Abstract
Cadherins play many diverse roles in the development of the nervous system of vertebrates. Far from being simple adhesion molecules, they also orchestrate cell generation, cell movements, and cell morphogenesis. Cadherins also regulate specificity of cell-to-cell interactions during neuronal circuit formation and function. Cadherin expression during neural development is also dynamic and highly regulated. At each phase of embryo development, cadherins emerge as key molecular determinants of neural function through their many diverse binding partners. Additionally, they play important roles in the plasticity of the nervous system, a key feature believed to underpin the ability of the brain to function. Many neurodevelopmental disorders also have cadherin disfunction at their heart indicating that cadherin-based therapies may emerge as future treatments for these devastating conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The nervous system of vertebrates is a functioning structure of awe-inspiring complexity. Many billions of neurons connect and communicate with one another via many trillions of structures known as synapses. Synapses are dynamic and can be strengthened or weakened, created or destroyed depending on the needs of the given neuronal circuit for information storage or processing. But these circuits are not born fully formed. During embryo development many thousands of different specialised subtypes of cells are generated which have to migrate to where they will assemble into a circuit. Concomitantly, neurons elaborate an axonal process that will grow towards and make contact with the other neurons in the circuit which may be some distance away from the neuron cell body. Cadherins are found expressed differentially in most areas of the developing nervous system and have been shown to play key roles in the assembly and functional plasticity of neuronal circuits. For neurons, cadherins are more than just cell adhesion proteins; they are essential to the formation and function of the entire brain. As the cadherin family consists of over 100 different subtypes, a whole textbook could be written on the role of cadherins in neural development alone. Consequently, we have decided to focus our attention on the roles of members of the classical cadherin family in vertebrate nervous system development. We discuss current understanding of cadherin function in neural development by first discussing their role in neural tissue morphogenesis. We then discuss cadherin function in the formation of individual postmitotic neurons with their characteristic morphology and finally their role in coordinating cell-to-cell assemblies which, when they go wrong, could underpin neurodevelopmental disorders. This latter field of endeavour is still in its infancy and much work remains to uncover the many varied roles that cadherins play in the lifetime of the nervous system.
2 Cadherins in Neural Tissue Morphogenesis
Tissue morphogenesis during neural development requires coordinated changes in cell shape, adhesion, and movement. As tissue morphogenesis involves the collective movement of cells together, cadherins have an obvious function in maintaining cell–cell adhesions throughout gross changes in embryo structure. However, this function is far from trivial, as the expression of multiple cadherin subtypes must be tightly regulated in time and space, and the dynamic assembly and disassembly of cadherin-mediated interaction accurately orchestrated in order to permit change without loss of tissue integrity. In this section, the involvement of cadherins in several processes of early embryo and neural tissue morphogenesis is outlined, and the role of cadherins in processes beyond simply cell adhesion is discussed.
