Abstract
Cytological and genetic studies using insects, performed in the first decades of the twentieth century, greatly contributed to establishing the notion that genotypic factors determine sexual fate. Since then, excellent studies of Drosophila have provided important clues to answering the question of how sex is determined. In Drosophila melanogaster, somatic sexual differentiation is regulated by a well-characterized genetic hierarchy composed of a primary genetic signal (X:A ratio), master regulator (Sex-lethal), subordinate regulator (transformer/transformer-2), and double-switch (dsx and fru). On the basis of the knowledge obtained from studies with Drosophila, scientists have gained understanding of molecular mechanisms of sex determination in a variety of insect species. Recent studies have revealed that several insect species, such as the silkworm and the mosquito, have a unique sex determination cascade, which is surprisingly different from that in Drosophila. The most characteristic feature of the sex-determining genes in insects so far identified is that their sex-specific expressions are controlled by alternative splicing. In this chapter, we give an overview of the sex-determining genes revealed thorough the studies of D. melanogaster and those homologues identified in either nondrosophilid insects or animal species other than insects. In particular, we provide a detailed description of the novel sex-determining genes identified in the silkworm on the basis of our recent studies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Sex determination
- Sexual differentiation
- Sex-determining gene
- Alternative splicing
- Insects
- Sex determination cascade
- Silkworm
1 Introduction
How is sex determined? Aristotle once answered this question as follows: “The heat of semen at the time of copulation determines the sexual fate.” According to his hypothesis, hot semen produces males, whereas cold semen yields females (Jacqart and Thomasset 1988). Similar hypotheses, where environmental conditions such as temperature and nutritional status are major factors in determining sexual fate, were believed until the end of nineteenth century. The first indication relevant to a sex chromosome came from cytological studies conducted by Hermann Henking in 1891. In the course of studying sperm formation in Pyrrhocoris fire wasps, Henking noticed that one chromosome did not have a counterpart, unlike all other chromosomes, during the stages of meiosis in sperm cells. He designated this curious chromosome as the “X element” because of its strange nature (Henking 1891). The X element could be found in males of a number of different species. On the basis of his findings, Henking speculated that the X element played a crucial role in sex determination, but he could not provide direct evidence to support his idea. Later the X element became known as the X chromosome after it was established to indeed be a chromosome. In 1902, as a consequence of his cytological studies using the long-horned grasshopper, Xiphidium fasciatum, Walter Sutton proposed the hypothesis that the Mendelian laws of inheritance could be applied to chromosomes at the cellular level of living organisms (Sutton 1903). More than a decade after Henking’s work, Nettie Stevens set out to investigate gamete formation in many insects—from termites to sand crickets to aphids and mealworms. In this last species, Tenebrio molitor, she noticed that fully formed spermatids showed something odd in the chromosome number and its size: half of them had ten large chromosomes, all of a similar size, while the other half had nine large chromosomes and one small one. On the other hand, the eggs had ten large chromosomes, without exception. She also found that somatic cells from the male always had a total of 19 large chromosomes and always one small “accessory” chromosome, while somatic cells from female mealworms always contained 20 large chromosomes. In 1905, she correctly concluded that the accessory chromosome determines male sex (Stevens 1905). The accessory chromosome is now known as the Y chromosome. In contrast to the male-specific Y chromosome, which determines male sex, Yoshimaro Tanaka identified a female-specific chromosome in the silkworm, Bombyx mori, and demonstrated by genetic analysis that the presence of the female-specific chromosome determines female sex (Tanaka 1916). This female-specific chromosome was designated as the W chromosome, which has also been found in frogs, snakes, and birds. In 1921, Calvin Bridges found that the primary genetic signal for sex determination in Drosophila melanogaster was the ratio of X chromosomes to sets of autosomes (Bridges 1921). A ratio of 1.0 leads to female development, while a ratio of 0.5 leads to male development. Thus, cytological and genetic studies using insects greatly contributed to establishing the notion that genotypic factors determine the sexual fate.
The primary genetic signal for sex determination observed in insects is manifested in a wide variety of ways. In Megaselia scalaris (Traut 1994; Sievert et al. 2000), Ceratitis capitata (Willhoeft and Franz 1996), Bactorocera tryoni (Shearman and Formmer 1998), Lucilia cuprina (Bedo and Foster 1985), and Chironomus thummi (Hägele 1985), an epistatic maleness factor is found on Y chromosomes. The mosquito Culex tritaeniorhynchus has no sex chromosome, and its male sex is determined by a dominant gene on an autosome (Baker and Sakai 1976). The diploid/haploid sex determination system is well known in Hymenoptera (Beukeboom 1995). In Lepidoptera, Lymantria disper has a Z-linked male determinant (M) and a maternally inherited female determinant factor (F) (Goldschmidt 1955). Thus, insects have continued to provide a deep understanding of the genotypic sex determination system. In particular, much has been learned about the sex determination cascade from molecular and genetic analyses of D. melanogaster. On the basis of the knowledge obtained from studies of Drosophila, scientists have gained understanding of molecular mechanisms of sex determination in a variety of insect species such as the Mediterranean fruit fly (medfly), C. capitata; the yellow fever mosquito, Aedes aegypti; the silkmoth, B. mori; the honeybee, Apis mellifera; and the jewel wasp Nasonia vitripennis. These insects are highly diverged from Drosophila; for example, A. aegypti and N. vitripennis are separated from D. melanogaster by about 250 and 300 million years, respectively (Gailey et al. 2006; Hasselmann et al. 2008). Therefore, comparisons of genes constituting the sex determination cascade in insects will be very helpful to gain insight into the evolutionary dynamics of the regulatory mechanisms that give rise to sexual dimorphisms. In this chapter, we give an overview of sex-determining genes revealed through studies of D. melanogaster and those homologues identified in either nondrosophilid insects or animal species other than insects. In particular, we provide a detailed description of the novel sex-determining genes identified in the silkworm on the basis of our recent studies.
