Abstract
What are the neural markers of encoding and retrieving memories of emotional events with increased efficacy? In recent years, this question has captured the attention of neuroscientists, who have been fervently engaged in addressing it using a multitude of approaches. The present chapter emphasizes evidence from brain imaging investigations regarding three emerging research directions in the field: the role of social information in emotional memory, the role of emotion regulation in the impact of emotion on memory, and the impact of emotion on associative or relational memory. Overall, this evidence provides insights into the brain mechanisms that make emotional memories special, points to possible alterations that may explain negative affective biases in encoding and retrieving emotional memories observed in affective disorders, and highlights specific aspects to be clarified in future research.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Emotion–cognition interactions
- Modulation hypothesis
- Social cognition
- Emotion regulation
- Associative/relational memory
- Neuroimaging
- Amygdala
- Hippocampus
- Prefrontal cortex
- Large-scale functional networks
1 Introduction
The memory-enhancing effect of emotion has long been documented, but elucidation of the neural mechanisms of this phenomenon has only relatively recently been the focus of intense research in humans, using functional neuroimaging tools. The present chapter builds upon our recent reviews (Dolcos et al. 2011, 2012), by incorporating new evidence regarding three emerging research directions in the fieldFootnote 1: the role of social information in emotional memory, the role of emotion regulation in the impact of emotion on memory, and the impact of emotion on associative or relational memory. The emphasis is on evidence from functional neuroimaging studies in neurologically intact humans investigating the role of the amygdala (AMY) and its interaction with memory-related medial temporal lobe (MTL) brain regions, along with the role of other brain regions (e.g., prefrontal cortex, PFC) in the effect of emotion during both the encoding and retrieval of episodic memories. Following a brief introduction to basic animal and human research regarding the neural mechanisms of emotional memory, along with an introduction to the important methodological developments that facilitated such findings, we discuss in detail new evidence from the literature circumscribed by the three emerging topics mentioned above. The chapter ends with concluding remarks and a brief presentation of open issues and future directions.
1.1 Basic Evidence from Animal Research
Historically, considerable evidence for the involvement of the AMY in emotional memory emerged from animal models, which provided the foundation for the investigation of neural mechanisms underlying emotional learning and memory in humans (Delgado et al. 2006; LeDoux 2000; McGaugh 2000, 2004; Phelps and LeDoux 2005). Animal research has primarily emphasized the role of the AMY in emotional learning and memory, but its specific role continues to be a matter of current debate. The two most influential hypotheses concerning its role are the modulation and the plasticity hypotheses, which posit different forms of involvement of the AMY in emotional memory (Dolcos and Denkova 2008). Specifically, the modulation hypothesis suggests a modulatory role of the AMY on the encoding and consolidation of memory processes occurring in other brain regions (McGaugh 2000, 2004), whereas the plasticity hypothesis proposes that the AMY itself is a site of plasticity Footnote 2 underlying learning and memory of fear conditioning, as well as reconsolidation of the memory trace following retrieval (LeDoux 2000, 2007). It should be noted that, although these hypotheses could be seen as conflicting (e.g., Cahill et al. 1999; Vazdarjanova and McGaugh 1998), in reality they are complementary (Fanselow and LeDoux 1999; Phelps and LeDoux 2005). The apparent contradiction seems to result from the fact that they tended to be tested with slightly different behavioral paradigms (i.e., inhibitory avoidance vs Pavlovian fear conditioning, respectively) that emphasize different aspects of the role of the AMY in mediating the impact of emotion on learning and memory (relatively more explicit/declarative vs more implicit/procedural forms of memory). However, given that real-life situations typically involve both declarative and nondeclarative aspects of behavior, and that emotion may influence memory not only during the initial stages, but also during the actual retrieval, it is more reasonable to consider both accounts when investigating the neural correlates of the memory-enhancing effect of emotion, rather than considering either one or the other of the mechanisms suggested by the two hypotheses. Evidence from functional neuroimaging studies in humans is actually consistent with this idea (Dolcos and Denkova 2008; Dolcos et al. 2004b, 2005, 2011, 2012).
1.2 Important Conceptual and Methodological Implementations That Facilitated Elucidation of the Neural Correlates of Emotional Memory in Human Research
Consistent with animal research, human research involving lesions (Adolphs et al. 1997, 2000; Cahill et al. 1995; LaBar et al. 1998; Phelps et al. 1998) and pharmacological methods (Cahill et al. 1994; Strange and Dolan 2004; Strange et al. 2003) highlighted the role of the AMY in emotional memory. More recently, human research has also greatly benefited from the advent of brain imaging methods, and from conceptual and methodological implementations that allowed identification with increased specificity of changes in brain activity linked to episodic memory processing and to dissociable effects of basic affective properties on emotional memory, in neurologically intact human brains.
Event-Related Designs
A significant conceptual and methodological contribution to our understanding of the neural mechanisms of memory has been made by the introduction of event-related designs as opposed to blocked designs (D’Esposito et al. 1999; Donaldson and Buckner 2001; Rosen et al. 1998). Different from blocked designs, constrained to averaging brain activity to several trials of the same type over time, event-related designs allow for analysis of brain imaging data on a stimulus-by-stimulus basis, so that the individual responses to single events can be specifically identified. An important advantage of the event-related data over the blocked data is that the former can be analyzed and categorized post-hoc according to the participant’s task performance. This advantage has proven particularly important in investigating the neural correlates of memory processes, as it allows comparison of brain activity for items that are subsequently remembered versus forgotten in a memory test, thereby allowing the possibility of establishing a direct link between brain activity and memory performance (Paller and Wagner 2002).
The Subsequent Memory Paradigm
One such event-related experimental paradigm that allows comparison of brain activity according to participants’ memory performance is the subsequent memory paradigm (SMP). For instance, as illustrated in Fig. 5.1, activity can be compared for items that are remembered versus those that are forgotten in a memory test, thereby establishing a direct link between brain activity and successful memory performance in individual participants. Comparing brain activity for remembered versus forgotten items can be done both during the encoding (learning) phase and during the retrieval (test) phase. By sorting brain activity recorded during encoding based on whether stimuli processed during the study phase are subsequently remembered (R) or forgotten (F) in a later memory test, the so-called difference in memory or Dm effect – i.e., greater encoding activity for remembered than for forgotten items – can be calculated (e.g., Paller et al. 1987; Paller and Wagner 2002). Brain regions showing a positive Dm effect (R > F) are assumed to mediate processes that lead to successful memory encoding, and are associated with encoding success (ES). In the case of retrieval, the contrast “remembered-minus-forgotten” identifies regions showing greater activity for items in which retrieval was successful (hits) than for items in which retrieval failed (misses). The activity difference between remembered and forgotten items during retrieval is known as retrieval success (RS) (Prince et al. 2005; Weis et al. 2004).
The subsequent memory paradigm (SMP) – measuring the impact of emotion on encoding success (ES) and retrieval success (RS) activity. (a) General procedure involved in the SMP. (b) Diagram of the comparisons that allow identification of the brain regions susceptible to emotional ES and RS. R subsequently remembered items, F subsequently forgotten items, fMRI functional magnetic resonance imaging, ERP event-related potential
In the context of investigating the impact of emotion on memory, one of the main advantages of Dm/ES and RS analyses is that they can eliminate the confounding effect of general emotion processing and specifically identify brain activity linked to memory processes and to the memory-enhancing effect of emotion (Shafer et al. 2011). By contrasting the emotional ES/RS with the neutral ES/RS, the brain regions whose memory-related activity is susceptible to emotional modulation during encoding and/or retrieval can be identified. Therefore, neuroimaging studies using event-related designs and the SMP have been highly influential in revealing brain regions that show an interaction between emotion and memory at different stages of memory processing; basic findings from such studies are reviewed below.
Manipulating Basic Affective Properties
Another important conceptual and methodological implementation concerns consideration of basic affective properties in experimental manipulations investigating the neural correlates of emotional memories. Emotional arousal and valence Footnote 3 are such affective dimensions (Lang et al. 1993; Russell 1980). Before the implementation of the proper manipulation of these dimensions (as illustrated in Shafer et al. 2011), the literature on emotion processing in general and on emotional memory in particular was dominated by the notion that negative stimuli produce stronger effects on memory than positive ones, which was linked to a negative bias in the involvement of basic emotion processing brain regions such as the AMY in these effects. However, subsequent studies directly comparing the effect of equally arousing positive and negative stimuli revealed similar memory-enhancing effects of such stimuli, regardless of their valence, and identified the sensitivity of AMY responses to processing positively valenced arousing stimuli (reviewed in Dolcos et al. 2012).
1.3 Basic Findings Regarding the Neural Correlates of Emotional Memory
Based on animal research, early human studies primarily emphasized the role of the AMY and its interaction with memory-related MTL regions in mediating the memory-enhancing effect of emotion, during encoding (e.g., Dolcos et al. 2004b; Kensinger and Corkin 2004; Kensinger and Schacter 2006a; Ritchey et al. 2008; Sergerie et al. 2006), consolidation (Ritchey et al. 2008), and retrieval (e.g., Dolcos et al. 2005; Kensinger and Schacter 2005; Sergerie et al. 2006) of emotional memories. Given its multiple connections with core memory structures, and with numerous cortical and subcortical regions involved in various aspects of information processing, such as perception, emotion, elaborative cognitive processes, and social cognition (see reviews by Dolcos and Denkova 2008; Dolcos et al. 2006b, 2012; Hamann 2001; LaBar and Cabeza 2006; LeDoux 2000; McGaugh 2004; Phelps 2004), the AMY together with the memory-related MTL regions and the PFC play a critical role in emotional memory. Most of these studies revealed that activity in the AMY, the MTL, and their interactions are primarily driven by the arousal and not the valence of the information, and that their interactions with the PFC contribute to valence-related effects in emotional memory (Dolcos et al. 2012).
Emotional Memory Encoding
Early studies suggested a link between AMY activity at encoding and later retrieval of emotionally arousing material (e.g., Cahill et al. 1996; Hamann et al. 1999). However, as they used blocked designs, these studies could not distinguish between brain activity for successfully and unsuccessfully encoded stimuli within participants, and thus could not specifically assess the role of the AMY in the successful encoding of emotional stimuli, as opposed to linking it to general emotion processing. By contrast, studies involving event-related designs in conjunction with the SMP have allowed identification of the role of the AMY and its interactions with the memory-related MTL brain regions (e.g., hippocampus, HC) in memory enhancement by emotion, in neurologically intact human brain (e.g., Canli et al. 2000; Dolcos et al. 2004b; Hamann et al. 1999; Kensinger and Corkin 2004; Kensinger and Schacter 2006a; Kilpatrick and Cahill 2003; Richardson et al. 2004; Ritchey et al. 2008; Sergerie et al. 2006), and that AMY–MTL interactions contribute to the persistence of emotional memories over time (Ritchey et al. 2008). More recently, using a combination of blocked and event-related designs, encoding of emotionally arousing stimuli over an extended period (>20 min) was found to induce a sustained arousal-related brain state (reflected in increased AMY–HC functional connectivity), which overall contributed to greater recollection of unrelated neutral items encoded minutes following the initial encoding of emotional items (Tambini et al. 2017). This suggests that transient exposure to emotional arousal can create persistent “carry-over” effects, resulting in similar memory-enhancing effects for subsequently encountered neutral items.
Although the main focus of the earlier neuroimaging studies investigating the facilitating effect of emotion on memory was on the AMY and its interaction with the memory-related MTL regions, increasing emphasis has been placed on the influence of emotion on memory through cognitive processes supported by other brain regions, such as the PFC (Dolcos et al. 2011). It has been suggested that, whereas the AMY and the MTL are part of basic/direct neurohormonal mechanisms underlying the memory enhancement effect of emotion, the PFC has an indirect/mediated involvement in the formation of emotional memories (e.g., by enhancing strategic, semantic, and working memory processes) (Dolcos and Denkova 2008; LaBar and Cabeza 2006). Moreover, whereas AMY–MTL mechanisms are modulated primarily by arousal, the involvement of the PFC also seems to be influenced by valence (Dolcos et al. 2004a; Kensinger 2004; Kensinger and Schacter 2006b), probably reflecting the PFC involvement in higher-order emotion processing associated with evaluation of emotional valence and/or cognitive control strategies (see Sect. 5.2.2 below). Finally, available evidence also shows that the interactions of the AMY and the MTL memory regions are linked to the valence of emotional information (Ritchey et al. 2011). Ritchey et al. (2011) showed that the intrinsic AMY–MTL interaction is stronger for encoding of negative stimuli, whereas the extrinsic interactions between the MTL and the PFC are stronger for encoding positive stimuli (see also Mickley Steinmetz et al. 2010).
Further supporting the idea of valence-related differences linked to differential connectivity, there is evidence that successful encoding of positive items involves a fronto-parietal network, whereas successful encoding of negative items involves a temporo-occipital network (Kensinger and Schacter 2008; Mickley Steinmetz and Kensinger 2009). These findings are consistent with those of other studies, revealing that encoding of positive information is associated with activity in specific PFC subregions (Botzung et al. 2010a; Dolcos et al. 2004a), probably because of more elaborative processing demanding cognitive resources, whereas encoding of negative information is associated with temporo-occipital regions (Mickley and Kensinger 2008), probably because of increased sensory processing. These findings suggest that successful encoding of positive and negative information might also depend on slightly different processing, with positive items involving more elaborate processing and engaging the PFC regions associated with semantic processing (Kapur et al. 1996; Poldrack et al. 1999; Shallice et al. 1994), working memory operations (D’Esposito et al. 2000; Owen et al. 1999; Petrides 1995), memory control (Anderson et al. 2004), and negative items involving perceptual processing and engaging posterior areas associated with the visual processing of emotional information (Vuilleumier et al. 2004).
Emotional Memory Retrieval
Although, as discussed above, involvement of the AMY in the formation of emotional memories has been well documented (for reviews see Dolcos and Denkova 2008; Dolcos et al. 2006b, 2012; LaBar and Cabeza 2006; Phelps 2004), until relatively recently it has been more difficult to demonstrate the involvement of the AMY in the retrieval of emotional memories (for review see Buchanan 2007). This may be partly due to memory testing methodology, using different kinds of events – e.g., laboratory micro-events versus autobiographical real-life events. Laboratory events are simpler experimenter-generated stimuli, such as lists of words or sets of pictures that are encoded in laboratory settings and retrieved at different intervals following encoding (e.g., minutes, hours, days, weeks, months). By contrast, autobiographical events are events from an individual’s own history that are encoded in real-world settings and may be retrieved after much longer intervals of years or even decades. Hence, retrieval of laboratory events is dissimilar to retrieval of autobiographical events in at least two aspects: time elapsed between encoding and retrieval, and self-relevance. However, testing memory with both types of events presents advantages: autobiographical events have the advantage of being close to personal real-life events and to cover temporally dispersed past episodes, whereas laboratory events have the advantage of being well controlled experimentally for neuroimaging applications (see also Cabeza et al. 2004).
