Abstract
Among vertebrates, teleost fish are of particular interest for their sexual diversity and plasticity. During ontogenesis in gonochoristic fish, the undifferentiated gonadal primordium develops into an ovary or testis, a process referred to as sex determination or sexual differentiation. After primary sex determination, the sexes of gonochoristic fish remain fixed for the remainder of their lives. Other fish are hermaphrodites, however, and can change their sex in adulthood, as either simultaneous hermaphrodites, which possess both functional ovarian and testicular tissue, or sequential hermaphrodites. Sequential hermaphrodite species can be divided into three groups. The first are protogynous (female-first) hermaphrodites; the fish begins its life as a female but later becomes a male. The reverse is the case for protandrous (male-first) species (the second group). A third group is composed of those few species that can change their sex serially (bidirectional sex change). This diversity of sexual plasticity is unique to fish and, as such, provides excellent model systems with which to investigate the mechanisms of sex determination and differentiation in vertebrates. In this chapter, we first discuss gonadal sex differentiation in hermaphroditic fish in comparison with gonochoristic fish and then describe various types of sex change in fish from the viewpoints of morphology and physiology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Sex change
- Gonad
- Testis
- Ovary
- Protandry
- Protogyny
- Bidirectional sex change
- Estrogen
- Androgen
- Sex determination
- Sex differentiation
- Wrasse
- Grouper
- Anemonefish
- Gobiid fish
- Tilapia
1 Introduction
Among vertebrates, teleost fish show the greatest range of sexuality. Fish are categorized mainly as either gonochoristic or hermaphroditic (Atz 1964; Yamamoto 1969). In gonochoristic fish—the more common type—sexes are determined genetically or by environmental factors, as in other vertebrates (see chapters 14 and 15). After sex determination, undifferentiated gonad primordia (germ and somatic cells) differentiate into an ovary (female) or testis (male), with the sex remaining fixed thereafter. In contrast, hermaphroditic fish can change sex during their lives. Among vertebrates, sex change in fish is a rare and fascinating phenomenon. Since sex change commonly occurs after pubertal maturity, it is possible to investigate the changes that occur in the sex of germ and somatic cells in the adult gonad. Research into sex change in fish makes a major contribution to the field of sex determination, differentiation, and sexual plasticity in vertebrates.
With respect to sex change, hermaphroditic fish are either simultaneous or sequential hermaphrodites (Atz 1964; Yamamoto 1969). Simultaneous hermaphrodites possess both functional ovarian and testicular tissue. In contrast, species that are sequential hermaphrodites first possess functional ovarian tissue and then functional testicular tissue, or vice versa. In this chapter, we focus on sequential hermaphrodites, which are broadly divided into three types: protogynous species, which change from female to male; protandrous species, which change from male to female; and species that can change sex in both directions, from male or female and back again multiple times (Devlin and Nagahama 2002).
Our major interest is in understanding the physiological mechanisms, especially the endocrinology, of sex change in hermaphroditic fish. We believe that studies of sex change in hermaphrodites provide a valuable complement to knowledge of sex differentiation in well-studied gonochoristic fish, such as tilapia and medaka, and thereby deepen understanding of the complicated mechanisms of sex determination and sexual plasticity in vertebrates. Thus, in thre first Sect. (16.2.1), we describe the process of gonadal sex differentiation in several types of hermaphrodites in comparison with gonochoristic species. Then we use five different species as examples to describe recent work on sex change. Finally we discuss four important conclusions arising from this work: (1) ovarian differentiation may be the primary state in all sequentially hermaphroditic species; (2) sex change is controlled mainly by estrogen; (3) gonadotropin hormone (GTH) is involved in sex change; and (4) adult ovaries of gonochoristic species exhibit sexual plasticity, as shown for hermaphroditic species.
2 Gonadal Sex Differentiation in Gonochoristic and Hermaphroditic Fish
Sequential hermaphroditism in fish has evolved independently multiple times; over 350 species in 23 teleost families are sequential hermaphrodites (Frisch 2004). As described in 16.3, the gonads of these fish exhibit a high degree of sexual plasticity, in some cases undergoing serial sex changes. In contrast, the gonads of gonochoristic fish remained fixed after sex determination, indicating a loss of sexual plasticity. To understand sexual plasticity in fish, it is essential to investigate the process of gonadal differentiation and formation during ontogeny. However, to date, information about gonadal differentiation in sex-changing fish remains limited. This limitation is due to the fact that the larvae of most of these fish—with the exceptions of groupers, anemonefish, and black porgy—are not robust and are consequently difficult to raise. We therefore first describe gonadal sex differentiation in a gonochoristic fish—the Nile tilapia—and the involvement of steroid sex hormones in gonadal differentiation. We then describe the process of gonadal sex differentiation in a protogynous grouper, in a protandrous anemonefish, in a protogynous wrasse and, finally, in a bidirectional sex-changing gobiid fish.
2.1 Gonadal Differentiation in the Gonochoristic Nile Tilapia
As seen in mammals, the sexes of the Nile tilapia, Oreochromis niloticus, are genetically determined XX/XY systems. All females (XX) and males (XY) may potentially be produced using sperm obtained from sex-reversed males (XX) or supermales (YY), respectively. Gonadal sex differentiation in the tilapia is summarized in Fig. 16.1 (Nakamura et al. 1998; Nakamura 2000, 2013). Until about 20 days posthatch (dph), the gonads of this fish are in an undifferentiated state; neither ovaries nor testes can be distinguished morphologically. Ovarian differentiation begins at 20–24 dph, as determined by changes in both germ cells and somatic cells. During this phase, a sudden proliferation in germ cells and cysts of oogonia occurs. The oogonia then immediately transition into meiotic prophase oocytes. At about the same time, differentiating somatic cells begin to form the ovarian cavity (from which future ova will be discharged). First, elongations of somatic cells form at the base and tip of the ovaries on the lateral walls. Then two elongations form and extend in the direction of the lateral wall; eventually the ends of the elongations join, forming a space enclosed by somatic cell tissue on the lateral wall. Next, from 50 to 70 dph, the oocytes in the ovaries develop rapidly up to the perinucleolus stage. Division and proliferation of germ cells during the formation of spermatozoa in the testes is sometimes quiescent, making it difficult to determine when testicular differentiation begins during the differentiation of germ cells. However, differentiation of somatic cells for sperm duct formation begins at about the same time as ovarian differentiation (Nakamura et al. 1998; Nakamura 2000). Active changes in germ cells—such as the transition to division, proliferation, and meiosis of spermatogonium to form spermatozoa—occur much later than in the ovaries, starting at about 70 dph.
Next we outline the physiological framework, including the activities of endogenous sex hormones, by which undifferentiated gonads differentiate into ovaries or testes. First, to determine directly whether endogenous steroid sex hormones play an essential role in the initiation of gonadal sex differentiation in fish, we examined the differentiation of steroid-producing cells (SPCs)—the site of steroid hormone production—during this process. A notable finding is that SPCs are already differentiated in the gonads at around the time when sex differentiation begins (Nakamura and Nagahama 1985). This timing suggests that endogenous steroid hormones contribute to sex differentiation of the gonads in the Nile tilapia. Next, to define the relationship between steroid hormones and sex differentiation, we used immunohistochemistry with antibodies against the cholesterol side-chain cleavage enzyme (P450scc), 17α-hydroxylase (P450c17), 3β-hydroxysteroid dehydrogenase (3β-HSD), and aromatase (P450arom); the expression and localization of these enzymes was examined during gonadal sex differentiation (Nakamura 2013; Strüssmann and Nakamura 2002). In genetic females, the expression of all of these enzymes occurred in undifferentiated gonads before ovarian differentiation. Furthermore, the intensity of the immunopositive reactions and the number of cells exhibiting reactivity increased as ovarian differentiation progressed. A particularly notable finding was that P450arom, an enzyme required for estrogen synthesis, is already detectable in the gonads before sex differentiation. This observation indicates that endogenous estrogen is involved in inducing ovarian differentiation in undifferentiated gonads. In genetic males, however, undifferentiated gonads did not exhibit immunopositive reactions to the tested enzyme antibodies until after testicular differentiation. Once testicular differentiation began, weak positive responses to enzymes P450scc, P450c17, and 3β-HSD (but not to P450arom) were detected, but strong responses were not observed until immediately before spermatozoon formation began. Thus, unlike ovarian differentiation, steroid sex hormones are not directly involved in testicular differentiation.