2 Sex Determination Cascade in Drosophila melanogaster
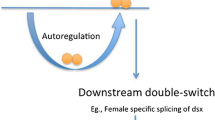
Sex determination in D. melanogaster is organized as a hierarchical order of genes designated as a sex determination cascade (Fig. 13.1). As described above, the first signal of sex determination is the ratio of X chromosomes to sets of autosomes (A) (Bridges 1921). The X:A ratio, a balance mechanism in which X chromosomal gene products are titrated against autosomal gene products, governs sex determination. Recent findings have indicated that the X:A ratio predicts the sexual fate but does not actively specify it. Instead, the instructive X chromosome signal is more appropriately seen as collective concentrations of several X-encoded signal element (XES) proteins in the early embryo (Erickson and Quintero 2007). The concentration of XES proteins determines the activity state of the Sex-lethal (Sxl) gene, which sits at the top of the sex determination cascade. A sufficient amount of XSE is supplied only when the animal has two X chromosomes (namely, XX female), leading to activation of transcription from the Sxl establishment promoter (SxlPe) at the early embryonic stage (Cline 1988; Keys et al. 1992; Estes et al. 1995; Erickson and Quintero 2007). The protein encoded by transcripts from SxlPe induces female-specific splicing of precursor messenger RNA (pre-mRNA) transcribed from the maintenance promoter, SxlPm, which is active in both sexes (Fig. 13.1). The female-specific splicing product from SxlPm encodes a functional SXL protein. The resulting SXL protein binds to its own pre-mRNA to maintain the female-specific mode of splicing (Fig. 13.1). The concentration of XSE is insufficient to activate SxlPe when the animal has only one X chromosome (namely, XY male). In the absence of protein products from SxlPe, pre-mRNA from SxlPm is spliced by default, yielding transcripts including an intervening exon that contains a stop codon (Fig. 13.1). As a result, SxlPm transcripts in males do not encode a full-length protein. Thus, Sxl acts as the memory device for female sexual development via its autoregulatory function (Cline 1984; Bell et al. 1991). SXL directs female-specific splicing of its downstream gene transformer (tra), giving rise to functional TRA protein (Boggs et al. 1987) (Fig. 13.1). TRA, together with the protein product of transformer-2 (tra-2), binds to an exonic splicing enhancer (ESE) element located within the female-specific exon of its downstream gene doublesex (dsx). The dsx ESE contains six copies of a 13-nucleotide (R1–R6) repeat sequence and is designated as dsxRE (Lynch and Maniatis 1995, 1996). TRA and TRA-2 are splicing factors belonging to the family of SR-type proteins whose amino acid sequences contain high frequencies of arginine (R) and serine (S) residues. The arginine- and serine-rich domain, which is known as the RS domain, mediates protein–protein interactions and regulates the recognition of specific splice sites (Manley and Tacke 1996). Proteins harboring the RS domain constitute a well-studied family of splicing regulators. An RS tetrapeptide (RSRS or SRSR) represents a functional unit in the RS domain of splicing activators (Graveley et al. 1998). Unlike other SR-type splicing factors, TRA lacks an RNA recognition motif (RRM) required for RNA binding. Therefore, TRA functions cooperatively with a factor such as TRA-2, which can bind to the cis-element in target pre-mRNAs. The TRA and TRA-2 (and one or more SR proteins) (Lynch and Maniatis 1995, 1996) complex binds to dsxRE functions to activate a weak 3′ splicing site preceding the female-specific exon, most likely by facilitating interactions of other general splicing factors with the RNA, to generate the female-type DSX protein (DSXF) (Kan and Green 1999; Li and Blencowe 1999). A lack of functional SXL protein causes male-specific splicing of tra, making its encoded product nonfunctional because of a premature stop codon (Fig. 13.1). In the absence of functional TRA protein, dsx pre-mRNA is spliced by default to generate the male-type DSX protein (DSXM). DSXF and DSXM regulate the sex-specific transcription of target genes that encode the sexual phenotype of the body (Baker and Wolfner 1988; Burtis and Baker 1989; Cline and Meyer 1996). To date, there are only a few validated direct gene targets for dsx: Yolk Proteins (yp-1 and yp-2), bric-à-brac 1, desatF (also known as Fad2), and Flavin-containing monooxygenase-2 (Fmos-2) (Burtis et al. 1991; Coschigano and Wensink 1993; Williams et al. 2008; Shirangi et al. 2009; Luo and Baker 2015). The regulation of yolk protein gene expression is by far the best characterized DSX function at the molecular level. DSXF and DSXM bind directly to several specific sites in an enhancer sequence designated as fat body enhancer (Burtis et al. 1991; Coschigano and Wensink 1993), which plays a role in directing the adult female fat body–specific transcription characteristic of the adjacent yolk protein genes, yp-1 and yp-2 (Garabedian et al. 1986). Recent genome-wide analyses by chromatin immunoprecipitation followed by sequencing (ChIP-seq) have revealed that DSXF and DSXM bind thousands of the same targets in multiple tissues in males and females, indicating that DSX action is regulated downstream from DSX binding (Clough et al. 2014).
Sex determination cascade and sex-determining genes in Drosophila melanogaster. The left panel shows the sex determination cascade, composed of Sxl, tra/tra-2, and dsx genes. The right panel shows sex-specific alternative splicing patterns in each gene. Transcripts from tra-2 do not undergo sex-specific splicing. The primary genetic signal for sex determination is the ratio of X chromosomes to sets of autosomes (X:A ratio). Transcription from the Sxl establishment promoter (SxlPe) in the early embryonic stage is activated when the animal has two X chromosomes (namely, XX female). The protein encoded by transcripts from SxlPe induces female-specific splicing of SxlPm precursor messenger RNA (pre-mRNA), yielding the functional SXL protein. SXL protein binds to its own pre-mRNA to maintain the female-specific mode of splicing (an autoregulatory loop). SXL directs the female-specific splicing of tra, giving rise to functional TRA protein. TRA, together with TRA-2, binds to an exonic splicing enhancer (ESE) element located within the female-specific exon of dsx. The TRA and TRA-2 complex activates the weak 3′ splicing site preceding the female-specific exon to generate the female-type DSX protein (DSXF). SxlPe is inactive when the animal has only one X chromosome (namely, XY male). In the absence of the protein products from SxlPe, SxlPm pre-mRNA is spliced by default, yielding transcripts including an intervening exon that contains a stop codon. A lack of functional SXL protein causes male-specific splicing of tra, making its encoded product nonfunctional because of a premature stop codon. In the absence of functional TRA protein, dsx pre-mRNA is spliced by default to generate the male-type DSX protein (DSXM). The DSXF and DSXM proteins regulate the sex-specific transcription of target genes that encode the sexual phenotype of the body
Another known downstream target of tra is fruitless (fru). In females, transcripts from the fru gene undergo female-specific splicing by TRA and TRA-2; in addition, these two factors prevent translation of the female-specific fru transcripts (Ryner et al. 1996; Heinrichs et al. 1998; Usui-Aoki et al. 2000). In males, because of the absence of TRA, default splicing produces male-specific fru transcripts, yielding male-specific protein isoforms (FRUM). Mutations in sex-specific fru transcripts disrupt both male courtship behavior and sexual orientation but have no effect on female behavior (Ito et al. 1996; Ryner et al. 1996; Anand et al. 2001; Lee and Hall 2001; Lee et al. 2001; Demir and Dickson 2005). The FRUM proteins play central roles in producing sexual differences in the mAL interneuron cluster containing a male-specific ipsilateral neurite in the central nervous system (CNS) (Kimura et al. 2005). In females, a subset of mAL neurons dies by programmed cell death. In males, FRUM proteins prevent the male counterparts from dying, allowing the development of mAL neurons. FRUM proteins are also necessary for the development of the muscle of Lawrence (MOL), an abdominal muscle present only in males (Usui-Aoki et al. 2000; Anand et al. 2001). All FRU isoforms contain a BTB (Broad-complex, Tramtrack, and Bric-a-brac) domain and a C-terminal C2H2 zinc finger domain for the DNA-binding function (Zollman et al. 1994). Recently, the binding sequences for different FRU isoforms have been identified (Dalton et al. 2013; Neville et al. 2014). However, to date, no direct target gene has been validated for fru.
3 Sxl Orthologues and Their Sex Determination Role in Nondrosophilid Species
Is Sxl, the master regulatory switch for sex determination in D. melanogaster, conserved among insects? Several Sxl orthologues thus far characterized in dipteran species other than D. melanogaster have no sex-determining role. For example, the Sxl orthologues in the medfly (C. capitata), housefly (Musca domestica), and scuttlefly M. scalaris encode proteins similar to the SXL of D. melanogaster but do not show sexual differences in those expression patterns (Saccone et al. 1998; Meise et al. 1998; Sievert et al. 2000). Furthermore, transgenic expressions of these orthologues in D. melanogaster failed to induce feminization in males (Saccone et al. 1998; Meise et al. 1998), suggesting an alteration of protein functions. In nondipteran insects, Sxl orthologues identified from the silkworm, B. mori, were expressed in both sexes equally (Niimi et al. 2006). Taken together with these findings, sex-specific expression and the protein role of Sxl in Drosophila are not conserved among insects.