2.1 Gastrulation
Cadherins play a central role in one of the earliest of morphogenetic processes in embryos, gastrulation. Gastrulation involves large-scale cell movements to reorganize the embryo from the blastula, a simple single-layered sphere of cells, into a trilayered structure known as the gastrula (Fig. 12.1a; Solnica-Krezel and Sepich 2012; Ozair et al. 2013). The endoderm, mesoderm, and ectoderm are the primary germ layers formed during gastrulation and will, amongst other tissues, give rise to the digestive system, muscles, and nervous system, respectively. Cadherins have been shown to be crucial mediators of cell–cell adhesions during the morphogenetic movements of gastrulation in vertebrates (Tepass et al. 2000; Nakaya and Sheng 2008; Nishimura and Takeichi 2009). In zebrafish, E-cadherin facilitates adhesion between the enveloping layer and deep cells, two cellular domains in the zebrafish blastula, and inhibition of E-cadherin expression significantly disrupts gastrulation processes such as epiboly movement and the thinning and spreading of the ectoderm (Babb and Marrs 2004; Shimizu et al. 2005; Lepage and Bruce 2010). Similarly, in Xenopus embryos C-cadherin adhesions are crucial for gastrulation movements as expression of dominant-negative C-cadherin results in failure to close the blastopore and impaired involution (Lee and Gumbiner 1995). Whereas cadherin-mediated adhesions are important for maintaining structural integrity of the tissue and facilitating collective cell migration, adhesions must also be downregulated in order to permit movement and changes in the tissue by promoting epithelial-to-mesenchymal transition (EMT) . For example, C-cadherin must be downregulated by mesoderm-inducing factor activin in order to permit convergent extension in Xenopus embryos, which is the anterior–posterior extension of the embryo as cells move towards and intercalate at the dorsal midline (Zhong et al. 1999). In zebrafish and mice, FGF signalling promotes EMT during gastrulation by Snail-mediated transcriptional downregulation of E-cadherin, and the mesoderm in mice deficient in Snail activity are unable to lose epithelial morphology and apicobasal cell polarity (Ciruna and Rossant 2001; Carver et al. 2001). Furthermore, disassembly of cadherin adhesions must occur rapidly in order to correlate with the gross movements of gastrulation, therefore cadherins are also regulated at the protein level. For example, EPB4.1 L5, p38 interacting protein and p38-MAP kinase all downregulate E-cadherin during EMT in gastrulation (Hirano et al. 2008; Zohn et al. 2006).
Cadherin roles in neural tissue morphogenesis. Cadherin cell–cell adhesions are important in maintaining tissue integrity of morphogenic structures, such as the gastrula (a). Dynamic regulation of cadherins is required for gross cellular rearrangements such as neurulation, where E-cadherin is replaced by N-cadherin in the invaginating neural plate (b). Cadherin subtype switching facilitates EMT during neural crest cell migration by permitting key changes in a cell’s adhesive interactions and phenotype (c)
2.2 Neurulation
Cadherin subtypes display a distinct spatiotemporal expression pattern throughout many morphogenetic processes in neural development (Hirano et al. 2012). During neurulation, the formation of the neural tube, E-cadherin expression is replaced with N-cadherin as well as other classical cadherin subtypes in the dorsal neural ectoderm (Fig. 12.1b; Hatta and Takeichi 1986). However, the purpose of this cadherin subtype switching and its correlation to the morphogenetic movements during neurulation is under debate. In N-cadherin mutant zebrafish, key cellular rearrangements such as convergent extension and intercalation are impaired during neurulation (Hong and Brewster 2006). However, in N-cadherin knockout mice, neural tube formation and closure occur normally with only some slight malformations in the tissue organization (Radice et al. 1997). Furthermore, close analysis of cadherin expression patterns during early morphogenesis in chick embryos revealed that the kinetics of E-to-N switching do not appear to be synchronised with the movements of neurulation (Dady et al. 2012). Instead, based on the fact that the transcriptional regulators involved are distinct from those in epithelial-to-mesenchymal transition (EMT), it is suggested that the switch from E-cadherin to N-cadherin during neurulation is more a reflection of the segregation of the neuroectoderm into its three main populations: ectoderm, neural crest, and neural tube (Dady et al. 2012). This is an interesting example where the loss of E-cadherin and gain of N-cadherin do not result in EMT, unlike during tumourigenesis and cancer metastasis (Hazan et al. 2004).
