression of goosecoid came to depend on dharma. This hypothesis could explain the variable expressivity and penetrance of the bozozok mutants (Fekany et al. 1999) because dharma may partly function redundantly with goosecoid.
6.2 Chordin and Other Bmp Antagonists Regulate Bmp Signaling
Chordin, which is a secreted Bmp inhibitor containing four cysteine-rich domains, binds Bmp dimers, thereby inhibiting Bmp’s binding to its receptors (Piccolo et al. 1996; Sasai et al. 1994; De Robertis 2009). Among the known Bmp inhibitors, Chordin functions nonredundantly in DV axis formation (Dal-Pra et al. 2006; Hammerschmidt et al. 1996; Schulte-Merker et al. 1997). The genes encoding the Bmp inhibitors Noggin1 and Follistatin-like 1b are also expressed in the dorsal organizer region at the blastula and early gastrula stages (Dal-Pra et al. 2006; Furthauer et al. 1999). These genes function redundantly with Chordin: knocking the genes down separately does not cause ventralization, but knockdown of the two genes along with chordin results in strong ventralization (Dal-Pra et al. 2006; Furthauer et al. 1999). Compared with Noggin1 and Follistatin-like 1b, Chordin has unique features: (1) chordin is expressed more broadly than other Bmp inhibitor genes and is negatively controlled by Bmp signaling (Miller-Bertoglio et al. 1997; Schulte-Merker et al. 1997); and (2) the stability of the Chordin protein is precisely regulated by a mechanism involving Tolloid/Bmp1 family proteinase, Sizzled, Twisted gastrulation, and other proteins (Blader et al. 1997; Connors et al. 1999, 2006; Jasuja et al. 2006; Little and Mullins 2004; Muraoka et al. 2006; Xie and Fisher 2005; Yabe et al. 2003) (Fig. 32.2b, c). These features make it likely that Chordin is a nonredundant factor that regulates Bmp signaling and DV axis formation.
In addition to Chordin, Crossveinless 2 (CV2)—a Chordin family protein that has von Willebrand factor C domains and is expressed ventrally—also modulates Bmp activity. Noncleaved CV2 functions as a Bmp inhibitor, but the cleaved form binds both Bmp and Chordin and suppresses Chordin-mediated Bmp inhibition (pro-Bmp activity) (Rentzsch et al. 2006; Zhang et al. 2010). The expression of the bmp genes bmp2b, bmp4, and bmp7a is regulated by the maternal factors Gdf6a (also known as Radar, a Bmp-related cytokine), Pou5f3 (also known as Pou2 or Spiel ohne grenzen, an Oct3/4 orthologue or paralogue) (Reim and Brand 2006; Sidi et al. 2003), and the zygotic SoxB1 transcription factors Sox2/3/19a/19b (Okuda et al. 2010) (Fig. 32.2a). bmp gene expression is also self-regulated by Bmp signaling (De Robertis 2009). Interactions between Chordin, the Chordin regulators, ventrally expressed Bmps (Bmp2b, Bmp4, and Bmp7a), and dorsally expressed Bmps (Bmp2b and Admp) define a clear Bmp signaling gradient that is required for cell differentiation along the DV axis (Fig. 32.3b). The control of Bmp signaling is beyond the scope of this chapter; for excellent reviews on the regulation of Bmp signaling in zebrafish axis formation, see Langdon and Mullins (2011) and Bier and De Robertis (2015).
6.3 Role of Wnt Inhibition in Forming the DV and AP Axes
Although maternal canonical Wnt signaling is involved in initiating dorsal axis formation, zygotic canonical Wnt signaling negatively regulates dorsal axis formation and positively regulates formation of posterior tissues. The mechanism by which maternal and zygotic Wnt signals regulate a different set of genes and exhibit opposite functions in axis formation has not been defined. During gastrulation, wnt8a and wnt3a are expressed throughout the blastoderm margin except in the most dorsal region, which corresponds to the embryonic shield; this expression persists in the tailbud at the end of gastrulation (Kelly et al. 1995; Lekven et al. 2001; Shimizu et al. 2005a). The expression of wnt8a is at least partly regulated by the maternal transcription factor Kzp (Kaiso zinc finger–containing protein) (Yao et al. 2010) (Fig. 32.2a). The zebrafish wnt8a gene has two open reading frames (ORFs), which are located tandemly in the zebrafish genome and can function as a bicistronic transcript (Lekven et al. 2001). Inhibition of both wnt8a ORFs reduces the ventrolateral mesoderm and tail structure, and enlarges the head structure (Lekven et al. 2001). Wnt3a functions redundantly with Wnt8a. Inhibition of both Wnt3a and the Wnt8a ORFs causes more severe phenotypes than inhibition of Wnt8a alone: the ventrolateral mesoderm is reduced, while the axial mesoderm expands, and the tail structure is lost, while the head structure expands (Shimcizu et al. 2005a; Thorpe et al. 2005). Inhibition of β-catenins 1 and 2 expands chordin expression and causes severe dorsalization (Varga et al. 2007). These data suggest that zygotic (gastrula) canonical Wnt signaling plays two roles in axis formation: (1) it controls the fate of the axial versus ventrolateral mesoderm for the DV axis, since Wnt induces the ventrolateral mesoderm; and (2) it functions as a posteriorizing signal for the AP axis (Fig. 32.3a, c). The ventral expression of vox, vent, and ved is regulated by Wnt8a/Wnt3a signaling, and wnt8a and wnt3a expression is regulated by Vox/Vent/Ved (Ramel and Lekven 2004; Shimizu et al. 2005a). The cross-regulation between Wnt signaling and Vox/Vent/Ved may refine the fate determination of axial versus ventrolateral mesoderm tissue; Dharma restricts the axial mesoderm by repressing these ventralizing signals (Figs. 32.2a and 32.3a). For posteriorization, the caudal-related genes cdx1a and cdx4 function downstream from Wnt signaling; inhibition of Cdx1a and Cdx4 severely truncates the posterior, as with inhibition of Wnt8a/Wnt3a (Shimizu et al. 2005a) (Fig. 32.3d). The loss of Cdx1a/Cdx4 also leads to ectopic formation of hindbrain tissue (Shimizu et al. 2006; Skromne et al. 2007), indicating that Cdx1a and Cdx4 function downstream from Wnt8a/3a to control posterior tissue formation and repress anterior tissues (Fig. 32.3d). The Sp1 family transcription factors Sp5a and Sp5l also function downstream from Wnt signaling, for both the DV and AP axes (Thorpe et al. 2005; Weidinger et al. 2005). Further studies are necessary to reveal the relationships between Sp5/5l and Vox/Vent/Ved, or Sp5/Sp5l and Cdx1a/Cdx4, for DV and AP axis formation (Table 32.2).