Hu antigen B (HuB), HuC, HuD, and HuR proteins belong to the ELAV/Hu protein family (Antic and Keene 1997; Wakamatsu and Weston 1997). HuR is expressed ubiquitously in various tissues, while the other ELAV/Hu proteins are expressed predominantly in neuronal cells and are involved in neuronal differentiation (Good 1995; Ma et al. 1996; Antic et al. 1999). The ELAV/Hu proteins have three copies of RNA-binding domains (RBDs). Interestingly, the amino acid sequences of the N-terminal two RBDs (RBD1 and RBD2) of ELAV/Hu proteins are significantly homologous (53% identity) to those of SXL (Szabo et al. 1991). NMR studies on RBDs of mouse HuC demonstrated that the two N-terminal RBD (RBD1 and RBD2) structures are quite similar to those of SXL (Inoue et al. 2000). In addition, RBD1-RBD2 binds specifically to a longer ARE RNA, UAUUUAUUUU, which is highly similar to the RNA sequence UGUUUUUUUU, which is preferentially bound by SXL RBD1-RBD2 (Handa et al. 1999). These findings indicate that the RNA-binding properties of the vertebrate HuC and SXL are similar, even though the target genes and the biological functions of the proteins are different. With regard to Sxl in Drosophila, it is postulated that not only the gene expression but also an altered protein function may have contributed to the gain of the sex determination function in Drosophila.
4 tra Orthologues and Their Sex Determination Role in Nondrosophilid Species
tra was considered to be a Drosophila genome–specific gene because tra orthologues showed an unusually high degree of evolutionary divergence when compared within Drosophila subgenus species (O’Neil and Belote 1992). The identification of tra orthologues from the medfly (C. capitata) and the olive fruit fly (Bactrocera oleae) changed this notion (Pane et al. 2002; Lagos et al. 2007). These tra homologues (Botra and Cctra) have a female-determining master function (Pane et al. 2002; Lagos et al. 2007). To date, the tra gene has been identified not only in dipteran and coleopteran species but also in more than a dozen hymenopteran species (ants and bees), which are the most basal order of the holometabolous insects (Schmieder et al. 2012; Geuverink and Beukeboom 2014). Six of seven sequenced ants have two copies of tra, resulting from an ancestral duplication rather than independent duplications in each of the six species (Privman et al. 2013). In three bee species where whole-genome information is available, the tra gene is also duplicated (Privman et al. 2013). One copy is named complementary sex-determiner (csd), which is the primary signal for sex determination in the honeybee, A. mellifera (Beye et al. 2003). It activates the second copy, designated as feminizer (fem), which is more conserved and retains the ancestral function to regulate its downstream target, dsx (Hasselmann et al. 2008). csd is considered to have arisen from duplication of the fem gene (Schmieder et al. 2012).
RNA interference (RNAi)–mediated knockdown of tra in Musca (Hediger et al. 2010), Ceratitis (Pane et al. 2002), Anastrepha (Ruiz et al. 2007), Lucilia (Concha and Scott 2009), Apis csd (Beye et al. 2003), Apis fem (Hasselmann et al. 2008), Nasonia (Verhulst et al. 2010), and Tribolium castaneum (Shukla and Palli 2012) caused disruption of endogenous tra function in these species and subsequent male-specific splicing of the endogenous dsx pre-mRNAs, leading to the transformation of chromosomally female embryos into adult pseudomales. On the other hand, several previous studies and recent genome-wide analysis in a wide range of insect species revealed that tra shows a distinctly patchy distribution among insects (Geuverink and Beukeboom 2014). For example, no tra homologues have been found in lepidopteran insects, including B. mori, Danaus plexippus, and Heliconius melpomene (Mita et al. 2004; Geuverink and Beukeboom 2014) (Fig. 13.2). The most interesting pattern of tra distribution is seen in the Diptera genus. The tra gene is found in brachycera species, including D. melanogaster, while several mosquito species, which belong to a basal dipteran lineage, do not possess tra (Geuverink and Beukeboom 2014) (Fig. 13.2). The situation is the same in Coleoptera. One of the coleopteran species, T. castaneum, carries a tra orthologue (Shukla and Palli 2012), while the tra gene has not to date been found in the genome of the coleopteran Dendroctonus ponderosae (Geuverink and Beukeboom 2014). Mengenilla moldrzyki, which belongs to Strepsiptera—a sister order to Coleoptera—appears to lack a tra homologue (Geuverink and Beukeboom 2014) (Fig. 13.2).
Schematic diagram of the phylogenetic distribution of tra. The name of each species is noted on the right side. Species with tra are squared, while species lacking tra are in the gray box. The phylogenetic tree simply indicates the comparative relationship between each species, thus the branch length in the tree is not precisely indicated
TRA protein contains several conserved domains; one is shared only among Diptera (the “dipteran domain”), and another domain is present only in Hymenoptera (the “hymenopteran domain”). The most conserved part of the TRA protein is the TRA-CAM (C, Ceratitis; A, Apis; and M, Musca) domain (Hediger et al. 2010). This domain is found in all TRA proteins, except for those in D. melanogaster. Another conserved feature of TRA is a proline-rich region located near the C-terminus. This region is also present in all tra genes so far characterized in insects (Geuverink and Beukeboom 2014). However, the functional importance of the TRA-CAM domain and the proline-rich region remains unknown. The splicing pattern of all insect tra transcripts shows sexual dimorphism, and the female-specific splice variant only encodes a functional protein (Bopp et al. 2014).
The tra orthologue has also been isolated from two noninsect species, Daphnia magna and Daphnia pulex. They belong to the subphylum Crustacea, which is regarded as a sister group to insects (Kato et al. 2010; Chen et al. 2014) (Fig. 13.2). Recently, we have isolated a tra homologue in the acorn worm Saccoglossus kowalevskii, which is a hemichordate belonging to the superphylum Deuterostomia (Suzuki et al. 2015). D. magna transformer (DmagTra), D. pulex transformer (Dptra), and S. kowalevskii tra (Sktra) also contain the highly conserved TRA-CAM domain and the arginine/serine-rich region near the N-terminus. Unlike insect tra genes, all of these tra orthologues show no sexual dimorphism in their splicing patterns (Kato et al. 2010; Chen et al. 2014; Suzuki et al. 2015). However, the expression level of Dptra has been shown to be significantly higher in sexually mature males than in ephippial females, implying that Dptra might play an important role in switching between reproduction modes and sexual differentiation in D. pulex, but this requires further confirmation (Chen et al. 2014). Similarly, the mRNA level of Sktra was approximately 7.5-fold greater in the testes than in the ovaries, suggesting that Sktra might be involved in sexual differentiation of S. kowalevskii (Suzuki et al. 2015).
These findings support the idea that the tra gene evolved independently in insect species and acquired a sex-determining gene function, especially as a dsx-splicing regulator. However, as described above, tra is present in the orders Hymenoptera, Coleotpera, and Diptera but, thus far, has not been found in Lepidoptera or in the basal lineages of Diptera (Geuverink and Beukeboom 2014), implying multiple independent losses or recruitment of tra into the sex determination cascade. Vertebrates and multiple metazoan phyla, including arthropods and nematodes, lack tra, which may be dispensable for embryogenesis, developmental processes, and some other biological processes. Interestingly, dsx genes in five Cladocera species (including D. magna and D. pulex), which are the closest relatives to the insects, are also expressed dominantly in males and do not exhibit the sex-specific splicing typical of insect dsx (Toyota et al. 2013). Unlike insect tra genes, both D. magna tra and D. pulex tra show no sexual dimorphism in their splicing patterns (Kato et al. 2010; Chen et al. 2014). These findings have led to the inference that the tra–dsx regulatory axis evolved independently in insect species.