2.3 Neural Crest Cell Migration
Epithelial-to-mesenchymal transition (EMT) is an extraordinary process in which cells undergo changes in cell shape and adhesion to transform from an epithelial phenotype into a migratory one (Thiery et al. 2009). EMT is required for multiple tissue morphogenic movements in neural development and cadherins play a major role in facilitating EMT, as cadherin subtype switching is required for key changes in a cell’s adhesive interactions and phenotype (Theveneau and Mayor 2012). Neural crest cells (NCCs) are a neural stem cell population located at the neural plate border that give rise to craniofacial structures, smooth muscles, cells of the cardiac system, and most of the neurons and glial of the peripheral nervous system (Dupin et al. 2006; Hall 2008). In a process called delamination, NCCs undergo EMT and detach from neighbouring neuroepithelial cells in the neural plate in order to migrate to various destinations in the embryo and differentiate (Fig. 12.1c; Thiery et al. 2009; Theveneau and Mayor 2012). During EMT, NCCs typically undergo a switch in cadherin expression, downregulating N-cadherin and upregulating Type II cadherins 6/7/11 (Nakagawa and Takeichi 1995; Vallin et al. 1998). At the initiation of EMT, N-cadherin expression is downregulated posttranslationally by the activation of metalloprotease ADAM10 by BMP/Wnt signalling in NCCs (Hall and Erickson 2003). Cleavage of N-cadherin aids NCC delamination firstly by loosening cell–cell adhesions, and secondly by the cytosolic cleavage-product of N-cadherin inducing transcription of cyclin-D1, which results in the activation of β-catenin signalling, an important promoter of NCC EMT (Shoval et al. 2007). Prior to delamination, premigratory NCCs express cadherin-6 (formally cadherin-6B in chick) and the expression of this cadherin is believed to play a role in segregating this population from other cells in the neuroepithelium, which do not express cadherin-6 (Coles et al. 2007; Theveneau and Mayor 2012). Following emigration from the neural tube, all populations of NCCs lack cadherin-6 expression. However, differences in the timing of downregulation suggest that cadherin-6 adhesions mediate different functions in the delamination of cranial and trunk NCC populations (Clay and Halloran 2014). As cranial NCCs undergo EMT, their cadherin-6 levels are rapidly reduced transcriptionally by Snail2 and posttranslationally via proteolytic cleavage by ADAM10, ADAM9, and γ-secretase (Taneyhill et al. 2007; Schiffmacher et al. 2014). Furthermore, evidence shows that this loss of cadherin-6 adhesion is critical for the transformation of cranial NCCs to the migratory state. In ovo knockout of cadherin-6 in chick embryos increases cranial NCC emigration from the neural plate and in vitro results support the conclusion that loss of cadherin-6 adhesions play a critical role in regulating the timing of cranial NCC delamination (Coles 2007; Schiffmacher et al. 2014). Trunk NCCs, on the other hand, maintain cadherin-6 expression throughout EMT and downregulation is only observed in chick and zebrafish embryos following delamination (Clay and Halloran 2014; Park and Gumbiner 2010). Furthermore, the current evidence actually points towards a pro-EMT role for cadherin-6 adhesions in trunk NCCs (Taneyhill and Schiffmacher 2013). Cadherin-6–mediated bone morphogenetic signalling has been shown to promote de-epithelialisation in chick trunk NCCs and in vivo live-cell imaging of zebrafish reveals a novel role for cadherin-6 in promoting apical detachment of NCCs by regulating F-actin dynamics (Park and Gumbiner 2010; Park and Gumbiner 2012; Clay and Halloran 2014). The involvement of cadherins in NCC development is complex, but the benefits of our understanding are great due to the number of NCC-related developmental disorders, neurocristopathies , and due to the remarkable similarity between the mechanisms used to regulate cadherin adhesions in NCCs and tumour cells during EMT (Theveneau 2012; Mayor and Theveneau 2013).
2.4 Retinal Morphogenesis
Retinal morphogenesis is a highly complex process in neural development that results in the astonishing organ that is the eye. Cadherin-mediated adhesions have been demonstrated to be important for retinal development in mice, chick, and zebrafish and in particular are crucial for appropriate patterning of the retina (Matsunaga et al. 1988b; Rungger-Brändle et al. 2010; Chen et al. 2012). Expression patterns of N-cadherin and R-cadherin are regulated by pax6 in the retina and zebrafish cadherin-mutants have severe disorders in retinal lamination (Rungger-Brändle et al. 2010). Additionally, cadherin adhesions have been shown to be important for optic fissure closure in both zebrafish and mice (Masai 2003, Chen 2012). Optic fissure closure is an important epithelial fusion event in eye development, and occurs when the growing edges of the optic cup at the optic fissure margins come together and fuse in order to form a continuous optic cup (Chow and Lang 2001). Cadherins are believed to play an important role in coordinating cell morphology changes in the optic fissure margins to ensure proper alignment and closure (Chen 2012). Furthermore, cadherin-defective mice develop coloboma, a congenital disease leading to childhood blindness. Therefore, there is hope that a better understanding of cadherin functions in optic fissure closure will help to pin down the cellular mechanisms responsible for coloboma development in humans (Chang et al. 2006).