The dorsal organizer expresses the Wnt inhibitor Dkk1 (Glinka et al. 1998), which binds the Wnt lipoprotein receptor proteins 5 and 6 (LRP5/6) and Kremen, and downregulates Wnt signaling (Davidson et al. 2002; Mao et al. 2002). Zebrafish have two dkk1 genes (Untergasser et al. 2011). Initially, dkk1b is expressed in the dorsal marginal blastoderm and the dorsal YSL at the blastula stage, and then in the prechordal plate at the gastrula stage (Hashimoto et al. 2000; Shinya et al. 2000). Canonical Wnt signaling also controls dkk1b, thus functioning as a negative feedback regulator (Shinya et al. 2000). In the blastoderm margin, dkk1b expression may be regulated by Wnt8a and Wnt3a (Hashimoto et al. 2000; Shinya et al. 2000). Thus, the Wnt8a/Wnt3–Dkk1 system is part of a reaction–diffusion mechanism that forms a Wnt signal gradient; a similar role has been proposed for Dkk in hair follicle formation (Sick et al. 2006). This mechanism may be similar to that in the Nodal–Lefty (Nodal inhibitor) system for mesoderm and endoderm differentiation (Muller et al. 2012; Schier 2009). As nonaxial mesendoderm expressing Wnt8a and Wnt3a is suggested to provide posteriorizing signals (Koshida et al. 1998; Woo and Fraser 1997), Dkk1b may generate a high Wnt signal area near the margin and a low Wnt signal area in the animal pole (Fig. 32.3c, d). This Wnt signaling gradient is required to establish AP embryonic polarity. At the late gastrula stage, Dkk1b from the prechordal plate may ensure the anterior neural fate. Since Dkk1b can rescue the formation of not only the anterior neuroectoderm but also the axial mesoderm in dharma-deficient bozozok embryos, Dkk1 may also be involved in axial mesendoderm formation (Hashimoto et al. 2000) (Fig. 32.3a). Although genetic analysis has revealed that Dkk1 is essential for head development in the mouse (Mukhopadhyay et al. 2001), the role of Dkk1 in formation of the AP axis in zebrafish has not been genetically proven. Combination knockouts of dkk family members expressed in zebrafish gastrula embryos (dkk1a, dkk1b, and dkk3) (Lu et al. 2011) should reveal the role of Dkk family proteins in body axis formation.
6.4 Nodal/Bmp/Fgf/Wnt Signaling Interactions for DV and AP Axis Formation
Zygotic Nodal, Bmp, Fgf, and Wnt signaling cooperatively regulate axis formation. DV patterning along the AP axis is reported to be temporally coordinated: inhibition of Bmp signaling at the onset of gastrulation controls the formation of the anterior neuroectoderm, whereas at a later stage it regulates the formation of the posterior neuroectoderm (Tucker et al. 2008). In this process, Bmp-mediated DV patterning is temporally coordinated with the posteriorizing signals Fgf, Wnt, and retinoic acid (Hashiguchi and Mullins 2013). Fgf negatively regulates Bmp signaling by phosphorylating Smad1/5, which are Bmp signal transducers; this mechanism is involved in coordination of Bmp and Fgf signaling, at least in part (Hashiguchi and Mullins 2013).
The ventral blastoderm margin is proposed to function as the tail organizer independently of the dorsal organizer. The activation of Nodal/Bmp/Wnt signaling mimics tail organizer activity (Agathon et al. 2003). The dorsal organizer, the tail organizer, and the entire blastoderm margin are proposed to function as an organizing center, which depends on the ratio of Nodal/Bmp activity: Nodal is high on the dorsal side, and Bmp is high on the ventral side (Fauny et al. 2009). Moreover, Nodal and Bmp alone are sufficient to organize uncommitted naïve cells of the blastula animal pole into a well-organized embryo, both in vivo and in vitro (Xu et al. 2014). These data suggest that Nodal and Bmp signaling are minimal requirements for providing embryonic cells with DV and AP axis information, and that Nodal/Bmp activity gradients play a pivotal role in axis formation. Although genetic evidence may be required to prove that endogenous Nodal and Bmp levels are sufficient to generate the embryonic axis, the data imply that Nodal and Bmp signaling function as hubs in the program for DV and AP axis formation. Other signaling pathways and transcription factors that control the expression of Nodal/Bmp molecules and inhibitors function downstream from (and possibly in parallel with) Nodal/Bmp signaling in axis formation, as discussed earlier. Mathematical modeling will help to explain the intricate programs that shape the DV and AP axes.
7 Evolution of Axis Formation in Ray-Finned Fish (Actinopterygii)
7.1 Bichir Provides a Good Model for Evolutionary Developmental Biology (Evo–Devo) Studies
Although the mechanisms controlling axis formation in zebrafish are relatively well understood, the degree to which these mechanisms are conserved among fish—and whether these mechanisms are also shared by other vertebrate species—is not clear. Ancient jawless fish (Agnatha) that existed about 600 million years ago were the ancestors of all vertebrates (Blair and Hedges 2005). The extant jawless fish are in the class Cyclostomata, which includes lampreys and hagfish; other vertebrates (Gnathostomata) are derived from an ancient lineage of jawed fish that diverged from the Agnatha. The extant jawed fish are categorized into two groups: cartilaginous fish (Chondrichthyes) and bony fish (Osteichthyes); the bony fish are further classified into two subgroups: lobe-finned fish (Sarcopterygii—e.g., coelacanths, lungfish, and tetrapods) and ray-finned fish (Actinopterygii). The ray-finned fish include several taxa: Polypteriformes (bichirs and reedfish), Chondrostei (sturgeons and paddlefishes), Lepisosteiformes (gars), Amiiformes (Amia calva), and Teleostei (teleosts, Fig. 32.4). Most extant fish, including zebrafish and medaka model fish, are teleosts. Early embryogenesis is similar in various teleosts, but teleost embryogenesis is quite different from that of nonteleost fish (Bolker 1993a, b; Cooper and Virta 2007). This difference may be rooted in the WGD that is proposed to have taken place in the teleosts (Amores et al. 1998; Jaillon et al. 2004). The teleost-specific WGD may have generated gene variations that contribute to teleost-specific developmental processes (Kuraku et al. 2009).
Vertebrate phylogenic tree and cleavage patterns. Basal chordate embryos undergo holoblastic cleavages. As the common ancestor of vertebrates evolutionarily acquired a massive yolk (indicated by the yellow color in the embryos), their cleavage patterns were highly diversified. The holoblastic cleavage pattern is retained in various vertebrate lineages (indicated by red lines). The transition from a holoblastic to a meroblastic cleavage pattern occurred independently several times in the vertebrate lineages (yellow circles). Bichirs (Polypteriformes)—the most primitive ray-finned fish (Actinopterygii)—undergo holoblastic cleavages, as do amphibians. Bichirs diverged from all other ray-finned fish about 400 million years ago (Mya) during the Devonian period, before the teleost-specific (TS) whole-genome duplication (WGD) and soon after an ancestral bony fish diverged into ray-finned and lobe-finned fish (Sarcopterygii)
Thus, to understand the evolution of fish embryogenesis, it is important to study the development of nonteleost fish. Bichirs (order: Polypteriformes, family; Polypteridae), which live in African rivers and lakes, have been used in recent studies of developmental biology (Takeuchi et al. 2009a). The bichir lineage diverged from ray-finned fish about 400 million years ago, soon after bony fish diverged into lobe-finned and ray-finned fish (Inoue et al. 2003). Thus, bichirs are considered to be one of the most primitive ray-finned fish. Furthermore, bichirs did not undergo teleost-specific WGD. Therefore, comparative analysis of zebrafish and bichir development should provide insights into the evolution of mechanisms for embryonic axis formation.
