Abstract
Many functions of amino acids, including protein synthesis, require that they be in the l-form. As a result, in most biological systems, the levels of free d-amino acids (DAAs) are enzymatically suppressed. However, the site-specific synthesis, accumulation, and release of DAAs do occur. In fact, the accumulation of DAAs in the nervous, exocrine, and endocrine systems suggests that they perform specific functions. The focus here is on the well-studied DAA, d-aspartate; we review the advancements in the analytical approaches used for its detection and characterization and discuss the role it plays in the structural and functional organization of numerous biological systems of nonmammalian animals. The view that d-Asp has specific functions is supported by a large body of experimental data showing its endogenous synthesis, accumulation, release, stimulation of follower cells, uptake, and enzymatic catabolism. A variety of biological models, each having distinct anatomies, morphologies, biochemistries, and behaviors, have been used to investigate the fundamental mechanisms of d-Asp involvement in the normal and pathological functioning of cells and organisms. Many physiological and behavioral effects induced by d-Asp have been documented, demonstrating it has neurotransmitter, hormonal, and neuromodulator roles. Similar to many classical neurotransmitters, d-Asp has physiological roles that are conserved throughout the evolutionary tree, with nearly all studied animals shown to possess and use d-Asp.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- d-aspartate
- d-amino acids
- Bioanalytical methods
- Neuromodulation
- Neurotransmission
- Signaling molecule
- Central nervous system
1 Introduction
Aspartic acid (Asp), or 2-aminobutanedioic acid, is one of the nonessential proteinogenic (l-stereoisomer) endogenous α-amino acids. Of the common amino acids, Asp exhibits the lowest isoelectric point of 2.77. There are two conjugate bases for Asp, aspartate(1-) and aspartate(2-). As is true for most of the other proteinogenic amino acids, Asp has two enantiomers, an l-form and a d-form. The physical properties of both are identical, with the exception of the direction the substance rotates plane-polarized light. Despite reports on the parameters of d-aspartate (d-Asp) as early as 1852 (Pasteur 1852; Dalton and Schmidt 1933), and the finding of the degradative enzyme d-aspartate oxidase (d-AspO) in rabbit kidney and liver in 1949 (Still et al. 1949), for more than 100 years, only l-aspartate (l-Asp) was considered as an endogenous form of Asp in animals. However, the detection of significant amounts of free d-Asp in the mollusk Octopus vulgaris in 1977 (D’Aniello and Giuditta 1977) challenged this view.
Nonmammalian animal models are often used to investigate d-Asp involvement in the structural organization and functional activities of biological systems. This diverse group of organisms unites a variety of animal species—insects, mollusks, amphibians, reptiles, and birds—offering countless options for investigating the fundamental aspects of organismal function and evolution on different levels of organization, including cellular, tissue, organ, organismal, and biota. For example, the mollusk Aplysia californica possesses a well-characterized neuronal network, allowing for the experimental determination of functional links between the activity of specific neurons and behavior. The insect Drosophila melanogaster and roundworm Caenorhabditis elegans have genomes and transcriptomes that are amenable to extensive molecular manipulation, as well as short life spans, making them ideal for high-throughput investigations. These and other extensively characterized model systems make possible experiments that target diverse fundamental questions under controlled conditions. Not surprisingly, nonmammalian animal models have been widely used in d-Asp-related research to determine its localization, metabolism, and function, as well as its role in cell-to-cell signaling (D’Aniello and Giuditta 1977, 1978; D’Aniello et al. 1993b; Shibata et al. 2003; Miao et al. 2006a; Spinelli et al. 2006; Di Fiore et al. 1998; Raucci and Di Fiore 2010, 2011; Assisi et al. 2001).
d-Asp is one of several endogenous d-amino acids (DAAs) found throughout the metazoan. d-Alanine (d-Ala), d-asparagine (d-Asn), d-serine, d-glutamate (d-Glu), and d-glutamine (d-Gln) are also detected in different nonmammalian vertebrates and invertebrates, including cephalopods (D’Aniello and Giuditta 1977, 1978; D’Aniello et al. 2010), gastropods (D’Aniello et al. 1993b; Shibata et al. 2003; Miao et al. 2006a; Spinelli et al. 2006), agnathans (Villar-Cerviño et al. 2010), amphibians (Di Fiore et al. 1998; Raucci and Di Fiore 2011), and reptiles (Assisi et al. 2001; Raucci and Di Fiore 2010).
d-Asp has been detected in representatives from many phyla (for a review, see D’Aniello 2007), including the roundworm C. elegans (Saitoh et al. 2012), the common cuttlefish Sepia officinalis (D’Aniello and Giuditta 1978), the Cape rock lobster Jasus lalandii (Okuma and Abe 1994), the amphioxus Branchiostoma lanceolatum (D’Aniello and Garcia-Fernandez 2007), the sea squirt Ciona intestinalis (D’Aniello et al. 1992, 2003), the edible frog Rana esculenta (Di Fiore et al. 1998; Di Giovanni et al. 2010; Raucci et al. 2004, 2005); Raucci and Di Fiore 2011, the Italian wall lizard Podarcis s. sicula (Santillo et al. 2006), the European hake Merluccius merluccius, the common sole Solea solea (D’Aniello et al. 1995), and the chicken Gallus gallus domesticus (Kera et al. 1996). d-Asp has also been found in a variety of mammalian species. There are several recent reviews describing different aspects of d-Asp physiology, biochemistry, and pathology in mammals, an area of d-Asp research where investigations by Homma and his group have played a crucial role (Errico et al. 2012; Homma 2007; Ota et al. 2012; Di Fiore et al. 2014; Katane and Homma 2011; 2010; Errico et al. 2013; Furuchi and Homma 2005; Errico et al. 2009; Tverdislov et al. 2011).
Detectable amounts of d-Asp are predominantly localized in the neuronal, endocrine, and exocrine (Baccari et al. 2003) tissues and associated with cells producing significant amounts of secreted biochemicals, including cell-to-cell signaling molecules. For example, d-Asp has been found in the retina of octopus (D’Aniello et al. 2005), as well as in the liver, kidney, and brain of squid (D’Aniello et al. 2005, 2010) and frog (Di Fiore et al. 1998; Raucci et al. 2005; Burrone et al. 2010; Di Giovanni et al. 2010; Raucci and Di Fiore 2011; Santillo et al. 2013) and neuroendocrine cells of the sea hare (Miao et al. 2005, 2006a; Fieber et al. 2010; Scanlan et al. 2010; Wang et al. 2011), and lizard (Raucci 2005; Santillo et al. 2006; Raucci and Di Fiore 2010; Raucci and Fiore 2009). The tissue levels of free d-Asp typically increase in the earlier stages of an individual’s development and decrease with age (Neidle and Dunlop 1990).
The success of d-Asp research is partially due to progress in the development and application of a variety of analytical tools. One of the main challenges in the investigation of amino acid enantiomers is the similarity of most of their physicochemical properties. However, the difference in the shape of these molecules allows their chiral separation via affinity recognition or enzymatic transformation, enabling the detection and characterization of individual enantiomers. Utilization of complex approaches based on chiral high-performance liquid chromatography (LC)-laser-induced fluorescence (LIF), capillary electrophoresis (CE)-LIF, and immunohistochemical staining, in conjunction with clever experimental designs such as radionuclide pulse-chase experiments and d-AspO sample treatment, has allowed the studies of d-Asp synthesis, uptake, localization, and degradation with greater sensitivity, selectivity, and confidence. Using these and other technologies, the roles of d-Asp in neurotransmission, neuromodulation, and hormonal regulation have been investigated (for a review, see Ota et al. 2012), and d-Asp neurotransmitter function has been asserted (D’Aniello et al. 2010). The schematic in Fig. 12.1 depicts experimentally suggested mechanisms reported to be involved in d-Asp neurotransmission, including d-Asp endogenous synthesis and known and putative transporters and receptors, as well as enzymes that are required for signal termination.
Schematic of potential d-Asp involvement in synaptic signaling. Biosynthesis of d-Asp from l-Asp in cell soma by the action of aspartate racemase, release from presynaptic terminals, and interactions with receptors and transporters are depicted. Additionally, d-Asp involvement in signal transduction through triggering of an increase in cyclic adenosine monophosphate (cAMP) concentration in a postsynaptic neuron and signal termination via the d-aspartate oxidase-mediated degradation of d-Asp are also shown
We begin with a discussion of the development and implementation of analytical techniques used to investigate d-Asp, followed by information on d-Asp localization and metabolism, as well as its physiological functions in nonmammalian animals.
2 Analytical Techniques for d-Asp Detection and Characterization in Biological Systems
The physical and chemical properties of l- and d-Asp are virtually identical, aside from the direction in which they rotate plane-polarized light. Therefore, d-Asp research requires methods that are capable of measuring enantiomeric species. A variety of suitable approaches based on enzymatic assays, affinity recognition, and chiral separations, including CE and LC, have been introduced (Konno 2007). Here, the emphasis will be on the more commonly employed methods for analysis of d-Asp in biological systems.