5 tra as a Memory Device for Female Sexual Development
In tephritid fruit flies—such as C. capitata, B. oleae, and 12 Anastrepha species—the Sxl gene is not regulated in a sex-specific manner and does not appear to play the key discriminating role (memory device) in sex determination that it plays in Drosophila (Saccone et al. 1998; Lagos et al. 2007). As in the drosophilids, the tephritid tra gene is constitutively expressed in both sexes and its pre-mRNA undergoes sex-specific alternative splicing. In contrast to the Drosophila situation in which Sxl regulates tra, in the tephritids the gene tra acts as the memory device for sex determination via its autoregulatory function, i.e., through the contribution of the TRA protein to the female-specific splicing of its own pre-mRNA. The tra gene in the tephritids has male-specific exons that contain translation stop codons. The inclusion of these exons in mature tra mRNA in males results in the production of a truncated, nonfunctional TRA protein. In females, the male-specific exons are spliced out because of the presence of TRA protein (Pane et al. 2002; Lagos et al. 2007; Ruiz et al. 2007). The presence of putative TRA-TRA2 binding sites in the male-specific exons and in the surrounding introns may suggest that the TRA interacts with its own pre-mRNA through TRA-2, resulting in skipping of the male-specific exons. It has been found that TRA-2 proteins in M. domestica and C. capitata are required for female-specific splicing of tra pre-mRNAs (Burghardt et al. 2005; Salvemini et al. 2009; Hediger et al. 2010). In Ceratitis and Musca, it is postulated that maternal tra mRNAs trigger the autoregulatory loop in XX (female) embryos. The zygotically produced TRA protein controls the maintenance of tra autoregulation and female-specific splicing of dsx pre-mRNA. The resulting DSXF protein induces female development. In XY (male) embryos, the male-determining M factor located on the Y chromosome prevents tra autoregulation through unknown mechanisms. Therefore, TRA protein is not produced in XY embryos, and thus the autoregulatory loop cannot initiate. Because of the absence of TRA protein, dsx pre-mRNA is spliced by default to yield DSXM protein, which induces male development (Pane et al. 2002; Hediger et al. 2010). A similar autoregulatory function is also thought to occur in the beetle T. castaneum (Shukla and Palli 2012).
The fem gene—a tra homologue in the honeybee, A. mellifera—needs its protein product to splice its own pre-mRNA into the productive female form, which establishes an autoregulatory feedback loop to maintain the female state throughout the bee’s life (Gempe et al. 2009). The csd gene, which is a paralogue of fem, initiates the autoregulatory loop (Hasselmann et al. 2008). The female mode of fem splicing occurs only when the csd presents in the heteroallelic condition. The female form of Fem protein is active and induces female splicing of A. mellifera dsx (Am-dsx) pre-mRNA, resulting in the production of Am-DSXF. In the absence of Csd protein activity in males, where the csd gene is homo- or hemiallelic, fem pre-mRNA is spliced into the male form, which includes the male-specific exons containing an intervening stop codon. The male form of Fem protein is inactive; therefore, Am-dsx pre-mRNA is spliced by default to produce Am-DSXM protein. As in the dipteran insects M. domestica and C. capitata, Am-TRA2 protein acts together with heteroallelic Csd proteins and/or Fem proteins to mediate female fem splicing by binding to fem pre-mRNAs (Nissen et al. 2012).
The jewel wasp N. vitripennis employs a similar but slightly different autoregulatory function in the regulation of tra gene (Nvtra) expression. In this species, sex is determined on the basis of the ploidy of embryos (Heimpel and de Boer 2008); males are haploid, developing from unfertilized eggs, whereas diploid females develop from fertilized eggs. In diploid eggs, maternally provided Nvtra mRNA initiates the female-specific autoregulatory loop required to maintain the female state throughout the wasp’s life (Verhulst et al. 2010). In haploid (unfertilized) eggs, it is postulated that zygotic transcription from the maternal Nvtra allele is prevented as a result of maternal imprinting, leading to male development.
6 dsx, the Most Conserved Regulatory Switch in Sex Determination
Orthologues of dsx have been found in each insect species examined, including Diptera, Lepidoptera, Coleoptera, and Hymenoptera species (Bopp et al. 2014). Primary transcripts from dsx genes thus far identified undergo sex-specific alternative splicing, producing either a male-specific (DSXM) or female-specific (DSXF) isoform (Schütt and Nöthiger 2000; Geuverink and Beukeboom 2014). The female-specific splicing of dsx transcripts in Diptera and Coleoptera is induced by TRA together with TRA-2 through canonical TRA-TRA2 binding sites, which are consistently present in the female-specific exon (Burghardt et al. 2005; Salvemini et al. 2009; Sarno et al. 2010; Shukla and Palli 2012). Interestingly, dsx pre-mRNAs in the honeybee, A. mellifera, lack the canonical binding sites of TRA-TRA2 proteins, even though its female-specific splicing needs Am-TRA2 proteins (Nissen et al. 2012). This finding suggests that the TRA-TRA2 protein-binding sites have evolved independently in the hymenopteran species. Consistent with this hypothesis, Am-TRA2 appears to have several amino acid replacements in the RNA-binding domain (RBD), one of which is a critical amino acid residue for female processing of dsx pre-mRNAs in D. melanogaster (Amrein et al. 1994). The dsx gene in the silkworm, B. mori—designated as Bmdsx—also lacks the canonical TRA-TRA2 binding sites (Suzuki et al. 2001). In contrast to dsx in other insect species, female-specific Bmdsx transcripts are produced by default splicing (Suzuki et al. 2001). TRA-2 proteins in this species are not relevant to sex-specific splicing of Bmdsx (Suzuki et al. 2012).
Geuverink and Beukeboom (2014) recently identified putative dsx orthologues in a number of primitive insect groups. For example, the human body louse, Pediculus humanus corporis, has a hypothetical protein composed of both a DM (Dsx/Mab-3 DNA-binding motif) domain and a dimerization domain (OD2). The DM domain is found in multiple genes of the DM superfamily group consisting of dsx, mab3, and related transcription factors (DMRT), while the dimerization domain is exclusively found in dsx. Five Cladocera species (Daphnia magna, Daphnia pulex, Daphnia galeata, Ceriodaphnia dubia, and Moina macrocopa), which are the closest relatives to the insects examined, also possess dsx genes (Kato et al. 2011; Toyota et al. 2013).
The DMRT genes appear to play a role in sex determination or sexual differentiation in all Metazoa; therefore, this gene family may represent the first example of sexual regulatory genes conserved across phyla (Hodgkin 2002; Kopp 2012). In vertebrates, orthologues of DMRT1 are expressed dominantly in males and are required only to guide male sex determination (Matson and Zarkower 2012). Therefore, it is conceivable that animal species in which DMRT1 is responsible for maleness do not necessarily need a dsx-splicing regulator such as an insect TRA. Interestingly, dsx genes in five Cladocera species (including D. magna and D. pulex), which are the closest relatives to the insects, are also expressed dominantly in males and do not exhibit the sex-specific splicing typical of insect dsx (Toyota et al. 2013). Unlike insect tra genes, both D. magna and D. pulex tra show no sexual dimorphism in their splicing patterns (Kato et al. 2010; Chen et al. 2014). These findings lead to the inference that the sex-specific splicing feature in dsx was acquired early in the evolution of insects and that the tra–dsx regulatory axis evolved independently in insect species.
7 fru, Another Downstream Regulator in the Sex Determination Cascade
In D. melanogaster, the male-specific splice isoform of the fru gene (FruM) encodes a set of transcription factors involved in the regulation of male courtship and copulation, as described above. Recent insights from nondrosophilid insects suggest a conserved evolutionary role for the transcription factor Fruitless. In the housefly, M. domestica, male-specific transcripts from the fru orthologue Md-tra are generated by a conserved mechanism of sex-specific splicing (Meier et al. 2013). As in Drosophila, Md-fru is similarly involved in controlling male courtship behavior. A male courtship behavioral function for Md-fru was revealed by behavioral and neuroanatomic analyses with a hypomorphic allele of Md-tra, which specifically disrupts the expression of Md-fru in males, leading to severely impaired male courtship behavior. In addition, expression of Md-fru is confined to neural tissues in the brain, most prominently in the optic neuropil and in peripheral sensory organs (Meier et al. 2013). In the mosquitoes Anopheles gambieae and A. aegypti, fru orthologues show conservation of sex-specific alternative splicing and male-specific protein expression in neural tissues (Gailey et al. 2006; Salvemini et al. 2013). The female-specific exons of both genes each contain short sequences resembling the TRA-TRA2 binding sites but showing degeneration and lack of a consensus. Therefore, it is postulated that sex-specific splicing of fru transcripts in the mosquitoes is most likely under the control of splicing regulatory factors, which are different from TRA and TRA-2 found in other dipteran insects. This is consistent with the fact that sex determination in A. aegypti is different from that in Drosophila, where the key male determiner M, located on one of a pair of homomorphic sex chromosomes, controls sex-specific splicing of the dsx and fru orthologue (Hall et al. 2015). Moreover, a tra orthologue seems to be absent from both Anopheles and Aedes genomes (Gailey et al. 2006; Nene et al. 2007). In the hymenopteran N. vitripennis, the fru architecture is essentially identical to that in Drosophila, and the P1 transcripts, derived from the most distal fru promoter, undergo a conserved sex-specific splicing regulation (Bertossa et al. 2009). These findings suggest that conserved fru sex-specific splicing evolved prior to the divergence between Hymenoptera and Diptera (250–300 million years ago) rather than being acquired independently in both lineages.
