Abstract
Primates, including humans, have great cognitive capability, can adapt to their environments, and have a brain is characterized by a large volume of prefrontal cortex. In this chapter, I provide an overview on how the primate prefrontal cortex differs from that of other species, and I discuss the structural similarities and differences of the prefrontal cortex among primate species. In particular, I discuss how the human prefrontal cortex has unique characteristics among primate species. I also provide an overview of the neural mechanisms of top-down control of visual attention and discuss how cognitive research in human and non-human primates is integrated to understand brain mechanisms. In summary, I will argue that comparative and integrative approaches aid the understanding of the biological basis of human cognition.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Attention
- Comparative anatomy
- Executive function
- Human brain
- Nonhuman primate
- Prefrontal cortex
- Top-down control
1 Introduction
Adaptive behavior requires flexible processing of external information for behavioral guidance according to the demands of environments (Miller and Cohen 2001; Real 1991). Organisms receive external information through sensory organs, but not all information received can be actively processed at the same time. For example, we cannot report two consecutively presented visual stimuli, called a phenomenon of attentional blink (Marois and Ivanoff 2005). The capacity of working memory is limited to five to seven items (Baddeley et al. 1974; Kane and Engle 2002). Therefore, the first step in achieving adaptive behavior is to select relevant information and ignore irrelevant information pertaining to the demand at hand. In the next step, the processed information is utilized and evaluated according to the current demand. In the last step, a relevant action is selected and executed. Higher cognitive functions, such as attention, working memory, planning, decision making, memory retrieval, motivated behavior, and social cognition, can be fractionated into these steps that are served by the prefrontal cortex (PFC) (Desimone and Duncan 1995; Fujii et al. 2009; Fuster 1988; Goldman-Rakic 1996; Grabenhorst and Rolls 2011; Iacoboni et al. 2004; Karafin et al. 2004; Miller and Cohen 2001; Passingham 2008; Passingham and Wise 2012; Rudebeck et al. 2006; Rushworth et al. 2007a, b; Sakai 2008; Sallet et al. 2011; Squire et al. 2013; Tsujimoto et al. 2011).
The PFC is a part of the cerebral cortex that is observed only in mammals. Amphibians and lower organisms do not have a PFC (Butler and Hodos 2005; Wilczynski 2009). Birds have a brain area analogous to the mammalian PFC, but the area does not have the columnar structure of the cerebral cortex that is found in mammals (Fuster 1988; Wilczynski 2009). Among mammalian species, primates have a considerably larger PFC compared to other species (Roth and Dicke 2005). Although the function of the PFC has been studied extensively in humans and nonhuman primates, rodents have recently been used to study PFC functions as well.
The goal of this chapter is to discuss human cognition by taking a comparative approach of brain architecture. To this end, in the first part of this chapter, I provide an overview of the anatomical architecture of the PFC in human and nonhuman primates and illustrate the differences from other nonprimate species. Then, I discuss the structural commonality and differences of the PFC among primate species. In the second part of this chapter, I review recent research on visual attention. I chose visual attention in this chapter, because researchers have investigated the neural mechanism of the top-down control of visual attention with human and nonhuman primates, and studies with different species have been nicely integrated to understand human cognition.
2 Anatomical Architecture of the PFC in Humans and Nonhuman Primates
2.1 The Definition of the PFC
First, I clarify the definition of the PFC. In this chapter, I define the PFC as a part of the cerebral cortex that covers cortical areas in the frontal lobe, except for motor areas, such as the primary motor area, premotor, and supplementary motor area. Therefore, the PFC is different from the frontal lobe; the frontal lobe is separated from the parietal lobe by the central sulcus and separated from the temporal lobe by the lateral sulcus (in humans, the Sylvian fissure). Within the primate PFC, distinct types of cytoarchitecture have been observed: granular, dysgranular, and agranular parts of the PFC (Fuster 1988; Passingham and Wise 2012). These three cytoarchitecture types are differentiated by the cell density of the internal granular layer; that is, layer IV. The granular PFC has an evident layer IV, whereas few neuronal cell bodies are observed in the agranular PFC. The dysgranular PFC shows an immature layer IV (Öngür and Price 2000; Petrides and Pandya 1999, 2007). Because of the subtle differences, the dysgranular PFC was included in Brodmann’s classical definition of the PFC (Brodmann 1909). Rodents are recognized to have a PFC, but no evidence has been shown for the existence of the granular PFC in rodents (Öngür and Price 2000; Preuss 1995; Uylings et al. 1990). Brodmann thus did not acknowledge the existence of the PFC in rodents, although the presence of a PFC in rodents is currently being considered (Preuss 1995; Uylings et al. 1990, 2003). Although the granular PFC is observed in a limited number of species, the agranular PFC is observed in lower mammals, such as rodents. Hence, the agranular PFC is considered to be an evolutionarily older PFC and the granular PFC is considered to contribute to the complex cognitive processes of primates.
