Abstract
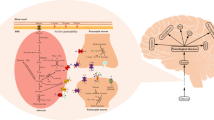
The discovery of endogenous d-serine and its brain-preferring and NMDA type glutamate receptor (NMDAR)-like distribution by the Nishikawa research group has become a milestone opening a new avenue in the research fields of not only d-amino acids but also the glutamate neurotransmission. This chapter consists of an overview and seven review articles describing the following current extension of our knowledge on different aspects of physiological functions and pathophysiology of d-serine in the central nervous system (CNS). In accordance with the finding preceded to the intrinsic d-serine detection that d-serine, but not l-serine, acts as a coagonist for the NMDAR by stimulating its strychnine-insensitive glycine site, d-serine, but not another coagonist glycine, has been shown to be required for full activation of the NMDAR in vitro and in vivo forebrain preparations. More recently, d-serine is suggested to be an endogenous ligand for the δ-type glutamate receptor in the cerebellum. These interactions have been considered to play a pivotal role in the regulation of the higher-order brain functions because glutamate neurotransmission is essential for their expression and control. The first half of this chapter depicts major progresses in the research about the molecular and cellular mechanisms that regulate the extracellular and intracellular concentrations and the target molecules of d-serine. The later half discusses pathophysiological consequences of disturbed d-serine metabolism and signaling and their relevance to clarify the biological basis of and to develop a novel therapy for neuropsychiatric disorders such as schizophrenia, ischemia, neurodegenerative disorders, and epilepsy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- N-Methyl-d-aspartate-type glutamate receptor
- δ2-type glutamate receptor
- d-Serine metabolism, function, and pathophysiology
- Neuropsychiatric disorders
The detection of the homeostatic presence of endogenous d-serine at high concentrations in mammalian brains by the Nishikawa research group (Hashimoto et al. 1992, 1993; Nishikawa 2011 for a review) was a groundbreaking discovery that required rethinking about the homochirality dogma that the free d-amino acid is uncommon in mammalian tissues. Until then, while d-amino acid residues of long-living proteins (Fujii et al. 2010 for a review) and the transient emergence of free d-aspartate during embryonic or early postnatal stages of development in brain and other organs in mammals (Dunlop et al. 1986; Neidle and Dunlop 1990) had been reported, these observations had been considered to be due to nonphysiological changes or an unusual phenomenon seen only in a very restricted developmental period. Endogenous d-serine has also attracted interests from the finding of its N-methyl-d-aspartate-type glutamate receptor (NMDAR)-like distribution in the brain that suggest the possibility of d-serine as a novel type of physiological modulator of the receptor (Hashimoto et al. 1992, 1993; Nishikawa 2011), because (1) d-serine had been known from earlier studies as a coagonist for the glycine modulatory site of the NMDAR that is needed for the activation of the NMDAR but does not produce the current of the receptor by itself (Danysz and Parsons 1998), (2) the NMDAR has been revealed to play essential roles in expression and regulation of the higher-order functions of the mammalian central nervous system (CNS) (Danysz and Parsons 1998), and (3) the pathophysiology of a variety of neuropsychiatric disorders such as brain ischemia, Alzheimer’s disease, and schizophrenia has been documented to be related to malfunction of the NMDAR (Nishikawa 2011). Since the first paper of the detection of endogenous brain d-serine published in 1992, enormous efforts have focused and advanced our knowledge on the molecular and cellular mechanisms underlying the metabolism and functions of d-serine and their pathological changes (Nishikawa 2011). It is notable that recent studies have revealed extra-NMDAR target molecules for d-serine (Nishikawa 2011). This chapter contains the seven review articles on the important contributions to the CNS free d-serine research by selected experts in this field.
The first three chapters will center the topics on the physiological metabolism and functions of d-serine. Synaptic dynamics and interactions with other CNS physiologically active substances of d-serine will also be reviewed in Chap. 6 in the second half of this chapter.
In the beginning Chap. 3, the Mothet group of CNRS of the Aix-Marseille University, France, will illustrate differential roles between d-serine and glycine in the glutamate synapse of the brain during postnatal development and in adult period. Before the advent of endogenous d-serine, glycine was believed to be the only NMDA receptor coagonist acting at its glycine modulatory site. In vitro experiments indicated that the allosteric regulation site of the NMDA was saturated by intrinsic glycine. However, this conjecture is challenged by the additional augmentation of the NMDA receptor function by exogenous application of glycine or d-serine based on both in vivo and in vitro experiments. On the other hand, the respective types of neurons and glia that synthesize, store, and release d-serine in the brain are still a debatable matter. To answer these fundamental questions, they will provide the data depicting how the two coagonists share the region-specific, NMDAR subunit-related, synaptic, or extrasynaptic area-directed, and development-dependent actions at the NMDAR in the rodent brains. The neuronal and glial components of these actions, their regulation mechanisms, and cognitive functional contexts will also be also extensively discussed.
In the next Chap. 4, the Konno group of the International University of Health and Welfare, Japan, will describe the significance of d-amino acid oxidase (DAO) in the degradation of d-serine and its behavioral consequences. Although the existence of d-amino acid oxidase in mammals was identified in 1935 (Krebs), indwelling d-amino acids as substrates for the enzyme had long been uncertain until the detection of various d-amino acids including d-serine following the advances in separation measurement technology for chiral amino acids. The spontaneously DAO activity-deficient mice that the Konno group found have significantly contributed to analyses to clarify the involvement of DAO in the decomposition of d-serine in vivo. They will review the changes in the d-serine concentrations in various CNS portions and peripheral organs and in motions and drug-induced and cognitive behavior in the DAO mutant mice.