In addition, various experimental findings suggest that endogenous estrogens are essential for gonadal sex differentiation. Administration of an aromatase inhibitor (AI)—which inhibits the synthesis of estrogen—to genetically female Nile tilapia during sex differentiation caused all individuals to change into males with testes. Simultaneous administration of an AI and estradiol-17β (E2) suppressed sex change and caused undifferentiated gonads to differentiate into ovaries (Nakamura 2000; Nakamura et al. 2000). Conversely, when a synthetic androgen, 17α-methyl testosterone (MT), was administered to genetically female Nile tilapia, the ovaries were transformed into testes. The expression of steroid-metabolizing enzymes during this MT-induced sex reversal was also examined immunohistochemically (Bhandari et al. 2006b). Almost no immunopositive reactions for any of the tested steroid hormone–metabolizing enzymes were observed in the MT-treated group. This result suggests that exogenous androgen can suppress the expression of several of these enzymes, thus inhibiting endogenous synthesis of estrogen during the sex differentiation. Taken together, these findings indicate that estrogens are inducers of ovarian differentiation and that the absence of estrogen is important for testicular differentiation. Therefore, the presence or absence of estrogen determines whether a fish becomes female or male.
2.2 Gonadal Differentiation in a Protogynous Grouper
Groupers (genus Epinephelus), which are protogynous, are important in fisheries throughout the world (Nakamura et al. 2005), and substantial efforts have been made to establish methods for their artificial breeding. As a part of this effort, we investigated the process of gonadal sex differentiation in the Malabar grouper (Epinephelus malabaricus) for 1 year after hatching (Fig. 16.2) (Murata et al. 2009). In fish at 11 dph, large primordial germ cells (PGCs) were evident in the primordial gonad tissue located below the mesonephric ducts on the dorsal side of the intestine. Up until 47 dph, neither the germ cells nor the somatic tissue showed any of the morphological characteristics of sexual differentiation. By 47 dph, the gonads had changed substantially, however, through an increase in the number of somatic cells. By 74 dph, the elongations indicating ovarian cavity formation (like those in tilapia ovarian differentiation) had developed further and were evident in the ovaries of all fish. However, ovarian germ cells had not yet begun active division. By 360 dph, oocytes were distributed within somatic tissue along the inner periphery of the ovarian cavity. These observations suggest that morphological changes associated with ovarian differentiation in the Malabar grouper begin at approximately 75 dph and that in all individuals the gonads differentiate directly into ovaries. No testicular differentiation was evident at the time of primary sex determination during ontogenesis.
To clarify the role of endogenous steroid hormones in sex differentiation in the grouper, we used immunohistochemistry to examine the expression of several steroidogenic enzymes—P450scc, P450arom, and P450 11β hydroxylase (P45011β), an important enzyme for androgen production in fish—during ovarian differentiation (Murata et al. 2011). P450scc and P450arom appeared first in the somatic cells surrounding the germ cells in undifferentiated gonads and were present throughout ovarian differentiation; in contrast, P45011β first appeared in the cluster of somatic cells in the ovary tunica near the dorsal blood vessels after ovarian differentiation. These observations suggest that endogenous estrogen is involved in ovarian differentiation. Treatment with androgen induced precocious spermatogenesis in the gonads of juveniles after ovarian differentiation (Murata et al. 2010). However, these testes reverted to ovaries upon withdrawal of androgen treatment (Murata et al. 2014).
2.3 Gonadal Sex Differentiation in a Protandrous Anemonefish
Anemonefishes (genus Amphiprion) are protandrous (male-first) fish. Histological analysis of the gonads of anemonefish has revealed that males and nonbreeding individuals possess bisexual gonads in which both mature testicular tissue and immature ovarian tissue coexist, whereas the gonads of females contain only ovarian tissues (Godwin 1994; Nakamura et al. 1994).
Gonadal sex differentiation in the anemonefish Amphiprion clarkii is shown in Fig. 16.3 (Miura et al. 2003, 2008a, b; Miura 2007) From hatching to 30 dph, the gonads are in a sexually undifferentiated state. Interestingly, the gonads of the fish differentiate into ovaries by 60 dph, and oocytes gradually develop and increase in number as the ovaries grow, through 183 dph. Then, by 214 dph, cysts of differentiated spermatogenic germ cells become evident in the ovaries, and by 273 dph, ambisexual gonads with both ovarian and testicular tissues have formed. Thus, no primary males exist, and all males derive from females in the anemonefish, as previously thought (Shapiro 1992).
Gonadal sex differentiation in the protandrous anemonefish Amphiprion clarkii. The gonads of all of the fish differentiate into ovaries by 60 days posthatch (dph). Between 60 and 183 dph the gonads of all of the fish contain oocytes at the perinucleolus stage, cysts of oocytes in the meiotic phase, and an ovarian cavity. Initial testicular differentiation, which occurs in the developed ovaries from 214 to 273 dph, is characterized by the appearance of spermatogenic germ cells among the oocytes. Direct differentiation of the gonads into testes is not observed in this fish
We have also examined the relationship between steroid hormones and gonadal differentiation in anemonefish (Miura et al. 2008a, b, 2013). Immunopositive cells reactive against P450scc, P45011β, and P450arom are present in sexually undifferentiated gonads by 30 dph, and they increase in activity around the time of ovarian differentiation at 60 dph. These results support the view that immature gonads around the time of sex differentiation have the potential to produce both androgen and estrogen. However, treatment with an AI and an estrogen antagonist (tamoxifen) around the time of primary ovarian differentiation had no effect on ovarian differentiation (e.g., differentiation and development of oocytes) and had no effect on ovarian cavity formation and induction of a seminal duct–like structure in the ovaries. These findings indicate that endogenous estrogen may not be required for primary ovarian differentiation in anemonefish. Androgen treatment during primary ovarian differentiation did not reverse differentiation of the ovary to testes. However, ovarian cavity development and differentiation of the seminal duct–like structure were delayed in the presence of androgen. The strength of immunoreactivity against P450scc and P45011β gradually increases, and testicular differentiation continues in the ovary until 210 dph; ambisexual gonads form by 270 dph. In contrast, P450arom-immunopositive cells with a weak signal intensity were seen in ambisexual gonads just after testicular differentiation. Moreover, cells positive against P450arom were not present in ambisexual gonads, either in testicular or ovarian tissue at 270 dph. Production of E2 was high in the ovaries before the appearance of any testicular tissue, and it decreased along with the differentiation of testicular tissue. Production of 11-keto testosterone (11KT) in the gonads gradually increased with testicular differentiation. E2 treatment suppressed the naturally occurring differentiation of testicular cells such that only ovarian tissues formed in the gonad in vivo. These results suggest that a shift from estrogen to androgen production occurs during testicular differentiation; this shift may induce testicular differentiation in the ovary (Miura et al. 2013).
2.4 Gonadal Sex Differentiation in a Protogynous Wrasse and in a Bidirectional Sex-Changing Gobiid Fish
As mentioned above, many of the fish that undergo sex change are difficult to raise. To circumvent this problem, specimens of various sizes of a protogynous wrasse and a bidirectional sex-changing gobiid fish were collected from the wild so the gonadal status of juveniles of these fish could be examined histologically (Kobayashi et al. 2013).