2.2 Subdivision of the PFC in Humans, Macaques, and Marmosets
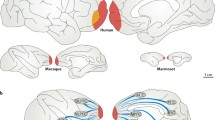
The PFC is not a homogeneous structure. Hence, the PFC is subdivided into subregions based on macroscopic and microscopic criteria. The cerebral cortex of mammals comprises six laminated layers based on distinct neuronal cell density layers. The patterns of cell densities in the six layers are not uniform across the entire cerebral cortex but are considerably different among different brain areas. Therefore, the cerebral cortex has been parcellated into subregions based on distinct patterns of cytoarchitecture. Brodmann’s initial parcellation of human brain areas is still of value (Brodmann 1909), and a similar naming rule has been adopted to label the brain areas of other primate species (Walker 1940). Figure 16.1 illustrates the parcellated brain areas of the PFC in humans, macaque monkeys, and marmoset monkeys (Burman et al. 2006; Burman and Rosa 2009; Öngür and Price 2000; Petrides and Pandya 1999, 2007). As shown in Fig. 16.1, the agranular PFC is observed in the more caudal part of the lateral and medial PFC, whereas the granular PFC is observed in the more rostral part of the lateral and medial PFC. The dysgranular PFC is located in between the two. In addition, the more rostral part of the orbital PFC has a thicker layer IV along with the anterior posterior line (Mackey and Petrides 2010). This rule is uniformly applied to humans, macaque monkeys, and marmoset monkeys. Although such comprehensive cytoarchitecture information is not available for other primate species, this rule could be applied to other primate species. The commonality in the naming rules among species helps to easily relate knowledge obtained from one species to another, in particular between humans and macaque monkeys, as both species have been extensively used to study the neural basis of cognition. The functional similarity of a particular area between human and nonhuman primates is ubiquitously reported. In addition, more recent studies have examined the pattern of anatomical and functional connectivity among brain areas and found certain similarities between human and primate brains (De Schotten et al. 2012; Margulies et al. 2009; Neubert et al. 2014, 2015; Ramnani et al. 2006; Rilling et al. 2008).
Cytoarchitectural subdivision of the prefrontal cortex (PFC): the subdivisions of the PFC in humans (a, d, g), macaque monkeys (b, e, h), and marmoset monkeys (c, f, i). Each lateral (a–c), medial (d–f), and orbital (g–i) surface is described. Subdivision of the surface of human prefrontal cortex is shown. Colors indicate the existence of granular layer IV. The granular, dysgranular, and agranular PFC areas are depicted by blue, purple, and yellow, respectively. As is the convention, the rostral ventral prefrontal area is described as area 47/12 or 45/12 in the lateral surface, whereas the area is labeled as area 12 in the orbital surface (Petrides and Pandya 2002) (Figures are adapted and modified from Petrides and Pandya (1999) (a, b, d, e), Öngür and Price (2000) (g, h), Burman et al. (2006) (c), and Burman and Rosa (2009) (f, i))
The second labeling rule of the PFC that is widely used is based on gross anatomical features, and the PFC is subdivided into five areas: the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), medial prefrontal cortex (MFPC), orbitofrontal cortex (OFC), and frontopolar cortex (otherwise called the anterior PFC or polar PFC). These definitions are convenient to roughly note the locus of activation, recording, or lesion. However, the nomenclature causes misunderstanding because each subregion covers broad cortical areas. For example, the DLPFC sometimes refers to the core area of executive control function, and BA 9 and BA 46 are involved in the process, but at the same time, the DLPFC in this definition includes BA 8, BA 9, BA 10, and BA 46 (Cieslik et al. 2012). Because of the confusion of the terminology, in particular in human neuroimaging studies, the foci of activation should be carefully confirmed to interpret the results. We sometimes encounter a study that refers to DLPFC activation as the control mechanism, but the activation was observed in the posterior extreme of area 6, which is less likely to be involved in executive control. Hence, the precise definition of brain areas is prerequisite for the interpretation of functional associations.
2.3 Evolutionarily Relevant Neural Index for Intelligence
What feature of the brain accounts for our intelligence? In this section, I overview potential neural indices that may account for the interspecies variation of intelligence: Total brain volume, total brain volume scaled by body size, PFC volume scaled by total cortex, and granular PFC size scaled by total cortex (Table 16.1). The brain volume of primates is generally larger than that of most other mammalian species (Roth and Dicke 2005). However, there are many species whose brain is larger than that of humans, such as elephants and whales. Hence, the total brain volume does not represent the uniqueness of primate intelligence, and another measure is needed to relate the structural variation among species with intelligence. If we scale total brain volume by body size, it may potentially account for the difference in intelligence across mammalian species (Fig. 16.2a) (Van Dongen 1998), but that is not yet an ideal measure to account for the association between brain structure and intelligence. In fact, the scaled total brain volume of humans is lower than that of mice and is comparable to that of marmosets (Fig. 16.2a). As we are discussing the PFC in this chapter, we consider the size of the PFC across species. If we look at the PFC volume scaled by total cerebral cortex, among primate species, humans have the highest relative PFC size, followed by apes, macaque monkeys, and marmosets (Semendeferi et al. 2002; Uylings et al. 1990). However, the relative PFC size of rats is considerably larger than that of humans (Uylings et al. 1990). Thus, PFC volume scaled by total cerebral cortex is not an appropriate measure to account for the association of brain size measures with primate intelligence. However, the relatively large PFC size in rats may account for the fact that rats have been widely used to study cognition.