Similar to the early studies of encoding, the early studies of emotional memory retrieval share many of the same limitations, as most of them either used blocked designs that did not allow assessment of the functional neuroimaging data on a trial-by-trial basis (e.g., Dolan et al. 2000; Kosslyn et al. 1996; Taylor et al. 1998), or they did not compare activity associated with successfully versus unsuccessfully retrieved items to distinguish brain activity specifically associated with RS (e.g., Fossati et al. 2004; Maratos et al. 2001). On the other hand, studies involving event-related designs in conjunction with the SMP have also proved influential in elucidating the role of the AMY during successful retrieval of emotional memory (e.g., Dolcos et al. 2005; Kensinger and Schacter 2005; Sergerie et al. 2006). These studies provided strong evidence that successful retrieval of emotional memories involves AMY engagement similar to that identified during successful encoding of emotional memories. Furthermore, refined examinations of AMY activity linked to a differential impact of emotion on recollection- versus familiarity-based retrievalFootnote 4 pointed to a specific role of the AMY in the enhancement of recollection rather than familiarity in retrieving emotional memories (Dolcos et al. 2005; Sharot et al. 2004; Talarico et al. 2004).
More recent neuroimaging evidence also suggests that the AMY might play a role in the successful retrieval of personally relevant autobiographical memories (AMs), following shorter retention intervals. By investigating basketball fans’ memories of specific basketball games, encoded a few days before the scanning session, Botzung et al. (2010b) showed that AMY activity was modulated by the emotional intensity of the recollected events, and was greater for extremely high- vs. low-intensity events. Hence, this study also points to the involvement of the AMY in the retrieval of memories for emotional and personally relevant events (see also Muscatell et al. 2010; Sharot et al. 2007a). Taken together, these studies provide evidence for the involvement of the AMY in the recollection of highly arousing and personally relevant recent events.
Unlike the involvement of the AMY in the retrieval of laboratory or relatively recent personally relevant events, which has been well documented, the engagement of the AMY during retrieval of remote autobiographical episodes temporally dispersed in an individual’s history was, until relatively recently, unclear (Buchanan 2007). Whereas some studies found increased activity in the AMY for emotional autobiographical events (Markowitsch et al. 2000, 2003), others did not observe such an effect (Oddo et al. 2008; Vandekerckhove et al. 2005). In the same vein, although some studies reported that AMY activity may be modulated by emotional intensity during the early stages of memory retrieval (Daselaar et al. 2008), others failed to observe a link between AMY activity and emotional intensity (Addis et al. 2004; Maguire and Frith 2003). One of the factors that may account for these inconsistencies in AMY engagement during retrieval of AMs (Denkova et al. 2006; Greenberg et al. 2005) may be related to differences in the engagement of effortful processing, which is increased when remembering temporally dispersed past events, and thus could divert the attentional resources from the emotional value of recollections (Phan et al. 2002). Hence, engagement of the AMY seems to depend on the task instructions (Smith et al. 2006), as discussed below.
The link between task instructions and AMY response was recently investigated by directing the participants’ attention focus either to emotional (emotion condition) or to other contextual (context condition) details during recollection of positive and negative AMs (Denkova et al. 2013). As expected, emotion compared with the context focus condition yielded increased activity in the left AMY for both positive and negative memories. Moreover, greater engagement of the AMY when focusing on emotional compared to the contextual details was associated with greater subjective re-experience of emotion of the recollected AMs. This finding is consistent with the emotion research, suggesting that the engagement of the AMY could be modulated by attention, current goals, and task demands (Lieberman et al. 2007; Shafer et al. 2012), and extends the available evidence by revealing that this effect also applies to the retrieval of both positive and negative AMs. In contrast to the left AMY, right AMY activity was not modulated by the current retrieval goals in the case of negative AMs, thus suggesting a hemispheric dissociation in the AMY as a function of retrieval focus and valence during AM recollection. One potential explanation for this AMY lateralization could be linked to Glascher and Adolph’s model (Glascher and Adolphs 2003), which suggests that the right AMY is involved in an initial, automatic detection of emotions, whereas the left AMY is involved in a more elaborate, cognitive representation of emotions (Morris et al. 1999; Phelps et al. 2001). Overall, these findings shed light on the involvement of the AMY during recollection of AMs as a function of retrieval focus and emotional valence.
In addition to the evidence highlighting the engagement of the AMY during emotional memory retrieval, further evidence showed increased interactions between an AMY-based emotion processing system and the memory-related MTL regions during retrieval (Dolcos et al. 2005; Greenberg et al. 2005). These findings are consistent with the idea that the AMY and the MTL memory system are part of a synergistic mechanism in which emotion enhances recollection and recollection enhances emotion. Emotion may enhance recollection because reinstating the affective context of the original episode is likely to facilitate the recovery of contextual details, such as where, when, and how the original events happened. In turn, recollection of the context surrounding an emotional effect is likely to augment the emotional arousal elicited by the event during retrieval (Dolcos et al. 2005).
There is also evidence that the interaction between the AMY and the MTL memory-related regions may be modulated by the involvement of the PFC. For instance, a study by Smith et al. (2006) used dynamic causal modeling to examine the effective connectivity among the AMY, HC, and PFC during retrieval, reporting increased bidirectional connectivity between the AMY and the HC, and increased engagement of the medial PFC. Moreover, the latter influenced activity in the AMY and the HC during a task requiring explicit recollection of the emotional information compared with a task not requiring explicit focus on emotional information. Consistent with the evidence discussed above, this finding suggests that the involvement of the AMY could be modulated by an individual’s goals (Cunningham et al. 2008), and points to the involvement of top–down processes through the medial PFC, during explicit retrieval of emotional information (see also Denkova et al. 2015). Finally, similar to evidence from encoding, studies of memory retrieval for emotional laboratory (Erk et al. 2005; Maratos et al. 2001; Smith et al. 2004b, 2006) and autobiographical (Botzung et al. 2010b; Markowitsch et al. 2003; Piefke et al. 2003) events also point to the involvement of the PFC linked to emotional valence. For instance, increased activity in the medial and/or the orbital PFC was associated with retrieval of positive contextual information (e.g., Erk et al. 2005) and of positive personal experiences (Markowitsch et al. 2003; Piefke et al. 2003). The medial orbital PFC has been associated with general emotion processing (Davidson and Irwin 1999; Phan et al. 2002), reward-related (Dolan 2007; O’Doherty 2004) processing, and self-referential processing (for review see Heinzel and Northoff 2009; Ochsner et al. 2004). Therefore, it is possible that the engagement of the medial orbital PFC during retrieval of positive emotional information is linked to the elaboration of self-relevant rewarding experience. Related to this, increased activity in the orbital PFC, together with its increased connectivity with the HC, was associated with the processing of socially rewarding stimuli (Tsukiura and Cabeza 2008) (see also Sect. 5.2.1 below).
In summary, the evidence discussed above supports the notion that the memory-enhancing effect of emotion is associated with increased activity and interaction between an emotion-based system involving the AMY and the memory-based MTL structures, during both encoding and retrieval. Whereas the MTL-based mechanism seems to be involved in arousal-dependent effects, valence effects could be revealed in the connectivity of these regions within and outside the MTL. It should also be noted that, although overall these findings provide strong evidence for the modulation hypothesis, the AMY findings, showing greater encoding and RS for emotional than for neutral stimuli, are also consistent with the plasticity hypothesis. Regarding the role of the PFC, the available evidence suggests that its involvement might be sensitive to the processing of valence and might reflect higher order cognitive processing (e.g., semantic memory, working memory, cognitive control, and self-referential processing), during both encoding and retrieval of emotional information.
2 Current Issues and Emerging Directions in the Impact of Emotion on Memory
This section discusses new evidence regarding three emerging research directions, as follows: the role of social information in emotional memory, the role of emotion regulation in the impact of emotion on memory, and the impact of emotion on associative or relational memory.
2.1 The Role of Social Information in Emotional Memory
Recent evidence suggests that the memory-enhancing effect of emotion might involve more complex interplays among the AMY, MTL, PFC, and other brain regions, when besides the typical basic affective dimensions (arousal and valence), other more abstract psychological dimensions, such as social relevance are considered. Evidence supporting these ideas is discussed in this section.
Elucidation of the complex interplay between emotion and social cognition and its impact on memory is important, as it may shed light on our understanding of emotional memory for more ecologically valid situations, involving interactions with other people and having direct relevance to our own social behavior. Knowledge acquired through previous social interactions is critical for our ability to navigate successfully the highly complex social world, guiding our adaptive behavior in a variety of social situations (Ciaramelli et al. 2013; Spreng and Andrews-Hanna 2015; Spreng and Mar 2012; Tsukiura 2012), which is influenced by personality traits affecting social behavior. For instance, trait empathy has been positively associated with recognition memory performance in healthy individuals (Wagner et al. 2015), and was significantly reduced in patients with hippocampal amnesia showing declarative memory impairments (Beadle et al. 2013), thus suggesting an important link between memory processes and healthy social behavior (see also Laurita and Spreng 2017). Moreover, recent neuroimaging evidence points to the existence of a network of brain regions subserving memory processes for stimuli with social relevance, such as other people’s faces and impressions formed based on them (e.g., Gilron and Gutchess 2012; Tsukiura 2012).
Given that social information may carry emotional significance, at a basic level, it should probably influence memory through similar mechanisms to those engaged by emotion. However, because of its complexity, it is also expected to engage additional brain systems. Consistent with this idea, there is evidence that social relevance requires more elaborative processing (e.g., interpretation of the stimulus meaning depending on the context and individual differences), and seems to enhance memory only when sufficient cognitive resources are available (Sakaki et al. 2012). Along these lines, Sakaki et al. (2012) suggested that, although basic processing of biologically emotional stimuli might be automatic (mediated by increased activity in and connectivity between the AMY and the visual cortex), processing of socially emotional stimuli might also depend on more elaborative processes involving enhanced activity in and connectivity between the AMY and the medial PFC. In support of this idea, neuroimaging studies have also demonstrated the involvement of the AMY and the medial/orbital PFC in complex social cognitive functions, such as detecting social cues in the environment, interpreting and monitoring affective reactions, engaging in self-referential processes, or processing reward and punishment (e.g., Botzung et al. 2010a; Gilron and Gutchess 2012; Harvey et al. 2007; Somerville et al. 2006; Tsukiura 2012; Yaoi et al. 2015). The subsections below discuss evidence regarding the neural mechanisms of encoding and retrieving socially relevant information.
Encoding of Socio-emotional Memories
Recent studies have suggested that the role of the AMY might extend beyond successful encoding of general emotional stimuli, to include successful encoding of socially (personally) relevant stimuli (Botzung et al. 2010a; Harvey et al. 2007; Kleinhans et al. 2007; Tsukiura 2012) and, more broadly, processing of stimuli with motivational significance (Adolphs 2010; Cunningham and Brosch 2012). For instance, by using personally relevant social stimuli (e.g., portions of basketball games), Botzung et al. (2010a, b) reported that AMY activity was preferentially sensitive to highly emotional memories, especially those regarding positively valenced plays. In this context, it is interesting to note that increased AMY activity was also observed when people imagined positive future events relative to negative ones (Sharot 2011; Sharot et al. 2007b). It is possible that basketball fans are more apt to consider positive (versus negative) plays as more personally significant, and thus this greater self-relevance could lead to increased AMY involvement in the encoding of positive personal episodes (but see Northoff et al. 2009). Overall, these findings suggest that the involvement of the AMY in emotional memory encoding might be influenced by personal involvement during encoding of positive episodes, which identifies the social belonging of the individual.
There is also evidence pointing to the role of the AMY in encoding both positive and negative information about social others (e.g., faces, traits) (Said et al. 2009; Schiller et al. 2009; Vrtička et al. 2012). It is possible that, owing to its intrinsic motivational value and importance for survival, social relevance in stimuli is detected by the AMY independently of the valence and arousal dimensions (Harvey et al. 2007; Vrtička et al. 2012). These findings are consistent with the involvement of the AMY in social cognition and behavior, and with a more general role of this region in tracking subjective significance or relevance in the environment based on the current goals (Adolphs 2010; Cunningham and Brosch 2012).
Besides the AMY, extant evidence also suggests the involvement of medial/orbital PFC regions in encoding socially relevant stimuli (Gilron and Gutchess 2012; Gutchess et al. 2015; Harvey et al. 2007; Tsukiura 2012; Vrtička et al. 2012; Yaoi et al. 2015). For instance, increased activity in the medial PFC has been associated with both enhanced memory for the information encoded with reference to oneself – i.e., the self-reference effect (Greenwald and Banaji 1989; Gutchess et al. 2015; Macrae et al. 2004; Yaoi et al. 2015) – and with encoding of impressions of other people, based on face–behavior associations (Cassidy et al. 2013; Gilron and Gutchess 2012; Mitchell et al. 2004). In addition, activity in the medial orbitofrontal cortex (OFC) was associated with encoding of faces signaling positive social cues (Tsukiura and Cabeza 2008, 2011a, b) and scenes with a social content in general (Harvey et al. 2007; Vrtička et al. 2012). These findings are consistent with the role of the medial PFC regions in the representations of an individual’s own and of others’ minds (Northoff et al. 2006; Wagner et al. 2012), in addition to the encoding and integration of the subjective value of the stimuli (Delgado et al. 2016).
Moreover, greater activity in the medial OFC, together with enhanced functional connectivity with the HC, have been observed during successful encoding of socially rewarding stimuli (i.e., smiling and attractive faces) (Tsukiura and Cabeza 2008, 2011a). These findings emphasize the interplay between the reward-related brain regions (medial PFC/OFC) and the memory-related regions (HC) during the formation of memory for positive social stimuli. In contrast, the enhancing effect of socially negative signals (e.g., untrustworthy or unattractive faces) on memory was mediated by increased activity in and connectivity between the insula and the HC (see also Botzung et al. 2010a; Tsukiura and Cabeza 2011b; Tsukiura et al. 2013). Finally, activity in the anterior temporal lobe (ATL) structures along with connectivity with the AMY, MTL, and medial PFC regions have also been implicated in socio-emotional memory encoding, particularly with regard to its role in representing and storing personal identity information (Collins and Olson 2014; Olson et al. 2013; Spreng and Andrews-Hanna 2015; Tsukiura et al. 2010).
Retrieval of Socio-emotional Memories
The involvement of the AMY and the memory-related MTL regions, together with the engagement of the PFC, have been revealed during the retrieval of socio-emotional memories. First, increased AMY activity was linked to retrieval of faces that had previously been encoded with emotional descriptions of behaviors in general (Somerville et al. 2006), and with social fairness learned in the context of an economic game (Singer et al. 2004). Second, increased activity in, and interactions between the AMY and the HC have been identified for socially induced memory errors during retrieval (Edelson et al. 2011), thus suggesting that social interaction could have long-lasting effects on memory through AMY–HC mechanisms. Regarding PFC regions, the right medial PFC appears to mediate retrieval of social contexts, whereas the left medial PFC seems to underlie retrieval of self-generated contexts (Mano et al. 2011), thus revealing that dissociable regions within the PFC mediate social and self-referential processing during episodic memory retrieval. Finally, increased activity in the ATL along with its enhanced connectivity with the HC have been associated with the retrieval of personal identity information (Collins and Olson 2014; Tsukiura et al. 2008, 2011).
