Abstract
The discovery of iPS indicated that overexpression of master transcriptional factors might change cell fate. Recent developments in reprogramming methods have shown that somatic cells can be directly reprogrammed to various kinds of neuronal cells directly. Moreover, overexpression of a neuron-specific transcriptional factor with a viral vector can change the fate of endogenous glial cells to neuronal cells in vivo. In this chapter, we discuss the advantages, issues, and possibility for clinical application of these reprogramming methods for cell transplantation/replacement therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
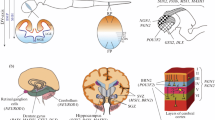
The number of elderly people is continuously increasing in the industrialized nations of the world, causing an increase in the number of patients that suffer from ischemic stroke. Stroke is the second leading cause of death in the world and results in a drastic reduction in the quality of life. On the other hand, effective therapeutic methods are currently very limited, especially in the chronic phase of a stroke; therefore, a novel therapeutic strategy for the chronic phase of a stroke is now urgently required. Recently, the discovery of ES and iPS seems to have opened the gate for stroke regenerative therapy. In addition, novel ways of inducing neuronal cells with direct reprogramming methods, such as induced neuronal stem cells (iNSCs) and induced neuronal cells (iNCs), have been reported (Fig. 4.1).
Summary of induction of iPSCs, iNSCs, and iNCs and direct in vivo reprogramming (Modified from Yamashita et al. [28]). (a) Overexpression of Oct3/4, Sox2, Klf4, and c-Myc can convert somatic cells such as skin fibroblasts into iPSCs. Neuronal cells can be obtained after differentiation in the cell culture system. (b) Overexpression of Sox2 with other factors can convert skin fibroblasts into iNSCs. Both neuronal and glial lineages can be obtained from iNSCs. (c) The combination of Asc1, Brn2, and Myt1l with other factors can directly convert skin fibroblasts into iNCs (direct reprogramming methods). (d) Overexpression of NeuroD1 with other factors can convert endogenous glial cells into neuronal cells in vivo (in vivo direct reprogramming methods)
In this chapter, we briefly review recent progress of cell transplantation/replacement therapy with iPSCs/iNSCs/iNCs alongside our recent findings.
2 Therapeutic Effect of Transplantation of Human IPS Cells in an Animal Model
In 2006, Prof. Yamanaka first established murine iPSCs by overexpressing four transcriptional factors (Oct3/4, Sox2, c-Myc, and Klf4) in mouse fibroblasts. Of note, they found that these key transcription factors (TFs) from 20 candidates were strongly expressed in embryonic stem cells (ESCs) [1]. iPSCs can retain high replication competence and pluripotency and can differentiate into various kinds of cells, similar to ESCs, indicating that overexpression of key TFs can change cell fate. Since iPSCs can be produced from a patient’s skin fibroblasts, there are no immunoreactive and/or ethical issues associated with ESCs. Therefore, iPSCs are believed to be a promising cell resource for cell transplantation/replacement therapy. Several scientific papers have demonstrated that human iPS-derived neuronal stem cells/neuronal progenitors, when transplanted into the stroke murine model brain, showed a therapeutic effect such as the recovery of motor function (Table 4.1). Notably, Oki et al. generated long-term self-renewing neuroepithelial-like stem cells from adult human fibroblast-derived iPSCs and transplanted them into the stroke mouse model. They found that motor function had already recovered by the first week after transplantation. They also confirmed that transplanted cells survived without forming tumors for at least 4 months. In their experiment, functional recovery was observed soon after cell transplantation, and the observed therapeutic effect was regarded to be derived from a neurotrophic effect caused by the release of transplanted cells [2].
3 Discovery of iN Cells
Some Japanese research groups have started or plan to conduct clinical transplantation therapy trials using iPS cells for age-related macular degeneration, spinal cord injury, and Parkinson disease [3]. However, iPS cells can form tumors, especially in pathological conditions such as poststroke [4]. In addition, it is likely to be difficult to monitor tumor formation for more than 2 years, even if iPS cells are transplanted into a mouse model. Therefore, a new technology and strategy to induce neuronal cells in damaged brains is required. Research findings using iPS suggest that master TFs regulating the overexpression of ES cells could convert fibroblasts to ES cell-like iPS cells. From this finding, many researchers have overexpressed neuron-specific TFs in skin/lung fibroblasts and tried to convert these fibroblasts into neuronal cells. In 2010, Wernig et al. first established murine-induced neuronal cells (iNCs) by introducing three neuron-specific TFs (Ascl1, Brn2, and Myt1l) into mouse fibroblasts. They found that these iNCs showed a glutamatergic neuronal phenotype with synapses and action potential, as recorded by electric patch-clump analysis [5]. Various kinds of iNCs, including dopaminergic neurons and motor neurons, have been reported (Table 4.2). Interestingly, Ascl1 appears to be a key factor in the induction of iN cells, and the specific combination of Ascl1 plus other factors can convert somatic cells to specific neuronal cells. In cell transplantation therapy, it has already been reported that induced dopaminergic neurons showed a therapeutic effect against 6-hydroxydopamine (6-OHDA)-treated rats by attenuating the level of striatal dopamine [6]. iNCs can be produced without passing through the multipotent stem cell linage as iPS cells can be regarded as safer and easier to induce within a relatively short time frame, compared with iPS cells. However, the cell cycle of iN cells stops during cell conversion, making it difficult to prepare sufficient quantities of iNCs for cell transplantation therapy. To overcome this problem, induced neuronal stem cells (iNSCs) were developed. In 2012, Han et al. demonstrated that a combination of TFs (Sox2, Brn4, Klf4, c-Myc) successfully induced mouse fibroblasts directly to iNSCs [7]. Han and collaborators evaluated the therapeutic effect of cell transplantation using iNSCs in the spinal cord injury rat model. They found that engrafted iNSCs could differentiate into neuronal lineages forming synapses and enhancing the recovery of locomotor function [8]. iNSCs can thus be regarded as a promising cell resource for cell transplantation/replacement therapy.