Relative brain and prefrontal size. (a) Relationship between the body weight and brain weight in mammalian species. The oblique line is drawn through the relative brain size equal to humans. The relative brain weight of marmoset monkeys is equal to humans, and the relative brain weight of the shrew is larger than for humans (Figure adapted and modified from Van Dongen 1998). (b) The relationship between the total cortical surface and fraction of the granular PFC in primates. In the human brain, the granular PFC occupies 80 % of the volume of the frontal lobe, whereas the granular PFC occupies between 40 and 60 % of the frontal lobe in other species. Among nonhuman primates, apes have a larger relative granular PFC area compared with other nonhuman primates (Figure adapted and modified from Elston et al. 2006)
Because the existence of granular cell layer IV in the primate PFC is a unique characteristic of the primate PFC, we can look at the size of the granular area of the PFC. The granular PFC is observed mostly in primates and among primate species, and the human granular PFC is extraordinarily large relative to the frontal lobe size (Elston et al. 2006) (Fig. 16.2b). Thus, granular PFC volume scaled by frontal lobe size is a promising neural index for the interspecies variation of intelligence in mammals. The granular part of the PFC is considered to characterize the uniqueness of the primate PFC. In primates, the granular PFC occupies a considerable part of the total cortex (from 8 % in marmosets to 29 % in humans), whereas in Pteropus (a bat) and Leporidae (rabbits), the granular area makes up approximately 2 % of the total cortex (Elston 2003). These results suggest that the larger granular PFC reflects the high intelligence of human and nonhuman primates but also results raises a new question: what is the function of the granular layer IV? From the microscopic perspective, neurons in layer IV consist of glutamatergic stellate neurons and GABAergic interneurons (de Almeida and Mengod 2008), which send excitatory and inhibitory inputs to layers II, III, and V. As layer IV receives input from the thalamus (Sherman 2007), layer IV in the granular PFC may integrate information from subcortical structures, local cortical inputs, and cortical inputs from other brain areas mediated via the thalamus. In sum, the existence and size of the granular PFC support the large capacity for processing information and may reflect the high intelligence of humans and nonhuman primates. Studying prefrontal functions of primates is thus important for the understanding not only of human intelligence but also of the biological origins of intelligence.
2.4 What Is Special About the Human Brain?
In this section, we consider the unique characteristics of the human PFC compared to that of other primate species. As already discussed, the human PFC has a considerably larger granular PFC (80 %) compared to that of other primates (40–55 %) (Elston et al. 2006). Other than the relative granular PFC size, the white matter volume is disproportionally larger in the human PFC than in that of other primates (Schoenemann et al. 2005). One point of caution when interpreting this study is that it adopted an uncommon definition of the PFC. Because it is hard to examine the cytoarchitecture of the cerebral cortex with MRI, the study specified the PFC as “all portions of the frontal cortex anterior to the genu of the corpus callosum, in a plane perpendicular to the line connecting the anterior and posterior commissures” (Schoenemann et al. 2005). The uncommon definition may create bias to estimate the PFC size across species. In addition to the size of the granular PFC area, the human PFC has area 44. Although area 44 is categorized as the dysgranular PFC, area 44 is observed in neither macaque monkeys nor marmoset monkeys and it is a part of Broca’s area, which is crucial for the production and comprehension of language. In humans, the volume of the left areas 44 and 45 is larger than that of right areas 44 and 45 (Amunts et al. 1999), which apparently reflects the dominance of language processing in the left side of the brain. Surprisingly, this asymmetry of the posterior part of the inferior frontal gyrus is observed not only in humans but also in great ape species (Cantalupo and Hopkins 2001), suggesting that the ape’s brain emerged with the anatomical capacity to process complex language systems. Other studies of the size of area 10 in humans and apes included that of Semendeferi et al. (2001), which found that the volume of area 10 relative to the total brain volume in humans is double that in other apes. Area 10 is located in the most rostral part of the PFC and is situated at the top of the prefrontal hierarchy (Badre and D’Esposito 2007; Botvinick 2008; Koechlin and Summerfield 2007). In fact, area 10 is involved in more complex processes of executive function, such as complex planning and decision making, metacognition, and lie telling (Burgess et al. 2007; Fleming et al. 2010; Karim et al. 2010; Passingham and Wise 2012; Ramnani and Owen 2004; Stuss and Knight 2013; Tsujimoto et al. 2011), and, in particular, the larger gray matter volume in area 10 is associated with better metacognitive ability (Fleming et al. 2010). Therefore, a large area 10 in the human PFC may reflect the capacity of human intelligence.