Functional Systems and Networks in Socio-emotional Memories
As discussed above, there is ample evidence supporting the idea that the encoding and retrieval of information with social relevance are subserved by the interaction of a host of regions broadly involved in emotion processing, social cognition, and memory processes. In integrating the differential contributions of brain regions to socioemotional memory, Tsukiura (2012) has posited that the impact of affective information on memory for faces may be mediated by the interaction between the two neural systems: affective (AMY, medial OFC, and insula) and memory (HC, fusiform face area [FFA]) systems (Fig. 5.2). In this context, the AMY may detect general emotional intensity in faces and work in concert with the medial OFC and the insula to process positive and negative signals from the faces respectively. These emotion processing regions may then modulate activity in the HC and the FFA, which have been implicated in encoding and retrieval of neutral faces (Prince et al. 2009), in enhancing memory for faces with affective information (Tsukiura 2012). This model is consistent with evidence that the memory-enhancing effect of emotion in general involves AMY-MTL interactions with top–down modulatory influences from the PFC regions (Dolcos et al. 2012), and with the emerging view of the AMY as a functional hub in the large-scale functional networks subserving various facets of social cognition and behavior in humans (Bickart et al. 2014). Moreover, evidence from the recent studies examining the dynamics of large-scale functional networks of the brain suggests that the complex processes involved in socio-emotional memory may be subserved by a network of regions whose activity represents a “default state” of the brain. These studies identified considerable overlap between the networks of regions typically activated in tasks involving emotion processing, social cognition, and episodic memory, and brain regions consistently activated when individuals are at rest – i.e., the default mode network (DMN) (Li et al. 2014; Schilbach et al. 2012; Spreng and Mar 2012). For instance, a recent meta-analysis which employed conjunction analyses to identify the common set of regions implicated consistently in studies of emotion processing, social cognition, and resting state confirmed the common involvement of a dorsal subregion of the medial PFC (dmPFC) along with the precuneus, suggesting that introspective processes may be a common denominator across the three processes (Schilbach et al. 2012). The dmPFC is considered part of the dorsomedial subsystem of the DMN (Spreng and Andrews-Hanna 2015), and more importantly, this region has been implicated in a broad range of social cognitive processes, including successful memory encoding of first impressions (Gilron and Gutchess 2012; Mitchell et al. 2004) and temporary storage and maintenance of information about others’ traits (Meyer and Lieberman 2012; Meyer et al. 2015).
A hypothetical model of the neural mechanisms underlying the effect of face-based affective signals on memory for faces. AMY amygdala, OFC orbitofrontal cortex, INS insular cortex, HIP hippocampus, FFA fusiform face area (Reproduced from Tsukiura 2012, with permission)
Interestingly, previous studies assessing resting-state connectivity within the DMN have shown that greater intrinsic functional connectivity among regions within this network is linked to more frequent retrieval of AMs in a social context (Yang et al. 2013) and higher scores in emotional intelligence (Takeuchi et al. 2013). Moreover, in support of these findings, and further extending the traditional view of the DMN, emerging evidence suggests that the AMY, through its high intrinsic and task-evoked connectivity with a subset of the DMN regions, might constitute a functional network involved in both maintenance of the brain’s default state and various socio-affective processes (i.e., the extended social-affective default network) (Amft et al. 2015; see also Bickart et al. 2014; Schilbach et al. 2008). Taken together, these findings identify the critical role of the DMN and associated regions in socio-emotional memory processes, and further demonstrate that variability in intrinsic connectivity among the DMN regions is linked to behavioral indices of emotional, social, and memory processes. Future research directly examining the relationships between task-evoked and intrinsic activity/connectivity in the (extended) DMN regions, in addition to behavioral performance, would be helpful in elucidating the role of large-scale networks in memory encoding and retrieval of information with social relevance.
In summary, recent neuroimaging studies examining the impact of social relevance on the emotional enhancing effect on memory emphasize the involvement of the AMY, HC, PFC, along with other regions involved in emotion processing and social cognition (e.g., insula, ATL), during both encoding and retrieval of socio-emotional memories. The engagement of the AMY/HC and the medial PFC was linked to memory for positive stimuli with increased personal or social relevance, whereas the HC and the insula were linked to memory for negative social stimuli, such as untrustworthy and unattractive faces. In addition, the AMY, medial PFC, and ATL were also associated with encoding and retrieval of social information, regardless of their valence, and these findings are consistent with the involvement of these regions in social cognition and behavior. Emerging evidence from the investigations of large-scale functional networks has also begun to reveal the link between the brain’s intrinsic functional architecture and various social-affective and cognitive processes subserving socio-emotional memory, with the dmPFC possibly serving as a “hub” of these processes. Therefore, memory encoding and retrieval of information with social relevance seem to involve complex interactions among both distinct and overlapping neural networks subserving basic emotion processing, social cognition, and memory processes, which may collectively allow us to integrate information from internal and external sources to adaptively navigate through the social landscape.
2.2 The Role of Emotion Regulation in the Impact of Emotion on Memory
The topic of emotion regulation (ER) has gained considerable interest, as the ability to cope adaptively with emotionally challenging situations is vital for physical and mental health (Gross 2008, 2015). Important progress has been made in understanding the neural correlates of ER associated with the immediate effects of engaging specific strategies, which are typically reflected in reduced emotional experiences, if down-regulated (e.g., Lieberman et al. 2011; Ray et al. 2010). However, less is known about the long-term effects of ER on memory for emotional events. These issues are discussed in the present section.
The two most widely studied ER strategies in brain imaging research are cognitive reappraisal, which involves attempts to change the meaning of stimuli/situations (by thinking for instance that the situation is not real), and expressive suppression, which involves attempts to decrease emotionally expressive behavior (Gross 2008). Extensive research suggests that reappraisal might have an advantage over suppression in reducing emotional experiences (Eippert et al. 2007; Kalokerinos et al. 2015; Olatunji et al. 2015), and a differential engagement of brain regions by these ER strategies (Goldin et al. 2008; Hermann et al. 2014). More recently, ER research has also considered the impact of attentional deployment strategies, such as focused attention (FA), which involves shifts in attention to or away from the emotional aspects of emotion eliciting stimuli or events (Gross 2008; Sheppes et al. 2014). The effectiveness of attentional deployment ER strategies has been confirmed by a recent meta-analysis (Webb et al. 2012), and the underlying neural mechanisms have also been investigated in some recent functional magnetic resonance imaging (fMRI) studies (Dorfel et al. 2014; Kanske et al. 2011; McRae et al. 2010). Recent studies also started clarifying how the engagement of various ER strategies affects the impact of emotion on memory (Ahn et al. 2015; Dillon et al. 2007; Richards and Gross 2000), and the underlying neural circuitry (Binder et al. 2012; Erk et al. 2010; Hayes et al. 2010). Better understanding of the impact of ER on emotional memories has important implications for understanding and treating affective disorders, which are characterized by an excessive focus on negative memories (Rubin et al. 2011; Williams and Moulds 2010) and emotional dysregulation (Gotlib and Joormann 2010; Mayberg 1997; Sheppes et al. 2015). Evidence emerging from the literature regarding the impact of ER on emotional memory is discussed below.
ER and Emotional Memory Encoding
Overall, available research reveals that engaging cognitive reappraisal during memory encoding enhances subsequent memory for the reappraised information (Dillon et al. 2007; Liu et al. 2015; Richards and Gross 2000), even after longer intervals (Ahn et al. 2015; Kim and Hamann 2012), whereas suppression tends to impair memory for the suppressed items (Richards and Gross 2000). One potential explanation that has been put forward to explain the beneficial effects of reappraisal on memory is linked to semantic elaboration processes involved during reappraisal, which may lead to a deeper level of encoding of the reappraised items (Dillon et al. 2007). Interestingly, the memory advantages of reappraisal have also been linked to increased bias toward enhancing positive memories (Levine et al. 2012). Regarding the neural correlates of reappraisal and suppression of emotional memory, recent neuroimaging studies point to a differential neural engagement of the HC and PFC for successfully encoded emotional items that were reappraised or suppressed (Binder et al. 2012; Hayes et al. 2010). Specifically, Hayes et al. (2010) showed that the memory enhancement by reappraisal was linked to increased engagement and co-activation of the HC and the left lateral PFC (Fig. 5.3), whereas Binder et al. (2012) revealed that the memory impairment by suppression was associated with decreased engagement and co-activation of the HC and the lateral PFC. These findings suggest that the core memory region (HC) and a higher-level cognitive processing region (PFC), along with their interplay during memory formation of “regulated” emotional items, are differently affected by the engagement of these ER strategies, which results in different effects on the subsequent emotional memory.
Stronger positive correlation between the left inferior frontal gyrus (IFG) and hippocampus (HC) when using reappraisal. Compared with passive viewing and suppression, reappraisal was associated with increased IFG–HC correlation for memory-related activity (Dm effect) as a function of the emotion regulation (ER) strategies used during encoding. Dm difference due to memory (Reproduced from Hayes et al. 2010, with permission)
ER and Emotional Memory Retrieval
Laboratory events. One of the most frequently studied strategies for inhibiting retrieval of laboratory-based episodic memories is thought suppression (Benoit et al. 2015; Depue et al. 2006, 2007; Kupper et al. 2014). It is referred to as an attentional deployment strategy (Sheppes and Gross 2012) and is typically studied using the think/no-think paradigm, during which participants first learn cue–target pair associations. They are then presented with the cues and instructed to suppress retrieval of the associated targets. In general, suppressing retrieval of unwanted emotionally neutral memories has been linked to top–down influences of the lateral PFC on the HC (Benoit et al. 2015; Gagnepain et al. 2014), and this effect was even stronger in participants who are better at suppressing memories (Benoit and Anderson 2012). More specifically, suppressing retrieval of emotional information has been linked to two-stage neural mechanisms, involving initial inhibition by cognitive control regions (inferior frontal gyrus) on visual regions (fusiform gyrus), followed by a subsequent inhibition by cognitive control regions (middle frontal gyrus) on emotional memory regions (AMY and HC) (Depue et al. 2007). This finding highlights the importance of considering the emotional content of unwanted memory and suggests that, although common mechanisms involving the PFC and the HC might play a role in suppressing both emotional and non-emotional memory retrieval, specific patterns of activity might be linked to suppressing the retrieval of emotional information.
Autobiographical Events. Investigation of the neural correlates underlying the impact of ER during autobiographical recollection is of particular relevance not only for everyday activities in healthy populations, but also for affective disorders. The very few studies investigating ER in the context of emotional AM retrieval focused particularly on reappraisal (Fabiansson et al. 2012; Holland and Kensinger 2013; Kross et al. 2009), and reported that it can lead to a decrease in emotional experience accompanying the retrieval of AMs. However, evidence regarding the associated neural correlates is not conclusive, particularly concerning the role of emotion-related brain regions. For instance, Holland and Kensinger (2013) reported increased activity in the lateral and the medial PFC, when participants down-regulated their emotional reactions using reappraisal during the (re)construction of AMs, but this was also associated with increased, rather than decreased, activity in emotion-related regions. The other two studies did not report specific activations related to reappraisal, which could be due to the use of only a few memories repeatedly presented across different conditions and/or to delayed instructions to regulate (i.e., after the engagement of memory retrieval) (Fabiansson et al. 2012; Holland and Kensinger 2013; Kross et al. 2009).
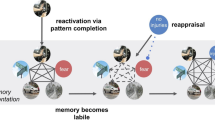
A recent study identified the neural underpinnings of FA, as an effective attentional deployment ER strategy during emotional autobiographical recollection (Denkova et al. 2015). This study revealed that focusing on the non-emotional contextual aspects (time, location, other persons present, etc.), and away from the emotional aspects of highly emotional personal memories led to decreased self-reported ratings of emotional experiences associated with those memories. This behavioral effect was accompanied by increased activity in the ventromedial PFC (vmPFC) and decreased activity in the AMY. Moreover, mediation analysis suggested that the vmPFC might play the role of a functional hub, integrating affective signals from the AMY and mediating their impact on the subjective re-experiencing of emotion according to the current retrieval focus (Fig. 5.4). Importantly, the finding regarding the role of the AMY (see also Dolcos et al. 2006a) challenges the view promoted by most of the functional neuroimaging studies of ER, which mainly emphasize top–down influences on the response of the AMY from the PFC regions involved in cognitive control, rather than reciprocal influences between these regions and the AMY.
Ventromedial PFC mediates the link between AMY and emotional ratings, when focusing away from the emotional aspects of autobiographical recollections. Mediation analysis identified a significant (p = 0.009) negative mediation effect of the ventromedial PFC (vmPFC) on the relation between the AMY and emotional ratings, while focusing on non-emotional aspects of personal memories, and a significant (p = 0.03) positive direct effect (path c′, X to Y controlling for M) between AMY and emotional ratings, when controlling for the influence of the vmPFC. Standardized coefficients and significance noted with asterisks are reported for each path. *p < 0.05; **p < 0.01; ***p < 0.001 (two-tailed); ns not significant (Reproduced from Denkova et al. 2015, with permission)
It is important to note that the manipulation used here involves simply switching the retrieval focus, whereas the main task remains the same (i.e., recollection of personal memories). This remarkably subtle manipulation is consistent with ER studies showing that the manipulation of attention focus either on emotional or non-emotional aspects during working memory tasks can alter the emotional responses (Thiruchselvam et al. 2011) and performance (Iordan 2016). Interestingly, this manipulation can also be linked to emerging evidence in the AM literature highlighting the beneficial impact of episodic specificity induction, which involves remembering events in great detail (Madore and Schacter 2016). This recent line of research revealed that focusing on very specific episodic details related to places and people may be beneficial to a range of cognitive tasks (Madore et al. 2015; McFarland et al. 2017), and to psychological well-being (Jing et al. 2016). These emerging lines of evidence are also consistent with previous studies showing that training people to be more concrete and specific in recollecting memories may have beneficial effects on depression (Raes et al. 2009; Watkins et al. 2009). The findings from the study by Denkova et al. (2015) extend such investigations to emotional AMs, by revealing that focusing on non-emotional aspects of AMs can influence the emotional (re)experience of such memories. These findings provide support for cognitive behavioral therapies involving ER training to “distract” from emotional aspects of personal memories, by focusing and elaborating on non-emotional contextual aspects of retrieved AMs, which in turn leads to reduced self-experienced emotions.
In summary, emerging evidence regarding the impact of ER on emotional memory suggests that the use of ER strategies influences both formation and retrieval of emotional memories at behavioral and neural levels. In particular, using different ER strategies while encoding or retrieving emotional information seems to result in differential effects on subsequent memory (enhanced versus impaired). More specifically, available evidence suggests that using cognitive reappraisal during encoding leads to enhanced emotional memory, whereas using suppression leads to impaired memory, and that reappraisal tends to favor positive memories. Using attentional deployment strategies, such FA, can have beneficial effects on the retrieval of emotional personal memories, because it can enhance the emotional impact of positive memories and reduce the impact of distressing ones. These behavioral effects are coupled with differential engagement of, and connectivity between, specific lateral and medial PFC regions, associated with cognitive control, and the MTL regions, associated with emotion (AMY) and memory (HC). Notably, the patterns of activity and connectivity linked to the engagement of ER are also consistent with the idea discussed in Sect. 5.1 that the PFC plays an indirect/mediating role in the formation of emotional memories, and further suggests that ER strategies might affect the basic MTL-related mechanisms underlying emotional memories through the involvement of the indirect PFC-related mechanisms.