7.2 Morphogenetic Processes of Bichir Embryos
A teleost embryo undergoes meroblastic cleavages, whereas a bichir embryo undergoes holoblastic cleavages, like the Xenopus embryo, cleaving from the animal to the vegetal pole. In Xenopus, the first cleavage furrow demarcates the DV axis. When one of the two blastomeres is labeled at the two-cell stage, the interface between the labeled and unlabeled halves coincides with the midsagittal plane (Klein 1987). In contrast, the first cleavage plane in zebrafish is not related to either the DV axis or the left–right (LR) axis, and the blastomeres are intermingled during the blastula and gastrula stages (Kimmel and Law 1985; Kimmel and Warga 1987), indicating that Xenopus and zebrafish use distinct developmental strategies for cleaving and mixing the blastomeres. In bichir embryos, injection of rhodamine dextran into one of the two blastomeres at the two-cell stage marks cells in either the left or right half of the embryo (Fig. 32.5a) (Takeuchi et al. 2009a). Therefore, the first cleavage plane in bichir embryos demarcates the DV axis, and the blastomeres are not intermingled as they are in Xenopus embryos. Holoblastic cleavage is associated with formation of embryonic cavities (the blastocoel and archenteron) and absence of the EVL and YSL. Bichir embryos have a blastocoel and an archenteron, and no EVL or YSL (Fig. 32.5), suggesting they have inherited an amphibian-type morphogenetic process. The embryonic structure of bichirs is also similar to that of the agnathan lamprey (Takeuchi et al. 2009b). It is tempting to speculate that the morphogenetic process of the bichir embryo may be similar to that of ancestral fish; the EVL and YSL might have evolved in the ray-finned fish lineage after the bichir had diverged from the ray-finned fish.
Early bichir and lamprey embryogenesis. (a) In bichirs, as in amphibians, the first cleavage plane of the embryo demarcates the dorsoventral (DV) axis. However, there is no correlation between the first cleavage and the body axes in teleost embryos. In bichirs, rhodamine injection into one of the blastomeres of a two-cell-stage embryo (left panel) results in fluorescence mostly in either the left or right half of the embryo at the tailbud stage (middle left panel: bright-field image; middle right panel: merged images from bright-field and fluorescent images; right panel: high-magnification view of the Fig. 32.5 (continued) midline region, fluorescent image). Dotted lines indicate the midline. (b) Germ layer patterning in the bichir and lamprey during gastrulation. Bichir and lamprey embryos have a blastocoel and an archenteron but no enveloping layer (EVL) or yolk syncytial layer (YSL). Both the mesoderm (red) and endoderm (green) are formed in the marginal zone of the bichir embryo and in the conical eminence of the lamprey embryo during early gastrulation. In the bichir embryo, the vegetal cell mass (VCM) is an extraembryonic structure, which does not contribute to the endoderm. The archenterons are marked by asterisks. (c) Evolution of endoderm and mesoderm induction in vertebrates. In the vertebrate ancestor, vegetal cells might have induced the endoderm (green arrow) and mesoderm (pink arrow) in the embryo; the bichir VCM retains this activity. With the holoblastic-to-meroblastic transition, this activity was retained in the YSL of teleost embryos. The activity was incorporated into the endoderm cells of amphibian embryos as the vegetal cells became the endoderm. In amniote embryos, the activity was retained in the visceral endoderm or the hypoblast, which are extraembryonic structures
7.3 Molecular Mechanisms Controlling Bichir Embryonic Development
It is thought that a cytoplasmic fusion of vegetal blastomeres during the evolution of ray-finned fish brought about the teleost YSL (Figs. 32.4 and 32.5). The zebrafish YSL and the Xenopus vegetal endoderm are strikingly similar in their ability to induce the mesoderm and endoderm. However, the cellular identity of the teleost YSL is distinct from that of the Xenopus vegetal endoderm. The Xenopus vegetal endoderm expresses endodermal markers and gives rise to endoderm tissues such as the pancreas, liver, and gut, whereas the teleost YSL is an extraembryonic tissue that does not express endoderm markers. Bichir embryos, like Xenopus embryos, have vegetal blastomeres, which raises the question of whether the vegetal blastomeres in bichir embryos are endoderm cells.
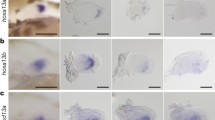
However, recent histological analyses of bichir and lamprey embryos, using molecular markers, has revealed that the vegetal blastomeres in bichirs and lampreys are extraembryonic nutritive cells that do not express endoderm markers (Takeuchi et al. 2009b). The endoderm and mesoderm form in the equatorial (marginal) zone (the conical eminence in lampreys). The Nodal-related gene ndr1, which is expressed in the zebrafish YSL, is also expressed in the equatorial zone of bichir embryos (Takeuchi et al. 2009b). Although bichir and lamprey embryos are morphologically similar to Xenopus embryos, the localization of the mesoderm and endoderm in bichir and lamprey embryos is similar to that in zebrafish (teleost) embryos (Fig. 32.5).
The T-box transcription factor Tbx16/VegT is required for endoderm formation and for vegetal cell induction of the mesoderm in Xenopus. Tbx16/VegT mRNA is maternally deposited at the vegetal pole of the Xenopus egg (Zhang et al. 1998). In contrast to Xenopus, bichirs do not express tbx16 maternally or in vegetal blastomeres (Takeuchi et al. 2009b). There is no tbx16 homologue in the lamprey genome. The mRNA of Eomesodermin homologue a (Eomesa, Eomes, Tbr2)—another T-box transcription factor involved in endoderm formation—is maternally deposited and zygotically expressed in the prospective endoderm in zebrafish (Bjornson et al. 2005). As with zebrafish, eomes transcripts are maternally deposited and zygotically expressed in the endoderm region in bichir and lamprey embryos (Takeuchi et al. 2009b). These data suggest that although bichirs and lampreys follow the amphibian-type morphogenetic process, they use the teleost-type mechanism for germ layer formation. The lamprey and bichir lineages are phylogenetically distant but use similar mechanisms for embryonic morphogenesis and germ layer formation. This developmental strategy—amphibian-type (holoblastic) morphogenesis and teleost-type germ layer formation—might have been used by the common ancestors of the vertebrates (the stem lineage). As maternal tbx16/VegT expression is common to at least some amphibians, Tbx16/VegT-dependent differentiation of the endoderm of the vegetal cells might have evolved in the amphibian lineage (Takeuchi et al. 2009b).