2.5 Summary
There has been significant progress in elucidating the complex cadherin expression patterns seen throughout tissue morphogenesis, and the development of live-cell microscopy techniques to follow cadherin dynamics in vivo has been invaluable. However, in understanding the roles of cadherin in a tissue morphogenetic process, having an accurate spatiotemporal map of cadherin expression is just the first step. It is clear that the resulting effect of a specific cadherin’s expression is strongly dependent on cellular context and cadherins can carry out multiple roles during neural development. Thus, as well as identifying the downstream effects of cadherin expression, it is equally important to elucidate the integration of upstream signals which regulate cadherin functions in a given morphogenetic process. Although challenging, obtaining an appreciation of the precise and various functions of cadherins in tissue morphogenesis will be crucial in advancing our knowledge of related neurodevelopmental disorders and cancer pathogenesis.
3 Cadherins in Neural Progenitor Cell Maintenance and Differentiation
During neural development, neural progenitor cells (NPCs) give rise to all of the neuronal cells that will populate the entire adult nervous system (Temple 2001). In this process called neurogenesis, it is essential that these self-renewing progenitor cells are sustained as well as undergo differentiation at the appropriate time and place in the embryo (Götz and Huttner 2005; Doe 2008). Cadherin molecules play a central role in this balance of NPC maintenance and differentiation, as many processes hinge on the appropriate assembly and disassembly of cadherin-mediated adhesions (Halbleib and Nelson 2006). For the maintenance of NPCs, cadherins have functions in organization, regulation of NPC proliferation , and preservation of NPC identity. Consequently, dynamic downregulation of cadherin-mediated adhesions is also necessary for NPC differentiation and migration during neurogenesis. This section provides an overview of cadherin roles in NPC maintenance and differentiation, and discusses how regulation of adhesion, signalling, and cell polarity is vital in eliciting these roles.
3.1 Cadherins in Organising the NPC Microenvironment
The appropriate structure and organization of the NPC microenvironment, known as the ventricular zone, is both essential to the maintenance of NPCs and to the process of neurogenesis (Fig. 12.2). This includes the controlled residence of NPCs, as removal of NPCs from the ventricular zone results in their exit from the cell cycle and terminal differentiation (Temple 2001). Cadherins facilitate many of the adhesions that are critical to the positioning of NPCs and maintenance of the NPC microenvironment. Early in development, cadherins in adherens junctions physically link neuroepithelial progenitors to each other and to the ventricular surface of the neuroepithelium (Chenn et al. 1998). Unlike in adult stem cell microenvironments, the neuroepithelium is made up entirely of precursor cells with no supporting cells present, thus the cell–cell adhesions mediated by cadherins are essential to maintaining the integrity of the NPC microenvironment (Takahashi et al. 1993). Further in development, as radial glial cells become the predominant NPC, cadherin adhesions also play an important role in organising radial glial cells and providing the architecture for neurogenesis. N-cadherin is responsible for the anchoring of radial glial cells to the ventricular surface of the developing cortex and loss of N-cadherin results in the failure of radial glial cells to extend processes from the apical surface to the basal lamina of the cortical layer, which provide the migrational track for newly formed neuronal cells during neurogenesis (Kadowaki et al. 2007). As well as being necessary for the formation of these processes, N-cadherin is also required for neuronal attachment to radial glial processes during migration (Shikanai et al. 2011). Therefore, deregulation of cadherin molecules has dire consequence for neurogenesis and results in improper population and layering of the cerebral cortex (Marín et al. 2010; Kadowaki et al. 2007).