2.3 The Impact of Emotion on Associative or Relational Memory
As highlighted in the previous section, there is abundant evidence that emotions play a critical modulatory role in episodic memory (Dolcos et al. 2012; Weymar and Hamm 2013). For instance, when emotional and neutral items (e.g., words, faces or scenes) are presented in isolation and memory is tested at a later time, emotional items are better remembered than neutral ones (e.g., Bradley et al. 1992; Dolcos et al. 2005; Weymar et al., 2009). This memory-enhancing effect of emotion has been attributed to the involvement of the AMY and its interaction with the memory-related MTL regions (HC, perirhinal and parahippocampal cortices) (Dolcos et al. 2012), which can also be influenced by social context and cognitive control (see also Liu et al. 2016). An important aspect of human memory, however, is the binding of contextual information (e.g., time, place, or associative cues), which constitutes many disparate features of a unified event (Davachi 2006; Ranganath 2010). This section discusses evidence regarding the impact of emotion on such associations, and the underlying neural mechanisms.
When experiencing a traffic accident, associated cues, such as the make of the car, the color of the surrounding vehicles, a radio song or a brake noise, that were part of such experience, are also able to reactivate strong emotional memories when exposed to them later. Understanding such fundamental associative mechanisms is crucial for everyday life, and is also relevant for clinical conditions. For instance, events associated with traumatizing contexts leading to post-traumatic stress disorder (PTSD) can involuntarily trigger vivid distressing memories in the form of intrusive thoughts, flashbacks, or nightmares (e.g., Flor and Nees 2014; Wilker et al. 2014), in which binding mechanisms seem to be compromised. Recent research has, therefore, focused on the effects of emotion on memory for items as a part of, or in relation to, other items (reviewed by Chiu et al. 2013). Both enhancing and impairing effects of emotion on memory binding have been observed and, as discussed below, these opposing effects have been explained in the context of different (but not mutually exclusive) views (Bisby et al. 2016; Chiu et al. 2013; Christianson 1992; Kensinger 2009; Mather and Sutherland 2011).
Behavioral Findings
It has long been known from the attention literature (Easterbrook 1959) that emotional arousal narrows attention to central cues, at the cost of peripheral, irrelevant cues. This prioritization of resources toward central aspects of information can result in better memory, compared with neutral peripheral or contextual information, which tends to be attended less (Buchanan and Adolphs 2002; Burke et al. 1992; Christianson 1992). For instance, memory is better for central aspects in a scenario (e.g., the weapon itself), but worse for other surrounding contextual details (e.g., about the perpetrator holding the weapon). Thus, emotional salience can weaken the integration of central aspects with peripheral contextual information into a unified memory representation (Mather 2007). This phenomenon is also referred to as emotion-induced memory trade-off (Kensinger 2009), in which memory is enhanced for emotional items but impaired for the associated neutral backgrounds.
Among the various accounts for these opposing effects of emotion on memory (e.g., Chiu et al. 2013; Kensinger 2009; Mather 2007), one suggests that emotional arousal has differential effects on memory binding depending on certain aspects related to an event. Mather (2007) proposes that, on the one hand, emotion can boost memory for within-binding features, but on the other hand, it impairs memory for between-object features, which do not take advantage of emotional arousal (see also Kensinger 2009, for relationship to the intrinsic versus extrinsic distinction). These assumptions were derived from a number of studies using either within-item or between-item binding tasks (for overview see Chiu et al. 2013). For instance, there is evidence that specific intrinsic features, such as color (e.g., D’Argembeau and Van der Linden 2004; Doerksen and Shimamura 2001) or location (Mather and Nesmith 2008; Nashiro and Mather 2011) of emotional stimuli, are better remembered than the same features associated with neutral events. By contrast, memory-imparing effects are observed for extrinsic aspects and contextual details of emotional stimuli, when emotional objects are accompanied by neutral scenes (Kensinger et al. 2007), when neutral stimuli (e.g., objects) are overlaid on emotional scenes (Touryan et al. 2007b), or when emotional scenes are associated with a colored frame (Rimmele et al. 2011).
Mather’s view regarding these effects has been recently postulated in the so-called arousal-biased competition (ABC) theory (Mather and Sutherland 2011), which highlights another important factor contributing to memory binding, namely, attentional priority. According to this model, emotional arousal enhances processing of whatever has the highest priority (because of bottom–up salience or top–down relevance), and impairs processing of information with lower priority (Mather and Sutherland 2011). Thus, emotional arousal may enhance associative memory for features of high-priority items (e.g., color or location of an item) and impair memory for neutral items, when presented at the same time (or almost the same time, see Sakaki et al. 2014) as emotional items. For instance, presentation of emotional images can enhance memory for preceding neutral objects when these objects receive high priority, but can impair memory when these objects are not prioritized (Sakaki et al. 2014).
This theory also addresses previous behavioral findings, in which better memory was found for neutral words presented in the context of emotional words (Guillet and Arndt 2009) or when neutral objects were placed on emotional scenes (e.g., Smith et al. 2004b, 2005; Ventura-Bort et al. 2016b), which should result in worse memory for the neutral event following the attention-narrowing (Easterbrook 1959) or between-binding concept (Mather 2007). In these studies, participants were instructed to learn word–word associations (Guillet and Arndt 2009) or to mentally integrate or connect objects and pictures at encoding (e.g., Smith et al. 2004b), which gives such associations high “attentional priority” that may facilitate binding of emotional and neutral information and diminish competition.
To summarize, behavioral findings point to both enhancing and impairing effects of emotion on memory binding, and these opposing effects can be explained by differences in attentional deployment toward, and prioritization of, emotional items and associated features (Easterbrook 1959; Kensinger 2009; Mather 2007; Mather and Sutherland 2011). The next subsections discuss evidence concerning the neural mechanisms of these effects.
Neural Findings: Encoding
At the neural level, the effect of emotion on associative memory remains largely unexplored. Available evidence from emotional memory research reviewed in the previous sections suggests that the effects of emotion on binding are mediated by the AMY (in interaction with regions supporting sensory and perceptual processing, e.g., Lang and Bradley 2010), which may modulate regions in the MTL (e.g., HC) that are important for relational representations, such the binding of item and context information (Davachi 2006; Ranganath 2010). Empirical evidence, however, has not been conclusive so far, partly because of differences in experimental designs and aspects tested (e.g., within versus between features) related to an event. In a study investigating the trade-off in memory, enhanced encoding activity was observed in emotional memory regions, such as the AMY, HC, and PFC regions (Waring and Kensinger 2011), when participants remembered emotional items better than neutral items presented in neutral scenes. Similarly, increased AMY activity was reported for emotional words that were subsequently remembered, compared with neutral words (Kensinger and Schacter 2006a). This is also in line with Dougal et al. (2007), who found AMY and MTL activation showing covariation with subsequent item memory for emotional words. However, such memory-enhancing effects in the AMY (and the MTL) were not observed for emotional events when associated contextual details (i.e., semantic judgment or color) were tested (Dougal et al. 2007; Kensinger and Schacter 2006a). This finding is consistent with evidence regarding the role of the AMY in emotional gist memory (e.g., Adolphs et al. 2005). Hippocampal activity, on the other hand, was related to correct memory of contextual details (Ranganath 2010) for both emotional and neutral events.
Together, these findings support the view that the AMY is specifically involved in memory-enhancing effects for aspects that are intrinsically linked to the emotional event itself (see also Thoresen et al. 2012), but not to extrinsic aspects. However, this prioritized processing of emotion-associated information, which could lead to better memory storage, is not restricted to intrinsic aspects of the emotional event. When participants are instructed to intentionally connect, for instance, objects and their locations with emotional or neutral background scenes, better memory is observed for the location of the objects when presented with emotional scenes, and this was associated with enhanced AMY activity during encoding (Luck et al. 2014). It remains to be clarified, however, whether or not such an emotion-enhancing effect is related to preferential processing due to high attentional prioritization, as suggested by Mather and Sutherland (2011). Extant evidence also points to a network consisting of the AMY and visual processing areas involved during encoding, depending on whether or not information is prioritized (Lee et al. 2014). This indicates that emotional arousal can in fact amplify visual processing of high-priority events, which may influence subsequent MTL-based binding into a long-lasting memory representation. This idea is consistent with event-related potential (ERP) findings of early enhanced perceptual and elaborative processing in conditioning studies using aversive electrical shocks (for review see Miskovic and Keil 2012) or emotional background pictures (Ventura-Bort et al. 2016a), as unconditioned stimuli, which provide support for the finding by Lee et al. (2014).
Neural Findings: Retrieval
Neural evidence for emotion effects on associative binding also comes from studies investigating memory retrieval. When measuring neural activity during a recognition memory test for words that had been encoded in the context of emotional and neutral sentences, enhanced activation was found for words from the emotional context in the AMY, HC, and PFC (Maratos et al. 2001), consistent with previous retrieval findings testing emotional item memory (e.g., Dolcos et al. 2005). Increased AMY activation during retrieval was also observed for recognized unpleasant scenes compared with forgotten ones, when cued by either an unpleasant or a neutral associated scene (Bisby et al. 2016). Moreover, increased retrieval activity was identified in the AMY, HC, and PFC when objects (and source information) from emotional background scenes were remembered (e.g., Smith et al. 2004b, 2005). Notably, both the AMY and the HC showed enhanced connectivity (Smith et al. 2006), during retrieval of objects that had been associated with emotional scenes during encoding, suggesting that the AMY and the HC support the retrieval of emotion-associated information from episodic memories when successfully integrated (see encoding instructions by Smith et al. 2004b, 2005, 2006; but see also Takashima et al. 2016 for HC involvement when emotional context memory is inaccurate).
The retrieval data from imaging studies, showing a memory-enhancing effect of emotion on associative binding, are also supported by a recent ERP study (Ventura-Bort et al. 2016b). Using a similar paradigm to the one used by Smith and colleagues (2004a, b, 2005, 2006), this study found larger parietal positivity during retrieval of neutral objects previously encoded with emotional, but not neutral, background scenes (Fig. 5.5a) (Smith et al. 2005; Ventura-Bort et al. 2016b). The observed parietal old/new effect (>400 ms after stimulus onset) has been related to recollection-based remembering (Rugg and Curran 2007; Weymar and Hamm 2013), suggesting that objects from emotional contexts were better recollected than those from neutral contexts. A similar ERP retrieval signature has been found for stimuli encoded under the threat of shock (Weymar et al. 2013, 2014). Specifically, when a painful electric shock was merely anticipated, signaled by the color of a stimulus (word; within-object binding), compared with a safety (no-shock) condition, words from the anticipatory threat condition were better recollected than words from the safety condition (Fig. 5.5b). Interestingly, the observed context effects on memory were most reliable for emotional events (Weymar et al. 2013, 2014), which fits with the ABC theory positing that arousal (e.g., threat of shock) during encoding may later facilitate the recollection of prioritized information (e.g., emotional salient words). These studies also show that a context that is bound to an event is not restricted to the actual experience, but also to its mere anticipation (see for example, cognitive contexts: Maren et al. 2013).
Retrieval-related ERP signature (old/new effect) of stimuli previously associated with emotional and neutral contexts. (a) Grand average ERP waveforms at a representative centro-parietal sensor cluster for correctly recognized objects that had been encoded in the context of an emotional background scene (red line) or neutral background scene (black line) and correctly classified new objects (grey line). the encoding sequence of the experiment is displayed upper left. In this experiment 144 objects were presented in the context of 144 background scenes (48 pleasant, 48 neutral, 48 unpleasant). Objects were presented first followed by the background scene, to avoid direct competition between emotional backgrounds and neutral objects. To facilitate memory binding, participants were instructed to imagine that the object is a part of the scene. The graph below illustrates the scalp topographies of the ERP difference (old minus new; 400–700 ms) separately for objects originally paired with emotional or neutral scenes (Adapted from Ventura-Bort et al. 2016b, with permission). (b) Grand average ERP waveforms at a representative centro-parietal sensor cluster for correctly remembered words encoded in a font color (see encoding sequence upper left) that signaled threat of shock (red line) or safety (black line) and correctly classified new words (gray line). The graph below illustrates the scalp topographies of the ERP difference (old minus new; 500–700 ms) separately for emotional words originally encoded under threat or safety (Adapted from Weymar et al. 2013, with permission)
The study by Ventura-Bort et al. (2016b) points to an important factor that may have an impact on memory binding – the retention interval. Specifically, enhanced ERP old/new effects were observed for objects from emotional contexts when tested 1 week after initial encoding, but not in previous studies using immediate or 24-h delayed testing (e.g., Jaeger et al. 2009; Jaeger and Rugg 2012; Smith et al. 2004a). A number of studies have demonstrated that longer retention periods facilitate consolidation processes, resulting in memory-enhancing effects for highly arousing emotional events, compared with less arousing neutral ones (e.g., Dolcos et al. 2005; LaBar and Cabeza 2006; Ritchey et al. 2008; Weymar and Hamm 2013; Yonelinas and Ritchey 2015). Thus, emotion may facilitate associative binding after longer delays, which is also supported by recent findings from Pierce and Kensinger (2011), using emotional and neutral word pairs. Hence, future research should also consider retention interval as an important factor when examining emotion effects on associative binding.
An Emerging Alternative Account: The Role of “Unitization”
Another interesting explanation for the opposing effects of emotion on memory binding has recently been proposed by Chiu et al. (2013), who suggest that enhancing or impairing effects might result from the way in which items are represented in the memory (unitized or not). This view was derived from a growing body of literature pointing to distinct MTL regions that support item (perirhinal cortex) and relational (parahippocampal cortex/HC) memory representations (e.g., Cohen et al. 1999; Davachi 2006; Ranganath 2010). Recent fMRI studies (Haskins et al. 2008; Staresina and Davachi 2010) revealed that the perirhinal cortex may also contribute to simpler forms of associative learning, based on unitization, which involves representation of separate components as a single unit (Graf and Schacter 1989), such as the association between an object and its color or location. Therefore, item memory and unitized items can be mediated by similar mechanisms, unlike memory representations that involve more complex associations (e.g., temporal, spatial, situational), which rely on HC-dependent mechanisms (Konkel and Cohen 2009). This finding is also supported by recent ERP data showing differential ERP old/new effects related to familiarity and recollection for highly unitized and less unitized associations (Diana et al. 2011).
Based on the extant evidence, Chiu et al. (2013) suggest that emotion might lead to enhancement of “item-only” or unitized memory representations, but it impairs more complex HC-dependent relational representations. Therefore, emotion leads to memory enhancement in tasks where the nature of the item–source association is more intrinsic (e.g., color or location) and allows a single representation, but it produces impairments when complex HC-dependent associations are required, for instance, in tasks using object–background associations (Kensinger et al. 2007) or item pairs (Mather and Knight 2008). However, when separate objects or features receive equivalent attention, for instance, by instructions to mentally integrate or connect certain items (e.g., Smith et al. 2004a, b; Ventura-Bort et al. 2016b), this can facilitate unitized processing and later remembering. A recent study by Murray and Kensinger (2014) using emotional and neutral word pairs found that such integrative mental imagery relies more on amygdalar and parietal processing, and less on frontal and hippocampal processing, indicating that unitized emotional associations may be less frequently mediated by HC-dependent mechanisms (see also Ventura-Bort et al. 2016b for a discussion on the involvement of familiarity-based recognition and valence). However, more research is required to substantiate this view, as to our knowledge it has not been empirically tested using systematic manipulations of unitization.