Protogynous Wrasse
The process of gonadal sex differentiation in the protogynous three-spot wrasse, Halichoeres trimaculatus, is shown in Fig. 16.4. Many of the smaller specimens, 10–30 mm in total length (TL), had undifferentiated gonads. Approximately 70–80% of specimens 30–40 mm in TL were observed to have ovaries containing oocytes in the perinucleolus stage. Approximately 20–25% of these larger fish were observed to have bisexual gonads, possessing both ovarian and testicular tissue. The remaining fish (4%) had fully differentiated testes. Among specimens of 40 mm in TL and greater, none was observed to have undifferentiated gonads; all of these specimens had begun to undergo sexual differentiation. Although 10–30% of the observed specimens possessed bisexual gonads, no such specimens were found among fish of 80 mm in TL and greater. Spermatids and sperm were observed within the bisexual gonads, and perinucleolus stage oocytes were distributed throughout the testicular tissue. Many specimens with bisexual gonads showed signs of oocyte degeneration. The bisexual organs of specimens of the large-size group were composed primarily of testicular tissue. These observations suggest that during the period of sexual differentiation, bisexual gonads differentiate into primary testes. Specifically, almost all primary males pass halfway through a female phase once. However, the relationship between gonadal sex differentiation and endogenous sex hormones in the wrasse remains unknown.
Gonadal sex differentiation in the protogynus three-spot wrasse, Halichoeres trimaculatus. The gonads of fish 30–40 mm in total length differentiate into three types. The gonads of 70–80% of the fish differentiate into normal ovaries with oocytes, 20–35% have ambisexual gonads with both oocytes and spermatogenic germ cells, and 4% differentiate directly into testes. Ambisexual gonads later differentiate into defined ovaries or testes
Bidirectional Sex-Changing Gobiid Fish
The gobiid fish Trimma okinawae—the adults of which undergo bidirectional sex change—possess an ovary and testis simultaneously (Kobayashi et al. 2005). However, the gonad of juveniles before puberty (less than 20 mm in TL) has no testicular tissue and consists only of ovarian tissue. It therefore seems likely that testicular tissue appears in the ovary after ovarian differentiation.
3 Morphology and Physiology of Sex Change in Hermaphroditic Fish
Fascination with the phenomenon of sex change in fish has led to numerous studies in the past. However, most of these studies have addressed sex change primarily from ecological, behavioral, or phylogenic perspectives (Erisman et al. 2013). Because most fish that undergo sex change are difficult to obtain or raise, the physiological and endocrinological mechanisms of their sex change remain unclear. Recently, observations of sex change in mature individuals after sex determination/differentiation have indicated that these fish would be good models for research into ovarian and testicular differentiation in vertebrates.
At an observational level, sex change in fish is triggered by social cues—typically loss of a larger male or larger female from a group or a harem. Upon receipt of the cue, responsive individuals quickly begin behaving like the opposite sex. Then, their gonads change to those of the opposite sex, and their body color and shape similarly become modified. In the 3.1–3.4.2, we describe the physiological changes during sex change in protogynous, protandrous, and bidirectional sex-changing fish.
3.1 Sex Change in Protogynous Wrasses
3.1.1 Visual Social Cues Affecting Sex Change
Wrasses exhibit diandric protogyny, with populations consisting of small initial-phase males (primary males), initial-phase females (primary females), and large terminal-phase males, which arise either from females that have undergone sex change to become males (secondary males) or from initial-phase males (terminal-phase primary males) (Ross 1982, 1983). Sex change from female to male in the saddleback wrasse, Thalassoma duperrey, occurs naturally in nature and can be induced by social factors under controlled experimental conditions. When a single large female is kept with a smaller conspecific of either sex, the large female will change sex, becoming a functional male within 8–12 weeks (Nakamura et al. 1989; Ross 1981, 1982, 1983). Social cues also induce sex change, from female to male, in the three-spot wrasse under captive conditions, as seen in the saddleback wrasse (Nozu et al. 2013).
3.1.2 Morphological and Physiological Changes During Sex Change in Wrasses
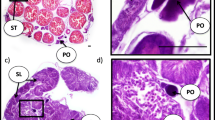
Histological analysis of gonadal transformations during sex change have been reported for many protogynous fish. These transformations follow a common process. First, ovarian tissue, including mature and immature oocytes, degenerates. Then, testicular tissue appears and develops. In the saddleback wrasse and three-spot wrasse, the histological process of gonadal sex change, which is similar in the two fish, is well described (Nakamura et al. 1989; Nozu et al. 2009). The process has been classified into six stages (Fig. 16.5).
Histology of gonadal sex change in the three-spot wrasse, Halichoeres trimaculatus. (A) Stage 1: ovary with previtellogenic oocytes (POs) and vitellogenic oocytes (VOs). (B) Stage 2: gonad including degenerate vitellogenic oocytes (DVOs), indicating the onset of sex change. (C) Stage 3: occurrence of degenerate previtellogenic oocytes (DPOs). (D) Stage 4: presumed spermatogonia (PSGs) proliferating actively in the peripheral region of the ovigerous lamella. (E) Stage 5: spermatogenesis occurring from the periphery of the ovigerous lamella. (F) Stage 6: mature secondary testis with an ovarian cavity (OC). SC spermatocyte, ST spermatid, SZ spermatozoon, VPO vestige of previtellogenic oocyte. Scale bars, 20 μm
Regardless of the direction of sex change, steroid hormones are known to play an important role in sex change (Devlin and Nagahama 2002; Frisch 2004). Upon the initiation of sex change, estrogen levels in the saddleback wrasse drop sharply, whereas during the second half of the process, androgen levels rise (Fig. 16.6) (Nakamura et al. 1989). This timing suggests that the fall in estrogen levels triggers the sex change. In support of this hypothesis, sex change has been induced in several varieties of protogynous fish via administration of an AI, which causes endogenous estrogen levels to decrease (Alam et al. 2006; Bhandari et al. 2004a, b; Higa et al. 2003; Nozu et al. 2009). Recently, sex change accompanied by falling estrogen levels was induced by administration of the stress hormone cortisol (Nozu and Nakamura 2015). This result suggests that cortisol plays a role in induction of sex change by regulating production of estrogen. On the other hand, administration of androgen also induces a female-to-male sex change (Bhandari et al. 2006a; Higa et al. 2003; Murata et al. 2014). It has also been reported for wrasses (Labridae) that a rise in androgen levels triggers sex change (Ohta et al. 2008a). From these kinds of findings, it is now thought that although the key steroid sex hormones may vary depending on the type of fish, disruption of the steroid sex hormone balance (estrogen/androgen) is essential for the initiation of sex change. In fact, it has been demonstrated that disruption of this balance via administration of exogenous sex hormones leads to opposite-directional sex changes not observed in the wild (Kojima et al. 2008; Miyake et al. 2008). Thus, because an individual’s sex is maintained through a balance of steroid hormones, fish that undergo sex change are endowed with an unusually high degree of sexual plasticity.
With the process of gonadal sex change defined, attention next focused on the expression of sex-related genes during sex change. Specifically, we examined the variation in the expression of sex-related genes in the three-spot wrasse. The genes examined include R-spondin 1 (rspo1) and Forkhead box protein L2 (foxl2), which are involved in ovarian differentiation and maintenance in vertebrates, and gonadal soma-derived factor (gsdf), doublesex and mab-3 related transcription factor 1 (dmrt1), anti-Müllerian hormone (amh), and sex-determining region Y box 9 (sox9), which play important roles in the differentiation and maintenance of testes. Changes in the expression of each of these genes during sex change were observed (Fig. 16.7). When sex change begins, the expression levels of rspo1 decline. Of great interest, during degeneration of ovarian tissue (stages 2–3), oocytes degenerate and disappear via apoptosis; apparently, however, granulosa cells surrounding the oocyte survive throughout the gonadal transformation (Nozu et al. 2013). Consistent with this result, expression levels of foxl2, a granulosa cell molecular marker, did not decline during sex change (Kobayashi et al. 2010c). Next, a distinct increase in spermatogonia-like cells occurs and spermatogenesis begins (stages 4–5). Before and after this period, the expression levels of gsdf, dmrt1, amh, and sox9 rise (Horiguchi et al. 2013; Nozu et al. 2015). These findings suggest that these genes contribute to testis formation during gonadal sex change. In particular, the expression levels of gsdf, a testis differentiation marker, rise early in the process (Horiguchi et al. 2013). With respect to messenger RNA (mRNA) localization, gsdf is localized to the ovary; weak expression can be observed in supporting cells adjacent to the gonial germ cells, and the level of expression increases with the progression of the sex change. At the next stage, gsdf is specifically expressed in cells of the Sertoli cell lineage. Apparently, therefore, Sertoli cells originate from the supporting cells adjacent to the gonial germ cells during sex change, i.e., the supporting cells seen in the ovary differentiate into Sertoli cells. Furthermore, although the rise in expression of dmrt1 is delayed in comparison with gsdf, changes in the expression localization of these genes support this hypothesis (Nozu et al. 2015). However, the specific origins of many of the types of cells that contribute to gonadal tissue during sex change are still unclear. We anticipate that through detailed descriptions of cellular behavior, the mechanisms governing properties such as individual cell sexual plasticity and irreversibility will be defined.