In Orthoptera, which belongs to a phylogenetically more basal insect lineage, fru orthologues have also been isolated, but the situation is different from that of the fru genes in Diptera (Salvemini et al. 2010). For example, fru orthologues thus far identified in several Chorthippus grasshopper species, the desert locust Schistocerca gregaria, and the cockroach Blatella germanica show no sexual dimorphism in those splicing patterns (Ustinova and Mayer 2006; Boerjan et al. 2011; Clynen et al. 2011). In spite of this, RNAi knockdown experiments have demonstrated that fru orthologues in S. gregaria and B. germanica play important roles in regulation of successful copulation in the adult male and in male sexual behavior, respectively (Boerjan et al. 2011; Clynen et al. 2011). In addition, the spermatheca of female copulation partners of fru knockdown males in both species contained fewer or no spermatozoids, resulting in a reduction in progeny numbers in their naïve female copulation partners. This seemed likely due to smaller numbers of spermatozoa in the male seminal vesicles, caused by fru knockdown (Boerjan et al. 2012). These findings suggest that the role of fruitless as a master regulator of male sexual behavior has been conserved in insect evolution, at least from cockroaches to flies. This raises the question of whether the fru gene exists not only in insect genomes but also in vertebrate genomes, like its counterpart dsx, which is at the bottom of the sex determination cascade. Homology searches of reported sequences using fru BTB and ZnF domains as virtual probes resulted in no hits outside insects (Gailey et al. 2006).
8 Sex Determination Mechanisms in Insects Lacking the tra Gene
As described above, the tra gene acts as a memory device for female sexual development and thus plays a central role in the sex determination cascade. Conservation of the tra orthologue in more than a dozen hymenopteran species, which are the most basal order of the holometabolous insects, strengthens the importance of this gene in insect sex determination. On the other hand, considerable numbers of insect species appear to lack a tra orthologue, even though these species have sex-specifically spliced dsx and fru orthologues, both of which are well known as tra-downstream targets (Suzuki et al. 2001; Gailey et al. 2006; Nene et al. 2007; Salvemini et al. 2013). How do they control sex-specific splicing of dsx and fru, and determine their sexual fate, without a tra gene?
Maleness in the mosquito A. aegypti is determined by an M factor located on the homomorphic sex-determining chromosome within a Y chromosome–like region called the M locus (Newton et al. 1974). To date, no tra orthologues have been found in the A. aegypti genome. Consistent with this, there are no well-conserved TRA-TRA2 binding sites in both fru and dsx pre-mRNAs (Salvemini et al. 2011, 2013). A. aegypti has four tra-2 paralogues, which phylogenetically form a single clade, apart from the other known dipteran and even nondipteran orthologues (Salvemini et al. 2013). It is therefore postulated that these tra-2 paralogues evolved to have different sequence binding specificity and novel functions under selective pressure relaxation after gene duplication. Recently, Hall et al. (2015) reported that an M locus gene, Nix, which is a distant homologue of tra-2, functions as an M factor in A. aegypti. Nix knockout resulted in largely feminized genetic males and the production of female forms of dsx and fru. These findings suggest the possibility that tra-2 paralogues may have acquired the function of a primary sex-determining gene during evolution in insects that lack a tra orthologue.
The silkworm, B. mori, also lacks a tra gene (Mita et al. 2004; Geuverink and Beukeboom 2014). Moreover, TRA-2 proteins in this species are not relevant to the sex-specific splicing of Bmdsx (Suzuki et al. 2012). In contrast to dsx in other insect species, female-specific Bmdsx transcripts are produced by default splicing (Suzuki et al. 2001). In accordance with these findings, Bmdsx lacks the canonical TRA-TRA2 binding sites (Suzuki et al. 2001). In this species, male splicing of Bmdsx transcripts requires the splicing inhibitor (BmPSI) and the male-specific isoform of IMP (IMPM) proteins. These proteins form a complex that binds to a cis-regulatory element called CE1, located in the female-specific exon, and inhibits the female mode of splicing in males (Suzuki et al. 2008, 2010). Our previous data suggested that ImpM maintains its male-specific mode of expression by an autoregulatory function and thus may function as a memory device for male development (Suzuki et al. 2014). Alternative splicing regulation by an autoregulatory function is not restricted to the female-specific splicing of tra and is also seen in common splicing regulators. For example, SRp20 and ASF/SF2, both of which belong to a highly conserved SR family of splicing factors, autoregulate the alternative splicing of their own pre-mRNAs (Ge et al. 1991; Jumaa and Nielsen 1997). It could therefore be expected that the autoregulatory function originally present in the Imp gene may have been recruited to the male-determining pathway and utilized as a memory device for male development during evolution. Imp is a Z chromosome–linked gene whose dosage is twice as high in males as in females (Suzuki et al. 2010). The Imp orthologue is located on the Z chromosome in another lepidopteran species, Samia cynthia ricini, which lacks a W chromosome (Yoshido et al. 2011). If the Imp orthologue in this species also produces a male-specific splice isoform, then it is highly possible that such a male isoform of Imp may function as a memory device for male sexual development.
It has been shown genetically that female sex in B. mori is determined by the presence of a dominant feminizing factor (Fem) on the W chromosome (Hashimoto 1933). The W chromosome in B. mori lacks protein-coding genes and is almost completely occupied by selfish repetitive elements such as transposons (Abe et al. 2005). The only transcripts produced from the W chromosome are PIWI-interacting RNAs (piRNAs) (Kawaoka et al. 2011). piRNAs are 23–30 nucleotides of small RNAs acting as sequence-specific guides for PIWI proteins that cleave target RNAs mainly to disrupt the activity of transposons in the gonads (Garabedian et al. 1986; Malone and Hannon 2009). Recently, one of the piRNAs originating from the sex-determining region of the W chromosome appeared to function as Fem (Kiuchi et al. 2014). Inhibiting the expression of Fem piRNA in female embryos not only induced ImpM expression (Sakai et al. 2015) but also shifted the splicing pattern of Bmdsx from the female to the male forms, suggesting that this piRNA is required for femaleness (Kiuchi et al. 2014; Sakai et al. 2015). Fem piRNA targets and cleaves mRNAs transcribed from the Z chromosome–linked gene that encodes a CCCH-tandem zinc finger protein. Knockdown of this gene expression in male embryos decreased the ImpM expression (Sakai et al. 2015) and led to the production of the female-type Bmdsx transcripts (Kiuchi et al. 2014; Sakai et al. 2015), indicating that this gene is essential for silkworm masculinization, and it was therefore named Masculinizer (Masc). Recently our studies using transgenic silkworms have demonstrated that forced expression of Masc in females inhibits the normal development of ovaries and induces the formation of spermatids in the abnormal tissues [manuscript in preparation].