2.1 Enzymatic Assays
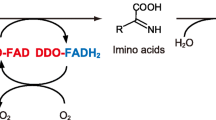
Due to their ability to alter the structure of d-Asp with high selectivity, enzymatic assays, often using the enzymes d-AspO and d-amino acid transaminase, are an effective approach for d-Asp analysis (D’Aniello and Giuditta 1977, 1978; Jones et al. 1994). For example, using a d-AspO-based colorimetric assay in which d-Asp was catabolized by d-AspO in the presence of flavin adenine dinucleotide, D’Aniello and Giuditta observed d-Asp in the nervous tissues of octopus and squid (D’Aniello and Giuditta 1977, 1978). This resulted in the formation of oxaloacetate, which was subsequently reacted with 2,4-dinitrophenylhydrazine to form a colored hydrazone compound, which was then quantified spectrophotometrically (D’Aniello et al. 1993c; Nagata et al. 1985). Enzymatic approaches are typically not specific for a single DAA, for example, d-AspO is known to also catabolize other DAAs and derivatives of DAAs, such as N-methyl-d-aspartate (NMDA), d-Glu, d-Asn, and d-Gln. Typically d-AspO exhibits different specificities for each substrate. Therefore, it is important to separate the DAAs prior to enzymatic analysis to prevent signal overlap, thus enabling the characterization and quantification of individual species.
2.2 Gas Chromatography (GC)
Due to the speed and resolving power of GC analysis, this rather mature separation technique has been widely used to separate amino acid enantiomers or their derivatives in complex mixtures. Chiral separations with GC are made possible by using columns where the common stationary phases are derivatized with compounds such as cyclodextrins (e.g., alkylated beta-cyclodextrins). Chiral columns in which DAAs elute before their l-forms, and vice versa, are commercially available and can be used with optically inactive derivatization reagents to facilitate detection (Brückner and Hausch 1993; Frank et al. 1977; Ali et al. 2010; Schieber et al. 1997). Additionally, traditional achiral columns can separate diastereomers formed by derivatization of d- and l-Asp with optically active reagents (Hoopes et al. 1978; Payan et al. 1985; Bertrand et al. 2008). However, it should be noted that derivatization with such reagents sometimes racemizes the amino acids (Payan et al. 1985).
Although there are a large number of detectors that can be utilized with GC, the flame ionization detector (FID) is one of the most widely used (Brückner and Hausch 1993; Frank et al. 1977; Hoopes et al. 1978; Payan et al. 1985; Skoog et al. 2007). More recently, the use of a mass spectrometer as a detector for GC, known as GC-mass spectrometry (MS), is an important approach. Compared to the FID, MS adds confidence to the analyte identification (Kaspar et al. 2008; Waldhier et al. 2010; Zampolli et al. 2007; Ali et al. 2010; Bertrand et al. 2008; Schieber et al. 1997; Waldhier et al. 2011). While providing an increased confidence in identification, it offers a poorer detectability compared to the FID; 300 nM is one example of GC-MS limits of detection (LODs) for d-Asp analysis (Kaspar et al. 2008).
2.3 Liquid Chromatography (LC)
Currently, LC is the most commonly used technique for DAA analyses due to its versatility, robustness, flexibility, and wide availability. As with GC, both chiral and achiral columns can be used. Chiral columns with stationary phases possessing optically active chemicals such as amino acids (Welch 1994), crown ethers (Shinbo et al. 1987), cyclodextrins (Armstrong et al. 1987), and proteins (Allenmark and Andersson 1991) are used in the separation of intact enantiomers. For separations with achiral columns, d- and l-Asp must first be derivatized with chemical modifiers such as o-phthalaldehyde (OPA) and/or chiral thiols like N-acyl-l-cysteine to form diastereomers (Aswad 1984; Brückner and Westhauser 2003; Buck and Krummen 1987; Nimura and Kinoshita 1986; Tsesarskaia et al. 2009; Saitoh et al. 2012). Two-dimensional high-performance LC separation using both achiral and chiral columns increases separation efficiency and simplifies analyte identification (Hamase et al. 2009; Miyoshi et al. 2012; Han et al. 2011).
Analyses of d-Asp by LC typically utilize fluorescence or MS for detection. Fluorescence detection allows for quantification with high sensitivity, for example, an LOD of 150 nM was achieved for OPA-derivatized compounds (Morikawa et al. 2001) and an LOD of 1 nM for 4-fluoro-7-nitrobenzofurazan-modified molecules (Han et al. 2011). Confidence in the identification of d-Asp in experiments relying on fluorescence-based detection can be enhanced by treating the sample with d-AspO, resulting in a specific decrease in the d-Asp peak area (Spinelli et al. 2006). Importantly, the use of MS detection adds information on the mass-to-charge ratios of the analytes. This information, along with retention time, aids analyte identification. The use of tandem MS (MS/MS) further enhances analyte identification capabilities (Song et al. 2008). The reported LOD for MS/MS detection of d-Asp is 1.20 μM (Song et al. 2007), which is poorer than obtained using LC with fluorescence-based detection or with GC-MS.
2.4 Capillary Electrophoresis (CE)
CE is yet another separation technique that has been used to analyze d-Asp in biological systems. This approach has a number of notable advantages, such as high separation efficiency, relatively short separation times, and low sample volumes. As with LC and GC, there are chiral and achiral separation modalities available (reviewed in (Wan and Blomberg 2000)). Chiral CE analyte separation, using micellar electrokinetic chromatography, for example, utilizes the interaction between enantiomers and chiral pseudostationary phases, with cyclodextrin and its various derivatives (Kitagawa and Otsuka 2011; Wan and Blomberg 2000) most commonly employed as the chiral selectors. Chiral surfactants such as deoxycholate (Terabe et al. 1989) and N-dodecoxycarbonylvaline (Swartz et al. 1995) are also capable of providing chiral separations in CE. The achiral separation is applicable in the analysis of diastereomeric derivatives of amino acids, which are produced by derivatization of analytes with optically pure reagents or chiral reagents like o-phthaldialdehyde with N-acetyl-cysteine (Kang and Buck 1992) or chiral thiols (Tivesten et al. 1997).
LIF has been the most commonly utilized detection modality in CE analyses of d-Asp. Ultraviolet absorption (Yang et al. 2010) and MS (Simo et al. 2005; Moini et al. 2003) are also used for d-Asp detection following CE separation, although with significantly lower sensitivity. When LIF detection is employed, the sample is initially derivatized with fluorescent reagents such as fluorescein isothiocyanate (Cheng and Dovichi 1988) or naphthalene-2,3-dicarboxaldehyde (Ueda et al. 1991). The typical LODs in CE-LIF analyses of d-Asp are on the pM level, which are sufficient for even subcellular analyses (Miao et al. 2005). In addition to comparing migration times using standards and enzymatic (d-AspO) treatments, immunoprecipitation of d-Asp increases the confidence in the d-Asp measurements (Miao et al. 2006b).
2.5 Immunohistochemistry
The previously described enzymatic assays, GC, LC, and CE, are useful for the characterization and quantification of endogenous free d-Asp; however, they do not allow for the determination of d-Asp’s distribution in a given system with high spatial resolution. In cases where details on analyte distribution are needed with high specificity, sensitivity, and spatial resolution, immunohistochemical staining is the method of choice. With proper tissue sample acquisition and fixation, and treatment with a selective antibody, information on the localization of d-Asp can be acquired. Accordingly, to enable the direct immunohistochemical analysis of d-Asp, selective anti-d-Asp antibodies have been developed using the glutaraldehyde conjugate of d-Asp as an antigen (Masuda et al. 2003; Lee et al. 1997; Schell et al. 1997). The obtained antibodies exhibit specificity only to the glutaraldehyde conjugate of d-Asp, and not to that of l-Asp, thus enabling specific immunohistochemical staining after tissue treatment with glutaraldehyde. The successful application and efficacy of the developed antibodies for the purpose of d-Asp visualization is shown in Fig. 12.2, which depicts immunostained neurons from Aplysia limacina showing differences in d-Asp localization from cell to cell. Specifically, the intensity of d-Asp immunostaining is distinct in the nucleus and the cytoplasm of different neurons, suggesting a unique function for d-Asp in different neuronal populations.
Paraffin section of a cerebral ganglion from Aplysia limacina immunostained with anti-d-Asp antibody. Immunohistochemical staining visualized the differential localization of d-Asp; some cells are stained in the (1) nucleus, others in the (2) cytoplasm, and others still in both the (3) cytoplasm and nucleus, and positive staining is also observed in the (arrows) neuropil (Modified with permission from Spinelli et al. 2006, copyright 2006, John Wiley & Sons)
2.6 Indirect Detection Approaches
The presence of d-Asp in a biological system can be inferred based on the presence of metabolic enzymes that are related to d-Asp, such as d-AspO and d-Asp racemase. These proteins can be detected with the use of affinity probes, which are commonly used in immunohistochemistry and related approaches such as immunoelectron microscopy, and the use of Western blotting and a range of molecular reporters such as protein coexpression with green fluorescent protein (Zaar 1996; Zaar et al. 2002; Yamamoto et al. 2011; Kim et al. 2010; Wang et al. 2011). These approaches are used to localize d-Asp-related proteins and hence, indirectly, the presence of d-Asp itself.