Changes in expression levels of sex-related genes during a sex change in Halichoeres trimaculatus. Female-related gene: rspo1; male-related genes: gsdf, dmrt1, amh, and sox9. The expression of rspo1 drops during the sex change. The expression of male-related genes increases from the midstage of the sex change
3.2 Sex Change in a Protogynous Grouper
Studies of groupers can be difficult because of the large size of the fish. We therefore chose a relatively small-sized species, the honeycomb grouper (E. merra), which is available in the wild, as an experimental model (Nakamura et al. 2005).
3.2.1 Morphology and Physiology of Sex Change
Sex change in groupers depends on age and body size, not on social factors (Murata et al. 2012; Nakamura et al. 2005). Therefore, to obtain baseline information on the sexuality of the honeycomb grouper, specimens were captured from the wild over a 1-year time span (Bhandari et al. 2003). Histological observations revealed that female-phase gonads consisted of oocytes in several developmental stages but lacked testicular tissue. At the beginning of the sex change, oocytes begin degenerating, followed by a proliferation of spermatogonia. Finally, no ovarian cells were observed, and the testis consisted of germ cells undergoing active spermatogenesis. Female and male fish were mostly smaller and larger, respectively, whereas fish undergoing sexual transition were in the intermediate size and weight ranges (Bhandari et al. 2003). During the breeding season, no specimens undergoing sex change were observed. During the nonbreeding season, in contrast, an overlap was present in the sex distribution of transitional individuals, indicating that sex change occurred during this time. Taken together, these observations indicate that sex change in the honeycomb grouper usually occurs in nonbreeding larger females (over 200 mm in TL).
As in the case of the protogynous wrasse, serum levels of E2 in the grouper are high in females but low in males and in individuals undergoing sex change (Bhandari et al. 2003, 2005). In contrast, 11KT levels are low in females and gradually increase in the transitional phase and in males (Bhandari et al. 2006a). These results suggest that low serum E2 levels and degeneration of oocytes, accompanied by a concomitant increase in levels of 11KT and a proliferation in spermatogenic germ cells, are the events mediating protogynous sex change in this species.
3.2.2 Involvement of Gonadotropin in Gonadal Sex Change
Since the signals for controlling gonadal sex change come from the brain, the hypothalamus–pituitary–gonad (HPG) axis is involved in this process (Godwin 2010). In teleosts, as in other vertebrates, gonadal steroidogenesis is largely controlled by two GTHs produced in the pituitary gland: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These GTHs contain a common glycoprotein hormone α-subunit (GPα), which forms a heterodimer with unique β-subunits (FSHβ and LHβ, respectively) (Swanson et al. 2003). In well-studied salmonids, FSH plays a significant role in puberty and gametogenesis, whereas LH is primarily involved in the final maturation of gametes in both sexes (Swanson et al. 2003). Variations in the expression profiles and roles of GTHs have been reported in teleosts, and several reports have suggested that GTHs are factors that control gonadal sex change (Godwin 2010). For example, sexually dimorphic expression of GTH subunit genes has been observed in the protogynous wrasse Pseudolabrus sieboldi (Ohta et al. 2008b). In the black porgy (Acanthopagrus schlegelii), which is protandrous, plasma LH levels were higher in males than in fish undergoing sex change (Lee et al. 2001). Furthermore, treatment with exogenous human chorionic gonadotropin (hCG) induced sex change in the protogynous wrasse Coris julis (Reinboth and Brusle-Sicard 1997). However, information on GTH expression patterns during the sex change process is lacking; as a consequence the specific biological roles of GTHs in sex change remain unresolved. This situation is beginning to change, however, as data indicating a role of GTHs in gonadal sex change have been reported recently, as described below.
The most comprehensive studies of the role of GTHs in sex change have been carried out in the protogynous honeycomb grouper (Kobayashi et al. 2010a). Although the control of sex change in this fish by social manipulation has not been possible, work on the different sexual phases has progressed using specimens captured from the wild (Bhandari et al. 2003). Specifically, transcripts of GTH subunit genes in the pituitary in different sexual phases of the honeycomb grouper have been quantified (Fig. 16.8). The relative levels of gpα and lhβ mRNA were higher in the breeding season than in the nonbreeding season. However, the levels did not differ significantly between different sexual phases during the nonbreeding season. In contrast, the expression pattern of fshβ transcripts showed marked sexual dimorphism. Transcripts of the fshβ subunit were low in breeding and nonbreeding female phases, and they substantially increased during female-to-male sex change, especially in the early transitional (ET) stage. Immunohistochemical analysis using antisera to the GTH subunit confirmed these results. Similarly, upregulation of the gonadal FSH receptor occurred during sex change (Alam et al. 2010). In addition, to identify the role of GTHs in gonadal sex change in this fish, in vivo treatments with bovine FSH and LH were carried out. After 3 weeks, FSH treatment had induced female-to-male sex change and had upregulated endogenous androgen levels and fshβ transcripts, whereas LH treatment had no effect on sex change. Taken together, these results strongly suggest that FSH triggers female-to-male gonadal sex change in the honeycomb grouper.
Changes in gonadotropin hormone (GTH) subunit transcripts in pituitaries in different sexual phases in the honeycomb grouper, Epinephelus merra. Quantification of the transcript abundances of the gp𝛼, fshβ, and lhβ genes in the pituitaries is determined by quantitative polymerase chain reaction (qPCR) and normalized to the abundance of glyceraldehyde-3-phosphate dehydrogenase (gapdh) transcripts. The values are expressed as fold changes in abundance relative to the means in the breeding female. The data are shown as means ± standard errors of the means (SEMs). Data points not sharing a letter differ significantly, according to a Tukey–Kramer multiple comparison test. ET early transitional, LT late transitional (Redrawn from Kobayashi et al. 2010a)
3.3 Protandrous Sex Change in Anemonefish
3.3.1 Visual Social Cues Effecting Sex Change
Anemonefish are hermaphrodites, which usually live in small social groups with isolated anemones. Each group consists of one breeding pair—a functional female and male—together with several subadults or juveniles (Fricke and Fricke 1977; Fricke 1979; Moyer and Nakazono 1978; Ross 1978). When the largest individual—which is always female—disappears, the largest remaining male changes its sex to female, and the largest subadult becomes a functional male. This pattern strongly suggests that a social factor, specifically the disappearance of the functional female from a group, triggers sex change in male anemonefish.
3.3.2 Morphological and Physiological Changes During Sex Change
Histological analysis of the gonads of anemonefish has revealed that males and nonbreeders possess bisexual gonads, with coexisting mature testicular and immature ovarian tissues, whereas the gonads of females contain only ovarian tissues (Fig. 16.9) (Godwin 1994; Nakamura et al. 1994). During sex change, the ovarian tissues develop and the testicular tissues regress in the bisexual gonads (Godwin 1994). The mechanisms of this process have been studied extensively. As in other sex-changing species (Nakamura et al. 2005), gonadal steroid sex hormones—especially estrogen—are the key regulators of sex change in anemonefish (Godwin and Thomas 1993; Kobayashi et al. 2010b). However, the upstream mechanisms controlling the production and activity of gonadal steroid hormones during sex change in anemonefish remain largely unknown.