At a microscopic level, several unique characteristics of the human PFC have been reported. First, Elston and colleagues studied the number of dendritic spines in seven primate species including humans (Elston et al. 2006). They showed that the number of spines is significantly larger in the human granular PFC than that in the granular PFC of other primates, although this difference was not observed in visual areas V1 and V2. Because spines are the locus for the reception of neural inputs to pyramidal neurons, these results suggested that human prefrontal neurons integrate more complex information. Second, a study by Sherwood and colleagues compared the density of glial cells relative to neurons in subregions of the PFC across 18 primate species including humans, gorillas, and chimpanzees (Sherwood et al. 2006). They showed that glial cell density relative to neuron density in the human PFC is considerably higher than in other primates. However, in that study, they did not examine glial cell densities other than in the PFC. Therefore, it was not clear whether the increased density of glial cells is specific to prefrontal areas. The third unique characteristic observed in the human PFC is a large number of bipolar spindle-shaped neurons, called von Economo neurons (VENs). We discuss VENs in the next section.
2.5 Von Economo Neurons
The seminal work by von Economo and Koskinas described large bipolar neurons in layer V of the anterior cingulate cortex (von Economo and Koskinas 1925). In humans, VENs are located in the anterior part of the insular cortex, the anterior cingulate cortex, and the rostral part of the middle cingulate cortex (Allman et al. 2010). VENs are mostly observed in anthropoids, such as humans, chimpanzees, gorillas, and bonobos, and, more recently, the existence of VENs was also reported in the anterior insular cortex of macaque monkeys (Evrard et al. 2012). Although the neurons were also reported as large spindle-shape neurons in other reports, including those of Betz and Cajal (Betz 1881; Cajal 1904), von Economo comprehensively described the morphology and location of these neurons, and these neurons are apt to be called VENs. The morphological characteristics of VENs are unique. VENs are projection neurons characterized as being elongated, gradually tapering, with large-sized somas, and are considerably larger than neighboring pyramidal neurons (Nimchinsky et al. 1995, 1999). The functional role of VENs is still elusive. It has been speculated that VENs might be involved in the processing of emotionally and socially salient information relevant to human awareness (Allman et al. 2010) because VENs are observed only in anthropoids and macaque monkeys and are located in the rostral part of the ACC and insula, areas highly associated with social cognition (Frith and Frith 2012). Although most of these speculations are not supported by experimental evidence, Santos and colleagues have reported the atypical characteristics of VENs in autistic children, suggesting the association of VENs with the theory of mind (Santos et al. 2011). The study investigated the number of VENs in the frontoinsular cortex of healthy and autistic children. Autistic spectrum disorder is a developmental psychiatric disorder characterized by difficulty in social interactions and atypical processing (mostly hypersensitive) of sensory information (American Psychiatric Association 2013; Frith 1991). That study showed that children with autism had a higher ratio of VENs to pyramidal neurons in the frontoinsular cortex than in healthy children, but the density of VENs was comparable between the two groups, indicating that the numbers of both VENs and other neurons were increased. In addition, the study reported that some VENs of autistic children had atypically swollen somata. The results suggested the association of VENs with autism, but it is still elusive how VENs contribute to social interactions, which are impaired in autism. One possible consideration that may be derived from comparative approaches is behavioral differences among species with VENs can be associated with differences in distributions of VENs among those species. In summary, we overviewed the unique characteristics of the human PFC; that is, larger granular PFC and area 10, and higher amounts of white matter volume, spines, and VENs. All those characteristics are quantitatively different from other primate species but not qualitatively different. Human cognition can be partly understood by interpolation from other species, but the complexity of brain networks may add new cognitive processes that can be studied only in humans.
3 Executive Function in Human and Nonhuman Primates
3.1 Top-Down Attentional Control Mechanism
In the second half of this chapter, we discuss the common neural mechanism of executive function in human and nonhuman primates. To discuss how studies of human and nonhuman primates are integrated to understand executive function, we consider neural mechanisms of attention in humans and macaque monkeys because attention has been extensively studied in both species. Attention refers to a cognitive process to enhance and suppress the processing of external information (Corbetta and Shulman 2002; Desimone and Duncan 1995; Kastner and Ungerleider 2000; Reynolds and Chelazzi 2004). Selective processing is important from an evolutionary perspective. First, living organisms need to process information relevant to survival according to environmental demands. Second, the processing of irrelevant information is not efficient from the perspective of energy consumption. Therefore, the prioritization of information processing is important for fitness in different environments. In addition, information relevant to a behavioral goal depends on the environmental demands at hand, and the brain therefore needs to flexibly control the focus of attention in favor of the processing of currently relevant information. In the following sections, I overview the neural mechanisms of top-down control of attention in humans and nonhuman primates and discuss the unified neural mechanisms of attentional systems.