Neurogenesis and the organisation of the developing cortex. Radial glial neural progenitor cells reside in the ventricular zone and extend processes to the basal lamina which form the migrational track for nascent neurons. Cadherin adhesions are important in providing the architecture for neurogenesis, attaching radial glial cells to each other and to the apical surface as well as facilitating neuronal attachment to radial glial processes
3.2 Cadherins in NPC Proliferation
Even subtle changes to NPC populations can result in dire developmental consequences. Insufficient proliferation can result in microcephaly, abnormally reduced brain size (Gilmore and Walsh 2013), whereas uncontrolled growth of NPCs has been linked to brain tumours such as astrocytomas (Perego et al. 2002) and medulloblastomas (Swartling et al. 2012). Cadherin adhesions within adherens junctions have been demonstrated to play an important role in the regulation of NPC proliferation during development. For example, mislocalisation of N-cadherin in the developing cortex and spinal cord results in hyperproliferation of NPCs and the formation of tumour-like rosettes (Teng et al. 2005).
The classic link between cadherin adhesion and cell proliferation has always been β-catenin. β-catenin connects cadherin molecules to the cell’s actin cytoskeleton and is a central signalling molecule in the Wnt-pathway, which is responsible for NPC growth and cell cycling (Chenn and Walsh 2003; Junghans et al. 2005). It is believed that cadherin adhesion sequesters β-catenin to the cell membrane, inhibiting its activity and thus cell proliferation pathways (Nelson and Nusse 2004). Some evidence for this mechanism in NPCs has been found in vitro, however, there is a growing body of in vivo evidence supporting a positive regulatory role of cadherin adhesion on β-catenin signalling and subsequent proliferation in neural progenitor cells (Noles and Chenn 2007; Zhang et al. 2010; Zhang et al. 2013). Most recently, N-cadherin was shown to be necessary for proper proliferation and cell cycling of NPCs in developing mice cortex by activating β-catenin signalling in a Wnt-mediated manner (Zhang et al. 2013). Furthermore, these findings also revealed N-cadherin positively regulates AKT activity, an inhibitor of NPC exit from the VZ and apoptosis, demonstrating an additional mechanism by which cadherin regulates NPC proliferation during development.
It is clear that further understanding of the complex relationship between cadherins and β-catenin in the context of NPC proliferation is required. Although, what is generally accepted is that it is the cell autonomous changes in β-catenin signalling and not changes in cadherin cell adhesion which primarily regulate NPC proliferation (Farkas and Huttner 2008). There is some suggestion, however, for a possible non-cell autonomous role for cadherins in controlling precursor behaviour in neural development. Earlier studies have shown a N-cadherin–dependent increase in β-catenin transcriptional activation when neural precursors are cultured at high density, suggesting a possible cell–cell contact (‘outside–in’) regulation mechanism (Zhang et al. 2010). However, recent attempts to test this non-cell autonomous regulation of cadherin adhesion on β-catenin and NPC proliferation in vitro were inconclusive (Zhang et al. 2013). Additional investigation will be required to evaluate a role for cadherins in transducing extracellular signals and will help to further understand the interplay of cadherin adhesion and signalling functions in regulating the proliferation of NPCs during development.