Male and female gonadal structure in the protandrous anemonefish Amphiprion clarkii. Males possess an ovotestis gonad in which mature testicular tissue and immature ovarian tissue are both present and incompletely delimited. The cysts of spermatogenic tissue are separated by a thin cellular barrier. In contrast, females show no testicular tissues in the gonad
3.4 Bidirectional Sex Change in a Gobiid Fish
In protandrous and protogynous species, sex change is generally not reversible, occurring only once in the fish’s life. In 1993, however, Sunobe and Nakazono first described bidirectional sex change in the Okinawa rubble goby, T. okinawae (family: Gobiidae) (Sunobe and Nakazono 1993). This gobiid fish has a polygynous mating system in which a group of individuals—a harem—normally consists of one dominant male and one or more females (Sunobe and Nakazono 1999). Removal of the dominant male from the harem results in female-to-male sex change by the largest female (protogyny). If the dominant male is returned to the harem, the fish that underwent the sex change transforms back into a female (protandry) (Fig. 16.10).
Bidirectional sex change in the gobiid fish Trimma okinawae. The mating system of T. okinawae is a harem consisting of a dominant male and two or three females. Removal of the dominant male (1) results in a protogynous sex change in the largest female in the harem (2). If placed back into the harem with a larger male (3), the male reverts back to a female (protandrous sex change) (4).
3.4.1 Visual Social Cues Affecting Bidirectional Sex Change
In T. okinawae, social interactions with conspecifics mediate bidirectional sex change. However, it is not clear how fish recognize their own social rank; changes in behavior at the beginning of a sex change have not been described in detail.
To investigate the temporal aspect of sex change in T. okinawae, we carried out laboratory experiments. When two males were kept together in an aquarium, the smaller male changed its sex to female (protandry). Conversely, when two females were kept together, the larger female changed its sex to male (protogyny). Protogynous sex change occurred within just 5 days, and protandrous sex change occurred within 10 days. These rates of sex change in T. okinawae are faster than those in other sex-changing fish species.
These observations indicate that the larger member of a pair is always male and the smaller member is always female (Fig. 16.11). However, it is not clear how the fish recognizes the body sizes of other fish or whether chemical cues might be involved. To address this question, we prepared a glass-separated aquarium (Fig. 16.11a) in which fish could see but not make physical contact with each other. As in the experiments described above, pairs of males or pairs of females were kept together in an aquarium but separated from each other by the glass. After 2 weeks, protandrous and protogynous sex changes were observed in pairs of females and pairs of males, respectively. This result indicates that sex change is induced by visual cues, not chemical or tactile cues. Interestingly, behavioral changes occurred within 30 min of social manipulation. After being placed together, the larger male or female attacked the smaller fish, which fled and often hid in a nest (Fig. 16.11b, c). After 30 min, however, the larger fish began to court the smaller fish. It is well known that sexual behaviors are dependent on brain sex (Godwin 2010). Thus, the rapid behavioral changes in T. okinawae after social manipulation suggest that, regardless of gonadal sex, visual cues quickly alter the brain sex of the fish.
Induction of a bidirectional sex change in the goby Trimma okinawae. (A) Two male or female fish are placed together in an aquarium, after which an intense competitive interaction occurs. (B) Two fish (males or females) are isolated in a glass-separated aquarium. The arrowheads indicate the experimental fish. (C) After pairing in the glass-separated aquarium, an intense competitive interaction occurs between the fish
3.4.2 The Physiological Mechanism of Bidirectional Sex Change
The structure of the gonad of T. okinawae differs fundamentally from that of other fish that undergo sex change (Fig. 16.12) (Kobayashi et al. 2005). All gonads that were examined, including those from female- and male-phase fish, unambiguously contained both ovaries and testes attached to an accessory gonadal structure. In addition, the oviduct and sperm duct are separate in this fish. In short, this fish, by having both ovaries and testes, is equipped to rapidly respond to its social status, even though only one gonad is active at a given time. This unique gonadal structure facilitates sex change in either direction in this species.
Gonadal structure of A functional female and B male Trimma okinawae. The female gonad contains vitellogenic oocytes but no spermatozoa in the testicular region. The male gonad has spermatozoa in the testicular region but no vitellogenic oocytes. AGS accessary gonadal structure. Bars, 200 μm (Redrawn from Kobayashi et al. 2005)
As mentioned above, GTHs are involved in gonadal sex change. GTHs, produced by the pituitary, are secreted into the blood and act primarily at the level of the gonads (Kobayashi et al. 2004). The actions of GTHs are mediated by GTH receptors (GTHRs) on the surface of the target cells in the gonads (Oba et al. 2001). The genes for two GTHRs—FSHR and LHR, which specifically bind FSH and LH, respectively—have been cloned from various vertebrates, including fish (Oba et al. 2001). Analysis of GTHR activity in the gonads of sex-changing fish presumably therefore would help reveal the mechanism by which GTHs function. This analysis has proven difficult, however, because the active and inactive regions of the gonads in sex-changing fish typically are difficult to separate. In this regard, we have found that T. okinawae, which can undergo bidirectional sex change, is a good model with which to investigate the activity of GTHRs in the gonad. In this fish, active and inactive gonad tissue can be separated and collected easily (Kobayashi et al. 2005). We have found that the earliest change in the gonads in this fish is a change in the expression levels of the GTHRs. The expression of both receptors was found to be confined to the active gonad of the corresponding sexual phase (Fig. 16.13). During a sex change from female to male, the ovary initially had high levels of fshr and lhr and eventually was transformed into testicular tissue. The gonads started to change with the switching on of GTHR expression, which was discernible within 8–12 h of a visual social cue (Kobayashi et al. 2009). These findings suggest that sex change is initiated through rapid changes in the brain and behavior, followed by changes, mediated by GTHRs, in the gonad.
4 Sexual Plasticity in Gonochoristic Fish
It has been assumed for some time that sexual plasticity of the ovaries in vertebrates is lost after sexual differentiation, except in some hermaphroditic species. In a contradiction of that view, however, we demonstrated recently that at least some gonochoristic fish maintain their sexual plasticity into adulthood. Specifically, depletion of estrogen by AI treatment induced functional testicular differentiation in the ovaries of tilapia, medaka, and zebrafish (Fig. 16.14) (Paul-Prasanth et al. 2013; Takatsu et al. 2013). We also induced testicular differentiation in the ovaries of adult specimens of other gonochoristic fish—the carp (Cyprinus carpio) and the golden rabbitfish (Siganus guttatus)—by AI treatment (Nakamura et al., unpublished data). It is evident from these results that E2 plays a critical role in maintaining the female phenotype. In addition, estrogen treatment induced a complete change from a testis to a mature ovary in males of the wrasse H. trimaculatus (Kojima et al. 2008). As mentioned above, AI treatment to artificially deplete estrogen brought about a complete change of ovaries into mature testes in the grouper E. merra and the wrasse H. trimaculatus, which are protogynous (Bhandari et al. 2004a, b; Nozu et al. 2009). The same treatment also induced the opposite sex change, from female to male, in the anemonefish A. clarkii (Nakamura et al. 2015). These results demonstrate clearly that some germ cells and somatic cells in the gonads of gonochoristic fish retain plasticity after sexual differentiation and maturation.