3 d-Asp Localization, Metabolism, and Fluxes
The application of the approaches described above has facilitated significant progress in characterizing and understanding the metabolism, concentration fluxes, and functions of d-Asp in neuronal, endocrine, and exocrine tissues. Information on the localization, fluxes, and levels of a given analyte in cells, tissues, and organs provides important clues on its putative function as well as its relation to various pathological states. The multiple mechanisms of d-Asp synthesis, uptake, spatial containment, release, and catabolism, as well as the different modes of regulation of extracellular and intracellular d-Asp levels, indicate that this molecule is an important player in the homeostatic balance of multicellular organisms (Homma 2007). These mechanisms also suggest the capability of d-Asp to mechanistically interfere with important biological processes such as protein synthesis.
3.1 Localization of d-Asp
Investigations of d-Asp localization have been carried out on vertebrate (Santillo et al. 2006; Di Giovanni et al. 2010) and invertebrate (D’Aniello et al. 2005; Scanlan et al. 2010) models as well as on cell cultures (Takigawa et al. 1998; Gadea et al. 2004; Adachi et al. 2004). These studies often involved immunohistochemical staining with anti-d-Asp antibodies in order to visualize d-Asp localization in cells and tissues and used a variety of analytical techniques to detect the presence of endogenous and radiolabeled d-Asp. Using these approaches, d-Asp has been detected in a large number of nervous, exocrine, and endocrine tissues of nonmammalian animals, including the gonads and Harderian glands of tiger prawn Penaeus esculentus (Di Fiore et al. 1998; Raucci et al. 2004; Di Giovanni et al. 2010) and the lizard P. s. sicula (Raucci 2005; Raucci and Fiore 2009), as well as the central nervous system of sea hare (Scanlan et al. 2010) and green frog (Santillo et al. 2013). Di Fiore et al. (2014) reviewed and summarized reports of d-Asp concentrations in endocrine and exocrine glands of several different species. Endogenous d-Asp concentrations in the exocrine Harderian glands of frog and lizard are 130 and 19 nmol/g tissue, respectively. Levels of d-Asp in endocrine glands are often similar to concentrations of the compound in exocrine glands. As examples (all in nmol/g tissue): the adrenal glands of chicken (23–30), pancreas of pigeon (15–18), chicken (6–10), frog (3–58), duck (11–23), and lizard (2–8 in ovaries and 3–30 in testes). Frog testes exhibit the highest levels of d-Asp at 140–236 nmol/g. d-Asp levels in the abovementioned glands are in the same concentration range as in the exocrine and endocrine glands of mammals, except for the rat pineal gland where its concentration may reach 3524 nmol/g tissue. Importantly, d-Asp concentrations in these glands may vary with age and functional state, as well as with the levels of d-Asp in the extracellular environment.
Free d-Asp formation and accumulation generally occur in a site-specific manner. For example, d-Asp was detected in the optic lobe of the cephalopod (D’Aniello et al. 2005), suggesting it may play a role in cephalopod vision. The detected d-Asp most likely had an endogenous origin. In other studies, d-Asp was found to form and accumulate in specific A. californica ganglia (Scanlan et al. 2010) and in the somata of selected neurons (D’Aniello et al. 2010).
3.2 Biosynthesis of d-Asp
There are several sources of d-Asp in organisms. Exogenous sources include the environment, e.g., seawater (Azua et al. 2014), food (Man and Bada 1987; Friedman and Levin 2012), and gut microbiota (Friedman 1999), whereas the endogenous sources are due to enzymatic conversion of l-Asp to d-Asp and the freeing of d-Asp from DAA-containing proteins. DAA residues of proteins are formed via enzymatic (Shikata et al. 1995) and nonenzymatic (Takahashi 2014; Stephenson and Clarke 1989) processes, which are often age dependent. The exogenous origin of some d-Asp is supported by results of experiments carried out in both mammalian and nonmammalian animals, which have demonstrated the absorption of this small molecule by the intestine and its transport to various solid tissues, including the brain, muscle, kidney, and liver (D’Aniello et al. 1993a). The enzymatic conversion of l- to d-Asp has been shown in a number of models, including pheochromocytoma cells (Long et al. 1998). The use of radiolabeled l-Asp in pharmacological, biological, and analytical studies has demonstrated that d-Asp can be formed from l-Asp via enzymatic activity in the nervous system, as shown in Fig. 12.3 (Scanlan et al. 2010). This observation corroborates findings of other studies performed on invertebrates such as European squid Loligo vulgaris (D’Aniello et al. 2005, 2010) and sea hare A. californica (Miao et al. 2006a) as well as vertebrates, e.g., the Italian wall lizard P. s. sicula (Raucci 2005; Raucci and Fiore 2009) and the edible frog Pelophylax esculentus (Raucci et al. 2004, 2005).
Biosynthesis of d-Asp in a cerebral ganglion of Aplysia californica. (a) Final percentages of radiolabeled Asp enantiomers after incubation of intact cerebral ganglion in [14C]-l-Asp, showing biosynthesis of d-Asp from l-Asp. (b) Final percentages of radiolabeled Asp enantiomers after incubation of desheathed cerebral hemiganglia and related sheath tissue in (1) [14C]-l-Asp and (2) [14C]-l-Asp, showing significantly larger conversion of [14C]-l-Asp to [14C]-d-Asp in the hemiganglion over the sheath tissue (Reprinted with permission from Scanlan et al. 2010, copyright 2010, Wiley-Blackwell)
In light of the findings from the aforementioned studies, it has been hypothesized that in nonmammalian animals, d-Asp is synthesized by enzymes with characteristics similar to known racemases and by DAA aminotransferases. One such d-Asp racemase has been cloned from the foot muscle of the clam Anadara broughtonii and subsequently purified and characterized (Shibata et al. 2003; Abe 2006). More recently, two pyridoxal 5′-phosphate-dependent d-Asp racemases were reported and characterized, one from the mouse brain (Kim et al. 2010). The other was from the head ganglia of A. californica (Wang et al. 2011); interestingly, this d-Asp racemase was found to also catalyze serine racemization.
In addition to d-Asp racemases, free d-Asp can be produced during the enzymatic degradation of DAA-containing peptides and proteins, perhaps where d-aspartyl endopeptidase (EC 3.4.99.B2) plays a role. DAA-containing peptides are typically formed by age-dependent posttranslational deamidation, isomerization, and racemization of l-aspartyl residues (Katane and Homma 2011; Geiger and Clarke 1987; Reissner and Aswad 2003). As a result, d-aspartyl and d-isoaspartyl residues are produced. While this mechanism may be responsible for only a small percentage of free d-Asp, it can impact d-Asp levels in some tissues and cells.
3.3 Catabolism of d-Asp
In addition to its formation, the ability to remove or degrade d-Asp is also important. For example, the catabolism of intercellular signals allows for signal termination and preparation for a new signaling event. d-Asp clearance combines diffusion away from the site of release, uptake by neurons or glia, and/or enzymatic modification, including breakdown. d-Asp catabolism is predominantly mediated by the enzyme d-AspO, a flavoenzyme which selectively catalyzes the oxidation of d-Asp, d-Glu, and NMDA to their corresponding α-keto acids (D’Aniello et al. 1993a). In contrast, the oxidative transformation of neutral and basic DAAs into α-keto acids is carried out by a different flavoenzyme, d-amino acid oxidase (DAAO). Both d-AspO and DAAO are reported in C. elegans (Katane et al. 2010; Saitoh et al. 2012) and L. vulgaris (D’Aniello et al. 2010) and are likely ubiquitous throughout the metazoan. Interesting distributions of these enzymes occur; for example, d-AspO is present in higher concentrations in L. vulgaris brain cytosol fractions.
d-AspOs from different species exhibit a variety of catalytic efficiencies. For example, multiple genes encoding functional d-AspOs have been found in C. elegans, each with unique specificities, kinetic parameters, and expected subcellular localizations (Katane et al. 2010). Although the physiological functions of the multiple d-AspOs may be redundant, the aforementioned differences in compartmentalization, concentration, and catalytic properties suggest the possibility of distinct roles for each enzyme. It is apparent that the existence of multiple d-AspOs with functional differences can allow for the modulation of local concentrations of d-Asp and thereby play integral roles in signaling, possibly by attenuating or terminating d-Asp signaling via its degradation.
Hypothetically, two other enzymes could influence the levels of free d-Asp. One is the protein-l-isoaspartate (d-aspartate) O-methyltransferase (EC 2.1.1.77), which is involved in the repair of age-related damage of protein aspartate and asparagine residues (Gomez et al. 2008). Another enzyme, d-amino acid transaminase (EC 2.6.1.21), catalyzes reactions in which d-Ala and d-Glu are formed from d-Asp. However, so far, the presence of this enzyme has only been reported in microorganisms and plants, e.g., Arabidopsis thaliana (Funakoshi et al. 2008).
The important role of d-AspO in the oxidative degradation of d-Asp as a consequence of its potential involvement in signaling pathways has led to investigations of the relationship between d-Asp levels and d-AspO activity in nonmammalian species. For example, intraperitoneal injection of d-Asp into green frog led to an increase of d-AspO activity in a variety of organs: brain, kidney, liver, pancreas, Harderian gland, heart, spleen, and testes (Di Giovanni et al. 2010; Burrone et al. 2010). In contrast, no increase in DAAO or l-amino acid oxidase activities were observed following d-Asp administration (Di Giovanni et al. 2010).