On the basis of these findings, we have proposed a genetic cascade regulating sex determination in B. mori, as follows (Fig. 13.3). In females (ZW), Fem piRNA disrupts the expression of Masc. Lower expression of Masc fails to induce expression of ImpM. In the absence of ImpM, Bmdsx pre-mRNA is spliced by default to generate the female-type BmDSX protein, which promotes female development. In males (ZZ), Masc expression remains at a higher level than in ZW individuals. This boosts the expression of Imp to a level sufficient to impose the male splicing mode on its own RNA. Once IMPM protein is produced, its male-specific expression is maintained by an autoregulatory function. IMPM protein, together with BmPSI, is altered to produce the male-specific splicing of Bmdsx. The male-type BmDSX protein promotes male development.
Proposed genetic cascade regulating sex determination in Bombyx mori. In females (ZW), transcripts from the Fem locus are cleaved by the maternally transmitted Masc PIWI-interacting RNA (piRNA)–BmAgo3 complex. The Fem piRNA–Siwi complex cleaves Masc mRNA, resulting in the accumulation of Masc piRNA and further enhancement of feminization (for more details, refer to the review described by Katsuma et al. in 2015). Lower expression of Masc fails to induce expression of ImpM. In the absence of ImpM, Bmdsx precursor messenger RNA is spliced by default to generate the female-type BmDSX protein (BmDSXF), which promotes female development. In males (ZZ), Masc expression remains at a higher level than in ZW individuals. This initiates the expression of ImpM. Once the IMPM protein is produced, its male-specific expression is maintained by an autoregulatory function. IMPM protein, together with BmPSI, is altered to produce the male-specific splicing of Bmdsx. The male-type BmDSX protein (BmDSXM) promotes male development
To date, Fem piRNA has been found only in Bombyx genomes, while its downstream target Masc is conserved among several lepidopteran species, some of which lack a W chromosome (Kiuchi et al. 2014). In such species, a Z chromosome–counting mechanism, such as XES factors in Drosophila, might regulate Masc expression, or a difference in the gene dosage of Masc itself may determine the sexual fate: ZZ animals with two genes develop into males, while ZO animals with only one Masc gene develop into females. A similar sex-determining mechanism has been reported in the chicken, which has a Z-linked dmrt1 gene. In this species, where the homogametic sex is male (ZZ) and the heterogametic sex is female (ZW), it is considered that two copies of the dmrt1 gene promote male differentiation, while a single copy of the dmrt1 gene promotes female differentiation (Smith et al. 2009).
B. mori presents the first known example of a sex-determining pathway controlled by the presence or absence of a piRNA. This tra-lacking insect also provides the first known example of a sex-specific splicing regulatory mechanism of dsx controlled by PSI and IMP orthologues. Whether this pathway is evolutionarily conserved among lepidopteran species will certainly be the subject of future research.
References
Abe H, Mita K, Yasukochi Y, Oshiki T, Shimada T (2005) Retrotransposable elements on the W chromosome of the silkworm, Bombyx mori. Cytogenet Genome Res 110:114–151
Amrein H, Hedley ML, Maniatis T (1994) The role of specific protein-RNA and protein–protein interactions in positive and negative control of pre-mRNA splicing by transformer-2. Cell 76:735–746
Anand A, Villella A, Ryner LC, Carlo T, Goodwin SF, Song HJ, Gailey DA, Morales A, Hall JC, Baker BS, Taylor BJ (2001) Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics 158:1569–1595
Antic D, Keene JD (1997) Embryonic lethal abnormal visual RNA-binding proteins involved in growth, differentiation, and posttranscriptional gene expression. Am J Hum Genet 61:273–278
Antic D, Lu N, Keene JD (1999) ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells. Genes Dev 13:449–461
Baker RH, Sakai RK (1976) Male determining factor on chromosome 3 in the mosquito, Culex tritaeniorhynchus. J Hered 67:289–294
Baker BS, Wolfner MF (1988) A molecular analysis of doublesex, a bifunctional gene that controls both male and female sexual differentiation in Drosophila melanogaster. Genes Dev 2:477–489
Bedo DG, Foster GG (1985) Cytogenotic mapping of the male-determining region of Lucilia cuprina (Diptera: Clliphoridae). Chromosoma 92:344–350
Bell LR, Horabin JI, Schedl P, Cline TW (1991) Positive autoregulation of Sexlethal by alternative splicing maintains the female determined state in Drosophila. Cell 65:229–239
Bertossa RC, van de Zande L, Beukeboom LW (2009) The Fruitless gene in Nasonia displays complex sex-specific splicing and contains new zinc finger domains. Mol Biol Evol 26:1557–1569
Beukeboom LW (1995) Sex determination in hymenoptera: a need for genetic and molecular studies. BioEssays 17:813–817
Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW (2003) The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114:419–429
Boerjan B, Tobback J, De Loof A, Schoofs L, Huybrechts R (2011) Fruitless RNAi knockdown in males interferes with copulation success in Schistocerca gregaria. Insect Biochem Mol Biol 41:340–347
Boerjan B, Tobback J, Vandersmissen HP, Huybrechts R, Schoofs L (2012) Fruitless RNAi knockdown in the desert locust, Schistocerca gregaria, influences male fertility. J Insect Physiol 58:265–269
Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M (1987) Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell 50:739–747
Bopp D, Saccone G, Beye M (2014) Sex determination in insects: variations on a common theme. Sex Dev 8:20–28
Bridges CB (1921) Triploid intersexes in Drosophila melanogaster. Science 54:252–254
Burghardt G, Hediger M, Siegenthaler C, Moser M, Dübendorfer A, Bopp D (2005) The transformer2 gene in Musca domestica is required for selecting and maintaining the female pathway of development. Dev Genes Evol 215:165–176
Burtis KC, Baker BS (1989) Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56:997–1010
Burtis KC, Coschigano KT, Baker BS, Wensink PC (1991) The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J 10:2577–2582
Chen P, Xu SL, Zhou W, Guo XG, Wang CL, Wang DL, Zhao YL (2014) Cloning and expression analysis of a transformer gene in Daphnia pulex during different reproduction stages. Anim Reprod Sci 146:227–237
Cline TW (1984) Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics 107:231–277
Cline TW (1988) Evidence that sisterless-a and sisterless-b are two of several discrete “numerator” elements of the X:A sex-determination signal in Drosophila that switch Sex-lethal between two alternative stable expression states. Genetics 119:829–862
Cline TW, Meyer BJ (1996) Vive la difference: males vs females in flies vs worms. Annu Rev Genet 30:637–702
Clough E, Jimenez E, Kim YA, Whitworth C, Neville MC, Hempel LU, Pavlou HJ, Chen ZX, Sturgill D, Dale RK, Smith HE, Przytycka TM, Goodwin SF, Van Doren M, Oliver B (2014) Sex- and tissue-specific functions of Drosophila doublesex transcription factor target genes. Dev Cell 31:761–773
Clynen E, Ciudad L, Bellés X, Piulachs MD (2011) Conservation of fruitless’ role as master regulator of male courtship behaviour from cockroaches to flies. Dev Genes Evol 221:43–48
Concha C, Scott MJ (2009) Sexual development in Lucilia cuprina (Diptera, Calliphoridae) is controlled by the transformer gene. Genetics 182:785–798
Coschigano KT, Wensink PC (1993) Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev 7:42–54
Dalton JE, Fear JM, Knott S, Baker BS, McIntyre LM, Arbeitman MN (2013) Male-specific fruitless isoforms have different regulatory roles conferred by distinct zinc finger DNA binding domains. BMC Genomics 14:659