Sexual plasticity of the ovaries in the gonochoristic Nile tilapia, Oreochromis niloticus. Differentiation and development of testicular tissues in the ovaries by treatment with an aromatase inhibitor (AI). (A) Normal immature ovary. (B) Immature ovary of a fish treated with an AI for more than 1 month; note the tissue structure changes near the edge of the ovarian cavity (OC) after the onset of AI treatment (arrow). (C) Initial testicular differentiation in the ovary; testicular tissue, including spermatogonia and spermatocytes (arrow), appears near the OC. (D) Development of spermatogenic tissues in the ovary; active spermatogenic tissue (arrows) spreads along the wall of the OC
5 Conclusion
Although the relationship between sex change and sex-determining genes has attracted considerable interest recently, the nature of this relationship remains poorly understood. Sex-changing fish undergo secondary sex determination after sexual maturation, so the same individual experiences life as both a female (with ovaries) and a male (with testes). Thus, it is evident that both sexes have the same genetic makeup. Previously for gonochoristic fish, it was believed that males possess sex-determining genes and that females do not; during primary sex determination, these genes are expressed in the somatic cells that surround the germ cells and cause them to differentiate into testes. In contrast to that view, however, we have shown—through detailed observation of the sexual differentiation processes in the grouper E. malabaricus, the anemone fish A. clarkii, and the gobiid fish T. okinawae—that primary males are not present. All males in these species arise only as secondary males that have originated from females. Experimentally, in gonochoristic fish such as tilapia, medaka, and zebrafish, females can be changed into males (artificial secondary males) by AI treatment (Paul-Prasanth et al. 2013; Takatsu et al. 2013). Offspring that arise by fertilization of normal female eggs with the sperm from artificial secondary male individuals (XX) are all female. Thus, in E. malabaricus and A. clarkii, which consist only of secondary males, the offspring should all be female. For these reasons, it is highly likely that monoandrous species of sex-changing fish do not possess sex-determining genes or that these genes have become dysfunctional. These species therefore depend on a physiological mechanism of sex determination to enable the change from female to male, the subsequent production of males, and the creation of female/male gonochorism—and to achieve sexual reproduction. The existence of physiological mechanisms of sex determination indicates that sex-determining genes are not essential.
Undifferentiated gonads in the protogynus wrasse H. trimaculatus differentiate into ambisexual gonads and into ovaries at the time of sex differentiation. In addition, direct differentiation into testes (primary sex differentiation) has been observed (unpublished data). Mature females later change into males. These findings strongly suggest that sex determination in the wrasse involves both sex-determining genes and a physiological mechanism. However, the observed percentage of direct testicular differentiation was extremely low (less than 5%). This fact suggests that functional sex-determining genes in this fish might be undergoing evolutionary loss. To examine this possibility, it will be necessary to define sex ratios through progeny tests such as mating of primary males with normal females or mating of secondary males with normal females.
Changes in the levels of endogenous steroid hormones provide a physiological mechanism for regulating sex change. Through research in saddleback wrasses, which undergo sex change in response to social factors, we have shown that endogenous estrogen is deeply involved in the transition from mature ovaries to functional testes. In wrasses, the levels of estrogen drop precipitously at the start of the sex change process. These fish have also been shown to transition from ovaries to testes when the levels of estrogen are artificially lowered with an AI (Higa et al. 2003; Nozu et al. 2009). Additionally, sex change was inhibited when estrogen was supplemented at the same time (Higa et al. 2003). A decrease in the levels of estrogen promotes the expression of genes involved in testicular differentiation (Horiguchi et al. 2013; Nozu et al. 2015). Artificial lowering of estrogen induces testicular differentiation not only in wrasses but also in the protogynous grouper E. merra and the protandrous anemonefish A. clarkii (Bhandari et al. 2004a, b; Nakamura et al. 2015). Although the processes of sex change vary in different fish, a commonality is the regulation of sex change through alterations in the levels of estrogen. This physiological mechanism of sex change—regulation by control of estrogen levels—is shared by the aforementioned fish species, which use only a physiological mechanism for primary sex determination, i.e., physiological sex differentiation.
By manipulating estrogen levels we have demonstrated that even mature ovaries can transform into testes in gonochoristic fish (e.g., tilapia, medaka, zebrafish, rabbit fish, and carp). This capability—previously considered impossible—confirms the hypothesis that a decrease in estrogen triggers sex change, and it shows that the ovaries of gonochoristic fish, like those of sex-changing fish, remain sexually plastic even after sex determination. In sex-changing fish, social stimuli induce a decrease in the production of estrogen. In contrast, ovaries of gonochoristic fish—despite their retention of sexual plasticity—do not transform into testes, because these fish lack the regulatory mechanism that lowers estrogen to the level necessary for induction of a sex change. These considerations suggest that a certain concentration of estrogen, which is synthesized and secreted in the follicular tissue surrounding the eggs in fish ovaries in gonochoristic and hermaphroditic species alike, is responsible for modulating the transformation of ovaries into testes. Sex-changing fish therefore are able to undergo sex change through a regulatory mechanism for modulating levels of estrogen. Gonochoristic fish, despite having apparently lost sex-determining genes, retain the ability to form males and carry out sexual reproduction by means of this physiological mechanism sex determination, thereby retaining the sexual plasticity of their ovaries.
6 Future Directions
Sex change in many kinds of fish is initiated by social factors. For example, the behavior of T. okinawae changes dramatically in response to visual cues well before gonadal transformation begins (Kobayashi et al. 2009). The presence of different-sized individuals serves as a social stimulus in this fish, one that reaches the brain via visual perception and subsequently influences the gonads. However, it remains unclear what types of changes are triggered in the brain and how those changes mediate gonadal transformation (Kobayashi et al. 2013). As discussed in 16.3.2.2, FSH from the pituitary gland plays a dominant role in grouper sex change (Kobayashi et al. 2010a). In T. okinawae as well, sex change is regulated by rapid and dramatic changes in the expression of GTHRs in the gonads (Kobayashi et al. 2009). Stimuli from the brain therefore are clearly involved in the sex change process. We therefore anticipate that pinning down the details of sex change will require an understanding of the relevant processes and mechanisms occurring within the brain.
References
Alam MA, Bhandari RK, Kobayashi Y, Soyano K, Nakamura M (2006) Induction of sex change within two full moons during breeding season and spawning in grouper. Aquaculture 255(1–4):532–535. https://doi.org/10.1016/j.aquaculture.2006.01.008