These data demonstrate that d-AspO may have multiple physiological purposes. It is possible that d-AspO is responsible for the general metabolism of endogenous and exogenous d-Asp to prevent the accumulation of high concentrations of d-Asp, which may have a negative impact on normal functioning of biological systems. Additionally, d-AspO may also be involved in the controlled degradation of d-Asp for the purposes of chemical signaling modulation, including signal termination.
3.4 d-Asp Flux
Understanding the movement of d-Asp into and out of specific locations, its flux, is important. Amino acid transporters such as solute carrier family 1 members 1–5 (also reported as excitatory amino acid transporters 1–5 (EAATs 1–5)) likely are involved in its import, mediated by the Na+/K+ electrochemical gradient (for reviews, see EMBL-EBI 2014; Boudko 2010; Stevens 2010; Krehbiel and Matthews 2003). For example, dEAAT1 and dEAAT2 are present in the nervous system of D. melanogaster (Umesh et al. 2003; Besson et al. 2000) (see Fig. 12.4). Drosophila sodium-dependent high-affinity aspartate transporter dEAAT2 has a much lower affinity to glutamate than d- or l-Asp. In another example, a single EAAT was expressed in the neuropil regions of the thoracic ganglia of the mosquito Aedes aegypti (Umesh et al. 2003). The transporter mediates high-affinity uptake of d-Asp as well as l-Asp and l-Glu, where both of the l-amino acids compete with d-Asp uptake. Therefore, the resulting excitatory amino acid import will depend on the extent and profile of transporter expression as well as levels of these compounds in the extracellular space, where d-Asp is typically present at lower concentrations. Not surprisingly, a large body of information on d-Asp transmembrane transport is available for mammalian models (for reviews, see Broer 2008; Saunders et al. 2013). This knowledge can be used in genomic and transcriptomic searches to aid the identification of molecular homologs in nonmammalian species.
Central nervous system expression patterns and transport properties of Drosophila melanogaster excitatory amino acid transporters (EAATs). Left panels: ventral views of Drosophila embryos stained with in situ hybridization with antisense (a) dEAAT1 or (b) dEAAT2 RNA probe. A differential expression pattern for dEAAT1 and dEAAT2 is apparent, with substantially higher expression levels for dEAAT1 in the embryonic nerve. Right panels: uptake of radiolabeled l-Glu and d-Asp into Drosophila S2 cells transfected with (a) dEAAT1 or (b) dEAAT2 cDNAs. Cells were transfected with (white bars, control) mock plasmid or (gray bars) Drosophila EAAT expression vectors. Selective transport of d-Asp relative to l-Glu is apparent for dEAAT2, whereas dEAAT1 shows comparable transport for the two excitatory amino acids (Reprinted with permission from Besson et al. 2000, copyright 2000, Elsevier)
d-Asp can be transported along nerves to release sites (Miao et al. 2006a; D’Aniello et al. 2010; Scanlan et al. 2010) and subsequently released upon stimulation. The vesicular nature of d-Asp release is also supported by the measurement of the subcellular localization of d-Asp in synaptosomes and synaptic vesicles of the nervous systems of A. limacina (Spinelli et al. 2006) and L. vulgaris (D’Aniello et al. 2010). Along with d-Asp, these synaptic vesicles possess relatively high levels of l-Asp and l-Glu, creating competitive conditions for neurotransmitter import by EAATs.
4 Physiological Functions of Free d-Asp
A number of roles for free d-Asp in central physiological processes on the cellular, tissue, organ, and organismal level have been suggested and in some cases experimentally confirmed. A large number of reports point to d-Asp operating as an environmental signal, neuromodulator, hormone, and neurotransmitter and being involved in mechanisms of the biosynthesis of hormones, endocrine and exocrine secretion (Monteforte et al. 2009), neurogenesis, memory (Topo et al. 2010), reproduction, vision, individual development, and stress (Erwan et al. 2012).
4.1 Neuronal Function
In neurons, d-Asp may generate an immediate cellular electrophysiological response through activation of the N-methyl-d-aspartate receptor (NMDAR), a type of ionotropic glutamate receptor (Foster and Fagg 1987), NMDAR-like receptors (Carlson and Fieber 2011), or sodium-dependent EAATs. The presence of other or unknown receptors and/or transporters that are specific to d-Asp is indicated by results of experiments where 25% of studied A. californica neurons exhibited d-Asp-induced currents but were not sensitive to l-Glu application (Carlson and Fieber 2011). Similarly, noticeable specificity to d-Asp is characteristic of Drosophila sodium-dependent dEAAT2 (Besson et al. 2000) (Fig. 12.4). Indeed, partial involvement of EAATs in the formation of d-Asp-induced l-Glu-independent cation currents in cultured A. californica neurons was demonstrated using a transporter blocker (Carlson et al. 2012).
The involvement of NMDA receptors in d-Asp-induced physiological and behavioral effects is supported by the results of experiments involving systemic in vivo intracerebroventricular administration of d-Asp to neonatal chicks. Specifically, when subjected to stressful conditions, d-Asp dose-dependent calming of the animals was observed, including a decrease in vocalization and an increase in the duration of time spent in resting positions with eyes open (Erwan et al. 2012). NMDA injection was also found to yield sedative effects (Yamane et al. 2009). The effects of l-Asp mimicked those of d-Asp; however, the periods of sleeplike state were observed to be longer, indicating possible mechanistic differences for the two enantiomers.
4.2 Environmental Signals
Decapod crustaceans such as the red rock crab (Cancer productus) and the Pacific rock crab (Romaleon antennarium) possess chemoreceptors located at their claw’s moveable fingers, or dactyls. Application of d-Asp induced an almost five times higher electrophysiological response in the corresponding nerve than application of l-Asp (Case 1964). Importantly, the response to d-Asp is pH dependent and not visibly modulated by the presence of l-Asp. It is plausible that d-Asp is present in seawater at nM concentrations; for example, the top layer of the ocean near Hawaii has been found to contain 8 nM of total dissolved d-Asp (Benner and Herndl 2011). This makes it apparent that d-Asp, along with other amino acids, may work as an environmental signal for marine animals. In the ocean environment, bacterioplankton are considered the main biotic source of d-Asp, and some other DAAs, and are found in the cell walls of these microorganisms (Perez et al. 2003; Azua et al. 2014).
4.3 Organism Development
d-Asp has been shown to play a role in development. In an early study, a transient increase in d-Asp levels during the embryonic stage in chicken was detected, with maximal concentration in the retina and brain between the 10th and 15th day of incubation (Neidle and Dunlop 1990). d-Asp in the chicken retina was observed to reach 20% of the total Asp levels at day 13. No similar changes in d-Asp content were found to occur in the heart, muscle, and liver during this stage of organism development. Additionally, C. elegans demonstrate a transient increase in d-Asp content, with a maximum level at the L3 larva stage (Saitoh et al. 2012). Interestingly, there is strong evidence to suggest that expression of the excitatory amino acid transporters dEAAT1 and dEAAT2 leads to formation of glia in the embryonic Drosophila central nervous system and may be involved in the generation of the functional diversity of these glial cells (Soustelle et al. 2002). The temporal alignment of such events may be significant for proper development of nervous system cellular circuitry.
4.4 Reproduction
The high concentration of d-Asp observed in the testes and ovaries of various nonmammalian animals suggests an involvement in reproduction (Di Fiore et al. 1998; Raucci et al. 2004; Raucci and Di Fiore 2011). Quantitative measurements of free d-Asp in the seasonal breeding green frog and Italian wall lizard uncovered a correlation between d-Asp levels and the phases of the reproductive cycle (Raucci and Di Fiore 2011) (Fig. 12.5). Moreover, d-Asp levels were found to correlate with androgen production in a sex- and species-dependent manner. d-Asp application in vivo enhanced expression of P450 aromatase mRNA and protein levels in male frog brain, which led to an increase in 17β-estradiol synthesis as well as a corresponding increase in expression of its receptor (Burrone et al. 2012). The modulatory effects of P450 aromatase expression were found to be a result of d-Asp participation in the CREB pathway. In a separate experiment, a d-Asp intraperitoneal injection was observed to modulate the levels of testosterone and 17β-estradiol in Italian wall lizard (Raucci 2005; Schell et al. 1997). The data made evident that d-Asp regulates steroidogenesis and spermatogenesis. The observed involvement of d-Asp in reproduction in a number of different species suggests that this particular functional role of d-Asp is likely evolutionarily conserved. For example, C. elegans has three functional d-AspOs: d-AspO-1, d-AspO-2, and d-AspO-3 (Katane et al. 2010). Modulation of d-AspO-1 expression alters d-Asp levels in animals, which correlates with changes in egg-laying capacity as well as hatching rate (Saitoh et al. 2012).