4 Development of iN Cell Technology
Recently, novel technologies and new findings in the field of iNCs are reported every year. In particular, in vivo direct conversion technology and chemical-induced neuronal cells are attracting the most attention. In a clinical setting, the culture medium, including bovine/calf serum, can be problematic as they may be infectious materials in the human body. Thus, if endogenous non-neuronal cells such as astroglia can be converted to required neurons, in vivo direct conversion technology could be a new, simple, and straightforward way of supplying required new neuronal cells to the human brain. Thus far, astroglia as well as pericytes have been reported to be directly reprogrammed into neuronal cells in cell culture systems [9, 10]. In 2013, Torper et al. showed that endogenous mouse astroglia could be converted into NeuN-positive neuronal cells in vivo [11]. In 2014, Guo et al. reported that reactive glial cells in the cortex of the stab-injured mice model could be directly reprogrammed into functional neurons in vivo by overexpressing a single neural TF, NeuroD1 [12]. These findings suggested that in vivo direct reprogramming technology is a hopeful method of supplying required neurons for the human central nervous system.
In 2015, two different research teams published that chemical-induced neuronal cells could be established using a cocktail of chemical compounds including forskolin (a cyclic AMP agonist) and CHIR99021 (a glycogen synthase kinase 3 beta inhibitor) [13, 14]. In this method, mouse/human skin fibroblasts were successfully converted to neuronal cells without virus vectors overexpressing TFs, suggesting that the chemical cocktail can replace previously reported reprogramming TFs, leading to easier and more stable reprogramming methods that supply neuronal cells.
5 Concluding Remarks
This chapter briefly highlights recent progress in the development of iPSCs, iNCs, and iNSCs for cell transplantation therapy of damaged brains following an ischemic stroke. Clinical trials using iPSCs are ongoing, but it is important to combine these technologies or to choose appropriate strategies depending on the target disease.
References
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–33.
Okamoto S, Takahashi M. Induction of retinal pigment epithelial cells from monkey iPS cells. Invest Ophthalmol Vis Sci. 2011;52:8785–90.
Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011;20:883–91.
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41.
Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9:413–9.
Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–72.
Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem. 2014;289:32512–25.
Addis RC, Hsu FC, Wright RL, Dichter MA, Coulter DA, Gearhart JD. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS ONE. 2011;6, e28719.
Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–6.
Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110:7038–43.
Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2013;14:188–202.
Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, et al. Direct conversion of normal and Alzheimer’s disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell. 2015;17:204–12.
Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, et al. Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell. 2015;17:195–203.
Gomi M, Takagi Y, Morizane A, Doi D, Nishimura M, Miyamoto S, et al. Functional recovery of the murine brain ischemia model using human induced pluripotent stem cell-derived telencephalic progenitors. Brain Res. 2012;1459:52–60.
Tornero D, Wattananit S, Gronning Madsen M, Koch P, Wood J, Tatarishvili J, et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–77.
Mohamad O, Drury-Stewart D, Song M, Faulkner B, Chen D, Yu SP, et al. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PLoS ONE. 2013;8, e64160.
Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–82.
Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–3.
Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–71.
Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–31.
Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–8.
Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–7.
Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–8.
Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–18.
Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2011;109:2527–32.
Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–9.
Yamashita T, Abe K. Direct reprogrammed neuronal cells as a novel resource for cell transplantation therapy. Cell Transplant. 2014;23:435–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Yamashita, T., Abe, K. (2017). iPS Cells and iN Cells. In: Houkin, K., Abe, K., Kuroda, S. (eds) Cell Therapy Against Cerebral Stroke. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56059-3_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-56059-3_4
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56057-9
Online ISBN: 978-4-431-56059-3
eBook Packages: MedicineMedicine (R0)