3.2 Existence of Top-Down Signals
Seminal work by Moran and Desimone has demonstrated that attention to a visual stimulus enhances the selectivity of neuronal response to the stimulus when distracting visual stimuli are simultaneously presented (Moran and Desimone 1985). This study suggested that attention creates control signals that enhance the selective processing of sensory information. However, it was not clear how the control signals that enhance the selective processing of visual stimuli are generated. One possibility is that these control signals are driven by the external sensory stimulus itself (bottom-up driven account). Another explanation is that the control signals were generated in the brain according to the demands of the environment without actual sensory input (top-down account). To distinguish between top-down and bottom-up processing, researchers examined the modulation of brain activity in sensory areas without any sensory stimuli. Kastner and colleagues have shown activation in the extrastriate cortex in the absence of visual stimuli when human subjects covertly directed attention to a peripheral location where visual stimuli were expected to be presented (Kastner et al. 1999) (Fig. 16.3a). In addition, they also observed activation in the PFC during orienting attention without a visual stimulus. Tomita and colleagues also demonstrated that neurons in the inferior temporal (IT) cortex, which selectively respond to a specific visual stimulus, increase the firing rate when monkeys retrieve a visual item during a paired-association memory retrieval task (Tomita et al. 1999). In that task, monkeys were first trained to learn the association of two visual items, and then they were presented with one of the visual items (stim 1) and were instructed to retrieve the other visual item (stim 2). The firing rate of IT neurons selectively responding to stim2 was increased after the presentation of the first visual item but before the presentation of the paired item, indicating an increase in the firing rate of the neurons without a visual stimulus. In addition, they also slit the posterior half of the corpus callosum and again presented the first visual stimulus in the single visual hemifield. Then, they showed that the firing rate of the ipsilateral IT neurons were increased during retrieval. Because a visual stimulus presented in one visual hemifield is processed in the contralateral side of the visual cortex, no bottom-up visual information will be transmitted to the ipsilateral side of the visual cortex. Therefore, the neuronal firing in the ipsilateral side is supposed to be driven by signals via the PFC. Those results clearly showed the existence of top-down control signals originating from the PFC.
Top-down signals during visual attention in humans and macaque monkeys. (a) Brain activity is measured by functional magnetic resonance imaging (MRI) in the human visual cortex. In one condition (EA), subjects were told to attend the forthcoming visual stimulus (E period, gray) and then an actual visual stimulus was presented (A period, yellow). In the other condition, the visual stimulus is presented, but subjects were not told to attend to the visual stimulus (UA period, blue). Note that the visual cortex was activated by the instruction to attend to the display without any visual stimulus (Figure is adapted and modified from Kastner et al. 1999). (b) Concurrent recording of visual neurons in area V4 and microstimulation of FEF. Neuronal firing of V4 neurons is recorded while presenting the visual stimulus to the receptive field of the V4 neurons. In the middle of the presentation of visual stimuli, microstimulation is briefly applied to FEF (red). The FEF microstimulation increased the firing rate of the V4 neurons after offset of microstimulation (Figure adapted from Moore and Armstrong 2003)
The studies left an open question of whether top-down signals originating from the PFC causally influence activity in the visual areas. Moore and colleagues addressed that question (Moore and Armstrong 2003) in a study in which they recorded neuronal activity from the V4 visual area while electronically stimulating the frontal eye field (FEF). The FEF is a subdivision of the PFC and is directly connected with V4 (Stanton et al. 1995). The FEF is also shown to have involvement in top-down attentional processes (Corbetta and Shulman 2002; Maunsell and Treue 2006). Electronic stimulation of the FEF increased the neuronal response of V4 neurons to visual stimuli (Fig. 16.3b), whereas the enhancement was not observed in the absence of visual stimulation. Another study by Ekstorm and colleagues reported that the electronic stimulation of the FEF increased the activation in visual areas only in the presence of visual stimuli (Ekstrom et al. 2008). The results suggested that the FEF causally enhances the activity of visual areas in the presence of visual stimuli. The results may sound contradictory to the initial report by Kaster et al., wherein there was activation in the extrastriate cortex in the absence of visual stimuli (Kastner et al. 1999). These results may be explained by the differences in underlying mechanisms between artificial electronic stimulation and endogenous top-down control. The other possible explanation is that both studies with monkeys did not instruct them to orient attention to a specific location during electronic stimulation in the absence of visual stimuli.