In Chap. 5, Yuzaki’s group in Department of Physiology at Keio University, Japan, will present the fact that the δ-type glutamate receptors are second physiological binding targets for d-serine besides the NMDAR. The ligand-binding portion of the δ2-type glutamate receptor has been shown to bind d-serine and glycine with a low affinity (10−4 M order) (Naur et al. 2007). This observation fits with the presence of a specific non-NMDAR glycine site binding of [3H]d-serine in the brain (Matoba et al. 1997). Extremely higher contents of d-serine in the cerebellum during infancy compared to the adult period and cerebellum-preferring localization of the δ2 receptor have predicted a developmentally regulated functional link between the two molecules. They will summarize their results demonstrating that d-serine, but not glycine, plays a pivotal role through its action at the δ2 receptor during the induction of long-term depression that is associated with motor learning only in the neonatal, but not adult, period and that d-serine also interacts with another δ-type glutamate receptor, the δ1 receptor that is widely distributed in the brain. These findings extend a perspective to the endogenous d-serine functions.
The subsequent four chapters of this chapter will discuss the relationships between d-serine and neuropsychiatric disorders with special attention on schizophrenia, neurodegenerative diseases of Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), and epilepsy. The increased and decreased d-serine signalings have been postulated to underline the pathological NMDAR hypofunction and hyperfunction in schizophrenia and the latter three disorders, respectively.
In Chap. 6, Nishikawa’s group of the Tokyo Medical and Dental University, Japan, will review the link of d-serine with schizophrenia to the detection of endogenous d-serine and their pioneering achievements about the neuroanatomical and biochemical nature of endogenous d-serine, its metabolism and related molecules, and mode of control of tissue and extracellular d-serine concentrations, chiefly in vivo. The potential modifications of d-serine signaling in schizophrenia will be pointed out in terms of the data of the above pieces of the d-serine system in the light of biological fluids, postmortem CNS tissues, or brain imaging of patients with schizophrenia. Moreover, the therapeutic efficacy of d-serine for schizophrenic symptoms will be outlined, and several target molecule candidates for the development of a new treatment will be mentioned.
In Chap. 7, Coyle’s group of the Harvard Medical School, USA, will introduce their systematic approach to elucidate the pathological consequences of reduced d-serine signaling in schizophrenia by creating total serine racemase knockout mice. They will clearly delineate the abnormalities in the serine racemase-deficient mice that mimic those seen in schizophrenia. Furthermore, they will present the data obtained from their genetically engineered mice with the neuron- or astroglia-specific disruption of serine racemase, which provides a powerful clue to resolving the current disputes about cellular localization of d-serine and its synthesis, and analyze the disturbances of cell circuits in schizophrenia. A critical review about the clinical trials of d-serine and other NMDAR glycine site agonists that the Coyle group pioneered will also be included.
In Chap. 8, Mori’s group of Toyama University, Japan, will purvey important lines of information concerning the modulatory roles of the basal level of the d-serine signal in the neurotoxicity and hyperexcitability mediated by the NMDAR in the brain based on the studies of their original serine racemase totally deficient mice. These studies provided a clue to clarifying the exact in vivo mechanisms of the excessive NMDAR activity that causes neuronal cell death or degeneration and convulsive seizures. Furthermore, their perspective will be extended to the molecular basis of control of serine racemase activity and the positioning of the serine racemase-null mutant mice.
Finally, in Chap. 9, Sasabe’s group from the Department of Anatomy at Keio University, Japan, will summarize the findings obtained from animal models of and patients with ALS that suggest involvement of the aberrant d-serine metabolism in the loss of the motoneurons in ALS. By integrating the increased d-serine concentrations and/or DAO activity in ALS and its model, the association of the mutation of DAO with familiar ALS, and the degeneration of motoneurons in the mice with diminished DAO activity, they will discuss the plausible pathological pathways and a future therapy targeting the d-serine metabolism.
References
Danysz W, Parsons CG (1998) Glycine and N-methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50(4):597–664
Dunlop DS, Neidle A, McHale D et al (1986) The presence of free d-aspartic acid in rodents and man. Biochem Biophys Res Commun 141(1):27–32
Fujii N, Kaji Y, Fujii N et al (2010) Collapse of homochirality of amino acids in proteins from various tissues during aging. Chem Biodivers 7(6):1389–1397
Hashimoto A, Nishikawa T, Hayashi T et al (1992) The presence of free d-serine in rat brain. FEBS Lett 296:33–36
Hashimoto A, Nishikawa T, Oka T et al (1993) Endogenous d-serine in rat brain: N-Methyl-d-aspartate receptor-related distribution and aging. J Neurochem 60:783–786
Matoba M, Tomita U, Nishikawa T (1997) Characterization of 5, 7-dichlorokynurenate-insensitive [3H] d-serine binding to synaptosomal fraction isolated from rat brain tissues. J Neurochem 69:399–405
Naur P, Hansen KB, Kristensen AS et al (2007) Ionotropic glutamate-like receptor. delta2 binds d-serine and glycine. Proc Natl Acad Sci U S A 104(35):14116–14121
Neidle A, Dunlop DS (1990) Developmental changes in free d-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci 46(21):1517–1522
Nishikawa T (2011) Analysis of free d-serine in mammals and its biological relevance. J Chromatogr B Anal Technol Biomed Life Sci 879:3169–3183
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Nishikawa, T. (2016). Overview. In: Yoshimura, T., Nishikawa, T., Homma, H. (eds) D-Amino Acids. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56077-7_2
Download citation
DOI: https://doi.org/10.1007/978-4-431-56077-7_2
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56075-3
Online ISBN: 978-4-431-56077-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)