To summarize, both enhancing and impairing effects of emotion on memory binding have been observed that can be explained by differences in attentional deployment toward emotional items and associated features during encoding (Easterbrook 1959; Kensinger 2009; Mather 2007; Mather and Sutherland 2011) that are processed in a unitized or complex manner (Chiu et al. 2013), and by differences in consolidation (retention retrieval) (Pierce and Kensinger 2011; Ventura-Bort et al. 2016b). The extant literature (e.g., Kensinger et al. 2011; Luck et al. 2014; Thoresen et al. 2012) shows that emotion may facilitate memory for contextual details (e.g., color or location) or other surrounding stimuli when viewed as intrinsic or united to the emotional event, via involvement of the AMY, which interacts with perceptual regions (see also Mather et al. 2015 for the possible role of the locus coeruleus) to promote binding in the MTL regions (Davachi 2006; Ranganath 2010). When context information is successfully bound to the emotional event, enhanced AMY and MTL activation (Smith et al. 2006), along with enhanced parietal electrophysiological processing (Ventura-Bort et al. 2016b) have been observed during memory retrieval of emotion-associated information.
3 Conclusions and Future Directions
The overarching goal of the present review was to discuss findings from brain imaging studies investigating the neural correlates of encoding and retrieving emotional memories, and how they are modulated by factors linked to social information, ER, and associative/relational memory processes. The available evidence points to the involvement of and interaction between direct MTL-based (i.e., AMY–MTL) and indirect (i.e., PFC, parietal) mechanisms for the memory-enhancing effect of emotion (Fig. 5.6), which seem to be differentially modulated by basic affective properties (arousal and valence) and by the three factors discussed here.
Diagram summarizing the neural correlates of the memory-enhancing effect of emotion, as resulted from brain imaging studies. Two main basic mechanisms involved in the memory-enhancing effect of emotion were identified: one based in the medial temporal lobe (MTL;(AMY and MTL memory system = HC and associated parahippocampal cortices) and the other also involving non-MTL regions, such as the medial and dorso-/ventrolateral prefrontal cortex (mPFC and dlPFC/vlPFC respectively), among others (e.g., parietal cortex). The AMY and the MTL memory regions interact through direct/automatic neurohormonal mechanisms that contribute to the memory enhancement effect of emotion (bottom–up mechanism), whereas PFC is part of a mechanism that has an indirect/mediated involvement in emotional memories, by enhancing strategic, semantic, working memory, and attentional processes (top–down mechanism). Moreover, investigation of emotional memory for social aspects identified valence-specific engagement of other brain regions that contribute to enhanced emotional memories in social contexts – i.e., memory for socially relevant information involves activity in and interactions between the medial OFC and the MTL in the case of items with positive connotations and between the insula and the MTL for items with negative connotations. Finally, investigation of the impact of ER on emotional memory identified bidirectional relations between the MTL and the PFC regions associated with specific emotion regulation strategies, involving the lateral/medial PFC in top–down modulation of the AMY–MTL mechanisms in emotional memory encoding and retrieval, and the AMY signaling the medial PFC the need to exert control over emotional stimuli, resulting in reduced emotional experience overall, during autobiographical retrieval (Adapted from Dolcos et al. 2011, 2012 and Denkova et al. 2015, with permission)
Regarding the role of social information, available evidence suggests that when such complex dimensions are taken into consideration, a spatially distributed yet functionally interconnected network of brain regions is engaged in concert with the bottom–up AMY–MTL mechanisms involved in emotional memory. The medial PFC seems to play an important role, possibly because of the need to integrate social and affective information from both external and internal sources. The involvement of other brain regions, such as the orbital PFC and the insula, has also been implicated in the enhancement of memory for socially positive and negative information respectively. Furthermore, more recently, emerging evidence linking these social–affective regions with the brain’s intrinsic functional architecture has started to paint a more comprehensive picture of the possible role of large-scale functional networks in socio-emotional memory.
Regarding the role of ER, emerging evidence identified the effect of specific ER strategies that can have an impact on the encoding and retrieval of emotional memories, for both laboratory micro-events and real-life autobiographical episodes. Overall, the evidence reviewed here suggests that the use of different ER strategies could lead to different patterns of emotional experience and memory (increased versus decreased), which are linked to differential interactions between the MTL and the PFC regions. For instance, reappraisal during encoding was associated with enhanced, whereas suppression was associated with impaired, subsequent emotional memory. These opposing effects are linked to differential activity in and connectivity between the HC and the lateral PFC (increased versus decreased respectively). Emerging evidence also highlights the efficacy of thought suppression and attentional deployment strategies in inhibiting recollection of unwanted memories and in modulating emotional re-experiencing of emotional personal events respectively. The successful suppression of unwanted emotional memories has been linked to top–down modulatory effects from the PFC on the AMY and the HC, whereas effectively switching attentional focus away from emotions during AM recollections has been linked to the central role of the vmPFC in integrating affective signals from the AMY and reducing the subjective re-experiencing of emotion.
Finally, available evidence regarding associative/relational memories points to opposing effects of emotion, which may be explained by differential effects of prioritization and to a possible role of operations allowing unitization of information. Emotion may enhance or impair memory for contextual details depending on whether such information is encoded as intrinsic/united or extrinsic to the emotional event, respectively (Chiu et al. 2013; Kensinger 2009; Murray and Kensinger 2013), possibly because of prioritized attentional processing for both item and contextual details (Mather and Sutherland 2011). Neuroimaging evidence regarding the enhancing effects of emotion in associative/relational memory points to the role of the AMY in influencing the activity of sensory/perceptual regions, the locus coeruleus, and memory-related MTL regions in facilitating memory-binding during encoding. ERP evidence has also identified enhanced parietal positivity during retrieval of emotion-associated information, which might point to the involvement of parietal brain regions (Vilberg & Rugg 2009; Weymar et al. 2011). Consolidation also seems to facilitate associative binding, as demonstrated by evidence showing enhanced ERP effects associated with recognition memory after longer versus shorter consolidation periods/retention intervals. Finally, opposing effects of emotion on memory binding may also be explained by the complex mechanisms involving the enhancement of item-based memory representations versus impairment of HC-dependent relational memory representations (Chiu et al. 2013). Overall, these findings highlight the important role of the AMY and the MTL regions and point to electrophysiological evidence indexing memory-enhancing effects of emotion-associated information.
Despite a rapidly growing body of literature elucidating the mechanisms underlying emotional memory in the context of these emerging directions, a number of issues are still unclear. Regarding the role of social information, one issue to be clarified in future investigations concerns the link with the other two factors emphasized in the present review. First, the mechanisms underlying the impact of ER on memory processes in a social context remain unclear, despite evidence from previous studies showing that the engagement of various cognitive control strategies modulates the impact of emotion on memory (e.g., Ahn et al. 2015; Dillon et al. 2007; Richards and Gross 2000) and the associated neural correlates (Binder et al. 2012; Erk et al. 2010; Hayes et al. 2010) (see Sect. 5.2.2). This is surprising, given the evidence that the ability to control and experience emotions appropriately is essential in successful social interaction, and has been associated with measures of social support and personal likeability (Gross 2002). A few behavioral studies have investigated the impact of ER on memory in a social context. For instance, Richards et al. (2003) showed that reappraisal and suppression were associated with enhanced memory for conversation content versus emotional reactions in the context of discussing relationship conflicts respectively. Also, Pasupathi (2003) found that talking about negative AMs was associated with a decreased negative emotional experience, particularly when the listeners agreed with the participants’ view of what had happened. More recently, it has been shown that social regulation (e.g., holding someone’s hand during an emotional experience) can lead to decreased memory for negative images (Flores and Berenbaum 2017). Complementing these findings, recent neuroimaging evidence suggests that the regulation of emotions experienced through interactions with others may be subserved by dissociable neural networks, compared with those involved in regulating emotions experienced individually (for a review see Grecucci et al. 2015). Thus, clarification of the mechanisms involved in regulating how we experience and interpret our own emotions and those of others, and how these processes influence our remembrance of social experience, may allow us to better understand the complex nature of human social interaction.
Second, it also remains unclear how association and integration of complex social information from different sources may influence the neural mechanisms underlying emotional memory for social context. Although most studies on social cognition to date have focused on examining the associated neural mechanisms using mainly facial stimuli (Adolphs 2010; Mende-Siedlecki et al. 2013; Tsukiura 2012), it is not clear whether similar or dissociable mechanisms are engaged when processing information about others from their faces only versus in the context of other information available (e.g., body language, social setting). When interacting with others in real-life situations, we rarely encounter their faces (or bodies) in isolation, but rather as an integrated whole (de Gelder et al. 2015). Therefore, to fully elucidate the mechanisms associated with memory processes in a social context, it is of particular importance to investigate these in the context of real-life social interactions.
A few behavioral studies investigating the impact of social contextual information on subsequent memory for faces have provided evidence for the role of social/affective context in face memory. For instance, Van den Stock and de Gelder (2012) presented compound stimuli consisting of faces with either whole-body expressions or scenes (categorized as fearful, happy, or neutral), and asked participants to remember only faces, regardless of contextual information. The authors identified poorer subsequent memory for faces that had been encoded with either emotional bodily expressions or scenes than those encoded with neutral ones, suggesting that social/emotional context hampers subsequent recognition of faces (Van den Stock and de Gelder 2012). On the other hand, in the Mattarozzi et al. (2015) study, participants were first presented with faces embedded in a newspaper layout, with a headline illustrating an action (negative, positive, or neutral) performed by the person depicted, and were later shown a series of faces to judge whether or not they had previously seen the faces. The authors found that faces encoded in emotional contexts were more likely to be remembered with episodic details, whereas those encoded in neutral context tended to be judged only as familiar (Mattarozzi et al. 2015). Although seemingly inconsistent findings from these two studies may, in part, be driven by the differences in task designs (e.g., instructed versus incidental encoding, nonverbal versus verbal context), these findings provide evidence that contextual information can have an impact on subsequent memory for faces. Future neuroimaging research addressing these issues would be useful to clarify the role of social context in memory encoding and retrieval of others’ faces.
Regarding the role of ER on emotional memory, given that this research is still in its infancy, several issues remain poorly understood. For instance, further investigations are needed to clarify the impact of various ER strategies that can be used to cope with emotional memories. Given that emerging research highlights the effectiveness of attentional deployment strategies (distraction, FA) (Denkova et al. 2015; Depue et al. 2007), future investigations of the impact of ER on memory should include more elaborate practices and training that rely on attentional control. Mindfulness, which involves attention to the present moment without emotional reactivity and elaboration, is such a technique that could be used as a training method to reduce the impact of distressing emotional memories. This line of future research seems promising, as there is preliminary evidence showing that mindfulness training, which has been associated with increased well-being, can have an impact on the neural correlates of emotional processing (Allen et al. 2012; Desbordes et al. 2012) and increase the specificity of AMs in healthy (Heeren et al. 2009) and formerly depressed (Williams et al. 2000) individuals. Second, because most studies focused on the impact of instructed ER, little is known about the role of habitual, spontaneous, or uninstructed use of ER strategies in emotional memories and the associated neural correlates (for a review, see Katsumi et al. 2017). Available evidence points to possible sex differences – e.g., engaging suppression as a habitual coping strategy in women may be inefficient and may come at a cost, as it is associated with overall increased retrieval of negative AMs and post-retrieval negative affect (Denkova et al. 2012). However, the neural mechanisms of these effects are not clear. Thus, further research is needed to advance our understanding of the neural correlates underlying the impact of the spontaneous use of ER strategies on emotional memory.
Regarding associative/relational emotional memory, the neural mechanisms of associative binding of emotional and neutral information are still not well understood. The following four issues are particularly unclear.
First, the engagement of and interaction between the MTL (AMY, HC) and the PFC regions when emotional and neutral information are successfully integrated into a unified representation or not (e.g., Murray and Kensinger 2014; Okada et al. 2011) needs further clarification. Recent data indicate that the AMY is involved not only during facilitated but also during disruptive encoding processes (Okada et al. 2011), coupled with reduced PFC and MTL activation (Murray and Kensinger 2014), but the mechanisms dissociating these opposing effects are not clear.
Second, the neural basis of ABC has not been sufficiently described for memory-binding processes, which is, in fact, difficult to test in an experimental environment. A novel approach that may help to overcome this issue is to use steady-state visually-evoked potentials, which allow time–frequency analyses (by frequency-tagging), when different stimuli are presented at the same time (Wieser and Keil 2014). This approach provides a continuous measure of the visual resource allocation to specific stimuli amid competing stimuli and thus makes it suitable to compare emotional and neutral information with regard to competition and memory binding.
Third, although arousal may influence associative memory to a greater extent, unpleasant valence can also influence the retention of associative information (Pierce and Kensinger 2011), which supports some previous findings showing a memory advantage for unpleasant stimuli over pleasant ones (Weymar et al. 2011). Valence may, therefore, be considered an important factor when examining the effect of emotion on associative processes.
Fourth, related to the third issue, the role of stress in neural memory binding has been less frequently explored. Recent lines of research (e.g., Hermans et al. 2014; Joels et al. 2011; van Ast et al. 2013; Vogel et al. 2016) emphasize two different corticosteroid actions critical for memory processes. On the one hand, to promote survival, shortly after stress, nongenomic corticosteroids interact with noradrenaline to enable immediate fight–flight responses and to focus attention on gist-based information at the cost of remembering contextual details. On the other hand, some hours after stress, slower, long-lasting gene-mediated corticosteroid actions are thought to facilitate restorative processes, involving enhanced executive control (Hermans et al. 2014) and remembering events in a more contextual manner (van Ast et al. 2013). Future studies investigating opposing effects of emotion on associative binding may consider these time-dependent stress actions (see also Dolcos 2014).
Finally, aside from these topic-related open questions, an important issue concerns the role of individual differences in emotional memory. As briefly discussed in the footnotes, investigation of how the neural mechanisms involved in memory-enhancing effects of emotion may vary between individuals (e.g., linked to personality, sex, and age; see also Dolcos et al., 2017) allows a better understanding of the underlying mechanism in both normal functioning and in clinical conditions, such as affective disorders, which are typically characterized by dysfunctional emotional memory. In this context, it is important to emphasize the need for future studies considering how individual differences in cognitive abilities, in addition to differences influencing emotion processing, modulate the impact of emotion on memory and the underlying neural mechanisms. Although investigation of individual differences in emotion-related dimensions seems promising to explain differences in the heightened sensitivity to emotional information and hence, enhanced encoding of emotional information (e.g., Haas and Canli 2008), little is known about the impact of individual differences in the cognitive domain. The latter may be of particular importance regarding the implementation and impact of ER strategies during memory formation. For instance, there is evidence that higher working memory capacity is associated with better suppression of negative emotions (Schmeichel et al. 2008) and that working memory training may lead to better emotion control through enhanced efficiency of the frontoparietal network (Schweizer et al. 2013). Hence, this seems a promising research avenue.