3.3 Flexible Control of Top-Down Signals
Studies with macaque monkeys have clearly shown that the top-down signals originating from the PFC can causally enhance the activity of visual areas in the presence of visual stimuli. However, to achieve adaptive behavior, the biased processing of sensory information needs to be flexibly controlled according to environmental demands at hand. This concept gives an assumption that top-down signals are flexibly controlled in favor of processing information relevant to behavioral goals. To address this question, our previous study measured neuronal signal transmission from the PFC to visual areas by concurrently using transcranical magnetic stimulation (TMS) and electroencephalography (EEG) (Morishima et al. 2009). The rationale of the TMS-EEG method is the following. A weak single-pulse TMS excites neurons in the stimulated area, and the impulse of stimulated neurons spreads along the anatomical connections. The spread of TMS-evoked potentials depends on the excitability of neuronal populations at the time of stimulation. In fact, other studies have shown that sleep state-dependent changes in neural transmission occur in cortical networks; neuronal signal transmission is broken down during the slow wave (deep stage) sleep (Massimini et al. 2005). We invented an experimental paradigm to study the flexible nature of top-down control signals during visual attention. Subjects were presented with visual stimuli comprising a face image and a moving stripe (Fig. 16.4a). The subjects were required to discriminate either the gender of the face image or the direction of the moving stripe according to a task instruction cue, which was presented at the beginning of each trial. Because the visual stimuli always comprised a face and moving stripe, the bottom-up information was equivalent in both tasks. In addition, we manipulated the level of preparation by changing the time interval between the task instruction cue and the target visual stimuli. Thus, the subject could fully prepare for the forthcoming target visual stimuli in long preparation trials, but they could not prepare in the short cue–target interval trials. We then applied TMS on the FEF (frontal eye field), shown as a source of top-down signals in primate studies (Ekstrom et al. 2008; Moore and Armstrong 2003) and examined TMS-evoked potentials in visual areas. We showed that TMS-evoked EEG potentials in the occipital area and neural transmission from the FEF to the visual cortex were changed depending on the task engaged, and the change was observed only when the subject had a long preparation time (long preparation trials) (Fig. 16.4b). We have also shown that TMS-evoked potentials spread to the middle temporal (MT) area, which is specialized to process visual motion, when performing a motion discrimination task, whereas TMS-evoked potentials spread to the fusiform face area, which is specialized to process face information, when performing the face discrimination task (Fig. 16.4c). In sum, the results suggested that the signal transmission from the FEF to visual areas is flexibly controlled according to the task demands at hand.
Flexible prefrontal transmission to visual areas. (a) Tasks used in experiments. Subjects were asked to indicate either the direction of the grating motion or the gender of a face image according to a task cue. Between the task cue and target stimulus, there were two types of preparation, long and short. This allows for the manipulation of the level of preparation for the target stimulus. After 134 ms of target onset, a TMS pulse was delivered to the FEF. (b) TMS-evoked EEG potentials from the visual cortex. A TMS was delivered to the FEF (orange dot in the head map), and its response was recorded at occipital area P8 (red dot in the head map). TMS-evoked potentials are different between the face (thick blue line) and motion (thick red line) conditions in the long preparation trials, whereas the difference disappeared in the short preparation trials (thin lines). (c) TMS-evoked source brain activity was estimated in the motion processing MT area and face processing FFA area. TMS-evoked source activity in the MT area was enhanced in the motion task, whereas activity in the FFA was enhanced in the face task (Figures adapted and modified from Morishima et al. 2009)
3.4 Prefrontal-Visual Interactions Through Neural Oscillations
In the previous two sections, I overviewed the causal evidence that top-down control signals originating from the PFC modulate selective processing in visual areas during visual attention. The studies with macaque monkeys have shown that the FEF causally influences the activity of visual areas in the presence of visual stimuli. The studies with human subjects have shown that the FEF can flexibly control the prefrontal influence over visual areas according to task demands. The next question arising from these studies is how top-down signals are triggered and how interactions between the FEF and visual areas are established.
A study by Gregoriou and colleagues addressed this question (Gregoriou et al. 2009). They simultaneously recorded from the FEF and V4 visual area of macaque monkeys while the monkeys were performing a visual attention task. Then, they identified the receptive field of each recording site. When the receptive fields of both FEF and V4 recording sites were overlapped, neuronal coherence at the gamma frequency band (40–60 Hz) between the FEF and V4 was significantly increased. By contrast, the coherence was not modulated when the two receptive fields were not overlapped. The results are in fact consistent with the human EEG study that demonstrated occipital-frontal gamma-band coherence (Doesburg et al. 2008). Gregoriou and colleagues also examined the direction of influence between the FEF and V4 as a function of time by using Granger causality analysis. Granger causality analysis calculates time-lagged correlations, and it therefore provides the direction of influence from one time series to another. They found that the Granger causality from the FEF to V4 was significantly modulated by visual attention at 110 ms after the onset of the visual stimulus, whereas Granger causality from V4 to the FEF was modulated at 160 ms after the onset of the visual stimulus. The results suggest that attentional effects on top-down influence precede bottom-up influence. In summary, the PFC, in particular the FEF, is a major source of top-down signals that enhance the processing of relevant information at hand. Prefrontal influence followed by bottom-up influence at a gamma band frequency establishes the prefrontal-visual interactions.
4 Conclusion
In this chapter, I first showed that the primate brain is characterized by a considerably large volume of the PFC and overviewed the commonality and difference of prefrontal anatomical architecture among human and nonhuman primates. Those studies indicate that the human PFC is not qualitatively different but rather is quantitatively different from that of other primate species, which suggests that the human PFC would have the capacity to address more complex information compared to other primates, and the study of other primate species will contribute to the understanding of human cognition. In fact, as I discussed in the second part, human and primate experiments are successfully integrated to understand the neural mechanisms of visual attention. However, to understand cognitive processes unique to humans, we need to study the cognitive processes that involve the brain areas unique to humans, such as area 10 and area 44.