7.4 Bichir DV Axis Formation
Injection of Xenopus β-catenin mRNA into both blastomeres of a two-cell-stage bichir embryo can induce a secondary axis, as in Xenopus (Takeuchi et al. 2009a) (Fig. 32.6). This observation indicates that the maternal canonical Wnt pathway also plays a key role in dorsal determination in the bichir embryo. What molecule(s) function downstream from the Wnt pathway to control the expression of dorsal-specific genes in bichirs? Nodal-related genes are regulated by the Wnt pathway in both Xenopus and zebrafish (Sect. 32.5.2). In bichir embryos, ndr1 is expressed in the dorsal equatorial zone (Takeuchi et al. 2009b), suggesting that ndr1 may also be involved in inducing the dorsal organizer, as proposed for zebrafish. The transcriptional repressor Dharma functions downstream from the Wnt pathway to induce dorsal tissues in zebrafish (Leung et al. 2003b; Ryu et al. 2001; Shimizu et al. 2000), whereas the transcriptional activator Siamois and its paralog Twin mediate this process in Xenopus (Ishibashi et al. 2008; Kessler 1997; Laurent et al. 1997; Lemaire et al. 1995). Injection of the mRNA of the bichir Siamois-related transcription activator into Xenopus embryos can induce a secondary axis (Masaki Takeuchi, unpublished data). These data suggest that bichirs use the amphibian-type mechanism—at least in part—for Wnt signal–mediated induction of dorsal tissues. Although siamois-related genes have not been identified in lampreys or other nonbichir fish, the presence of the siamois-related gene in bichirs suggests that common ancestors of the bony fish might have used Siamois-related transcription factor(s) for dorsal axis formation (Fig. 32.6). During teleost evolution, the siamois-related gene was lost, and dharma might have evolved (or diverged from goosecoid) in the teleost lineage (Leung et al. 2003b; Ryu et al. 2001; Shimizu et al. 2000).
Axis formation in bichirs and its evolution in vertebrates. A siamois-related gene might have mediated the canonical Wnt pathway to induce the dorsal axis in the common ancestor of the bony fish. During evolution, the siamois-related gene was retained in the amphibian and bichir genomes, but it was lost in the amniote and teleost genomes. In contrast, dharma evolved to function as a Wnt target for dorsal axis formation in teleosts
8 Perspectives
Although we understand many aspects of the molecular mechanisms that control axis formation in zebrafish, there are many unanswered questions, including how the oocyte AV polarity is initially established during oogenesis, how the vegetal pole mRNAs are transferred through the Balbiani body to the vegetal pole, what initiates the formation of the vegetal microtubules, how the vegetal microtubules are oriented to the prospective dorsal side, and how patterning signals (Wnt, Bmp, Nodal, Fgf, etc.) are coordinated to control axis formation.
Many loss-of-function studies of zygotic genes have used antisense morpholinos. Recent genome-editing techniques, such as the CRISPR/Cas9 and TALEN systems, can generate mutants of genes of interest, which will allow us to determine the function of genes whose mutants were not isolated by forward genetic screening. Germline replacement with the genetic mutants will enable us to understand the role of maternal factors (Ciruna et al. 2002). It has been reported that antisense morpholino-mediated knockdown and genetic knockout (mutation) often lead to different phenotypes (Kok et al. 2015; Stainier et al. 2015). Although genetic compensation (upregulation of genes that compensate for loss of target genes) may explain such discrepancies (Rossi et al. 2015), we may need to reevaluate data obtained from antisense morpholino experiments.
In dorsal determination, regulation of microtubule formation and microtubule-dependent transport play essential roles. Visualization of the components involved in dorsal determination, along with time-lapse analysis, will reveal the molecular dynamics associated with DV axis formation. Mathematical modeling with precise transcriptome (single-cell transcriptome) data should help us understand the intricate processes that coordinate multiple signals.
There are also questions with respect to evolution, including to what degree the axis formation mechanisms are conserved among fish; whether the bichir-type developmental mechanism (amphibian-type morphogenetic process, teleost-type germ layer formation, and amphibian-type dorsal axis formation) is used by Cyclostomata (lamprey and hagfish), cartilaginous fish, lobe-finned fish, and nonteleost ray-finned fish; and how stem lineage axis formation is adapted for the amniote lineage. Sequencing with next- and third-generation sequencers enables us to reveal the genome sequences and transcriptomes of nonmodel animals, and whole-genome sequencing of bichir is in progress. Genome-editing techniques also allow us to study gene function in these animals. In the future, we will be able to discuss the details of the molecular mechanisms that form the body axes in many different vertebrate species, and find the blueprint for the evolution of axis formation in vertebrates.
References
Agathon A, Thisse C, Thisse B (2003) The molecular nature of the zebrafish tail organizer. Nature 424(6947):448–452. https://doi.org/10.1038/nature01822
Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282(5394):1711–1714
Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N (2004) Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA 101(35):12792–12797. https://doi.org/10.1073/pnas.0403929101
Bier E, De Robertis EM (2015) Embryo development. BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science 348(6242):aaa5838. https://doi.org/10.1126/science.aaa5838
Bjornson CR, Griffin KJ, Farr GH 3rd, Terashima A, Himeda C, Kikuchi Y, Kimelman D (2005) Eomesodermin is a localized maternal determinant required for endoderm induction in zebrafish. Dev Cell 9(4):523–533. https://doi.org/10.1016/j.devcel.2005.08.010
Blader P, Rastegar S, Fischer N, Strahle U (1997) Cleavage of the BMP-4 antagonist chordin by zebrafish tolloid. Science 278(5345):1937–1940
Blair JE, Hedges SB (2005) Molecular phylogeny and divergence times of deuterostome animals. Mol Biol Evol 22(11):2275–2284. https://doi.org/10.1093/molbev/msi225
Bolker JA (1993a) Gastrulation and mesoderm morphogenesis in the white sturgeon. J Exp Zool 266(2):116–131. https://doi.org/10.1002/jez.1402660206
Bolker JA (1993b) The mechanism of gastrulation in the white sturgeon. J Exp Zool 266(2):132–145. https://doi.org/10.1002/jez.1402660207
Bontems F, Stein A, Marlow F, Lyautey J, Gupta T, Mullins MC, Dosch R (2009) Bucky ball organizes germ plasm assembly in zebrafish. Curr Biol 19 (5):414–422. doi:S0960-9822(09)00610-1, pii: https://doi.org/10.1016/j.cub.2009.01.038
Cai Q, Gerwin C, Sheng ZH (2005) Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol 170 (6):959–969. doi:jcb.200506042, pii: https://doi.org/10.1083/jcb.200506042
Campbell PD, Heim AE, Smith MZ, Marlow FL (2015) Kinesin-1 interacts with Bucky ball to form germ cells and is required to pattern the zebrafish body axis. Development 142(17):2996–3008. https://doi.org/10.1242/dev.124586
Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J (2008) Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and noncanonical signaling in Xenopus axis formation. Development 135 (22):3719–3729. doi:dev.029025, pii: https://doi.org/10.1242/dev.029025
Cha SW, Tadjuidje E, White J, Wells J, Mayhew C, Wylie C, Heasman J (2009) Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol 19(18):1573–1580. https://doi.org/10.1016/j.cub.2009.07.062
Chen S, Kimelman D (2000) The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development 127(21):4681–4689
Cho KW, Blumberg B, Steinbeisser H, De Robertis EM (1991) Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67(6):1111–1120
Ciruna B, Weidinger G, Knaut H, Thisse B, Thisse C, Raz E, Schier AF (2002) Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci U S A 99(23):14919–14924. https://doi.org/10.1073/pnas.222459999
Colozza G, De Robertis EM (2014) Maternal syntabulin is required for dorsal axis formation and is a germ plasm component in Xenopus. Differentiation 88(1):17–26. https://doi.org/10.1016/j.diff.2014.03.002
Connors SA, Trout J, Ekker M, Mullins MC (1999) The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development 126(14):3119–3130
Connors SA, Tucker JA, Mullins MC (2006) Temporal and spatial action of tolloid (mini fin) and chordin to pattern tail tissues. Dev Biol 293(1):191–202. https://doi.org/10.1016/j.ydbio.2006.01.029
Cooper MS, Virta VC (2007) Evolution of gastrulation in the ray-finned (actinopterygian) fishes. J Exp Zool B Mol Dev Evol 308(5):591–608. https://doi.org/10.1002/jez.b.21142
Dal-Pra S, Furthauer M, Van-Celst J, Thisse B, Thisse C (2006) Noggin1 and Follistatin-like2 function redundantly to chordin to antagonize BMP activity. Dev Biol 298(2):514–526. https://doi.org/10.1016/j.ydbio.2006.07.002
Davidson G, Mao B, del Barco Barrantes I, Niehrs C (2002) Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development 129(24):5587–5596
De Robertis EM (2009) Spemann’s organizer and the self-regulation of embryonic fields. Mech Dev. doi:S0925–4773(09)01446–4, pii: https://doi.org/10.1016/j.mod.2009.08.004
Dixon Fox M, Bruce AE (2009) Short- and long-range functions of Goosecoid in zebrafish axis formation are independent of Chordin, Noggin 1 and Follistatin-like 1b. Development 136(10):1675–1685. https://doi.org/10.1242/dev.031161
Dosch R, Wagner DS, Mintzer KA, Runke G, Wiemelt AP, Mullins MC (2004) Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell 6(6):771–780. https://doi.org/10.1016/j.devcel.2004.05.002
Dougan ST, Warga RM, Kane DA, Schier AF, Talbot WS (2003) The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development 130(9):1837–1851
Elinson RP, Rowning B (1988) A transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specifies the dorso-ventral axis. Dev Biol 128(1):185–197
Erter CE, Solnica-Krezel L, Wright CV (1998) Zebrafish nodal-related 2 encodes an early mesendodermal inducer signaling from the extraembryonic yolk syncytial layer. Dev Biol 204(2):361–372
Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L (2001) Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development 128(18):3571–3583
Fauny JD, Thisse B, Thisse C (2009) The entire zebrafish blastula–gastrula margin acts as an organizer dependent on the ratio of nodal to BMP activity. Development 136(22):3811–3819. https://doi.org/10.1242/dev.039693
Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, Schier AF, Driever W, Hirano T, Talbot WS, Solnica-Krezel L (1999) The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development 126(7):1427–1438
Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS (1998) Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395(6698):181–185
Flores MV, Lam EY, Crosier KE, Crosier PS (2008) Osteogenic transcription factor Runx2 is a maternal determinant of dorsoventral patterning in zebrafish. Nat Cell Biol 10(3):346–352. https://doi.org/10.1038/ncb1697
Furthauer M, Thisse C, Thisse B (1997) A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development 124(21):4253–4264
Furthauer M, Thisse B, Thisse C (1999) Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol 214(1):181–196. https://doi.org/10.1006/dbio.1999.9401
Furthauer M, Lin W, Ang SL, Thisse B, Thisse C (2002) Sef is a feedback-induced antagonist of Ras/MAPK-mediated FGF signalling. Nat Cell Biol 4(2):170–174. https://doi.org/10.1038/ncb750
Furthauer M, Van Celst J, Thisse C, Thisse B (2004) Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development 131(12):2853–2864. https://doi.org/10.1242/dev.01156
Ge X, Grotjahn D, Welch E, Lyman-Gingerich J, Holguin C, Dimitrova E, Abrams EW, Gupta T, Marlow FL, Yabe T, Adler A, Mullins MC, Pelegri F (2014) Hecate/Grip2a acts to reorganize the cytoskeleton in the symmetry-breaking event of embryonic axis induction. PLoS Genet 10(6):e1004422. https://doi.org/10.1371/journal.pgen.1004422
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391(6665):357–362. https://doi.org/10.1038/34848
Gore AV, Maegawa S, Cheong A, Gilligan PC, Weinberg ES, Sampath K (2005) The zebrafish dorsal axis is apparent at the four-cell stage. Nature 438 (7070):1030–1035. doi:nature04184, pii: https://doi.org/10.1038/nature04184
Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF (1999) The EGF–CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97(1):121–132
Gupta T, Marlow FL, Ferriola D, Mackiewicz K, Dapprich J, Monos D, Mullins MC (2010) Microtubule actin crosslinking factor 1 regulates the Balbiani body and animal–vegetal polarity of the zebrafish oocyte. PLoS Genet 6(8):e1001073. https://doi.org/10.1371/journal.pgen.1001073
Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C (1996) Dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123:95–102
Hashiguchi M, Mullins MC (2013) Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development 140(9):1970–1980. https://doi.org/10.1242/dev.088104
Hashimoto H, Itoh M, Yamanaka Y, Yamashita S, Shimizu T, Solnica-Krezel L, Hibi M, Hirano T (2000) Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev Biol 217(1):138–152. https://doi.org/10.1006/dbio.1999.9537
Heisenberg CP, Nusslein-Volhard C (1997) The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol 184(1):85–94. https://doi.org/10.1006/dbio.1997.8511
Hibi M, Hirano T, Dawid IB (2002) Organizer formation and function. Results Probl Cell Differ 40:48–71
Hoppler S, Brown JD, Moon RT (1996) Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10(21):2805–2817
Houliston E, Elinson RP (1991) Evidence for the involvement of microtubules, ER, and kinesin in the cortical rotation of fertilized frog eggs. J Cell Biol 114(5):1017–1028
Imai Y, Gates MA, Melby AE, Kimelman D, Schier AF, Talbot WS (2001) The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development 128(12):2407–2420
Inoue JG, Miya M, Tsukamoto K, Nishida M (2003) Basal actinopterygian relationships: a mitogenomic perspective on the phylogeny of the “ancient fish”. Mol Phylogenet Evol 26(1):110–120
Ishibashi H, Matsumura N, Hanafusa H, Matsumoto K, De Robertis EM, Kuroda H (2008) Expression of Siamois and twin in the blastula chordin/noggin signaling center is required for brain formation in Xenopus laevis embryos. Mech Dev 125(1–2):58–66. https://doi.org/10.1016/j.mod.2007.10.005