In sum, the evidence reviewed here shed light on the mechanisms through which emotion influences how we remember or forget certain information in different contexts. Emphasis is on evidence from brain imaging investigations regarding three emerging research directions: The role of social information in emotional memory; The role of emotion regulation in the impact of emotion on memory; and The impact of emotion on associative or relational memory. This emerging evidence also opens up new avenues for future research, including intervention studies aimed at facilitating adaptive emotional memory encoding and retrieval, both in healthy and pathological functioning.
Notes
- 1.
It is important to note that besides the emerging aspects discussed here, aspects emphasizing the importance of understanding individual differences in the research of emotional memory are also important. However, because of space limitations, these aspects are not the focus of the present discussion (but see Dolcos et al. 2012, 2017; Katsumi et al. 2017). It should be mentioned, however, that investigation of how the neural mechanisms involved in the memory-enhancing effects of emotion vary among individuals allows a better understanding of the underlying mechanisms in both normal functioning and in clinical conditions, such as affective disorders, which are typically characterized by dysfunctional emotional memory. Thus, it is crucial to understand the factors that influence individual variation in the engagement of and interactions among brain regions during the encoding and retrieval of emotional memories. Such factors could be linked to personality, sex, and age-related differences, among others, in basic emotional responses and in emotion regulation (reviewed in Dolcos et al. 2017).
- 2.
As previously noted, this nomenclature does not refer to the notion of neuronal plasticity sensu stricto, as is generally accepted in neuroscience linked to memory processes (see Martin et al. 2000), but rather to the place where plasticity is expected in relation to processing, leading to increased emotional memories, as predicted by the two views. Both the modulation and the plasticity hypotheses predict neural plasticity, but although the former view predicts that the AMY influences plasticity occurring in other brain regions, the latter view posits that the AMY itself is a main site of plasticity that contributes to the memory-enhancing effect of emotion.
- 3.
Arousal refers to a continuum that varies from calm to excitement, whereas valence refers to a continuum that varies from pleasant to unpleasant, with neutral as an intermediate value (for methods of assessing these dimensions, see Bradley and Lang 1994).
- 4.
Recollection-based retrieval refers to remembering specific contextual details of an event (e.g., about the time and place of its occurrence), whereas familiarity-based retrieval refers to only knowing that certain events occurred, without retrieving specific contextual details (Tulving 1985).
References
Addis DR, Moscovitch M, Crawley AP, McAndrews MP (2004) Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus 14(6):752–762
Adolphs R (2010) What does the amygdala contribute to social cognition? Ann N Y Acad Sci 1191(1):42–61
Adolphs R, Cahill L, Schul R, Babinsky R (1997) Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem 4(3):291–300
Adolphs R, Tranel D, Denburg N (2000) Impaired emotional declarative memory following unilateral amygdala damage. Learn Mem 7(3):180–186
Adolphs R, Tranel D, Buchanan TW (2005) Amygdala damage impairs emotional memory for gist but not details of complex stimuli. Nat Neurosci 8(4):512–518
Ahn HM, Kim SA, Hwang IJ, Jeong JW, Kim HT, Hamann S, Kim SH (2015) The effect of cognitive reappraisal on long-term emotional experience and emotional memory. J Neuropsychol 9(1):64–76
Allen M, Dietz M, Blair KS, van Beek M, Rees G, Vestergaard-Poulsen P, Lutz A, Roepstorff A (2012) Cognitive-affective neural plasticity following active-controlled mindfulness intervention. J Neurosci 32(44):15601–15610
Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB (2015) Definition and characterization of an extended social-affective default network. Brain Struct Funct 220(2):1031–1049
Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JDE (2004) Neural systems underlying the suppression of unwanted memories. Science 303(5655):232–235
Beadle JN, Tranel D, Cohen NJ, Duff M (2013) Empathy in hippocampal amnesia. Front Psychol 4:69
Benoit RG, Anderson MC (2012) Opposing mechanisms support the voluntary forgetting of unwanted memories. Neuron 76(2):450–460
Benoit RG, Hulbert JC, Huddleston E, Anderson MC (2015) Adaptive top-down suppression of hippocampal activity and the purging of intrusive memories from consciousness. J Cogn Neurosci 27(1):96–111
Bickart KC, Dickerson BC, Barrett LF (2014) The amygdala as a hub in brain networks that support social life. Neuropsychologia 63:235–248
Binder J, de Quervain DJ, Friese M, Luechinger R, Boesiger P, Rasch B (2012) Emotion suppression reduces hippocampal activity during successful memory encoding. NeuroImage 63(1):525–532
Bisby JA, Horner AJ, Horlyck LD, Burgess N (2016) Opposing effects of negative emotion on amygdalar and hippocampal memory for items and associations. Soc Cogn Affect Neurosci 11(6):981–990
Botzung A, LaBar KS, Kragel P, Miles A, Rubin DC (2010a) Component neural systems for the creation of emotional memories during free viewing of a complex, real-world event. Front Hum Neurosci 4:34
Botzung A, Rubin DC, Miles A, Cabeza R, Labar KS (2010b) Mental hoop diaries: emotional memories of a college basketball game in rival fans. J Neurosci 30(6):2130–2137
Bradley MM, Lang PJ (1994) Measuring emotion: i self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25(1):49–59
Bradley MM, Greenwald MK, Petry MC, Lang PJ (1992) Remembering pictures: pleasure and arousal in memory. J Exp Psychol Learn Mem Cogn 18(2):379–390
Buchanan TW (2007) Retrieval of emotional memories. Psychol Bull 133(5):761–779
Buchanan TW, Adolphs R (2002) The role of the human amygdala in emotional modulation of long-term declarative memory. In: Moore SC, Oaksford M (eds) Emotional cognition: from brain to behaviour, Advances in consciousness research, vol. 44. John Benjamins Publishing Company, Amsterdam, pp 9–34
Burke A, Heuer F, Reisberg D (1992) Remembering emotional events. Mem Cogn 20(3):277–290
Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC (2004) Brain activity during episodic retrieval of autobiographical and laboratory events: aN fMRI study using a novel photo paradigm. J Cogn Neurosci 16(9):1583–1594
Cahill L, Prins B, Weber M, McGaugh JL (1994) Beta-adrenergic activation and memory for emotional events. Nature 371(6499):702–704
Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL (1995) The amygdala and emotional memory. Nature 377(6547):295–296
Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL (1996) Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci 93(15):8016–8021
Cahill L, Weinberger NM, Roozendaal B, McGaugh JL (1999) Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron 23(2):227–228
Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L (2000) Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20(19):RC99
Cassidy BS, Leshikar ED, Shih JY, Aizenman A, Gutchess AH (2013) Valence-based age differences in medial prefrontal activity during impression formation. Soc Neurosci 8(5):462–473
Chiu Y-C, Dolcos F, Gonsalves BD, Cohen NJ (2013) On opposing effects of emotion on contextual or relational memory. Front Psychol 4:103
Christianson SA (1992) Emotional stress and eyewitness memory: a critical review. Psychol Bull 112(2):284–309
Ciaramelli E, Bernardi F, Moscovitch M (2013) Individualized Theory of Mind (iToM): when memory modulates empathy. Front Psychol 4:4
Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C (1999) Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus 9(1):83–98
Collins JA, Olson IR (2014) Beyond the FIthe role of the ventral anterior temporal lobes in face processing. Neuropsychologia 61:65–79
Cunningham WA, Brosch T (2012) Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr Dir Psychol Sci 21(1):54–59
Cunningham WA, Van Bavel JJ, Johnsen IR (2008) Affective flexibility: evaluative processing goals shape amygdala activity. Psychol Sci 19(2):152–160
D’Argembeau A, Van der Linden M (2004) Influence of affective meaning on memory for contextual information. Emotion 4(2):173–188
D’Esposito M, Zarahn E, Aguirre GK (1999) Event-related functional MRI: implications for cognitive psychology. Psychol Bull 125(1):155–164
D’Esposito M, Postle BR, Rypma B (2000) Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res 133(1):3–11
Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC (2008) The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex 18(1):217–229
Davachi L (2006) Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16(6):693–700
Davidson RJ, Irwin W (1999) The functional neuroanatomy of emotion and affective style. Trend Cogn Sci 3(1):11–21
De Gelder B, de Borst AW, Watson R (2015) The perception of emotion in body expressions. Wiley Interdiscip Rev Cogn Sci 6(2):149–158
Delgado MR, Olsson A, Phelps EA (2006) Extending animal models of fear conditioning to humans. Biol Psychol 73(1):39–48
Delgado MR, Beer JS, Fellows LK, Huettel SA, Platt ML, Quirk GJ, Schiller D (2016) Viewpoints: dialogues on the functional role of the ventromedial prefrontal cortex. Nat Neurosci 19(12):1545–1552
Denkova E, Botzung A, Scheiber C, Manning L (2006) Implicit emotion during recollection of past events: a nonverbal fMRI study. Brain Res 1078(1):143–150
Denkova E, Dolcos S, Dolcos F (2012) Reliving emotional personal memories: affective biases linked to personality and sex-related differences. Emotion 12(3):515–528
Denkova E, Dolcos S, Dolcos F (2013) The effect of retrieval focus and emotional valence on the medial temporal lobe activity during autobiographical recollection. Front Behav Neurosci 7:109
Denkova E, Dolcos S, Dolcos F (2015) Neural correlates of ‘distracting’ from emotion during autobiographical recollection. Soc Cogn Affect Neurosci 10(2):219–230
Depue BE, Banich MT, Curran T (2006) Suppression of emotional and nonemotional content in memory: effects of repetition on cognitive control. Psychol Sci 17(5):441–447
Depue BE, Curran T, Banich MT (2007) Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 317(5835):215–219
Desbordes G, Negi LT, Pace TW, Wallace BA, Raison CL, Schwartz EL (2012) Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front Hum Neurosci 6:292
Diana RA, Van den Boom W, Yonelinas AP, Ranganath C (2011) ERP correlates of source memory: unitized source information increases familiarity-based retrieval. Brain Res 1367:278–286
Dillon DG, Ritchey M, Johnson BD, LaBar KS (2007) Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion 7(2):354–365
Doerksen S, Shimamura AP (2001) Source memory enhancement for emotional words. Emotion 1(1):5–11
Dolan RJ (2007) The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond Ser B Biol Sci 362(1481):787–799
Dolan RJ, Lane R, Chua P, Fletcher P (2000) Dissociable temporal lobe activations during emotional episodic memory retrieval. NeuroImage 11(3):203–209
Dolcos F (2014) The fast and the slow sides of cortisol’s effects on emotional interference and sustained attention. Front Neurosci 8:268
Dolcos F, Denkova E (2008) Neural correlates of encoding emotional memories: a review of functional neuroimaging evidence. Cell Sci Rev 5:78–122
Dolcos F, LaBar KS, Cabeza R (2004a) Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage 23(1):64–74
Dolcos F, LaBar KS, Cabeza R (2004b) Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42(5):855–863
Dolcos F, LaBar KS, Cabeza R (2005) Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci 102(7):2626–2631
Dolcos F, Kragel P, Wang L, McCarthy G (2006a) Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport 17(15):1591–1594
Dolcos F, LaBar KS, Cabeza R (2006b) The memory-enhancing effect of emotion: functional neuroimaging evidence. In: Uttl B, Ohta N, Siegenthaler AL (eds) Memory and emotion: interdisciplinary perspectives. Blackwell Publishing, Malden, pp 105–134
Dolcos F, Iordan AD, Dolcos S (2011) Neural correlates of emotion-cognition interactions: a review of evidence from brain imaging investigations. J Cogn Psychol 23(6):669–694
Dolcos F, Denkova E, Dolcos S (2012) Neural correlates of emotional memories: a review of evidence from brain imaging studies. Psychologia 55(2):80–111
Dolcos F, Katsumi Y, Weymar M, Moore M, Tsukiura T, Dolcos S (2017) Emerging directions in emotional episodic memory. Front Psychol. 8:1867
Donaldson DI, Buckner RL (2001) Effective paradigm design. In: Jezzard P, Matthews PM, Smith SM (eds) Functional MRI: an introduction to methods. Oxford University Press, New York, pp 177–198
Dorfel D, Lamke JP, Hummel F, Wagner U, Erk S, Walter H (2014) Common and differential neural networks of emotion regulation by detachment, reinterpretation, distraction, and expressive suppression: a comparative fMRI investigation. NeuroImage 101:298–309
Dougal S, Phelps EA, Davachi L (2007) The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cogn Affect Behav Neurosci 7(3):233–242
Easterbrook JA (1959) The effect of emotion on cue utilization and the organization of behavior. Psychol Rev 66(3):183–201
Edelson M, Sharot T, Dolan RJ, Dudai Y (2011) Following the crowd: brain substrates of long-term memory conformity. Science 333(6038):108–111
Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S (2007) Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp 28(5):409–423
Erk S, Martin S, Walter H (2005) Emotional context during encoding of neutral items modulates brain activation not only during encoding but also during recognition. NeuroImage 26(3):829–838
Erk S, von Kalckreuth A, Walter H (2010) Neural long-term effects of emotion regulation on episodic memory processes. Neuropsychologia 48(4):989–996
Fabiansson EC, Denson TF, Moulds ML, Grisham JR, Schira MM (2012) Don’t look back in anger: neural correlates of reappraisal, analytical rumination, and angry rumination during recall of an anger-inducing autobiographical memory. NeuroImage 59(3):2974–2981
Fanselow MS, LeDoux JE (1999) Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23(2):229–232
Flor H, Nees F (2014) Learning, memory and brain plasticity in posttraumatic stress disorder: context matters. Restor Neurol Neurosci 32(1):95–102
Flores LE, Berenbaum H (2017) The effect of the social regulation of emotion on emotional long-term memory. Emotion 17(3):547–556
Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H (2004) Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. NeuroImage 22(4):1596–1604
Gagnepain P, Henson RN, Anderson MC (2014) Suppressing unwanted memories reduces their unconscious influence via targeted cortical inhibition. Proc Natl Acad Sci 111(13):E1310–E1319
Gilron R, Gutchess AH (2012) Remembering first impressions: effects of intentionality and diagnosticity on subsequent memory. Cogn Affect Behav Neurosci 12(1):85–98
Glascher J, Adolphs R (2003) Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci 23(32):10274–10282
Goldin PR, McRae K, Ramel W, Gross JJ (2008) The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 63(6):577–586
Gotlib IH, Joormann J (2010) Cognition and depression: current status and future directions. Annu Rev Clin Psychol 6:285–312
Graf P, Schacter DL (1989) Unitization and grouping mediate dissociations in memory for new associations. J Exp Psychol Learn Mem Cogn 15(5):930–940