Demir E, Dickson BJ (2005) Fruitless splicing specifies male courtship behavior in Drosophila. Cell 1215:785–794
Erickson JW, Quintero JJ (2007) Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol 5:2821–2830
Estes PA, Keys LN, Schedl P (1995) Multiple response elements in the Sex-lethal early promoter ensure its female-specific expression pattern. Mol Cell Biol 15:904–917
Gailey DA, Billeter JC, Liu JH, Bauzon F, Allendorfer JB, Goodwin SF (2006) Functional conservation of the fruitless male sex-determination gene across 250 Myr of insect evolution. Mol Biol Evol 23:633–643
Garabedian MJ, Shepherd BM, Wensink PC (1986) A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell 45:859–867
Ge H, Zuo P, Manley JL (1991) Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell 66:373–382
Gempe T, Hasselmann M, Schiøtt M, Hause G, Otte M, Beye M (2009) Sex determination in honeybees: two separate mechanisms induce and maintain the female pathway. PLoS Biol 7:e1000222
Geuverink E, Beukeboom LW (2014) Phylogenetic distribution and evolutionary dynamics of the sex determination genes doublesex and transformer in insects. Sex Dev 8:38–49
Goldschmidt RB (1955) Theoretical genetic. University of California Press, Berkeley
Good PJ (1995) A conserved family of elav-like genes in vertebrates. Proc Natl Acad Sci U S A 92:4557–4561
Graveley BR, Hertel KJ, Maniatis T (1998) A systematic analysis of the factors that determine the strength of pre-mRNA splicing enhancers. EMBO J 17:6747–6756
Hägele K (1985) Identification of a polytene chromosome band containing a male sex determiner of Chironomus thummi thummi. Chromosoma 91:167–171
Hall AB, Basu S, Jiang X, Qi Y, Timoshevskiy VA, Biedler JK, Sharakhova MV, Elahi R, Anderson MA, Chen XG, Sharakhov IV, Adelman ZN, Tu Z (2015) A male-determining factor in the mosquito Aedes aegypti. Science 2348:1268–1270
Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S (1999) Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature 398:579–585
Hashimoto H (1933) The role of the W chromosome for sex determination in the silkworm, Bombyx mori. Jpn J Genet 8:245–258
Hasselmann M, Gempe T, Schiøtt M, Nunes-Silva CG, Otte M, Beye M (2008) Evidence for the evolutionary nascence of a novel sex determination pathway in honeybees. Nature 454:519–522
Hediger M, Henggeler C, Meier N, Perez R, Saccone G, Bopp D (2010) Molecular characterization of the key switch F provides a basis for understanding the rapid divergence of the sex-determining pathway in the housefly. Genetics 184:155–170
Heimpel GE, de Boer JG (2008) Sex determination in the Hymenoptera. Annu Rev Entomol 53:209–230
Heinrichs V, Ryner LC, Baker BS (1998) Regulation of sex-specific selection of fruitless 5′ splice sites by transformer and transformer-2. Mol Cell Biol 18:450–458
Henking H (1891) Unlersuehungen ueber die ersten Entwicklungsvorgänge in den Eiern der Insekten. II. Ueber Spermatogenese und deren Beziehung zur Entwickelung bei Pyrrhocoris apterus L. Z Wiss Zool 51:685–736
Hodgkin J (2002) The remarkable ubiquity of DM domain factors as regulators of sexual phenotype: ancestry or aptitude? Genes Dev 16:2322–2326
Inoue M, Muto Y, Sakamoto H, Yokoyama S (2000) NMR studies on functional structures of the AU-rich element-binding domains of Hu antigen C. Nucleic Acids Res 28:1743–1750
Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D (1996) Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci U S A 93:9687–9692
Jacqart D, Thomasset C (1988) Sexuality and medicine in the Middle Ages (trans: Adamson M). Princeton University Press, Princeton
Jumaa H, Nielsen PJ (1997) The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J 16:5077–5085
Kan JL, Green MR (1999) Pre-mRNA splicing of IgM exons M1 and M2 is directed by a juxtaposed splicing enhancer and inhibitor. Genes Dev 13:462–471
Kato Y, Kobayashi K, Oda S, Tatarazako N, Watanabe H, Iguchi T (2010) Sequence divergence and expression of a transformer gene in the branchiopod crustacean, Daphnia magna. Genomics 95:160–165
Kato Y, Kobayashi K, Watanabe H, Iguchi T, Kopp A (2011) Environmental Sex Determination in the Branchiopod Crustacean Daphnia magna: deep conservation of a doublesex gene in the sex-determining pathway. PLoS Genet 7:e1001345
Katsuma S, Kawamoto M, Kiuchi T (2015) Guardian small RNAs and sex determination. RNA Biol 11:1238–1242
Kawaoka S, Kadota K, Arai Y, Suzuki Y, Fujii T, Abe H, Yasukochi Y, Mita K, Sugano S, Shimizu K et al (2011) The silkworm W chromosome is a source of female enriched piRNAs. RNA 17:2144–2151
Keys LN, Cline TW, Schedl P (1992) The primary sex determination signal of Drosophila acts at the level of transcription. Cell 68:933–943
Kimura K, Ote M, Tazawa T, Yamamoto D (2005) fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature 438:229–233
Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, Arai Y, Ishihara G, Kawaoka S, Sugano S, Shimada T, Suzuki Y, Suzuki MG, Katsuma S (2014) A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature 2014(509):633–636
Kopp A (2012) Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet 28:175–184
Lagos D, Koukidou M, Savakis C, Komitopoulou K (2007) The transformer gene in Bactrocera oleae: the genetic switch that determines its sex fate. Insect Mol Biol 16:221–230
Lee G, Hall JC (2001) Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. J Neurosci 21:513–526
Lee G, Villella A, Taylor BJ, Hall JC (2001) New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J Neurobiol 47:121–149
Li Y, Blencowe BJ (1999) Distinct factor requirements for exonic splicing enhancer function and binding of U2AF to the polypyrimidine tract. J Biol Chem 274:35074–35079
Luo SD, Baker BS (2015) Constraints on the evolution of a doublesex target gene arising from doublesex’s pleiotropic deployment. Proc Natl Acad Sci U S A 112:E852–E861
Lynch KW, Maniatis T (1995) Synergistic interactions between two distinct elements of a regulated splicing enhancer. Genes Dev 9:284–293
Lynch KW, Maniatis T (1996) Assembly of specific SR protein complexes on distinct regulatory elements of the Drosophila doublesex splicing enhancer. Genes Dev 10:2089–2101
Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H (1996) Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem 271:8144–8151
Malone CD, Hannon GJ (2009) Small RNAs as guardians of the genome. Cell 136:656–668
Manley JL, Tacke R (1996) SR proteins and splicing control. Genes Dev 10:569–1579
Matson CK, Zarkower D (2012) Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet 13:163–174
Meier N, Käppeli SC, Hediger Niessen M, Billeter JC, Goodwin SF, Bopp D (2013) Genetic control of courtship behavior in the housefly: evidence for a conserved bifurcation of the sex-determining pathway. PLoS One 8:e62476
Meise M, Hilfiker-Kleiner D, Dubendorfer A, Brunner C, Nothiger R, Bopp D (1998) Sex-lethal, the master sex-determining gene in Drosophila, is not sex-specifically regulated in Musca domestica. Development 125:1487–1494
Mita K, Kasahara M, Sasaki S, Nagayasu Y, Yamada T et al (2004) The genome sequence of silkworm, Bombyx mori. DNA Res 11:27–35
Nene V, Wortman JR, Lawson D, Haas B, Kodira C et al (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316:1718–1723
Neville MC, Nojima T, Ashley E, Parker DJ, Walker J, Southall T, Van de Sande B, Marques AC, Fischer B, Brand AH, Russell S, Ritchie MG, Aerts S, Goodwin SF (2014) Male-specific fruitless isoforms target neurodevelopmental genes to specify a sexually dimorphic nervous system. Curr Biol 24:229–241
Newton ME, Southern DI, Wood RJ (1974) X and Y chromosomes of Aedes aegypti (L.) distinguished by Giemsa C-banding. Chromosoma 49:41–49