Jaillon O, Aury JM, Brunet F, Petit JL, Stange-Thomann N, Mauceli E, Bouneau L, Fischer C, Ozouf-Costaz C, Bernot A, Nicaud S, Jaffe D, Fisher S, Lutfalla G, Dossat C, Segurens B, Dasilva C, Salanoubat M, Levy M, Boudet N, Castellano S, Anthouard V, Jubin C, Castelli V, Katinka M, Vacherie B, Biemont C, Skalli Z, Cattolico L, Poulain J, De Berardinis V, Cruaud C, Duprat S, Brottier P, Coutanceau JP, Gouzy J, Parra G, Lardier G, Chapple C, McKernan KJ, McEwan P, Bosak S, Kellis M, Volff JN, Guigo R, Zody MC, Mesirov J, Lindblad-Toh K, Birren B, Nusbaum C, Kahn D, Robinson-Rechavi M, Laudet V, Schachter V, Quetier F, Saurin W, Scarpelli C, Wincker P, Lander ES, Weissenbach J, Roest Crollius H (2004) Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431(7011):946–957. https://doi.org/10.1038/nature03025
Jasuja R, Voss N, Ge G, Hoffman GG, Lyman-Gingerich J, Pelegri F, Greenspan DS (2006) bmp1 and mini fin are functionally redundant in regulating formation of the zebrafish dorsoventral axis. Mech Dev 123(7):548–558. https://doi.org/10.1016/j.mod.2006.05.004
Jesuthasan S, Stahle U (1997) Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol 7(1):31–42
Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB (2000a) Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc Natl Acad Sci U S A 97(22):12121–12126. https://doi.org/10.1073/pnas.97.22.12121
Kawahara A, Wilm T, Solnica-Krezel L, Dawid IB (2000b) Functional interaction of vega2 and goosecoid homeobox genes in zebrafish. Genesis 28(2):58–67
Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT (1995) Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development 121(6):1787–1799
Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES (2000) Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127(18):3899–3911
Kessler DS (1997) Siamois is required for formation of Spemann's organizer. Proc Natl Acad Sci U S A 94(24):13017–13022
Kimmel CB, Law RD (1985) Cell lineage of zebrafish blastomeres. III. Clonal analyses of the blastula and gastrula stages. Dev Biol 108(1):94–101
Kimmel CB, Warga RM (1987) Indeterminate cell lineage of the zebrafish embryo. Dev Biol 124(1):269–280
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203(3):253–310. https://doi.org/10.1002/aja.1002030302
Klein SL (1987) The first cleavage furrow demarcates the dorsal–ventral axis in Xenopus embryos. Dev Biol 120(1):299–304
Kloc M, Etkin LD (1995) Two distinct pathways for the localization of RNAs at the vegetal cortex in Xenopus oocytes. Development 121(2):287–297
Kloc M, Larabell C, Etkin LD (1996) Elaboration of the messenger transport organizer pathway for localization of RNA to the vegetal cortex of Xenopus oocytes. Dev Biol 180 (1):119–130. doi:S0012-1606(96)90289-4, pii: https://doi.org/10.1006/dbio.1996.0289
Kloc M, Bilinski S, Chan AP, Allen LH, Zearfoss NR, Etkin LD (2001) RNA localization and germ cell determination in Xenopus. Int Rev Cytol 203:63–91
Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND (2015) Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell 32(1):97–108. https://doi.org/10.1016/j.devcel.2014.11.018
Koos DS, Ho RK (1998) The nieuwkoid gene characterizes and mediates a Nieuwkoop-center-like activity in the zebrafish. Curr Biol 8(22):1199–1206
Koos DS, Ho RK (1999) The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula. Dev Biol 215(2):190–207. https://doi.org/10.1006/dbio.1999.9479
Kosaka K, Kawakami K, Sakamoto H, Inoue K (2007) Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mech Dev 124 (4):279–289. doi:S0925-4773(07)00004-4, pii: https://doi.org/10.1016/j.mod.2007.01.003
Koshida S, Shinya M, Mizuno T, Kuroiwa A, Takeda H (1998) Initial anteroposterior pattern of the zebrafish central nervous system is determined by differential competence of the epiblast. Development 125(10):1957–1966
Koshida S, Shinya M, Nikaido M, Ueno N, Schulte-Merker S, Kuroiwa A, Takeda H (2002) Inhibition of BMP activity by the FGF signal promotes posterior neural development in zebrafish. Dev Biol 244(1):9–20. https://doi.org/10.1006/dbio.2002.0581
Kudoh T, Wilson SW, Dawid IB (2002) Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 129(18):4335–4346
Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW (2004) Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development 131(15):3581–3592. https://doi.org/10.1242/dev.01227
Kuraku S, Meyer A, Kuratani S (2009) Timing of genome duplications relative to the origin of the vertebrates: did cyclostomes diverge before or after? Mol Biol Evol 26(1):47–59. https://doi.org/10.1093/molbev/msn222
Langdon YG, Mullins MC (2011) Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet 45:357–377. https://doi.org/10.1146/annurev-genet-110410-132517
Laurent MN, Blitz IL, Hashimoto C, Rothbacher U, Cho KW (1997) The Xenopus homeobox gene twin mediates Wnt induction of goosecoid in establishment of Spemann's organizer. Development 124(23):4905–4916
Lekven AC, Thorpe CJ, Waxman JS, Moon RT (2001) Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1(1):103–114
Lemaire P, Garrett N, Gurdon JB (1995) Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81(1):85–94
Lessman CA (2009) Oocyte maturation: converting the zebrafish oocyte to the fertilizable egg. Gen Comp Endocrinol 161(1):53–57. https://doi.org/10.1016/j.ygcen.2008.11.004
Leung T, Bischof J, Soll I, Niessing D, Zhang D, Ma J, Jackle H, Driever W (2003a) Bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development 130(16):3639–3649
Leung T, Soll I, Arnold SJ, Kemler R, Driever W (2003b) Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastula-transition activation of boz expression. Dev Dyn 228(3):424–432
Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM (1997) Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88(6):747–756
Lim S, Kumari P, Gilligan P, Quach HN, Mathavan S, Sampath K (2012) Dorsal activity of maternal squint is mediated by a noncoding function of the RNA. Development 139(16):2903–2915. https://doi.org/10.1242/dev.077081
Little SC, Mullins MC (2004) Twisted gastrulation promotes BMP signaling in zebrafish dorsal–ventral axial patterning. Development 131(23):5825–5835. https://doi.org/10.1242/dev.01464
Lu FI, Thisse C, Thisse B (2011) Identification and mechanism of regulation of the zebrafish dorsal determinant. Proc Natl Acad Sci U S A 108(38):15876–15880. https://doi.org/10.1073/pnas.1106801108
Maegawa S, Varga M, Weinberg ES (2006) FGF signaling is required for β-catenin-mediated induction of the zebrafish organizer. Development 133(16):3265–3276. https://doi.org/10.1242/dev.02483
Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417(6889):664–667. https://doi.org/10.1038/nature756
Marlow FL (2010). Maternal control of development in vertebrates: my mother made me do it! Morgan & Claypool Life Sciences, San Rafael
Marlow FL, Mullins MC (2008) Bucky ball functions in Balbiani body assembly and animal–vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev Biol 321 (1):40–50. doi:S0012-1606(08)00916-0, pii: https://doi.org/10.1016/j.ydbio.2008.05.557
Mei W, Lee KW, Marlow FL, Miller AL, Mullins MC (2009) hnRNP I is required to generate the Ca2+ signal that causes egg activation in zebrafish. Development 136 (17):3007–3017. doi:136/17/3007, pii: https://doi.org/10.1242/dev.037879