Free d-Asp is present in the ovary extract of Rana esculenta in each phase of the sexual cycle. The variations in d-Asp concentration depend on the phase of the reproductive cycle, with the highest d-Asp concentrations observed during the prereproductive period, at which time testosterone is at its minimum level. During the reproduction phase, d-Asp concentration decreases substantially, while the testosterone levels in the ovary and plasma increase greatly. During the post-reproductive period, the concentration of d-Asp moderately increases relative to the reproductive period, and the ovarian and plasma testosterone levels reduce to moderately low concentrations (Modified with permission from Raucci and Di Fiore 2011, copyright 2011 from Elsevier)
4.5 Vision
As mentioned earlier, another physiological function in which d-Asp may play a role relates to vision. This potential role for d-Asp is supported by its abundance in the retina of multiple animals, including the chicken (Neidle and Dunlop 1990) and common cuttlefish (D’Aniello et al. 2005). d-Asp was also found to be present in the optic lobe of S. officinalis (D’Aniello et al. 2005). There are several origins of d-Asp in the retina of this animal, including transport from the optical lobe as well as endogenous synthesis by the action of d-Asp racemase. The levels of d-Asp, l-Asp, l-Glu, and d-Asp racemase all decrease in both the retina and optic lobe after sustained light deprivation. However, other amino acids exhibit no change with regard to concentration. These observations strongly point toward an important role for d-Asp in vision.
5 Summary
d-Asp is an enigmatic molecule that has been observed throughout the metazoan, with a number of studies helping to define its formation, localization, and degradation. Uncovering the different roles of d-Asp has been made possible by the development and application of a number of measurement approaches that provide information on the specific chiral form of Asp in an animal tissue. The measurement approaches have included immunohistochemical staining, LC-LIF, and CE-LIF.
Now that such approaches exist, studies have turned to more functional questions. The use of nonmammalian animal models has enabled the characterization of d-Asp in organisms with well-understood nervous systems, such as A. californica, and in organisms with short life spans and well-characterized genomes, such as C. elegans. The result of the d-Asp research has been the thorough investigation of the biosynthesis, localization, metabolism, controlled degradation, and concentration fluxes of this DAA. Together, these findings provide a more complete understanding of free d-Asp and its involvement in important physiological processes, from the cellular to the organismal level. For example, the cell-to-cell signaling roles of free d-Asp have been demonstrated in a number of nonmammalian animals. In addition, several physiological and behavioral effects have been linked to d-Asp. Specifically, relationships to cell-to-cell and environment-to-organism signaling, as well as neurogenesis, reproduction, and vision, have been revealed.
References
Abe K (2006) Cloning and expression of the pyridoxal 5′-phosphate-dependent aspartate racemase gene from the bivalve mollusk Scapharca broughtonii and characterization of the recombinant enzyme. J Biochem 139(2):235–244. doi:10.1093/jb/mvj028
Adachi M, Koyama H, Long Z, Sekine M, Furuchi T, Imai K, Nimura N, Shimamoto K, Nakajima T, Homma H (2004) l-Glutamate in the extracellular space regulates endogenous d-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Arch Biochem Biophys 424(1):89–96. doi:10.1016/j.abb.2004.01.016
Ali H, Pätzold R, Brückner H (2010) Gas chromatographic determination of amino acid enantiomers in bottled and aged wines. Amino Acids 38(3):951–958. doi:10.1007/s00726-009-0304-1
Allenmark S, Andersson S (1991) Chiral amino acid microanalysis by direct optical resolution of fluorescent derivatives on BSA-based (resolvosil) columns. Chromatographia 31(9-10):429–433. doi:10.1007/BF02262384
Armstrong DW, Yang X, Han SM, Menges RA (1987) Direct liquid chromatographic separation of racemates with an alpha-cyclodextrin bonded phase. Anal Chem 59(21):2594–2596. doi:10.1021/ac00148a014
Assisi L, Botte V, D’Aniello A, Di Fiore MM (2001) Enhancement of aromatase activity by d-aspartic acid in the ovary of the lizard Podarcis s. Sicula. Reproduction 121(5):803–808. doi:10.1530/rep.0.1210803
Aswad DW (1984) Determination of d- and l-aspartate in amino acid mixtures by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. Anal Biochem 137(2):405–409. http://dx.doi.org/10.1016/0003-2697(84)90106-4
Azua I, Goiriena I, Bana Z, Iriberri J, Unanue M (2014) Release and consumption of d-amino acids during growth of marine prokaryotes. Microb Ecol 67(1):1–12. doi:10.1007/s00248-013-0294-0
Baccari G, Di Fiore M, Monteforte R, Raucci F, D’Aniello A (2003) d-aspartic acid induces merocrine secretion in the frog harderian gland. Rend Fis Acc Lincei 14(3):205–215. doi:10.1007/bf02904524
Benner R, Herndl GJ (2011) Bacterially derived dissolved organic matter in the microbial carbon pump. In: Jiao N, Azam F, Sanders S (eds) Microbial carbon pump in the ocean. Science/AAAS, Washington, DC, pp 46–48. doi:10.1126/science.opms.sb0001
Bertrand M, Chabin A, Brack A, Westall F (2008) Separation of amino acid enantiomers VIA chiral derivatization and non-chiral gas chromatography. J Chromatogr 1180(1–2):131–137. http://dx.doi.org/10.1016/j.chroma.2007.12.004
Besson MT, Soustelle L, Birman S (2000) Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr Biol 10(4):207–210
Boudko DY (2010) Molecular ontology of amino acid transport. In: Gerencser GA (ed) Epithelial transport physiology. Humana Press, Totowa, NJ, pp 379–472. doi:10.1007/978-1-60327-229-2_16
Broer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88(1):249–286. doi:10.1152/physrev.00018.2006
Brückner H, Hausch M (1993) Gas chromatographic characterization of free d-amino acids in the blood serum of patients with renal disorders and of healthy volunteers. J Chromatogr 614(1):7–17. http://dx.doi.org/10.1016/0378-4347(93)80218-S
Brückner H, Westhauser T (2003) Chromatographic determination of l- and d-amino acids in plants. Amino Acids 24(1–2):43–55. doi:10.1007/s00726-002-0322-8
Buck RH, Krummen K (1987) High-performance liquid chromatographic determination of enantiomeric amino acids and amino alcohols after derivatization with o-phthaldialdehyde and various chiral mercaptans: application to peptide hydrolysates. J Chromatogr 387(0):255–265. http://dx.doi.org/10.1016/S0021-9673(01)94529-7
Burrone L, Di Giovanni M, Di Fiore MM, Chieffi Baccari G, Santillo A (2010) Effects of d-aspartate treatment on d-aspartate oxidase, superoxide dismutase, and caspase 3 activities in frog (Rana esculenta) tissues. Chem Biodivers 7:1459–1466. doi:10.1002/cbdv.200900331
Burrone L, Santillo A, Pinelli C, Baccari GC, Di Fiore MM (2012) Induced synthesis of P450 aromatase and 17β-estradiol by d-aspartate in frog brain. J Exp Biol 215(20):3559–3565. doi:10.1242/jeb.073296
Carlson SL, Fieber LA (2011) Physiological evidence that d-aspartate activates a current distinct from ionotropic glutamate receptor currents in Aplysia californica neurons. J Neurophysiol 106(4):1629–1636. doi:10.1152/jn.00403.2011
Carlson SL, Kempsell AT, Fieber LA (2012) Pharmacological evidence that d-aspartate activates a current distinct from ionotropic glutamate receptor currents in Aplysia californica. Brain Behav 2(4):391–401. doi:10.1002/brb3.60
Case J (1964) Properties of the dactyl chemoreceptors of Cancer antennarius stimpson and C productus randall. Biol Bull 127(3):428–446. doi:10.2307/1539246
Cheng Y, Dovichi N (1988) Subattomole amino acid analysis by capillary zone electrophoresis and laser-induced fluorescence. Science 242(4878):562–564. doi:10.1126/science.3140381
D’Aniello A (2007) d-aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res Rev 53(2):215–234. doi:10.1016/j.brainresrev.2006.08.005
D’Aniello S, Garcia-Fernandez J (2007) d-aspartic acid and l-amino acids in the neural system of the amphioxus Branchiostoma lanceolatum. Amino Acids 32(1):21–26