References
Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR (2010) The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214:495–517
Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Association, Washington, DC
Baddeley AD, Hitch GJ, et al (1974) Working memory. Psychol Learn Motiv 8:47–89
Badre D, D’Esposito M (2007) Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci 19:2082–2099
Betz W (1881) Ueber die feinere Structur der Gehirnrinde des Menschen. Zentralbl Med Wiss 19:193–195
Botvinick MM (2008) Hierarchical models of behavior and prefrontal function. Trends Cogn Sci 12:201–208
Brodmann K (1909) Vergleichende Lokalisationslehre der Groshirnrinde. Barth, Leipzig
Burgess PW, Gilbert SJ, Dumontheil I (2007) Function and localization within rostral prefrontal cortex (area 10). Philos Trans R Soc B Biol Sci 362:887–899
Burman KJ, Rosa MG (2009) Architectural subdivisions of medial and orbital frontal cortices in the marmoset monkey (Callithrix jacchus). J Comp Neurol 514:11–29
Burman KJ, Palmer SM, Gamberini M, Rosa MG (2006) Cytoarchitectonic subdivisions of the dorsolateral frontal cortex of the marmoset monkey (Callithrix jacchus), and their projections to dorsal visual areas. J Comp Neurol 495:149–172
Butler AB, Hodos W (2005) Comparative vertebrate neuroanatomy: evolution and adaptation. Wiley, New York
Cajal R (1904) Textura del sistema nervioso del hombre y de los vertebrados. Alicante Instituto de Neurociencias, Madrid
Cantalupo C, Hopkins WD (2001) Asymmetric Broca’s area in great apes. Nature 414:505–505. doi:10.1038/35107134
Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2012) Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex 23(11):2677–2689
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
de Almeida J, Mengod G (2008) Serotonin 1A receptors in human and monkey prefrontal cortex are mainly expressed in pyramidal neurons and in a GABAergic interneuron subpopulation: implications for schizophrenia and its treatment. J Neurochem 107:488–496
De Schotten MT, Dell’Acqua F, Valabregue R, Catani M (2012) Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48:82–96
Desimone R, Duncan J (1995) Neural mechanisms of selective visual attention. Annu Rev Neurosci 18:193–222
Doesburg SM, Roggeveen AB, Kitajo K, Ward LM (2008) Large-scale gamma-band phase synchronization and selective attention. Cereb Cortex 1991(18):386–396
Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W (2008) Bottom-up dependent gating of frontal signals in early visual cortex. Science 321:414–417
Elston GN (2003) Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex 13:1124–1138
Elston GN, Benavides-Piccione R, Elston A, Zietsch B, Defelipe J, Manger P, Casagrande V, Kaas JH (2006) Specializations of the granular prefrontal cortex of primates: implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol 288:26–35
Evrard HC, Forro T, Logothetis NK (2012) Von Economo neurons in the anterior insula of the macaque monkey. Neuron 74:482–489
Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G (2010) Relating introspective accuracy to individual differences in brain structure. Science 329:1541–1543
Frith U (1991) Autism and asperger syndrome. Cambridge University Press, Cambridge/New York
Frith CD, Frith U (2012) Mechanisms of social cognition. Annu Rev Psychol 63:287–313
Fujii N, Hihara S, Nagasaka Y, Iriki A (2009) Social state representation in prefrontal cortex. Soc Neurosci 4:73–84
Fuster JM (1988) Prefrontal cortex. Springer, Berlin
Goldman-Rakic PS (1996) The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci 351:1445–1453
Grabenhorst F, Rolls ET (2011) Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci 15:56–67
Gregoriou GG, Gotts SJ, Zhou H, Desimone R (2009) High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324:1207–1210
Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, Fiske AP (2004) Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage 21:1167–1173
Kane MJ, Engle RW (2002) The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev 9:637–671
Karafin MS, Tranel D, Adolphs R (2004) Dominance attributions following damage to the ventromedial prefrontal cortex. J Cogn Neurosci 16:1796–1804
Karim AA, Schneider M, Lotze M, Veit R, Sauseng P, Braun C, Birbaumer N (2010) The truth about lying: inhibition of the anterior prefrontal cortex improves deceptive behavior. Cereb Cortex 1991(20):205–213
Kastner S, Ungerleider LG (2000) Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23:315–341
Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999) Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–761
Koechlin E, Summerfield C (2007) An information theoretical approach to prefrontal executive function. Trends Cogn Sci 11:229–235
Mackey S, Petrides M (2010) Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. Eur J Neurosci 32:1940–1950
Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M (2009) Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106:20069–20074
Marois R, Ivanoff J (2005) Capacity limits of information processing in the brain. Trends Cogn Sci 9:296–305
Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G (2005) Breakdown of cortical effective connectivity during sleep. Science 309:2228–2232
Maunsell JH, Treue S (2006) Feature-based attention in visual cortex. Trends Neurosci 29:317–322
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202
Moore T, Armstrong KM (2003) Selective gating of visual signals by microstimulation of frontal cortex. Nature 421:370–373
Moran J, Desimone R (1985) Selective attention gates visual processing in the extrastriate cortex. Science 229:782–784