3.3 Cadherins in the Maintenance of NPC Identity
In order to give rise to the millions of cells in the nervous system, NPCs must be maintained in the undifferentiated, continuously dividing state during neural development. Premature differentiation of NPCs and loss of their stem cell-like identity will result in the depletion of the progenitor pool and underdevelopment of the nervous system (Zhu et al. 2006; Hatakeyama et al. 2004). Central to the identity of NPCs is the maintenance of their epithelial apicobasal cell polarity. This is demonstrated by the disruption of cell polarity complexes in NPCs, which leads to loss of neuroepithelial markers and premature differentiation (Cappello et al. 2006; Costa et al. 2008). Cadherins in adherens junctions facilitate apicobasal polarity by positioning important determinants and adhering processes of radial glial and neuroepithelial cells to the ventricular surface and basal lamina (Götz and Huttner 2005; Martin-Belmonte and Perez-Moreno 2011). Indeed, multiple groups have demonstrated that the disruption of cadherin adhesions leads to the loss of apicobasal polarity in NPCs and subsequent premature differentiation (Zhadanov et al. 1999; Kadowaki et al. 2007; Lein et al. 2006). Cadherins also function to maintain the undifferentiated NPC population by influencing outcomes of individual mitotic divisions; promoting self-renewing divisions, and inhibiting terminally differentiating divisions which would deplete the progenitor pool (Götz and Huttner 2005; Noles and Chenn 2007). Additionally, cadherins have also been shown to maintain NPC identity by facilitating communication between NPCs and differentiating cells in an ‘outside–in’ regulation mechanism (Hatakeyama et al. 2014). In vitro and in vivo evidence in chick and mice embryos demonstrates that cadherin-mediated adhesions in adherens junctions of the apical end-feet of differentiating cells keep Notch signalling active in neighbouring NPCs, preventing premature differentiation in a non-cell autonomous manner. It is often believed that adherens junctions simply mediate physical contact between cells, but discoveries such as the one above have led to a growing appreciation for cadherin-mediated adherens junctions as sites for intercellular signalling, which have important roles in regulating spatiotemporal maintenance and differentiation of NPCs.
3.4 Cadherins in NPC Differentiation and Migration of Differentiated Cells
Cadherin adhesions are crucial in maintaining the self-renewing NPC population, and consequently dynamic disassembly of their adhesive contacts is important for the eventual differentiation of NPCs and detachment from the ventricular zone (Doe 2008). However, the loss of cadherin adhesions must be tightly regulated in order not to disrupt the careful balance between NPC maintenance and differentiation, as aberrant disruption of cadherin adhesions has dire consequences for the NPC population. Although loss of cadherin adhesions does not appear to affect the ability of differentiated cells to arise in N-cadherin–deficient mice, it is becoming clear that the precise regulation of cadherin adhesions is important for the successful formation of differentiated cells during neural development (Kadowaki et al. 2007; Kostetskii et al. 2001). At the end of the neurogenic period, retinal ganglion cells undergo changes in cell polarity and adhesive contacts in order to differentiate into the required cell types (Götz and Huttner 2005). Some RGCs downregulate adherens junctions and lose apical contacts to differentiate into multipolar parenchymal astrocytes, whereas others maintain adherens junctions and retract basal processes to form the ependymal lining of ventricles (Rakic 2003; Schmid et al. 2003). Downregulation of N-cadherin is also required for apical abscission, the process where differentiated NPCs detach and migrate away from the ventricular surface during neurogenesis. High-resolution live-cell imaging in chick neural tubes reveals disassembly of cadherin adhesions is essential for the retraction of apical processes during apical abscission, likely by loosening cell–cell junctions and actin–myosin tension (Das and Storey 2014). Recent work has focused on understanding the signalling networks which regulate cadherin adhesions in order to control the balance between NPC self-renewal and differentiation. Numb and Numb-like, regulators of Notch signalling, are required for the maintenance of adherens junctions in cortical progenitor cells in mice and consequently dictate NPC cell fate and polarity in a cadherin-dependent manner (Rasin et al. 2007). Additionally, a transcription factor network involving Sox2 and two Forkhead proteins (Foxp2 and Foxp4) has been identified, which regulates the expression of N-cadherin in order to control the balance of NPC self-renewal and differentiation in the developing neuroepithelium (Rousso et al. 2013). Foxp2 and Foxp4 are potent suppressors of N-cadherin expression, and disruption of the Foxp proteins inhibits NPC differentiation and migration from the VZ in the spinal cords of chicks and mice (Rousso et al. 2013). Sox2 acts in opposition to activate N-cadherin expression, and together with the Foxp proteins it helps to establish the level of cadherin expression in the developing nervous system in order to regulate NPC self-renewal and differentiation. Further work such as those outlined above will give us a complete understanding of the genetic circuits that dictate cadherin expression, and how the embryo is able to regulate the behaviour of NPCs spatiotemporally with exquisite precision.