D’Aniello A, Giuditta A (1977) Identification of d-aspartic acid in the brain of Octopus vulgaris Lam. J Neurochem 29(6):1053–1057. doi:10.1111/j.1471-4159.1977.tb06508.x
D’Aniello A, Giuditta A (1978) Presence of d-aspartate in squid axoplasm and in other regions of the cephalopod nervous system. J Neurochem 31(4):1107–1108. doi:10.1111/j.1471-4159.1978.tb00155.x
D’Aniello A, Vetere A, Padula L (1992) Occurrence of free d-amino acids in the gametes, embryos, larvae and adults of the sea-squirt Ciona intestinalis. Comp Biochem Phys B 102(4):795–797. doi:10.1016/0305-0491(92)90082-3
D’Aniello A, D’Onofrio G, Pischetola M, D’Aniello G, Vetere A, Petrucelli L, Fisher G (1993a) Biological role of d-amino acid oxidase and d-aspartate oxidase. Effects of d-amino acids. J Biol Chem 268:26941–26949
D’Aniello A, Nardi G, Vetere A, Ferguson GP (1993b) Occurrence of free d-aspartic acid in the circumsoesophageal ganglia of Aplysia fasciata. Life Sci 52(8):733–736. doi:10.1016/0024-3205(93)90235-u
D’Aniello A, Vetere A, Petrucelli L (1993c) Further study on the specificity of d-amino acid oxidase and of d-aspartate oxidase and time course for complete oxidation of d-amino acids. Comp Biochem Phys B 105(3–4):731–734. http://dx.doi.org/10.1016/0305-0491(93)90113-J
D’Aniello A, Nardi G, DeSantis A, Vetere A, diCosmo A, Marchelli R, Dossena A, Fisher G (1995) Free l-amino acids and d-aspartate content in the nervous system of Cephalopoda. A comparative study. Comp Biochem Phys B 112(4):661–666. doi:10.1016/0305-0491(95)00227-8
D’Aniello A, Spinelli P, De Simone A, D’Aniello S, Branno M, Aniello F, Fisher GH, Di Fiore MM, Rastogi RK (2003) Occurrence and neuroendocrine role of d-aspartic acid and N-methyl-d-aspartic acid in Ciona intestinalis. FEBS Lett 552(2–3):193–198. doi:10.1016/s0014-5793(03)00921-9
D’Aniello S, Spinelli P, Ferrandino G, Peterson K, Tsesarskia M, Fisher G, D’Aniello A (2005) Cephalopod vision involves dicarboxylic amino acids: d-aspartate, l-aspartate and l-glutamate. Biochem J 386:331–340. doi:10.1042/BJ20041070
D’Aniello S, Somorjai I, Garcia-Fernandez J, Topo E, D’Aniello A (2010) d-aspartic acid is a novel endogenous neurotransmitter. FASEB J 25(3):1014–1027. doi:10.1096/fj.10-168492
Dalton JB, Schmidt CLA (1933) The solubilities of certain amino acids in water, the densities of their solutions at twenty-five degrees, and the calculated heats of solution and partial molal volumes. J Biol Chem 103(2):549–578
Di Fiore MM, Assisi L, Botte V, D’Aniello A (1998) d-aspartic acid is implicated in the control of testosterone production by the vertebrate gonad. Studies on the female green frog, Rana esculenta. J Endocrinol 157(2):199–207. doi:10.1677/joe.0.1570199
Di Fiore MM, Santillo A, Chieffi Baccari G (2014) Current knowledge of d-aspartate in glandular tissues. Amino Acids 46(8):1805–1818. doi:10.1007/s00726-014-1759-2
Di Giovanni M, Burrone L, Chieffi Baccari G, Topo E, Santillo A (2010) Distribution of free d-aspartic acid and d-aspartate oxidase in frog Rana esculenta tissues. J Exp Zool A Ecol Genet Physiol 303:137–143. doi:10.1002/jez.585
EMBL-EBI (2014) GO:0070779 d-aspartate import. http://www.ebi.ac.uk/QuickGO/GTerm?id=GO:0070779#info=2&term=info. Accessed 30 Nov 2014
Errico F, Napolitano F, Nistico R, Centonze D, Usiello A (2009) d-aspartate: an atypical amino acid with neuromodulatory activity in mammals. Rev Neurosci 20(5–6):429–440
Errico F, Napolitano F, Nistico R, Usiello A (2012) New insights on the role of free d-aspartate in the mammalian brain. Amino Acids 43(5):1861–1871. doi:10.1007/s00726-012-1356-1
Errico F, Di Maio A, Marsili V, Squillace M, Vitucci D, Napolitano F, Usiello A (2013) Bimodal effect of d-aspartate on brain aging processes: insights from animal models. J Biol Regul Homeost Agents 27(2):49–59
Erwan E, Tomonaga S, Yoshida J, Nagasawa M, Ogino Y, Denbow DM, Furuse M (2012) Central administration of l- and d-aspartate attenuates stress behaviors by social isolation and CRF in neonatal chicks. Amino Acids 43(5):1969–1976. doi:10.1007/s00726-012-1272-4
Fieber LA, Carlson SL, Capo TR, Schmale MC (2010) Changes in d-aspartate ion currents in the Aplysia nervous system with aging. Brain Res 1343:28–36. doi:10.1016/j.brainres.2010.05.001
Foster AC, Fagg GE (1987) Comparison of l-[3H]glutamate, d-[3H]aspartate, dl-[3H]AP5 and [3H]NMDA as ligands for NMDA receptors in crude postsynaptic densities from rat brain. Eur J Pharmacol 133(3):291–300
Frank H, Nicholson GJ, Bayer E (1977) Rapid gas chromatographic separation of amino acid enantiomers with a novel chiral stationary phase. J Chromatogr Sci 15(5):174–176. doi:10.1093/chromsci/15.5.174
Friedman M (1999) Chemistry, nutrition, and microbiology of d-amino acids. J Agric Food Chem 47(9):3457–3479
Friedman M, Levin CE (2012) Nutritional and medicinal aspects of d-amino acids. Amino Acids 42(5):1553–1582. doi:10.1007/s00726-011-0915-1
Funakoshi M, Sekine M, Katane M, Furuchi T, Yohda M, Yoshikawa T, Homma H (2008) Cloning and functional characterization of Arabidopsis thaliana d-amino acid aminotransferase - d-aspartate behavior during germination. FEBS J 275(6):1188–1200. doi:10.1111/j.1742-4658.2008.06279.x
Furuchi T, Homma H (2005) Free d-aspartate in mammals. Biol Pharm Bull 28(9):1566–1570
Gadea A, López E, López-Colomé A (2004) Glutamate-induced inhibition of d-aspartate uptake in Müller glia from the retina. Neurochem Res 29(1):295–304. doi:10.1023/B:NERE.0000010458.45085.e8
Geiger T, Clarke S (1987) Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem 262(2):785–794
Gomez TA, Banfield KL, Clarke SG (2008) The protein l-isoaspartyl-O-methyltransferase functions in the Caenorhabditis elegans stress response. Mech Ageing Dev 129(12):752–758. doi:10.1016/j.mad.2008.09.019
Hamase K, Morikawa A, Etoh S, Tojo Y, Miyoshi Y, Zaitsu K (2009) Analysis of small amounts of d-amino acids and the study of their physiological functions in mammals. Anal Sci 25(8):961–968. doi:10.2116/analsci.25.961
Han H, Miyoshi Y, Ueno K, Okamura C, Tojo Y, Mita M, Lindner W, Zaitsu K, Hamase K (2011) Simultaneous determination of d-aspartic acid and d-glutamic acid in rat tissues and physiological fluids using a multi-loop two-dimensional HPLC procedure. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3196–3202. http://dx.doi.org/10.1016/j.jchromb.2011.01.023
Homma H (2007) Biochemistry of d-aspartate in mammalian cells. Amino Acids 32(1):3–11. doi:10.1007/s00726-006-0354-6
Hoopes EA, Peltzer ET, Bada JL (1978) Determination of amino acid enantiomeric ratios by gas liquid chromatography of the N-trifluoroacetyl-l-prolyl-peptide methyl esters. J Chromatogr Sci 16(11):556–560. doi:10.1093/chromsci/16.11.556
Jones WM, Ringe D, Soda K, Manning JM (1994) Determination of free d-amino acids with a bacterial transaminase: their depletion leads to inhibition of bacterial growth. Anal Biochem 218(1):204–209. http://dx.doi.org/10.1006/abio.1994.1161
Kang L, Buck RH (1992) Separation and enantiomer determination of OPA-derivatised amino acids by using capillary zone electrophoresis. Amino Acids 2(1–2):103–109. doi:10.1007/BF00806080
Kaspar H, Dettmer K, Gronwald W, Oefner PJ (2008) Automated GC–MS analysis of free amino acids in biological fluids. J Chromatogr B Analyt Technol Biomed Life Sci 870(2):222–232. http://dx.doi.org/10.1016/j.jchromb.2008.06.018
Katane M, Homma H (2010) d-aspartate oxidase: the sole catabolic enzyme acting on free d-aspartate in mammals. Chem Biodivers 7(6):1435–1449. doi:10.1002/cbdv.200900250
Katane M, Homma H (2011) d-aspartate – an important bioactive substance in mammals: a review from an analytical and biological point of view. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3108–3121. doi:10.1016/j.jchromb.2011.03.062
Katane M, Saitoh Y, Seida Y, Sekine M, Furuchi T, Homma H (2010) Comparative characterization of three d-aspartate oxidases and one d-amino acid oxidase from Caenorhabditis elegans. Chem Biodivers 7(6):1424–1434. doi:10.1002/cbdv.200900294
Kera Y, Aoyama H, Watanabe N, Yamada RH (1996) Distribution of d-aspartate oxidase and free d-glutamate and d-aspartate in chicken and pigeon tissues. Comp Biochem Physiol B: Biochem Mol Biol 115(1):121–126