Melby AE, Beach C, Mullins M, Kimelman D (2000) Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol 224(2):275–285. https://doi.org/10.1006/dbio.2000.9780
Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME (1997) Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol 192(2):537–550. https://doi.org/10.1006/dbio.1997.8788
Mizuno T, Yamaha E, Wkahara A, Kuroiwa A, Takeda H (1996) Mesoderm induction in zebrafish. Nature 383(6596):131–132
Mizuno T, Yamaha E, Kuroiwa A, Takeda H (1999) Removal of vegetal yolk causes dorsal deficencies and impairs dorsal-inducing ability of the yolk cell in zebrafish. Mech Dev 81(1–2):51–63
Mo S, Wang L, Li Q, Li J, Li Y, Thannickal VJ, Cui Z (2010) Caveolin-1 regulates dorsoventral patterning through direct interaction with beta-catenin in zebrafish. Dev Biol 344(1):210–223. https://doi.org/10.1016/j.ydbio.2010.04.033
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Izpisua Belmonte JC, Westphal H (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1(3):423–434
Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF (2012) Differential diffusivity of nodal and lefty underlies a reaction-diffusion patterning system. Science 336(6082):721–724. https://doi.org/10.1126/science.1221920
Muraoka O, Shimizu T, Yabe T, Nojima H, Bae YK, Hashimoto H, Hibi M (2006) Sizzled controls dorso-ventral polarity by repressing cleavage of the Chordin protein. Nat Cell Biol 8(4):329–338. https://doi.org/10.1038/ncb1379
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Develop Growth Differ 50(Suppl 1):S195–S219. https://doi.org/10.1111/j.1440-169X.2008.01019.x
Nojima H, Shimizu T, Kim CH, Yabe T, Bae YK, Muraoka O, Hirata T, Chitnis A, Hirano T, Hibi M (2004) Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev 121(4):371–386. https://doi.org/10.1016/j.mod.2004.02.003
Nojima H, Rothhamel S, Shimizu T, Kim CH, Yonemura S, Marlow FL, Hibi M (2010) Syntabulin, a motor protein linker, controls dorsal determination. Development 137 (6):923–933. doi:dev.046425, pii: https://doi.org/10.1242/dev.046425
Ober EA, Schulte-Merker S (1999) Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev Biol 215(2):167–181
Okuda Y, Ogura E, Kondoh H, Kamachi Y (2010) B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet 6(5):e1000936. https://doi.org/10.1371/journal.pgen.1000936
Olson DJ, Oh D, Houston DW (2015) The dynamics of plus end polarization and microtubule assembly during Xenopus cortical rotation. Dev Biol 401(2):249–263. https://doi.org/10.1016/j.ydbio.2015.01.028
Piccolo S, Sasai Y, Lu B, De Robertis EM (1996) Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86(4):589–598
Ramel MC, Lekven AC (2004) Repression of the vertebrate organizer by Wnt8 is mediated by vent and Vox. Development 131(16):3991–4000. https://doi.org/10.1242/dev.01277
Rebagliati MR, Toyama R, Fricke C, Haffter P, Dawid IB (1998a) Zebrafish nodal-related genes are implicated in axial patterning and establishing left–right asymmetry. Dev Biol 199(2):261–272
Rebagliati MR, Toyama R, Haffter P, Dawid IB (1998b) Cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci U S A 95(17):9932–9937
Reim G, Brand M (2006) Maternal control of vertebrate dorsoventral axis formation and epiboly by the POU domain protein Spg/Pou2/Oct4. Development 133(14):2757–2770. https://doi.org/10.1242/dev.02391
Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M (2006) Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133(5):801–811. https://doi.org/10.1242/dev.02250
Ro H, Dawid IB (2009) Organizer restriction through modulation of Bozozok stability by the E3 ubiquitin ligase Lnx-like. Nat Cell Biol 11(9):1121–1127. https://doi.org/10.1038/ncb1926
Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, Patient R, Holder N (1999) Induction of the mesendoderm in the zebrafish germ ring by yolk cell–derived TGF-beta family signals and discrimination of mesoderm and endoderm by FGF. Development 126(14):3067–3078
Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, Stainier DY (2015) Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524(7564):230–233. https://doi.org/10.1038/nature14580
Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA (1997) Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci U S A 94(4):1224–1229
Ryu SL, Fujii R, Yamanaka Y, Shimizu T, Yabe T, Hirata T, Hibi M, Hirano T (2001) Regulation of dharma/bozozok by the Wnt pathway. Dev Biol 231(2):397–409. https://doi.org/10.1006/dbio.2000.0150
Sakaguchi T, Mizuno T, Takeda H (2002) Formation and patterning roles of the yolk syncytial layer. Results Probl Cell Differ 40:1–14
Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV (1998) Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature 395(6698):185–189
Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79(5):779–790
Schier AF (2009) Nodal morphogens. Cold Spring Harb Perspect Biol 1(5):a003459. https://doi.org/10.1101/cshperspect.a003459
Schier AF, Talbot WS (2005) Molecular genetics of axis formation in zebrafish. Annu Rev Genet 39:561–613. https://doi.org/10.1146/annurev.genet.37.110801.143752
Schneider S, Steinbeisser H, Warga RM, Hausen P (1996) Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57(2):191–198
Schroeder MM, Gard DL (1992) Organization and regulation of cortical microtubules during the first cell cycle of Xenopus eggs. Development 114(3):699–709
Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M (1997) The zebrafish organizer requires chordino. Nature 387(6636):862–863. https://doi.org/10.1038/43092
Shih YH, Kuo CL, Hirst CS, Dee CT, Liu YR, Laghari ZA, Scotting PJ (2010) SoxB1 transcription factors restrict organizer gene expression by repressing multiple events downstream of Wnt signalling. Development 137(16):2671–2681. https://doi.org/10.1242/dev.054130
Shimizu T, Yamanaka Y, Ryu S, Hashimoto H, Yabe T, Hirata T, Bae Y, Hibi M, Hirano T (2000) Cooperative roles of Bozozok/Dharma and nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech Dev 91(1–2):293–303
Shimizu T, Yamanaka Y, Nojima H, Yabe T, Hibi M, Hirano T (2002) A novel repressor-type homeobox gene, ved, is involved in dharma/bozozok-mediated dorsal organizer formation in zebrafish. Mech Dev 118 (1–2):125–138. doi:S0925477302002435
Shimizu T, Bae YK, Muraoka O, Hibi M (2005a) Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol 279(1):125–141. https://doi.org/10.1016/j.ydbio.2004.12.007
Shimizu T, Yabe T, Muraoka O, Yonemura S, Aramaki S, Hatta K, Bae YK, Nojima H, Hibi M (2005b) E-cadherin is required for gastrulation cell movements in zebrafish. Mech Dev 122 (6):747–763. doi:S0925-4773(05)00053-5, pii: https://doi.org/10.1016/j.mod.2005.03.008
Shimizu T, Bae YK, Hibi M (2006) Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue. Development 133(23):4709–4719. https://doi.org/10.1242/dev.02660
Shinya M, Eschbach C, Clark M, Lehrach H, Furutani-Seiki M (2000) Zebrafish Dkk1, induced by the pre-MBT Wnt signaling, is secreted from the prechordal plate and patterns the anterior neural plate. Mech Dev 98(1–2):3–17
Sick S, Reinker S, Timmer J, Schlake T (2006) WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314(5804):1447–1450. https://doi.org/10.1126/science.1130088
Sidi S, Goutel C, Peyrieras N, Rosa FM (2003) Maternal induction of ventral fate by zebrafish radar. Proc Natl Acad Sci U S A 100(6):3315–3320. https://doi.org/10.1073/pnas.0530115100