3.5 Summary
Cadherins sit at the centre of the balance between NPC maintenance and differentiation during neural development. In vitro investigations and invaluable embryo models have revealed the diverse functions of cadherin molecules in maintaining NPCs and the pathways involved in their regulation for mediating neurogenesis. In the future, there is hope to elucidate fully the network of interactions dictating cadherin adhesions and this will set the foundation for advancements in treating neural developmental diseases and utilising stem cell technology to its full potential.
4 Cadherins in Neuronal Form and Function
Once generated from a progenitor cell population, postmitotic cells of the nervous system still undergo many phases of development to become integral parts of neural circuits. This includes their taking on the characteristic neuronal morphology of having a single axon and an elaboration of dendrites, the growth and patterning of those processes, assembly of the neuronal soma into their mature position, and formation of synapses between axons and dendrites of neurons within the circuit.
4.1 Cadherins in Axon Formation
A key first step in postmitotic neuronal development is to break symmetry of a new-born cell to generate the characteristic neuronal polarity of a long thin axon emanating from the neuronal cell body (Fig. 12.3; Dotti et al. 1988). This asymmetry is generated around the time of the differentiation of the neuron where the localisation of the centrosome predicts the location of the first neurite process that will become the axon (de Anda et al. 2005, 2008; Bradke and Dotti 2000; Powell et al. 1997). Clusters of cadherins, most notably N-cadherin and E-cadherin are required for the positioning of the centrosome (Gärtner et al. 2012a, b, 2014a, b; Pollarolo et al 2011). Interestingly, in vitro studies suggest that this asymmetry of cadherins is cell autonomous, in other words an intrinsic function of the cell and thus not related to the binding of the cadherin to an extracellular substrate. It may be that cadherin clustering from the plane of progenitor division in the last mitosis that generates the postmitotic neuron may define the neurite precursor of the axon. In Xenopus retinal ganglion cells , expression of an N-cadherin construct lacking its extracellular region inhibits axon elongation (Riehl et al. 1996). It is thought that cytoplasmic interactions of the cadherin with members of the armadillo family of catenins, β-catenin, or p120 regulate cytosolic levels of the catenins. Each catenin has different roles in activating or inactivating members of the Rho GTPase family that may be critical for the cell autonomous regulation of axon elongation (Hirano and Takeichi 2012; Gärtner et al. 2014a, b).
Neuron structure and cadherin function. Neurons consist of a cell soma with a single axon and multiple dendrites. Each of these structures branches to varying degrees. Dendritic spines are found on excitatory neurons. Many different neuronal morphologies can be observed in the central nervous system. Two examples are shown here
For excitatory cells of the cerebral cortex, the definition of the axon is predictive of the orientation of the first dendrite processes. The centrosome moves around from the axon to the opposite side of the neuron and a dendrite is elaborated (Kadowaki et al. 2007; de Anda et al. 2010; Bellion et al. 2005; Gregory and Edmondson 1988; Higginbotham and Gleeson 2007; Solecki et al. 2004; Tanaka et al. 2004; Zmuda and Rivas 1998). This orientation then subsequently predicts the direction of migration of the cortical neuron along radial glial cell processes towards the pial surface of the developing brain. Thus, for excitatory cortical neurons, the asymmetry of axon and dendrite formation and direction of initial migration are hardwired and depend on cadherin localisation within the new-born neuron.
4.2 Cadherins in Axon Patterning
The contact and subsequent formation of synapses with other cells requires the axon to grow towards its synaptic target and for dendrites to elaborate ready for the axon–dendrite contact which will generate the beginnings of a neuronal circuit. Cadherin function is also implicated in aspects of axon growth and branching (Bixby et al. 1988; Matsunaga et al. 1988a; Tomaselli et al. 1988; Masai et al. 2003; Riehl et al. 1996; Tanabe et al. 2006; Andrews and Mastick 2003; Barnes et al. 2010; Borchers et al. 2001; Oblander et al. 2007, Oblander and Brady-Kalnay 2010; Redies and Takeichi 1993) as well as in the function and plasticity of synapses and the specificity of neuronal circuit formation.