Kim PM, Duan X, Huang AS, Liu CY, Ming GL, Song HJ, Snyder SH (2010) Aspartate racemase, generating neuronal d-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci U S A 107(7):3175–3179. doi:10.1073/pnas.0914706107
Kitagawa F, Otsuka K (2011) Recent progress in capillary electrophoretic analysis of amino acid enantiomers. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3078–3095, http://dx.doi.org/10.1016/j.jchromb.2011.03.016
Konno R (2007) d-amino acids: a new frontier in amino acids and protein research: practical methods and protocols. Nova Biomedical Books, New York
Krehbiel CR, Matthews JC (2003) Absorption of amino acids and peptides. In: D’Mello JPF (ed) Amino acids in animal nutrition, 2nd edn. CABI Publishers, Wallingford/Cambridge, MA, pp 41–70
Lee J-A, Homma H, Sakai K, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Yoshikawa M, Imai K (1997) Immunohistochemical localization of d-aspartate in the rat pineal gland. Biochem Biophys Res Commun 231(2):505–508, http://dx.doi.org/10.1006/bbrc.1996.5902
Long Z, Homma H, Lee J, Fukushima T, Santa T, Iwatsubo T, Yamada R, Imai K (1998) Biosynthesis of d-aspartate in mammalian cells. FEBS Lett 434(3):231–235
Man EH, Bada JL (1987) Dietary d-amino acids. Annu Rev Nutr 7:209–225. doi:10.1146/annurev.nu.07.070187.001233
Masuda W, Nouso C, Kitamura C, Terashita M, Noguchi T (2003) Free d-aspartic acid in rat salivary glands. Arch Biochem Biophys 420(1):46–54, http://dx.doi.org/10.1016/j.abb.2003.09.032
Miao H, Rubakhin SS, Sweedler JV (2005) Subcellular analysis of d-aspartate. Anal Chem 77(22):7190–7194. doi:10.1021/ac0511694
Miao H, Rubakhin SS, Scanlan CR, Wang LP, Sweedler JV (2006a) d-aspartate as a putative cell-cell signaling molecule in the Aplysia californica central nervous system. J Neurochem 97(2):595–606. doi:10.1111/j.1471-4159.2006.03891.x
Miao H, Rubakhin SS, Sweedler JV (2006b) Confirmation of peak assignments in capillary electrophoresis using immunoprecipitation. Application to d-aspartate measurements in neurons. J Chromatogr A 1106(1–2):56–60. doi:10.1016/j.chroma.2005.09.037
Miyoshi Y, Koga R, Oyama T, Han H, Ueno K, Masuyama K, Itoh Y, Hamase K (2012) HPLC analysis of naturally occurring free d-amino acids in mammals. J Pharm Biomed Anal 69:42–49, http://dx.doi.org/10.1016/j.jpba.2012.01.041
Moini M, Schultz CL, Mahmood H (2003) CE/electrospray ionization-MS analysis of underivatized d/l-amino acids and several small neurotransmitters at attomole levels through the use of 18-crown-6-tetracarboxylic acid as a complexation reagent/background electrolyte. Anal Chem 75(22):6282–6287. doi:10.1021/ac034708i
Monteforte R, Santillo A, Di Giovanni M, D’Aniello A, Di Maro A, Chieffi Baccari G (2009) d-aspartate affects secretory activity in rat Harderian gland: molecular mechanism and functional significance. Amino Acids 37(4):653–664. doi:10.1007/s00726-008-0185-8
Morikawa A, Hamase K, Inoue T, Konno R, Niwa A, Zaitsu K (2001) Determination of free d-aspartic acid, d-serine and d-alanine in the brain of mutant mice lacking d-amino-acid oxidase activity. J Chromatogr B Biomed Sci Appl 757(1):119–125, http://dx.doi.org/10.1016/S0378-4347(01)00131-1
Nagata Y, Akino T, Ohno K (1985) Microdetermination of serum d-amino acids. Anal Biochem 150(1):238–242, http://dx.doi.org/10.1016/0003-2697(85)90465-8
Neidle A, Dunlop DS (1990) Developmental changes in free d-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci 46(21):1517–1522. doi:10.1016/0024-3205(90)90424-P
Nimura N, Kinoshita T (1986) O-Phthalaldehyde—N-acetyl-L-cysteine as a chiral derivatization reagent for liquid chromatographic optical resolution of amino acid ernantiomers and its application to conventional amino acid analysis. J Chromatogr 352:169–177, http://dx.doi.org/10.1016/S0021-9673(01)83377-X
Okuma E, Abe H (1994) Simultaneous determination of d- and l-amino acids in the nervous tissues of crustaceans using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase ion-pair high-performance liquid chromatography. J Chromatogr B Biomed Appl 660(2):243–250. doi:10.1016/0378-4347(94)00304-1
Ota N, Shi T, Sweedler J (2012) d-aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 43(5):1873–1886. doi:10.1007/s00726-012-1364-1
Pasteur L (1852) Untersuchungen über Asparaginsäure und Aepfelsäure. Justus Liebigs Ann Chem 82(3):324–335. doi:10.1002/jlac.18520820306
Payan IL, Cadilla-Perezrios R, Fisher GH, Man EH (1985) Analysis of problems encountered in the determination of amino acid enantiomeric ratios by gas chromatography. Anal Biochem 149(2):484–491, http://dx.doi.org/10.1016/0003-2697(85)90603-7
Perez MT, Pausz C, Herndl GJ (2003) Major shift in bacterioplankton utilization of enantiomeric amino acids between surface waters and the ocean’s interior. Limnol Oceanogr 48(2):755–763
Raucci F (2005) Endocrine roles of d-aspartic acid in the testis of lizard Podarcis s. sicula. J Endocrinol 187(3):347–359. doi:10.1677/joe.1.06115
Raucci F, Di Fiore MM (2010) The maturation of oocyte follicular epithelium of Podarcis s. sicula is promoted by d-aspartic acid. J Histochem Cytochem 58(2):157–171. doi:10.1369/jhc.2009.954636
Raucci F, Di Fiore MM (2011) d-Asp: a new player in reproductive endocrinology of the amphibian Rana esculenta. J Chromatogr B Analyt Technol Biomed Life Sci 879(29):3268–3276. doi:10.1016/j.jchromb.2011.04.007
Raucci F, Fiore MMD (2009) The reproductive activity in the testis of Podarcis s. sicula involves d-aspartic acid: a study on c-kit receptor protein, tyrosine kinase activity and PCNA protein during annual sexual cycle. Gen Comp Endocrinol 161(3):373–383. doi:10.1016/j.ygcen.2009.02.002
Raucci F, Assisi L, D’Aniello S, Spinelli P, Botte V, Di Fiore MM (2004) Testicular endocrine activity is upregulated by d-aspartic acid in the green frog, Rana esculenta. J Endocrinol 182(2):365–376
Raucci F, Santillo A, D’Aniello A, Chieffi P, Baccari GC (2005) d-aspartate modulates transcriptional activity in Harderian gland of frog, Rana esculenta: morphological and molecular evidence. J Cell Physiol 204(2):445–454. doi:10.1002/jcp.20316
Reissner K, Aswad D (2003) Deamidation and isoaspartate formation in proteins: unwanted alterations or surreptitious signals? Cell Mol Life Sci 60(7):1281–1295
Saitoh Y, Katane M, Kawata T, Maeda K, Sekine M, Furuchi T, Kobuna H, Sakamoto T, Inoue T, Arai H, Nakagawa Y, Homma H (2012) Spatiotemporal localization of d-amino acid oxidase and d-aspartate oxidases during development in Caenorhabditis elegans. Mol Cell Biol 32(10):1967–1983. doi:10.1128/MCB.06513-11
Santillo A, Monteforte R, Raucci F, D’Aniello A, Baccari GC (2006) Occurrence of d-aspartate in the harderian gland of Podarcis s. sicula and its effect on gland secretion. J Exp Zool A Comp Exp Biol 305A(8):610–619. doi:10.1002/jez.a.301
Santillo A, Pinelli C, Burrone L, Chieffi Baccari G, Di Fiore MM (2013) d-aspartic acid implication in the modulation of frog brain sex steroid levels. Gen Comp Endocrinol 181:72–76. doi:10.1016/j.ygcen.2012.11.003
Saunders NR, Daneman R, Dziegielewska KM, Liddelow SA (2013) Transporters of the blood-brain and blood-CSF interfaces in development and in the adult. Mol Aspects Med 34(2–3):742–752. doi:10.1016/j.mam.2012.11.006
Scanlan C, Shi T, Hatcher NG, Rubakhin SS, Sweedler JV (2010) Synthesis, accumulation, and release of d-aspartate in the Aplysia californica CNS. J Neurochem 115(5):1234–1244. doi:10.1111/j.1471-4159.2010.07020.x
Schell MJ, Cooper OB, Snyder SH (1997) d-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci U S A 94(5):2013–2018
Schieber A, Brückner H, Rupp-Classen M, Pecht W, Nowitzki-Gfimm S, Classen HG (1997) Evaluation of d-amino acid levels in rat by gas chromatography-selected ion monitoring mass spectrometry: no evidence for subacute toxicity of orally fed d-proline and d-aspartic acid. J Chromatogr B Biomed Sci Appl 691(1):1–12, http://dx.doi.org/10.1016/S0378-4347(96)00378-7
Shibata K, Watanabe T, Yoshikawa H, Abe K, Takahashi S, Kera Y, Yamada RH (2003) Purification and characterization of aspartate racemase from the bivalve mollusk Scapharca broughtonii. Comp Biochem Physiol B: Biochem Mol Biol 134(2):307–314. doi:10.1016/s1096-4959(02)00267-1