Niimi T, Sahara K, Oshima H, Yasukochi Y, Ikeo K, Traut W (2006) Molecular cloning and chromosomal localization of the Bombyx Sex-lethal gene. Genome 2006(49):263–268
Nissen I, Müller M, Beye M (2012) The Am-tra2 gene is an essential regulator of female splice regulation at two levels of the sex determination hierarchy of the honeybee. Genetics 192:1015–1026
O’Neil MT, Belote JM (1992) Interspecific comparison of the transformer gene of Drosophila reveals an unusually high degree of evolutionary divergence. Genetics 131:113–128
Pane A, Salvemini M, Delli Bovi P, Polito C, Saccone G (2002) The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 129:3715–3725
Privman E, Wurm Y, Keller L (2013) Duplication and concerted evolution in a master sex determiner under balancing selection. Proc Biol Sci 280:20122968
Ruiz MF, Milano A, Salvemini M, Eirín-López JM, Perondini AL, Selivon D, Polito C, Saccone G, Sánchez L (2007) The gene transformer of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. PLoS One 28:e1239
Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA (1996) Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell 87:1079–1089
Saccone G, Peluso I, Artiaco D, Giordano E, Bopp D, Polito LC (1998) The Ceratitis capitata homologue of the Drosophila sex-determining gene Sex-lethal is structurally conserved, but not sex-specifically regulated. Development 125:1495–1500
Sakai H, Sakaguchi H, Aoki F, Suzuki MG (2015) Functional analysis of sex-determination genes by gene silencing with LNA-DNA gapmers in the silkworm, Bombyx mori. Mech Dev 137:45–52
Salvemini M, Robertson M, Aronson B, Atkinson P, Polito LC, Saccone G (2009) Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int J Dev Biol 53:109–120
Salvemini M, Polito C, Saccone G (2010) Fruitless alternative splicing and sex behaviour in insects: an ancient and unforgettable love story? J Genet 89:287–299
Salvemini M, Mauro U, Lombardo F, Milano A, Zazzaro V, Arcà B, Polito LC, Saccone G (2011) Genomic organization and splicing evolution of the doublesex gene, a Drosophila regulator of sexual differentiation, in the dengue and yellow fever mosquito Aedes aegypti. BMC Evol Biol 11:41
Salvemini M, D’Amato R, Petrella V, Aceto S, Nimmo D, Neira M, Alphey L, Polito LC, Saccone G (2013) The orthologue of the fruitfly sex behaviour gene fruitless in the mosquito Aedes aegypti: evolution of genomic organisation and alternative splicing. PLoS One 8:e48554
Sarno F, Ruiz MF, Eirín-López JM, Perondini AL, Selivon D, Sánchez L (2010) The gene transformer-2 of Anastrepha fruit flies (Diptera, Tephritidae) and its evolution in insects. BMC Evol Biol 10:140
Schmieder S, Colinet D, Poirié M (2012) Tracing back the nascence of a new sex-determination pathway to the ancestor of bees and ants. Nat Commun 3:895
Schütt C, Nöthiger R (2000) Structure, function and evolution of sex-determining systems in Dipteran insects. Development 127:667–677
Shearman DCA, Formmer M (1998) The Bactrocera tryoni homolog of the Drosophila melanogaster sex-determination gene doublesex. Insect Mol Biol 7:355–366
Shirangi TR, Dufour HD, Williams TM, Carroll SB (2009) Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol 7:e1000168
Shukla JN, Palli SR (2012) Sex determination in beetles: production of all male progeny by parental RNAi knockdown of transformer. Sci Rep 2:602
Sievert V, Kuhn S, Paululat A, Traut W (2000) Sequence conservation and expression of the sex-lethal homologue in the fly Megaselia scalaris. Genome 43:382–390
Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH (2009) The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461:267–271
Stevens N (1905) Studies in spermatogenesis with special reference to the accessory chromosome. 36th Carnegie Institution of Washington Publication, Washington, DC
Sutton WS (1903) The chromosomes in heredity. Biol Bull 4:231–251
Suzuki MG, Ohbayashi F, Mita K, Shimada T (2001) The mechanism of sex-specific splicing at the doublesex gene is different between Drosophila melanogaster and Bombyx mori. Insect Biochem Mol Biol 31:1201–1211
Suzuki MG, Imanishi S, Dohmae N, Nishimura T, Shimada T, Matsumoto S (2008) Establishment of a novel in vivo sex-specific splicing assay system to identify a trans-acting factor that negatively regulates splicing of Bombyx mori dsx female exons. Mol Cell Biol 28:333–343
Suzuki MG, Imanishi S, Dohmae N, Asanuma M, Matsumoto S (2010) Identification of a male-specific RNA binding protein that regulates sex-specific splicing of Bmdsx by increasing RNA binding activity of BmPSI. Mol Cell Biol 30:5776–5786
Suzuki MG, Suzuki K, Aoki F, Ajimura M (2012) Effect of RNAi-mediated knockdown of the Bombyx mori transformer-2 gene on the sex-specific splicing of Bmdsx pre-mRNA. Int J Dev Biol. 56:693–699
Suzuki MG, Kobayashi S, Aoki F (2014) Male-specific splicing of the silkworm Imp gene is maintained by an autoregulatory mechanism. Mech Dev 131:47–56
Suzuki MG, Tochigi M, Sakaguchi H, Aoki F, Miyamoto N (2015) Identification of a transformer homolog in the acorn worm, Saccoglossus kowalevskii, and analysis of its activity in insect cells. Dev Genes Evol 225:161–169
Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner JB, Furneaux HM (1991) HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell 67:325–333
Tanaka Y (1916) Genetic studies in the silkworm. J Coll Agric Sapporo 6:1–33
Toyota K, Kato Y, Sato M, Sugiura N, Miyagawa S, Miyakawa H, Watanabe H, Oda S, Ogino Y, Hiruta C, Mizutani T, Tatarazako N, Paland S, Jackson C, Colbourne JK, Iguchi T (2013) Molecular cloning of doublesex genes of four cladocera (water flea) species. BMC Genomics 14:239
Traut W (1994) Sex determination in the fly Megaselia scalaris, a model system for primary steps of sex chromosome evolution. Genetics 136:1097–1104
Ustinova J, Mayer F (2006) Alternative starts of transcription, several paralogues, and almost-fixed interspecific differences of the gene fruitless in a hemimetabolous insect. J Mol Evol 63:788–800
Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, Nakata H, Piao ZF, Nilsson EE, Tomida J, Yamamoto D (2000) Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat Cell Biol 2:500–506
Verhulst EC, Beukeboom LW, van de Zande L (2010) Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 328:620–623
Wakamatsu Y, Weston JA (1997) Sequential expression and role of Hu RNA-binding proteins during neurogenesis. Development 124:3449–3460
Willhoeft U, Franz G (1996) Identification of the sex-determining region of the Ceratitis capitata Y chromosome by deletion mapping. Genetics 144:737–745
Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, Carroll SB (2008) The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134:610–623
Yoshido A, Yasukochi Y, Sahara K (2011) Samia cynthia versus Bombyx mori: comparative gene mapping between a species with a low-number karyotype and the model species of Lepidoptera. Insect Biochem Mol Biol 41:370–377
Zollman S, Godt D, Prive GG, Couderc JL, Laski FA (1994) The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc Natl Acad Sci U S A 91:10717–10721
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Japan KK, part of Springer Nature
About this chapter
Cite this chapter
Suzuki, M.G. (2018). Sex Determination Cascade in Insects: A Great Treasure House of Alternative Splicing. In: Kobayashi, K., Kitano, T., Iwao, Y., Kondo, M. (eds) Reproductive and Developmental Strategies. Diversity and Commonality in Animals. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56609-0_13
Download citation
DOI: https://doi.org/10.1007/978-4-431-56609-0_13
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56607-6
Online ISBN: 978-4-431-56609-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)