Grecucci A, Theuninck A, Frederickson J, Job R (2015) Mechanisms of social emotion regulation: from neuroscience to psychotherapy. In: Bryant ML (ed) Emotion regulation: processes, cognitive effects and social consequences. Nova Science Publishers, New York, pp 57–84
Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS (2005) Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 43(5):659–674
Greenwald AG, Banaji MR (1989) The self as a memory system: powerful, but ordinary. J Pers Soc Psychol 57(1):41
Gross JJ (2002) Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 39(3):281–291
Gross JJ (2008) Emotion regulation. In: Lewis M, Haviland-Jones JM, Barrett LF (eds) Handbook of emotions. Guilford, New York, pp 497–512
Gross JJ (2015) Emotion regulation: current status and future prospects. Psychol Inq 26(1):1–26
Guillet R, Arndt J (2009) TabooIds: the effect of emotion on memory for peripheral information. Mem Cogn 37(6):866–879
Gutchess AH, Sokal R, Coleman JA, Gotthilf G, Grewal L, Rosa N (2015) Age differences in self-referencing: evidence for common and distinct encoding strategies. Brain Res 1612:118–127
Haas BW, Canli T (2008) Emotional memory function, personality structure and psychopathology: a neural system approach to the identification of vulnerability markers. Brain Res Rev 58(1):71–84
Hamann S (2001) Cognitive and neural mechanisms of emotional memory. Trends Cogn Sci 5(9):394–400
Hamann SB, Ely TD, Grafton ST, Kilts CD (1999) Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci 2(3):289–293
Harvey PO, Fossati P, Lepage M (2007) Modulation of memory formation by stimulus content: specific role of the medial prefrontal cortex in the successful encoding of social pictures. J Cogn Neurosci 19(2):351–362
Haskins AL, Yonelinas AP, Quamme JR, Ranganath C (2008) Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron 59(4):554–560
Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, LaBar KS (2010) Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front Hum Neurosci 4:230
Heeren A, Van Broeck N, Philippot P (2009) The effects of mindfulness on executive processes and autobiographical memory specificity. Behav Res Ther 47(5):403–409
Heinzel A, Northoff G (2009) Emotional feeling and the orbitomedial prefrontal cortex: theoretical and empirical considerations. Philos Psychol 22(4):443–464
Hermann A, Bieber A, Keck T, Vaitl D, Stark R (2014) Brain structural basis of cognitive reappraisal and expressive suppression. Soc Cogn Affect Neurosci 9(9):1435–1442
Hermans EJ, Henckens MJ, Joels M, Fernandez G (2014) Dynamic adaptation of large-scale brain networks in response to acute stressors. Trend Neurosci 37(6):304–314
Holland AC, Kensinger EA (2013) The neural correlates of cognitive reappraisal during emotional autobiographical memory recall. J Cogn Neurosci 25(1):87–108
Iordan AD (2016) The impact of emotional distraction on cognition: from basic brain responses to large-scale network interactions. Doctoral dissertation, University of Illinois at Urbana-Champaign
Jaeger A, Rugg MD (2012) Implicit effects of emotional contexts: an ERP study. Cogn Affect Behav Neurosci 12(4):748–760
Jaeger A, Johnson JD, Corona M, Rugg MD (2009) ERP correlates of the incidental retrieval of emotional information: effects of study-test delay. Brain Res 1269:105–113
Jing HG, Madore KP, Schacter DL (2016) Worrying about the future: an episodic specificity induction impacts problem solving, reappraisal, and well-being. J Exp Psychol Gen 145(4):402–418
Joels M, Fernandez G, Roozendaal B (2011) Stress and emotional memory: a matter of timing. Trends Cogn Sci 15(6):280–288
Kalokerinos EK, Greenaway KH, Denson TF (2015) Reappraisal but not suppression downregulates the experience of positive and negative emotion. Emotion 15(3):271–275
Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M (2011) How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 21(6):1379–1388
Kapur S, Tulving E, Cabeza R, McIntosh AR, Houle S, Craik FI (1996) The neural correlates of intentional learning of verbal materials: a PET study in humans. Brain Res Cogn Brain Res 4(4):243–249
Katsumi Y, Denkova E, Dolcos S (2017) Personality and memory. In: Zeigler-Hill V, Shackelford TK (eds) Encyclopedia of personality and individual differences. Springer International Publishing, New York
Kensinger EA (2004) Remembering emotional eIiences: the contribution of valence and arousal. Rev Neurosci 15(4):241–251
Kensinger EA (2009) Remembering the details: effects of emotion. Emot Rev 1(2):99–113
Kensinger EA, Corkin S (2004) Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci 101(9):3310–3315
Kensinger EA, Schacter DL (2005) Retrieving accurate and distorted memories: neuroimaging evidence for effects of emotion. NeuroImage 27(1):167–177
Kensinger EA, Schacter DL (2006a) Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci 26(9):2564–2570
Kensinger EA, Schacter DL (2006b) Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci 6(2):110–126
Kensinger EA, Schacter DL (2008) Neural processes supporting young and older adults’ emotional memories. J Cogn Neurosci 20(7):1161–1173
Kensinger EA, Garoff-Eaton RJ, Schacter DL (2007) Effects of emotion on memory specificity: memory trade-offs elicited by negative visually arousing stimuli. J Mem Lang 56(4):575–591
Kensinger EA, Addis DR, Atapattu RK (2011) Amygdala activity at encoding corresponds with memory vividness and with memory for select episodic details. Neuropsychologia 49(4):663–673
Kilpatrick L, Cahill L (2003) Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage 20(4):2091–2099
Kim SH, Hamann S (2012) The effect of cognitive reappraisal on physiological reactivity and emotional memory. Int J Psychophysiol 83(3):348–356
Kleinhans NM, Johnson LC, Mahurin R, Richards T, Stegbauer KC, Greenson J, Dawson G, Aylward E (2007) Increased amygdala activation to neutral faces is associated with better face memory performance. Neuroreport 18(10):987–991
Konkel A, Cohen NJ (2009) Relational memory and the hippocampus: representations and methods. Front Neurosci 3(2):166–174
Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, Alpert NM (1996) Neural effects of visualizing and perceiving aversive stimuli: a PET investigation. Neuroreport 7(10):1569–1576
Kross E, Davidson ML, Weber M, Ochsner K (2009) Coping withItions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry 65(5):361–366
Kupper CS, Benoit RG, Dalgleish T, Anderson MC (2014) Direct suppression as a mechanism for controlling unpleasant memories in daily life. J Exp Psychol Gen 143(4):1443–1449
LaBar KS, Cabeza R (2006) Cognitive neuroscience of emotional memory. Nat Rev Neurosci 7(1):54–64
LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998) Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20(5):937–945
Lang PJ, Bradley MM (2010) Emotion and the motivational brain. Biol Psychol 84(3):437–450
Lang PJ, Greenwald MK, Bradley MM, Hamm AO (1993) Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 30:261–273
Laurita AC, Spreng RN (2017) The hippocampus and social cognition. In: Hannula DE, Duff MC (eds) The hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition. Springer International Publishing, Cham, pp 537–558
LeDoux J (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
LeDoux J (2007) The amygdala. Curr Biol 17(20):R868–R874
Lee TH, Sakaki M, Cheng R, Velasco R, Mather M (2014) Emotional arousal amplifies the effects of biased competition in the brain. Soc Cogn Affect Neurosci 9(12):2067–2077
Levine LJ, Schmidt S, Kang HS, Tinti C (2012) Remembering the silver lining: reappraisal and positive bias in memory for emotion. Cognit Emot 26(5):871–884
Li W, Mai X, Liu C (2014) The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci 8:74
Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM (2007) Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci 18(5):421–428
Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ (2011) Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion 11(3):468–480
Liu F, Cui L, Zhang Q (2015) The influences of reappraisal and suppression instructions on memory for neutral words in negative background. Neuroreport 26(17):1023–1031
Liu ZX, Grady C, Moscovitch M (2016) Effects of prior-knowledge on brain activation and connectivity during associative memory encoding. Cereb Cortex 27(3):1991–2009
Luck D, Leclerc M-E, Lepage M (2014) The potentiation of associative memory by emotions: an event-related fMRI study. Adv Neurosci 2014:9
Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM (2004) Medial prefrontal activity predicts memory for self. Cereb Cortex 14(6):647–654
Madore KP, Schacter DL (2016) Remembering the past and imagining the future: selective effects of an episodic specificity induction on detail generation. Q J Exp Psychol 69(2):285–298
Madore KP, Addis DR, Schacter DL (2015) Creativity and memory: effects of an episodic-specificity induction on divergent thinking. Psychol Sci 26(9):1461–1468
Maguire EA, Frith CD (2003) Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci 23(12):5302–5307
Mano Y, Sugiura M, Tsukiura T, Chiao JY, Yomogida Y, Jeong H, Sekiguchi A, Kawashima R (2011) The representation of social interaction in episodic memory: a functional MRI study. NeuroImage 57(3):1234–1242
Maratos EJ, Dolan RJ, Morris JS, Henson RN, Rugg MD (2001) Neural activity associated with episodic memory for emotional context. Neuropsychologia 39(9):910–920
Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14(6):417–428
Markowitsch HJ, Thiel A, Reinkemeier M, Kessler J, Koyuncu A, Heiss WD (2000) Right amygdalar and temporofrontal activation during autobiographic, but not during fictitious memory retrieval. Behav Neurol 12(4):181–190
Markowitsch HJ, Vandekerckhove MM, Lanfermann H, Russ MO (2003) Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex 39(4–5):643–665
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
Mather M (2007) Emotional arousal and memory binding: an object-based framework. Perspect Psychol Sci 2(1):33–52
Mather M, Knight M (2008) The emotional harbinger effect: poor context memory for cues that previously predicted something arousing. Emotion 8(6):850–860
Mather M, Nesmith K (2008) Arousal-enhanced location memory for pictures. J Mem Lang 58(2):449–464
Mather M, Sutherland MR (2011) Arousal-biased competition in perception and memory. Perspect Psychol Sci 6(2):114–133
Mather M, Clewett D, Sakaki M, Harley CW (2015) Norepinephrine ignites local hot spots of neuronal excitation: how arousal amplifies selectivity in perception and memory. Behav Brain Sci 39:e200
Mattarozzi K, Todorov A, Codispoti M (2015) Memory for faces: the effect of facial appearance and the context in which the face is encountered. Psychol Res 79(2):308–317
Mayberg HS (1997) Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9(3):471–481
McFarland CP, Primosch M, Maxson CM, Stewart BT (2017) Enhancing memory and imagination improves problem solving among individuals with depression. Mem Cogn 45(6):932–939
McGaugh JL (2000) Memory—a century of consolidation. Science 287(5451):248–251
McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28
McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN (2010) The neural bases of distraction and reappraisal. J Cogn Neurosci 22(2):248–262
Mende-Siedlecki P, Said CP, Todorov A (2013) The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Soc Cogn Affect Neurosci 8(3):285–299
Meyer ML, Lieberman MD (2012) Social working memory: neurocognitive networks and directions for future research. Front Psychol 3:571
Meyer ML, Taylor SE, Lieberman MD (2015) Social working memory and its distinctive link to social cognitive ability: an fMRI study. Soc Cogn Affect Neurosci 10(10):1338–1347
Mickley KR, Kensinger EA (2008) Emotional valence influences the neural correlates associated with remembering and knowing. Cogn Affect Behav Neurosci 8(2):143–152
Mickley Steinmetz KR, Kensinger EA (2009) The effects of valence and arousal on the neural activity leading to subsequent memory. Psychophysiology 46(6):1190–1199
Mickley Steinmetz KR, Addis DR, Kensinger EA (2010) The effect of arousal on the emotional memory network depends on valence. NeuroImage 53(1):318–324
Miskovic V, Keil A (2012) Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology 49(9):1230–1241
Mitchell JP, Macrae CN, Banaji MR (2004) Encoding-specific effects of social cognition on the neural correlates of subsequent memory. J Neurosci 24(21):4912–4917
Morris JS, Ohman A, Dolan RJ (1999) A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci 96(4):1680–1685
Murray BD, Kensinger EA (2013) A review of the neural and behavioral consequences for unitizing emotional and neutral information. Front Behav Neurosci 7:42
Murray BD, Kensinger EA (2014) The route to an integrative associative memory is influenced by emotion. PLoS One 9(1):e82372
Muscatell KA, Addis DR, Kensinger EA (2010) Self-involvement modulates the effective connectivity of the autobiographical memory network. Soc Cogn Affect Neurosci 5(1):68–76
Nashiro K, Mather M (2011) How arousal affects younger and older adults’ memory binding. Exp Aging Res 37(1):108–128
Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006) Self-referential processing in our brain – a meta-analysis of imaging studies on the self. NeuroImage 31(1):440–457
Northoff G, Schneider F, Rotte M, Matthiae C, Tempelmann C, Wiebking C, Bermpohl F, Heinzel A, Danos P, Heinze HJ, Bogerts B, Walter M, Panksepp J (2009) Differential parametric modulation of self-relatedness and emotions in different brain regions. Hum Brain Mapp 30(2):369–382
O’Doherty JP (2004) Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol 14(6):769–776
Ochsner KN, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Mackey S (2004) Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16(10):1748–1772
Oddo S, Lux S, Weiss PH, Schwab A, Welzer H, Markowitsch HJ, Fink GR (2008) Specific role of medial prefrontal cortex in retrieving recent autobiographical memories: an fMRI study of young female subjects. Cortex 46(1):29–39
Okada G, Okamoto Y, Kunisato Y, Aoyama S, Nishiyama Y, Yoshimura S, Onoda K, Toki S, Yamashita H, Yamawaki S (2011) The effect of negative and positive emotionality on associative memory: an fMRI study. PLoS One 6(9):e24862
Olatunji BO, Berg HE, Zhao Z (2015) Emotion regulation of fear and disgust: differential effects of reappraisal and suppression. Cognit Emot 31(2):403–410
Olson IR, McCoy D, Klobusicky E, Ross LA (2013) Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci 8(2):123–133
Owen AM, Herrod NJ, Menon DK, Clark JC, Downey SP, Carpenter TA, Minhas PS, Turkheimer FE, Williams EJ, Robbins TW, Sahakian BJ, Petrides M, Pickard JD (1999) Redefining the functional organization of working memory processes within human lateral prefrontal cortex. Eur J Neurosci 11(2):567–574
Paller KA, Wagner AD (2002) Observing the transformation of experience into memory. Trends Cogn Sci 6(2):93–102
Paller KA, Kutas M, Shimamura AP, Squire LR (1987) Brain responses to concrete and abstract words reflect processes that correlate with later performance on a test of stem-completion priming. Electroencephalogr Clin Neurophysiol Suppl 40:360–365
Pasupathi M (2003) Emotion regulation during social remembering: differences between emotions elicited during an event and emotions elicited when talking about it. Memory 11(2):151–163
Petrides M (1995) Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci 769:85–96
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16(2):331–348
Phelps EA (2004) Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 14(2):198–202
Phelps EA, LeDoux J (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48(2):175–187