Alam MA, Kobayashi Y, Hirai T, Nakamura M (2010) Isolation, characterization and expression analyses of FSH receptor in protogynous grouper. Comp Biochem Physiol A Mol Integr Physiol 156(3):364–371. https://doi.org/10.1016/j.cbpa.2010.03.001
Atz JW (1964) Intersexuality in fishes. In: Armstrong CN, Marshall AJ (eds) Intersexuality in vertebrates including man. Academic, London, pp 145–232
Bhandari RK, Komuro H, Nakamura S, Higa M, Nakamura M (2003) Gonadal restructuring and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper ( Epinephelus merra). Zool Sci 20(11):1399–1404. https://doi.org/10.2108/zsj.20.1399
Bhandari RK, Higa M, Nakamura S, Nakamura M (2004a) Aromatase inhibitor induces complete sex change in the protogynous honeycomb grouper (Epinephelus merra). Mol Reprod Dev 67(3):303–307. https://doi.org/10.1002/mrd.20027
Bhandari RK, Komuro H, Higa M, Nakamura M (2004b) Sex inversion of sexually immature honeycomb grouper (Epinephelus merra) by aromatase inhibitor. Zool Sci 21(3):305–310. https://doi.org/10.2108/zsj.21.305
Bhandari RK, Alam MA, Higa M, Soyano K, Nakamura M (2005) Evidence that estrogen regulates the sex change of honeycomb grouper (Epinephelus merra), a protogynous hermaphrodite fish. J Exp Zool A Comp Exp Biol 303(6):497–503. https://doi.org/10.1002/jez.a.178
Bhandari RK, Alam MA, Soyano K, Nakamura M (2006a) Induction of female-to-male sex change in the honeycomb grouper (Epinephelus merra) by 11-ketotestosterone treatments. Zool Sci 23(1):65–69. https://doi.org/10.2108/zsj.23.65
Bhandari RK, Nakamura M, Kobayashi T, Nagahama Y (2006b) Suppression of steroidogenic enzyme expression during androgen-induced sex reversal in Nile tilapia (Oreochromis niloticus). Gen Comp Endocrinol 145(1):20–24. https://doi.org/10.1016/j.ygcen.2005.06.014
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208(3–4):191–364. https://doi.org/10.1016/s0044-8486(02)00057-1
Erisman BE, Petersen CW, Hastings PA, Warner RR (2013) Phylogenetic perspectives on the evolution of functional hermaphroditism in teleost fishes. Integr Comp Biol 53(4):736–754. https://doi.org/10.1093/icb/ict077
Fricke HW (1979) Mating system, resource defence and sex change in the anemonefishAmphiprion akallopisos. Z Tierpsychol 50(3):313–326. https://doi.org/10.1111/j.1439-0310.1979.tb01034.x
Fricke H, Fricke S (1977) Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266(5605):830–832. https://doi.org/10.1038/266830a0
Frisch A (2004) Sex-change and gonadal steroids in sequentially-hermaphroditic teleost fish. Rev Fish Biol Fish 14(4):481–499. https://doi.org/10.1007/s11160-005-3586-8
Godwin J (1994) Historical aspects of protandrous sex change in the anemonefish Amphiprion melanopus (Pomacentridae, Teleostei). J Zool 232(2):199–213. https://doi.org/10.1111/j.1469-7998.1994.tb01569.x
Godwin J (2010) Neuroendocrinology of sexual plasticity in teleost fishes. Front Neuroendocrinol 31(2):203–216. https://doi.org/10.1016/j.yfrne.2010.02.002
Godwin J, Thomas P (1993) Sex change and steroid profiles in the protandrous anemonefish Amphiprion melanopus (Pomacentridae, Teleostei). Gen Comp Endocrinol 91(2):144–157. https://doi.org/10.1006/gcen.1993.1114
Higa M, Ogasawara K, Sakaguchi A, Nagahama Y, Nakamura M (2003) Role of steroid hormones in sex change of protogynous wrasse. Fish Physiol Biochem 28(1–4):149–150. https://doi.org/10.1023/B:FISH.0000030505.28138.d1
Horiguchi R, Nozu R, Hirai T, Kobayashi Y, Nagahama Y, Nakamura M (2013) Characterization of gonadal soma-derived factor expression during sex change in the protogynous wrasse,Halichoeres trimaculatus. Dev Dyn 242(4):388–399. https://doi.org/10.1002/dvdy.23929
Kobayashi Y, Kobayashi T, Sunobe T, Nagahama Y, Nakamura M (2004) Expression of gonadotropin receptors in the serial sex changing goby, Trimma okinawae. Zool Sci 21(12):1333
Kobayashi Y, Sunobe T, Kobayashi T, Nagahama Y, Nakamura M (2005) Gonadal structure of the serial-sex changing gobiid fish Trimma okinawae. Develop Growth Differ 47(1):7–13. https://doi.org/10.1111/j.1440-169x.2004.00774.x
Kobayashi Y, Nakamura M, Sunobe T, Usami T, Kobayashi T, Manabe H, Paul-Prasanth B, Suzuki N, Nagahama Y (2009) Sex change in the gobiid fish is mediated through rapid switching of gonadotropin receptors from ovarian to testicular portion or vice versa. Endocrinology 150(3):1503–1511. https://doi.org/10.1210/en.2008-0569
Kobayashi Y, Alam MA, Horiguchi R, Shimizu A, Nakamura M (2010a) Sexually dimorphic expression of gonadotropin subunits in the pituitary of protogynous honeycomb grouper (Epinephelus merra): evidence that follicle-stimulating hormone (FSH) induces gonadal sex change. Biol Reprod 82(6):1030–1036. https://doi.org/10.1095/biolreprod.109.080986
Kobayashi Y, Horiguchi R, Miura S, Nakamura M (2010b) Sex- and tissue-specific expression of P450 aromatase (cyp19a1a) in the yellowtail clownfish, Amphiprion clarkii. Comp Biochem Physiol A Mol Integr Physiol 155(2):237–244. https://doi.org/10.1016/j.cbpa.2009.11.004
Kobayashi Y, Horiguchi R, Nozu R, Nakamura M (2010c) Expression and localization of forkhead transcriptional factor 2 (Foxl2) in the gonads of protogynous wrasse, Halichoeres trimaculatus. Biol Sex Differ 1:3. https://doi.org/10.1186/2042-6410-1-3
Kobayashi Y, Nagahama Y, Nakamura M (2013) Diversity and plasticity of sex determination and differentiation in fishes. Sex Dev 7(1–3):115–125. https://doi.org/10.1159/000342009
Kojima Y, Bhandari RK, Kobayashi Y, Nakamura M (2008) Sex change of adult initial-phase male wrasse, Halichoeres trimaculatus by estradiol-17 beta treatment. Gen Comp Endocrinol 156(3):628–632. https://doi.org/10.1016/j.ygcen.2008.02.003
Lee YH, Du JL, Yueh WS, Lin BY, Huang JD, Lee CY, Lee MF, Lau EL, Lee FY, Morrey C, Nagahama Y, Chang CF (2001) Sex change in the protandrous black porgy, Acanthopagrus schlegeli: a review in gonadal development, estradiol, estrogen receptor, aromatase activity and gonadotropin. J Exp Zool 290(7):715–726. https://doi.org/10.1002/jez.1122
Miura S (2007) Morphological and experimental studies on sex differentiation and sex change in the protandrous anemonefish Amphiprion clarkii. Doctoral thesis, University of the Ryukyus, Nishihara
Miura S, Komatsu T, Bhandari RK, Nakamura S, Nakamura M (2003) Gonadal sex differentiation in protandrous anemone fish, Amphiprion clarkii. Fish Physiol Biochem 28(1–4):165–166. https://doi.org/10.1023/B:FISH.0000030513.05061.88
Miura S, Horiguchi R, Nakamura M (2008a) Immunohistochemical evidence for 11beta-hydroxylase (P45011beta) and androgen production in the gonad during sex differentiation and in adults in the protandrous anemonefish Amphiprion clarkii. Zool Sci 25(2):212–219. https://doi.org/10.2108/zsj.25.212
Miura S, Nakamura S, Kobayashi Y, Piferrer F, Nakamura M (2008b) Differentiation of ambisexual gonads and immunohistochemical localization of P450 cholesterol side-chain cleavage enzyme during gonadal sex differentiation in the protandrous anemonefish, Amphiprion clarkii. Comp Biochem Physiol B Biochem Mol Biol 149(1):29–37. https://doi.org/10.1016/j.cbpb.2007.08.002
Miura S, Kobayashi Y, Bhandari RK, Nakamura M (2013) Estrogen favors the differentiation of ovarian tissues in the ambisexual gonads of anemonefish Amphiprion clarkii. J Exp Zool A Ecol Genet Physiol 319(10):560–568. https://doi.org/10.1002/jez.1818
Miyake Y, Fukui Y, Kuniyoshi H, Sakai Y, Hashimoto H (2008) Examination of the ability of gonadal sex change in primary males of the diandric wrasses Halichoeres poecilopterus and Halichoeres tenuispinis: estrogen implantation experiments. Zool Sci 25(2):220–224. https://doi.org/10.2108/zsj.25.220
Moyer JT, Nakazono A (1978) Protandrous hermaphroditism, in six species of the Anemonefish genus Amphiprion in Japan. Jpn J Ichthyol 25(2):101–106