Morishima Y, Akaishi R, Yamada Y, Okuda J, Toma K, Sakai K (2009) Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci 12:85–91
Neubert F-X, Mars RB, Thomas AG, Sallet J, Rushworth MF (2014) Comparison of human ventral frontal cortex areas for cognitive control and language with areas in monkey frontal cortex. Neuron 81:700–713
Neubert F-X, Mars RB, Sallet J, Rushworth MFS (2015) Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc Natl Acad Sci USA 112(20):E2695–E2704
Nimchinsky EA, Vogt BA, Morrison JH, Hof PR (1995) Spindle neurons of the human anterior cingulate cortex. J Comp Neurol 355:27–37
Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR (1999) A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA 96:5268–5273
Öngür D, Price JL (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219
Passingham R (2008) What is special about the human brain? Oxford University Press, Oxford/New York
Passingham RE, Wise SP (2012) The neurobiology of the prefrontal cortex: anatomy, evolution, and the origin of insight. Oxford University Press, Oxford
Petrides M, Pandya DN (1999) Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11:1011–1036
Petrides M, Pandya DN (2002) Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16:291–310
Petrides M, Pandya DN (2007) Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci 27:11573–11586
Preuss T (1995) Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci 7:1–24
Ramnani N, Owen AM (2004) Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci 5:184–194
Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, Rudebeck P, Ciccarelli O, Richter W, Thompson AJ, et al (2006) The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from macaque monkeys and humans. Cereb Cortex 16:811–818
Real LA (1991) Animal choice behavior and the evolution of cognitive architecture. Science 253:980–986
Reynolds JH, Chelazzi L (2004) Attentional modulation of visual processing. Annu Rev Neurosci 27:611–647
Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TEJ (2008) The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11:426–428
Roth G, Dicke U (2005) Evolution of the brain and intelligence. Trends Cogn Sci 9:250–257
Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF (2006) A role for the macaque anterior cingulate gyrus in social valuation. Science 313:1310–1312
Rushworth MF, Behrens TE, Rudebeck PH, Walton ME (2007a) Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 11:168–176
Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM (2007b) Functional organization of the medial frontal cortex. Curr Opin Neurobiol 17:220–227
Sakai K (2008) Task set and prefrontal cortex. Annu Rev Neurosci 31:219–245
Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MFS (2011) Social network size affects neural circuits in macaques. Science 334:697–700
Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, Heinsen H, Schmitz C, Hof PR (2011) von Economo neurons in autism: a stereologic study of the frontoinsular cortex in children. Brain Res 1380:206–217
Schoenemann PT, Sheehan MJ, Glotzer LD (2005) Prefrontal white matter volume is disproportionately larger in humans than in other primates. Nat Neurosci 8:242–252. doi:10.1038/nn1394
Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW (2001) Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol 114:224–241
Semendeferi K, Lu A, Schenker N, Damasio H (2002) Humans and great apes share a large frontal cortex. Nat Neurosci 5:272–276. doi:10.1038/nn814
Sherman SM (2007) The thalamus is more than just a relay. Curr Opin Neurobiol 17:417–422
Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, et al (2006) Evolution of increased glia–neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA 103:13606–13611
Squire RF, Noudoost B, Schafer RJ, Moore T (2013) Prefrontal contributions to visual selective attention. Annu Rev Neurosci 36:451–466
Stanton GB, Bruce CJ, Goldberg ME (1995) Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol 353:291–305
Stuss DT, Knight RT (2013) Principles of frontal lobe function. Oxford University Press, Oxford/New York
Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y (1999) Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401:699–703
Tsujimoto S, Genovesio A, Wise SP (2011) Frontal pole cortex: encoding ends at the end of the endbrain. Trends Cogn Sci 15:169–176
Uylings HB, van Eden CG, et al (1990) Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res 85:31–62
Uylings HB, Groenewegen HJ, Kolb B (2003) Do rats have a prefrontal cortex? Behav Brain Res 146:3–17
Van Dongen PAM (1998) Brain size in vertebrates. In: The central nervous system of vertebrates. Springer, Berlin, pp 2099–2134
von Economo CF, Koskinas GR (1925) Die Cytoarchitektonik der Hirnrinde der erwachsenen Menschen. Springer, Berlin
Walker AE (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73:59–86
Wilczynski W (2009) Evolution of the brain in amphibians. In: Encyclopedia of neuroscience. Springer, Berlin, pp 1301–1305
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Morishima, Y. (2017). Prefrontal Anatomical Architecture and Top-Down Behavioral Control in Human and Nonhuman Primates. In: Shigeno, S., Murakami, Y., Nomura, T. (eds) Brain Evolution by Design. Diversity and Commonality in Animals. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56469-0_16
Download citation
DOI: https://doi.org/10.1007/978-4-431-56469-0_16
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56467-6
Online ISBN: 978-4-431-56469-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)