Sirotkin HI, Dougan ST, Schier AF, Talbot WS (2000) Bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. Development 127(12):2583–2592
Skromne I, Thorsen D, Hale M, Prince VE, Ho RK (2007) Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development 134(11):2147–2158. https://doi.org/10.1242/dev.002980
Solnica-Krezel L, Driever W (1994) Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development 120(9):2443–2455
Solnica-Krezel L, Sepich DS (2012) Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol 28:687–717. https://doi.org/10.1146/annurev-cellbio-092910-154043
Stachel SE, Grunwald DJ, Myers PZ (1993) Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development 117(4):1261–1274
Stainier DY, Kontarakis Z, Rossi A (2015) Making sense of anti-sense data. Dev Cell 32(1):7–8. https://doi.org/10.1016/j.devcel.2014.12.012
Su Q, Cai Q, Gerwin C, Smith CL, Sheng ZH (2004) Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol 6(10):941–953. https://doi.org/10.1038/ncb1169
Takeuchi M, Okabe M, Aizawa S (2009a) Whole-mount in situ hybridization of bichir (Polypterus) embryos. Cold Spring Harbor protocols 2009 (5):pdb prot5158. doi:https://doi.org/10.1101/pdb.prot5158
Takeuchi M, Takahashi M, Okabe M, Aizawa S (2009b) Germ layer patterning in bichir and lamprey; an insight into its evolution in vertebrates. Dev Biol 332(1):90–102. https://doi.org/10.1016/j.ydbio.2009.05.543
Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J (2005) Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120 (6):857–871. doi:S0092-8674(05)00086-3, pii: https://doi.org/10.1016/j.cell.2005.01.013
Tarbashevich K, Koebernick K, Pieler T (2007) XGRIP2.1 is encoded by a vegetally localizing, maternal mRNA and functions in germ cell development and anteroposterior PGC positioning in Xenopus laevis. Dev Biol 311(2):554–565. https://doi.org/10.1016/j.ydbio.2007.09.012
Thorpe CJ, Weidinger G, Moon RT (2005) Wnt/beta-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish. Development 132(8):1763–1772. https://doi.org/10.1242/dev.01733
Tran LD, Hino H, Quach H, Lim S, Shindo A, Mimori-Kiyosue Y, Mione M, Ueno N, Winkler C, Hibi M, Sampath K (2012) Dynamic microtubules at the vegetal cortex predict the embryonic axis in zebrafish. Development 139(19):3644–3652. https://doi.org/10.1242/dev.082362
Tsang M, Friesel R, Kudoh T, Dawid IB (2002) Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol 4(2):165–169. https://doi.org/10.1038/ncb749
Tsang M, Maegawa S, Kiang A, Habas R, Weinberg E, Dawid IB (2004) A role for MKP3 in axial patterning of the zebrafish embryo. Development 131(12):2769–2779. https://doi.org/10.1242/dev.01157
Tucker JA, Mintzer KA, Mullins MC (2008) The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell 14(1):108–119. https://doi.org/10.1016/j.devcel.2007.11.004
Untergasser G, Martowicz A, Hermann M, Tochterle S, Meyer D (2011) Distinct expression patterns of dickkopf genes during late embryonic development of Danio Rerio. Gene Expr Pattern GEP 11(8):491–500. https://doi.org/10.1016/j.gep.2011.08.005
Varga M, Maegawa S, Bellipanni G, Weinberg ES (2007) Chordin expression, mediated by nodal and FGF signaling, is restricted by redundant function of two beta-catenins in the zebrafish embryo. Mech Dev 124(9–10):775–791. https://doi.org/10.1016/j.mod.2007.05.005
Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr (1997) Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88(6):757–766
Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT (2005) The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/beta-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol 15(6):489–500. https://doi.org/10.1016/j.cub.2005.01.041
Wilk K, Bilinski S, Dougherty MT, Kloc M (2005) Delivery of germinal granules and localized RNAs via the messenger transport organizer pathway to the vegetal cortex of Xenopus oocytes occurs through directional expansion of the mitochondrial cloud. Int J Dev Biol 49 (1):17–21. doi:041906kw, pii: https://doi.org/10.1387/ijdb.041906kw
Woo K, Fraser SE (1997) Specification of the zebrafish nervous system by nonaxial signals. Science 277(5323):254–257
Xie J, Fisher S (2005) Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development 132(2):383–391. https://doi.org/10.1242/dev.01577
Xiong B, Rui Y, Zhang M, Shi K, Jia S, Tian T, Yin K, Huang H, Lin S, Zhao X, Chen Y, Chen YG, Lin SC, Meng A (2006) Tob1 controls dorsal development of zebrafish embryos by antagonizing maternal beta-catenin transcriptional activity. Dev Cell 11(2):225–238. https://doi.org/10.1016/j.devcel.2006.06.012
Xu PF, Houssin N, Ferri-Lagneau KF, Thisse B, Thisse C (2014) Construction of a vertebrate embryo from two opposing morphogen gradients. Science 344(6179):87–89. https://doi.org/10.1126/science.1248252
Yabe T, Shimizu T, Muraoka O, Bae YK, Hirata T, Nojima H, Kawakami A, Hirano T, Hibi M (2003) Ogon/secreted frizzled functions as a negative feedback regulator of Bmp signaling. Development 130(12):2705–2716
Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T (1998) A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev 12(15):2345–2353
Yao S, Qian M, Deng S, Xie L, Yang H, Xiao C, Zhang T, Xu H, Zhao X, Wei YQ, Mo X (2010) Kzp controls canonical Wnt8 signaling to modulate dorsoventral patterning during zebrafish gastrulation. J Biol Chem 285(53):42086–42096. https://doi.org/10.1074/jbc.M110.161554
Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J (1998) The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94(4):515–524
Zhang JL, Patterson LJ, Qiu LY, Graziussi D, Sebald W, Hammerschmidt M (2010) Binding between Crossveinless-2 and chordin von Willebrand factor type C domains promotes BMP signaling by blocking chordin activity. PLoS One 5(9):e12846. https://doi.org/10.1371/journal.pone.0012846
Note
We have recently reported that maternal wnt8a is dispensable for the initial dorsal determination but cooperates with zygotic wnt8a for ventrolateral and posterior tissue formation. Maternal wnt6a is an alternative dorsal determinant candidate (Hino et al. Dev Biol 434(1), 96–107, 2018). The data suggest that Wnt8a, Wnt6a, and possibly other Wnts that are expressed maternally may cooperate to activate the canonical Wnt pathway for the dorsal axis formation.
AcknowledgementsThe authors thank the past and current members of the Hibi Laboratory for their contribution to the work cited here, and thank Shin-ichi Aizawa for his advice on bichir studies. This work was partially supported by the Kawasaki University of Medical Welfare Scientific Research Fund (2012, 2014).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK, part of Springer Nature
About this chapter
Cite this chapter
Hibi, M., Takeuchi, M., Hashimoto, H., Shimizu, T. (2018). Axis Formation and Its Evolution in Ray-Finned Fish. In: Kobayashi, K., Kitano, T., Iwao, Y., Kondo, M. (eds) Reproductive and Developmental Strategies. Diversity and Commonality in Animals. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56609-0_32
Download citation
DOI: https://doi.org/10.1007/978-4-431-56609-0_32
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56607-6
Online ISBN: 978-4-431-56609-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)