Murata R, Karimata H, Alam MA, Nakamura M (2009) Gonadal sex differentiation in the Malabar grouper, Epinephelus malabaricus. Aquaculture 293(3–4):286–289. https://doi.org/10.1016/j.aquaculture.2009.04.031
Murata R, Karimata H, Alam MA, Nakamura M (2010) Precocious sex change and spermatogenesis in the underyearling Malabar grouper Epinephelus malabaricus by androgen treatment. Aquac Res 41(2):303–308. https://doi.org/10.1111/j.1365-2109.2009.02332.x
Murata R, Karimata H, Kobayashi Y, Horiguchi R, Kishimoto K, Kimura M, Kobayashi T, Soyano K, Nakamura M (2011) Differentiation of steroid-producing cells during ovarian differentiation in the protogynous Malabar grouper, Epinephelus malabaricus. Int J Dev Biol 55(6):619–625. https://doi.org/10.1387/ijdb.103181rm
Murata R, Kobayashi Y, Karimata H, Kishimoto K, Kimura M, Shimizu A, Nakamura M (2012) The role of pituitary gonadotropins in gonadal sex differentiation in the protogynous Malabar grouper, Epinephelus malabaricus. Gen Comp Endocrinol 178(3):587–592. https://doi.org/10.1016/j.ygcen.2012.07.012
Murata R, Kobayashi Y, Karimata H, Kishimoto K, Kimura M, Nakamura M (2014) Transient sex change in the immature Malabar grouper, Epinephelus malabaricus, androgen treatment. Biol Reprod 91(1):25. https://doi.org/10.1095/biolreprod.113.115378
Nakamura M (2000) Endocrinological studies on sex differentiation and reproduction in fish. Nippon Suisan Gakkaishi 66(3):376–379. https://doi.org/10.2331/suisan.66.376
Nakamura M (2013) Morphological and physiological studies on gonadal sex differentiation in teleost fish. Aqua BioSci Monogr 6(1):1–47. https://doi.org/10.5047/absm.2013.00601.0001
Nakamura M, Nagahama Y (1985) Steroid producing cells during ovarian differentiation of the tilapia, Sarotherodon niloticus. Develop Growth Differ 27(6):701–708. https://doi.org/10.1111/j.1440-169X.1985.00701.x
Nakamura M, Hourigan TF, Yamauchi K, Nagahama Y, Grau EG (1989) Histological and ultrastructural evidence for the role of gonadal steroid hormones in sex change in the protogynous wrasse Thalassoma duperrey. Environ Biol Fish 24(2):117–136. https://doi.org/10.1007/bf00001282
Nakamura M, Mariko T, Nagahama Y (1994) Ultrastructure and in vitro steroidogenesis of the gonads in the protandrous anemonefish Amphiprion frenatus. Jpn J Ichtyol 41(1):47–56. https://doi.org/10.11369/jji1950.41.47
Nakamura M, Kobayashi T, Chang XT, Nagahama Y (1998) Gonadal sex differentiation in teleost fish. J Exp Zool 281(5):362–372. https://doi.org/10.1002/(SICI)1097-010X(19980801)281:5<362::AID-JEZ3>3.0.CO;2-M
Nakamura M, Kobayashi T, Yoshiura Y, Nagahama Y (2000) Role of endogenous steroid hormones on gonadal sex differentiation in fish. Proc 6th Int Symp Rep Physiol Fish 1:247–249
Nakamura M, Kobayashi Y, Miura S, Alam MA, Bhandari RK (2005) Sex change in coral reef fish. Fish Physiol Biochem 31(2–3):117–122. https://doi.org/10.1007/s10695-006-7595-x
Nakamura M, Miura S, Nozu R, Kobayashi Y (2015) Opposite-directional sex change in functional female protandrous anemonefish, Amphiprion clarkii: effect of aromatase inhibitor on the ovarian tissue. Zool Lett 1:30. https://doi.org/10.1186/s40851-015-0027-y
Nozu R, Nakamura M (2015) Cortisol administration induces sex change from ovary to testis in the protogynous wrasse, Halichoeres trimaculatus. Sex Dev. https://doi.org/10.1159/000373902
Nozu R, Kojima Y, Nakamura M (2009) Short term treatment with aromatase inhibitor induces sex change in the protogynous wrasse, Halichoeres trimaculatus. Gen Comp Endocrinol 161(3):360–364. https://doi.org/10.1016/j.ygcen.2009.01.024
Nozu R, Horiguchi R, Murata R, Kobayashi Y, Nakamura M (2013) Survival of ovarian somatic cells during sex change in the protogynous wrasse, Halichoeres trimaculatus. Fish Physiol Biochem 39(1):47–51. https://doi.org/10.1007/s10695-012-9632-2
Nozu R, Horiguchi R, Kobayashi Y, Nakamura M (2015) Expression profile of doublesex/male abnormal-3-related transcription factor-1 during gonadal sex change in the protogynous wrasse, Halichoeres trimaculatus. Mol Reprod Dev. https://doi.org/10.1002/mrd.22527
Oba Y, Hirai T, Yoshiura Y, Kobayashi T, Nagahama Y (2001) Fish gonadotropin and thyrotropin receptors: the evolution of glycoprotein hormone receptors in vertebrates. Comp Biochem Physiol B Biochem Mol Biol 129(2–3):441–448. https://doi.org/10.1016/S1096-4959(01)00374-8
Ohta K, Hirano M, Mine T, Mizutani H, Yamaguchi A, Matsuyama M (2008a) Body color change and serum steroid hormone levels throughout the process of sex change in the adult wrasse, Pseudolabrus sieboldi. Mar Biol 153(5):843–852. https://doi.org/10.1007/s00227-007-0856-0
Ohta K, Mine T, Yamaguchi A, Matsuyama M (2008b) Sexually dimorphic expression of pituitary glycoprotein hormones in a sex-changing fish (Pseudolabrus sieboldi). J Exp Zool A Ecol Genet Physiol 309(9):534–541. https://doi.org/10.1002/jez.485
Paul-Prasanth B, Bhandari RK, Kobayashi T, Horiguchi R, Kobayashi Y, Nakamoto M, Shibata Y, Sakai F, Nakamura M, Nagahama Y (2013) Estrogen oversees the maintenance of the female genetic program in terminally differentiated gonochorists. Sci Rep 3:2862. https://doi.org/10.1038/srep02862
Reinboth R, Brusle-Sicard S (1997) Histological and ultrastructural studies on the effects of hCG on sex inversion in the protogynous teleost Coris julis. J Fish Biol 51(4):738–749. https://doi.org/10.1111/j.1095-8649.1997.tb01995.x
Ross RM (1978) Reproductive behavior of the anemonefish Amphiprion melanopus on Guam. Copeia 1978(1):103–107. https://doi.org/10.2307/1443829
Ross RM (1981) Experimental evidence for stimulation and inhibition of sex change in the Hawaiian reef fish Thalassoma duperrey. Proc 4th Int Coral Reef Symp 2:575–580
Ross RM (1982) Sex change in the endemic Hawaiian labridThalassoma duperrey (Quoy and Gaimard): a behavioral and ecological analysis. PhD dissertation, University of Hawaii, Honolulu
Ross RM (1983) Annual, semilunar, and diel reproductive rhythms in the Hawaiian labrid Thalassoma duperrey. Mar Biol 72(3):311–318. https://doi.org/10.1007/BF00396837
Shapiro DY (1992) Plasticity of gonadal development and protandry in fishes. J Exp Zool 261(2):194–203. https://doi.org/10.1002/jez.1402610210
Strüssmann CA, Nakamura M (2002) Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol Biochem 26(1):13–29
Sunobe T, Nakazono A (1993) Sex change in both directions by alteration of social dominance in Trimma okinawae (Pisces: Gobiidae). Ethology 94(4):339–345. https://doi.org/10.1111/j.1439-0310.1993.tb00450.x
Sunobe T, Nakazono A (1999) Mating system and hermaphroditism in the gobiid fish, Priolepis cincta, at Kagoshima, Japan. Ichthyol Res 46(1):103–105. https://doi.org/10.1007/BF02674954
Swanson P, Dickey JT, Campbell B (2003) Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem 28(1–4):53–59. https://doi.org/10.1023/B:FISH.0000030476.73360.07
Takatsu K, Miyaoku K, Roy SR, Murono Y, Sago T, Itagaki H, Nakamura M, Tokumoto T (2013) Induction of female-to-male sex change in adult zebrafish by aromatase inhibitor treatment. Sci Rep 3:3400. https://doi.org/10.1038/srep03400
Yamamoto T (1969) Sex differentiation. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 3. Academic, New York, pp 117–175
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK, part of Springer Nature
About this chapter
Cite this chapter
Kobayashi, Y., Nozu, R., Horiguchi, R., Nakamura, M. (2018). Variety of Sex Change in Tropical Fish. In: Kobayashi, K., Kitano, T., Iwao, Y., Kondo, M. (eds) Reproductive and Developmental Strategies. Diversity and Commonality in Animals. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56609-0_16
Download citation
DOI: https://doi.org/10.1007/978-4-431-56609-0_16
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56607-6
Online ISBN: 978-4-431-56609-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)