Phelps EA, LaBar KS, Anderson AK, O’Connor KJ, Fulbright RK, Spencer DD (1998) Specifying the contributions of the human amygdala to emotional memory: a case study. Neurocase 4(6):527–540
Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M (2001) Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4(4):437–441
Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR (2003) Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 126(3):650–668
Pierce BH, Kensinger EA (2011) Effects of emotion on associative recognition: valence and retention interval matter. Emotion 11(1):139–144
Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999) Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10(1):15–35
Prince SE, Daselaar SM, Cabeza R (2005) Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci 25(5):1203–1210
Prince SE, Dennis NA, Cabeza R (2009) Encoding and retrieving faces and places: distinguishing process- and stimulus-specific differences in brain activity. Neuropsychologia 47(11):2282–2289
Raes F, Williams JM, Hermans D (2009) Reducing cognitive vulnerability to depression: a preliminary investigation of MEmory Specificity Training (MEST) in inpatients with depressive symptomatology. J Behav Ther Exp Psychiatry 40(1):24–38
Ranganath C (2010) Binding items and contexts the cognitive neuroscience of episodic memory. Curr Dir Psychol Sci 19(3):131–137
Ray RD, McRae K, Ochsner KN, Gross JJ (2010) Cognitive reappraisal of negative affect: converging evidence from EMG and self-report. Emotion 10(4):587–592
Richards JM, Gross JJ (2000) EmoI regulation and memory: the cognitive costs of keeping one’s cool. J Pers Soc Psychol 79(3):410–424
Richards JM, Butler EA, Gross JJ (2003) Emotion regulatiIn romantic relationships: the cognitive consequences of concealing feelings. J Soc Pers Relat 20(5):599–620
Richardson MP, Strange BA, Dolan RJ (2004) Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci 7(3):278–285
Rimmele U, Davachi L, Petrov R, Dougal S, Phelps EA (2011) Emotion enhances the subjective feeling of remembering, despite lower accuracy for contextual details. Emotion 11(3):553–562
Ritchey M, Dolcos F, Cabeza R (2008) Role of amygdala connectivity in the persistence of emotional memories over time: an event-related fMRI investigation. Cereb Cortex 18(11):2494–2504
Ritchey M, LaBar KS, Cabeza R (2011) Level of processing modulates the neural correlates of emotional memory formation. J Cogn Neurosci 23(4):757–771
Rosen BR, Buckner RL, Dale AM (1998) Event-related functional MRI: past, present, and future. Proc Natl Acad Sci 95(3):773–780
Rubin DC, Dennis MF, Beckham JC (2011) AutobiograIal memory for stressful events: the role of autobiographical memory in posttraumatic stress disorder. Conscious Cogn 20(3):840–856
Rugg MD, Curran T (2007) Event-related potentials and recognition memory. Trends Cogn Sci 11(6):251–257
Russell J (1980) A circumplex model of affect. J Pers Soc Psychol 39(6):1161–1178
Said CP, Baron SG, Todorov A (2009) Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. J Cogn Neurosci 21(3):519–528
Sakaki M, Niki K, Mather M (2012) Beyond arousal and valence: the importance of the biological versus social relevance of emotional stimuli. Cogn Affect Behav Neurosci 12(1):115–139
Sakaki M, Fryer K, Mather M (2014) Emotion strengthens high-priority memory traces but weakens low-priority memory traces. Psychol Sci 25(2):387–395
Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K (2008) Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn 17(2):457–467
Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB (2012) Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 7(2):e30920
Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA (2009) A neural mechanism of first impressions. Nat Neurosci 12(4):508–514
Schmeichel BJ, Volokhov RN, Demaree HA (2008) Working memory capacity and the self-regulation of emotional expression and experience. J Pers Soc Psychol 95(6):1526–1540
Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T (2013) Training the emotional brain: improving affective control through emotional working memory training. J Neurosci 33(12):5301–5311
Sergerie K, Lepage M, Armony JL (2006) A process-specific functional dissociation of the amygdala in emotional memory. J Cogn Neurosci 18(8):1359–1367
Shafer A, Iordan A, Cabeza R, Dolcos F (2011) Brain imaging investigation of the memory-enhancing effect of emotion. J Vis Exp 51:2433
Shafer AT, Matveychuk D, Penney T, O’Hare AJ, Stokes J, Dolcos F (2012) Processing of emotional distraction is both automatic and modulated by attention: evidence from an event-related fMRI investigation. J Cogn Neurosci 24(5):1233–1252
Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ (1994) Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368(6472):633–635
Sharot T (2011) The optimism bias. Curr Biol 21(23):R941–R945
Sharot T, Delgado MR, Phelps EA (2004) How emotion enhances the feeling of remembering. Nat Neurosci 7(12):1376–1380
Sharot T, Martorella EA, Delgado MR, Phelps EA (2007a) How personal experience modulates the neural circuitry of memories of September 11. Proc Natl Acad Sci 104(1):389–394
Sharot T, Riccardi AM, Raio CM, Phelps EA (2007b) Neural mechanisms mediating optimism bias. Nature 450(7166):102–105
Sheppes G, Gross JJ (2012) Emotion regulation effectiveness: what works when. In: Tennen HA, Suls JM (eds) Handbook of psychology: personality and social psychology, vol 5. Wiley, New York
Sheppes G, Scheibe S, Suri G, Radu P, Blechert J, Gross JJ (2014) Emotion regulation choice: a conceptual framework and supporting evidence. J Exp Psychol Gen 143(1):163–181
Sheppes G, Suri G, Gross JJ (2015) Emotion regulation and psychopathology. Annu Rev Clin Psychol 11:379–405
Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD (2004) Brain responses to the acquired moral status of faces. Neuron 41(4):653–662
Smith AP, Dolan RJ, Rugg MD (2004a) Event-related potential correlates of the retrieval of emotional and nonemotional context. J Cogn Neurosci 16(5):760–775
Smith AP, Henson RN, Dolan RJ, Rugg MD (2004b) fMRI correlates of the episodic retrieval of emotional contexts. NeuroImage 22(2):868–878
Smith AP, Henson RN, Rugg MD, Dolan RJ (2005) Modulation of retrieval processing reflects accuracy of emotional source memory. Learn Mem 12(5):472–479
Smith AP, Stephan KE, Rugg MD, Dolan RJ (2006) Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron 49(4):631–638
Somerville LH, Wig GS, Whalen PJ, Kelley WM (2006) Dissociable medial temporal lobe contributions to social memory. J Cogn Neurosci 18(8):1253–1265
Spreng RN, Andrews-Hanna JR (2015) The default network and social cognition. In: Brain mapping: an encyclopedic reference, vol 3. Elsevier/Academic Press, Amsterdam, pp 165–169
Spreng RN, Mar RA (2012) I remember you: a role for memory in social cognition and the functional neuroanatomy of their interaction. Brain Res 1428:43–50
Staresina BP, Davachi L (2010) Object unitization and associative memory formation are supported by distinct brain regions. J Neurosci 30(29):9890–9897
Strange BA, Dolan RJ (2004) Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci 101(31):11454–11458
Strange BA, Hurlemann R, Dolan RJ (2003) An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proc Natl Acad Sci 100(23):13626–13631
Takashima A, van der Ven F, Kroes MCW, Fernández G (2016) Retrieved emotional context influences hippocampal involvement during recognition of neutral memories. NeuroImage 143:280–292
Takeuchi H, Taki Y, Nouchi R, Sekiguchi A, Hashizume H, Sassa Y, Kotozaki Y, Miyauchi CM, Yokoyama R, Iizuka K, Nakagawa S, Nagase T, Kunitoki K, Kawashima R (2013) Resting state functional connectivity associated with trait emotional intelligence. NeuroImage 83:318–328
Talarico JM, LaBar KS, Rubin DC (2004) Emotional intensity predicts autobiographical memory experience. Mem Cogn 32(7):1118–1132
Tambini A, Rimmele U, Phelps EA, Davachi L (2017) Emotional brain states carry over and enhance future memory formation. Nat Neurosci 20(2):271–278
Taylor SF, Liberzon I, Fig LM, Decker LR, Minoshima S, Koeppe RA (1998) The effect of emotional content on visual recognition memory: a PET activation study. NeuroImage 8(2):188–197
Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, Gross JJ (2011) The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biol Psychol 87(1):84–92
Thoresen C, Jensen J, Sigvartsen NP, Bolstad I, Server A, Nakstad PH, Andreassen OA, Endestad T (2012) Arousal modulates activity in the medial temporal lobe during a short-term relational memory task. Front Hum Neurosci 5:177
Touryan SR, Marian DE, Shimamura AP (2007b) Effect of negative emotional pictures on associative memory for peripheral information. Memory 15(2):154–166
Tsukiura T (2012) Neural mechanisms underlying the effects of face-based affective signals on memory for faces: a tentative model. Front Integr Neurosci 6:50
Tsukiura T, Cabeza R (2008) Orbitofrontal and hippocampal contributionI memory for face-name associations: the rewarding power of a smile. Neuropsychologia 46(9):2310–2319
Tsukiura T, Cabeza R (2011a) Remembering beauty: roles of orbitofrontal and hippocampal regions in successful memory encoding of attractive faces. NeuroImage 54(1):653–660
Tsukiura T, Cabeza R (2011b) Shared brain activity for aesthetic and moral judgments: implications for the beauty-is-good stereotype. Soc Cogn Affect Neurosci 6(1):138–148
Tsukiura T, Suzuki C, Shigemune Y, Mochizuki-Kawai H (2008) Differential contributions of the anterior temporal and medial temporal lobe to the retrieval of memory for person identity information. Hum Brain Mapp 29(12):1343–1354
Tsukiura T, Mano Y, Sekiguchi A, Yomogida Y, Hoshi K, Kambara T, Takeuchi H, Sugiura M, Kawashima R (2010) Dissociable roles of the anterior temporal regions in successful encoding of memory for person identity information. J Cogn Neurosci 22(10):2226–2237
Tsukiura T, Sekiguchi A, Yomogida Y, Nakagawa S, Shigemune Y, Kambara T, Akitsuki Y, Taki Y, Kawashima R (2011) Effects of aging on hippocampal and anterior temporal activations during successful retrieval of memory for face-name associations. J Cogn Neurosci 23(1):200–213
Tsukiura T, Shigemune Y, Nouchi R, Kambara T, Kawashima R (2013) Insular and hippocampal contributions to remembering people with an impression of bad personality. Soc Cogn Affect Neurosci 8(5):515–522
Tulving E (1985) Memory and consciousness. Can Psychol 26(1):1–12
Van Ast VA, Cornelisse S, Marin MF, Ackermann S, Garfinkel SN, Abercrombie HC (2013) Modulatory mechanisms of cortisol effects on emotional learning and memory: novel perspectives. Psychoneuroendocrinology 38(9):1874–1882
Van den Stock J, de Gelder B (2012) Emotional information in body and background hampers recognition memory for faces. Neurobiol Learn Mem 97(3):321–325
Vandekerckhove MM, Markowitsch HJ, Mertens M, Woermann FG (2005) Bi-hemispheric engagement in the retrieval of autobiographical episodes. Behav Neurol 16(4):203–210
Vazdarjanova A, McGaugh JL (1998) Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. Proc Natl Acad Sci 95(25):15003–15007
Ventura-Bort C, Löw A, Wendt J, Dolcos F, Hamm AO, Weymar M (2016a) When neutral turns significant: brain dynamics of rapidly formed associations between neutral stimuli and emotional contexts. Eur J Neurosci 44(5):2176–2183
Ventura-Bort C, Löw A, Wendt J, Molto J, Poy R, Dolcos F, Hamm AO, Weymar M (2016b) Binding neutral information to emotional contexts: brain dynamics of long-term recognition memory. Cogn Affect Behav Neurosci 16(2):234–247
Vilberg KL, Rugg MD (2009) Functional significance of retrieval-related activity in lateral parietal cortex: Evidence from fMRI and ERPs. Hum Brain Mapp 30(5): 1490-1501
Vogel S, Fernandez G, Joels M, Schwabe L (2016) Cognitive adaptation under stress: a case for the mineralocorticoid receptor. Trends Cogn Sci 20(3):192–203
Vrtička P, Sander D, Vuilleumier P (2012) Lateralized interactive social content and valence processing within the human amygdala. Front Hum Neurosci 6:358
Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ (2004) Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci 7(11):1271–1278
Wagner DD, Haxby JV, Heatherton TF (2012) The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdiscip Rev Cogn Sci 3(4):451–470
Wagner U, Handke L, Walter H (2015) The relationship between trait empathy and memory formation for social vs. non-social information. BMC Psychol 3(1):2
Waring JD, Kensinger EA (2011) How emotion leads to selective memory: neuroimaging evidence. Neuropsychologia 49(7):1831–1842
Watkins ER, Baeyens CB, Read R (2009) Concreteness training reduces dysphoria: proof-of-principle for repeated cognitive bias modification in depression. J Abnorm Psychol 118(1):55–64
Webb TL, Miles E, Sheeran P (2012) Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol Bull 138(4):775–808
Weis S, Klaver P, Reul J, Elger CE, Fernandez G (2004) Temporal and cerebellar brain regions that support both declarative memory formation and retrieval. Cereb Cortex 14(3):256–267
Weymar M, Löw A, Melzig CA, Hamm AO (2009) Enhanced long-term recollection for emotional pictures: evidence from high-density ERPs. Psychophysiology 46(6):1200–1207
Weymar M, Hamm AO (2013) Electrophysiological signature of emotional memories. In: Linden M, Rutkowski K (eds) Hurting memories and beneficial forgetting: posttraumatic stress disorders, biographical developments and social conflicts. Elsevier, Saint Louis, pp 21–35
Weymar M, Löw A, Hamm AO (2011) Emotional memories are resilient to time: evidence from the parietal ERP old/new effect. Hum Brain Mapp 32(4):632–640
Weymar M, Bradley MM, Hamm AO, Lang PJ (2013) When fear forms memories: threat of shock and brain potentials during encoding and recognition. Cortex 49(3):819–826
Weymar M, Bradley MM, Hamm AO, Lang PJ (2014) Encoding and reinstatement of threat: recognition potentials. Neurobiol Learn Mem 107:87–92
Wieser MJ, Keil A (2014) Fearful faces heighten the cortical representation of contextual threat. NeuroImage 86:317–325
Wilker S, Elbert T, Kolassa IT (2014) The downside of strong emotional memories: how human memory-related genes influence the risk for posttraumatic stress disorder—a selective review. Neurobiol Learn Mem 112:75–86
Williams AD, Moulds ML (2010) The impact of ruminative processing on the experience of self-referent intrusive memories in dysphoria. Behav Ther 41(1):38–45
Williams JM, Teasdale JD, Segal ZV, Soulsby J (2000) Mindfulness-based cognitive therapy reduces overgeneral autobiographical memory in formerly depressed patients. J Abnorm Psychol 109(1):150–155
Yang XF, Bossmann J, Schiffhauer B, Jordan M, Immordino-Yang MH (2013) Intrinsic default mode network connectivity predicts spontaneous verbal descriptions of autobiographical memories during social processing. Front Psychol 3:592
Yaoi K, Osaka M, Osaka N (2015) Neural correlates of the self-reference effect: evidence from evaluation and recognition processes. Front Hum Neurosci 9:383
Yonelinas AP, Ritchey M (2015) The slow forgetting of emotional episodic memories: an emotional binding account. Trends Cogn Sci 19(5):259–267
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Dolcos, F., Katsumi, Y., Denkova, E., Weymar, M., Dolcos, S. (2017). Current Issues and Emerging Directions in the Impact of Emotion on Memory: A Review of Evidence from Brain Imaging Investigations. In: Tsukiura, T., Umeda, S. (eds) Memory in a Social Context. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56591-8_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-56591-8_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56589-5
Online ISBN: 978-4-431-56591-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)