Shikata Y, Watanabe T, Teramoto T, Inoue A, Kawakami Y, Nishizawa Y, Katayama K, Kuwada M (1995) Isolation and characterization of a peptide isomerase from funnel-web spider venom. J Biol Chem 270(28):16719–16723. doi:10.1074/jbc.270.28.16719
Shinbo T, Yamaguchi T, Nishimura K, Sugiura M (1987) Chromatographic separation of racemic amino acids by use of chiral crown ether-coated reversed-phase packings. J Chromatogr 405:145–153, http://dx.doi.org/10.1016/S0021-9673(01)81756-8
Simo C, Rizzi A, Barbas C, Cifuentes A (2005) Chiral capillary electrophoresis-mass spectrometry of amino acids in foods. Electrophoresis 26(7–8):1432–1441. doi:10.1002/elps.200406199
Skoog DA, Holler FJ, Crouch SR (2007) Principles of instrumental analysis, 6th edn. Thomson Brooks/Cole, Belmont
Song Y, Liang F, Liu Y-M (2007) Quantification of d-amino acids in the central nervous system of Aplysia californica by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 21(1):73–77. doi:10.1002/rcm.2803
Song Y, Feng Y, Lu X, Zhao S, Liu CW, Liu YM (2008) d-amino acids in rat brain measured by liquid chromatography/tandem mass spectrometry. Neurosci Lett 445(1):53–57. doi:10.1016/j.neulet.2008.08.058
Soustelle L, Besson MT, Rival T, Birman S (2002) Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev Biol 248(2):294–306
Spinelli P, Brown ER, Ferrandino G, Branno M, Montarolo PG, D’Aniello E, Rastogi RK, D’Aniello B, Baccari GC, Fisher G, D’Aniello A (2006) d-aspartic acid in the nervous system of Aplysia limacina: possible role in neurotransmission. J Cell Physiol 206(3):672–681. doi:10.1002/jcp.20513
Stephenson RC, Clarke S (1989) Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J Biol Chem 264(11):6164–6170
Stevens BR (2010) Amino acid transport by epithelial membranes. In: Gerencser GA (ed) Epithelial transport physiology. Humana Press, Totowa, NJ, pp 353–378. doi:10.1007/978-1-60327-229-2_15
Still JL, Buell MV, Knox WE, Green DE (1949) Studies on the cyclophorase system; d-aspartic oxidase. J Biol Chem 179(2):831–837
Swartz ME, Mazzeo JR, Grover ER, Brown PR (1995) Separation of amino acid enantiomers by micellar electrokinetic capillary chromatography using synthetic chiral surfactants. Anal Biochem 231(1):65–71, http://dx.doi.org/10.1006/abio.1995.1504
Takahashi O (2014) Just three water molecules can trigger the undesired nonenzymatic reactions of aspartic acid residues: new insight from a quantum-chemical study. J Phys Conf Ser 490:012147. doi:10.1088/1742-6596/490/1/012147
Takigawa Y, Homma H, Lee JA, Fukushima T, Santa T, Iwatsubo T, Imai K (1998) d-aspartate uptake into cultured rat pinealocytes and the concomitant effect on l-aspartate levels and melatonin secretion. Biochem Biophys Res Commun 248(3):641–647. doi:10.1006/bbrc.1998.8971
Terabe S, Shibata M, Miyashita Y (1989) Chiral separation by electronkinetic chromatography while bile salt micelles. J Chromatogr 480:403–411, http://dx.doi.org/10.1016/S0021-9673(01)84309-0
Tivesten A, Lundqvist A, Folestad S (1997) Selective chiral determination of aspartic and glutamic acid in biological samples by capillary electrophoresis. Chromatographia 44(11–12):623–633. doi:10.1007/Bf02466666
Topo E, Soricelli A, Di Maio A, D’Aniello E, Di Fiore MM, D’Aniello A (2010) Evidence for the involvement of d-aspartic acid in learning and memory of rat. Amino Acids 38(5):1561–1569. doi:10.1007/s00726-009-0369-x
Tsesarskaia M, Galindo E, Szókán G, Fisher G (2009) HPLC determination of acidic d-amino acids and their N-methyl derivatives in biological tissues. Biomed Chromatogr 23(6):581–587. doi:10.1002/bmc.1156
Tverdislov VA, Yakovenko LV, Ivlieva AA, Tverdislova IL (2011) Ionic and chiral asymmetries as physical factors of biogenesis and ontogenesis. Mosc U Phys B+ 66(2):105–115. doi:10.3103/S0027134911020184
Ueda T, Kitamura F, Mitchell R, Metcalf T, Kuwana T, Nakamoto A (1991) Chiral separation of naphthalene-2,3-dicarboxaldehyde-labeled amino acid enantiomers by cyclodextrin-modified micellar electrokinetic chromatography with laser-induced fluorescence detection. Anal Chem 63(24):2979–2981. doi:10.1021/ac00024a033
Umesh A, Cohen BN, Ross LS, Gill SS (2003) Functional characterization of a glutamate/aspartate transporter from the mosquito Aedes aegypti. J Exp Biol 206(Pt 13):2241–2255
Villar-Cerviño V, Barreiro-Iglesias A, Rodicio MC, Anadón R (2010) d-serine is distributed in neurons in the brain of the sea lamprey. J Comp Neurol 518(10):1688–1710. doi:10.1002/cne.22296
Waldhier MC, Dettmer K, Gruber MA, Oefner PJ (2010) Comparison of derivatization and chromatographic methods for GC–MS analysis of amino acid enantiomers in physiological samples. J Chromatogr B Analyt Technol Biomed Life Sci 878(15–16):1103–1112, http://dx.doi.org/10.1016/j.jchromb.2010.03.021
Waldhier MC, Almstetter MF, Nürnberger N, Gruber MA, Dettmer K, Oefner PJ (2011) Improved enantiomer resolution and quantification of free d-amino acids in serum and urine by comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J Chromatogr 1218(28):4537–4544, http://dx.doi.org/10.1016/j.chroma.2011.05.039
Wan H, Blomberg LG (2000) Chiral separation of amino acids and peptides by capillary electrophoresis. J Chromatogr 875(1–2):43–88, http://dx.doi.org/10.1016/S0021-9673(99)01209-1
Wang LP, Ota N, Romanova EV, Sweedler JV (2011) A novel pyridoxal 5′-phosphate-dependent amino acid racemase in the Aplysia californica central nervous system. J Biol Chem 286(15):13765–13774. doi:10.1074/jbc.M110.178228
Welch CJ (1994) Evolution of chiral stationary phase design in the Pirkle laboratories. J Chromatogr 666(1–2):3–26, http://dx.doi.org/10.1016/0021-9673(94)80367-6
Yamamoto A, Tanaka H, Ishida T, Horiike K (2011) Immunohistochemical localization of d-aspartate oxidase in porcine peripheral tissues. Amino Acids 41(2):529–536. doi:10.1007/s00726-010-0785-y
Yamane H, Tsuneyoshi Y, Denbow DM, Furuse M (2009) N-methyl-d-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors involved in the induction of sedative effects under an acute stress in neonatal chicks. Amino Acids 37(4):733–739. doi:10.1007/s00726-008-0203-x
Yang L, Chen CJ, Liu X, Shi J, Wang GA, Zhu LD, Guo LP, Glennon JD, Scully NM, Doherty BE (2010) Use of cyclodextrin-modified gold nanoparticles for enantioseparations of drugs and amino acids based on pseudostationary phase-capillary electrochromatography. Electrophoresis 31(10):1697–1705. doi:10.1002/elps.200900541
Zaar K (1996) Light and electron microscopic localization of d-aspartate oxidase in peroxisomes of bovine kidney and liver: an immunocytochemical study. J Histochem Cytochem 44(9):1013–1019. doi:10.1177/44.9.8773567
Zaar K, Köst H-P, Schad A, Völkl A, Baumgart E, Fahimi HD (2002) Cellular and subcellular distribution of d-aspartate oxidase in human and rat brain. J Comp Neurol 450(3):272–282. doi:10.1002/cne.10320
Zampolli MG, Basaglia G, Dondi F, Sternberg R, Szopa C, Pietrogrande MC (2007) Gas chromatography–mass spectrometry analysis of amino acid enantiomers as methyl chloroformate derivatives: application to space analysis. J Chromatogr 1150(1–2):162–172. http://dx.doi.org/10.1016/j.chroma.2006.12.033
Acknowledgments
This work was supported by the National Science Foundation (NSF), Division of Chemistry, under grant CHE-11-11705 (with co-funding from the Division of Biological Infrastructure), by Award No. RO1 NS031609 from the National Institute of Neurological Disorders and Stroke (NINDS) and Award Number P30 DA018310 from the National Institute on Drug Abuse (NIDA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSF, NINDS, NIDA, or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Patel, A.V., Kawai, T., Rubakhin, S.S., Sweedler, J.V. (2016). Free d-Aspartate in Nonmammalian Animals: Detection, Localization, Metabolism, and Function. In: Yoshimura, T., Nishikawa, T., Homma, H. (eds) D-Amino Acids. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56077-7_12
Download citation
DOI: https://doi.org/10.1007/978-4-431-56077-7_12
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56075-3
Online ISBN: 978-4-431-56077-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)