Within cranial motor neurons, temporal differential cadherin expression has been shown to regulate axon outgrowth or branching (Barnes et al. 2010). For example, cadherin-7 is expressed in the motor neurons early in their development and cadherin-7 interactions are important for the growth of the axon from the neuron cell body. In contrast, cadherin-6b, which is expressed later in motor neuron development, is important for regulating the branching of the cranial motor neurons. This later branching is important for the subsequent arborisation of the motor axons when they reach their muscle target. Of note is that both cadherin-7 and cadherin-6b actions seem to require binding to substrate cadherins. In other words, in contrast to the cell autonomous role for cadherins in the initial specification of the axon, subsequent phases of axon development require extracellular cadherin–cadherin interactions. Also of note is that the effect of cadherin-6b on branching requires the PI3Kinase/AKT pathway. Both β-catenin and γ-catenin can bind to the PI3Kinase and so it seems likely that the effect of the cadherin is, intracellularly, through catenin binding.
These data suggest the differential actions of cadherin-7 and cadherin-6b on different aspects of cranial motor axonogenesis presumably through cadherin action at different times. As a family, classical cadherins have also been shown to be differentially expressed throughout the developing nervous system (Hirano and Takeich 2012; Matsunaga et al. 2013; Bekirov et al. 2008; Tsuchiya et al. 2006; Redies et al. 1993; Inuzuka et al. 1991; Takeichi et al. 1990). There are two likely ways that these expression patterns could operate in nervous system development. In one scheme, different functions of the cadherins could operate in different neurons, necessitating differential expression. In a related scheme, different combinations of cadherin expression could further refine the actions of the cadherins, particularly through specificity of cadherin function within a defined subset of neurons.
A recent example of the first scheme shows that differential expression of cadherin-8 and cadherin-9 in mouse retinal bipolar cells controls connectivity in different types of direction-selective visual circuits (Duan et al. 2014). Cadherin-8 and cadherin-9 are expressed in different classes of bipolar cells and each cadherin directs specificity of axonal lamina targeting in the inner plexiform layer of the retina. In the absence of either cadherin, the retinal cells’ axonal arbours target both the correct and incorrect lamina. The inappropriate targeting of these axons disrupted the visual responses of the neurons with the synapses formed being highly attenuated in their synaptic transmission. This suggests that differential cadherin function in the retina targets axonal arborisation in the correct lamina and is important for the function of synapses. Interestingly, the functions of cadherin-8 and cadherin-9 appear to act heterophilically as introduction of either cadherin sparsely into each respective mutant mouse was sufficient to rescue the lamina targeting of the retinal bipolar cells. Should each cadherin be acting through cadherin–cadherin interactions then the presumption must be that they act through binding to additional cadherins. Cadherin-6 has been shown to be expressed in the retina and it may be that heterophilic cadherin interactions are mediated by that family member (Kay et al. 2011).
4.3 Cadherins in Neuronal Clustering
Differential expression of multiple cadherins within neuronal subsets has also been described (Liu et al. 2004). For example, within spinal and cranial motor neurons up to four different cadherins are expressed in defined functional groupings of motor neurons (Price et al. 2002; Demireva et al. 2011; Bello et al. 2012; Astick et al. 2014). Within the spinal cord, the motor neurons that project axons to an individual muscle in the limb cluster in groupings known as motor neuron pools. Different motor pools segregate from one another with little mixing of neurons of different pools. In order to form these pools, motor neurons pass through a migratory phase followed by a pool coalescence phase (Fig. 12.4). Each motor neuron pool expresses a different combination of cadherins and this combinatorial expression is instructive for the clustering of the motor neurons into pools. For example, the adductor motor neuron pool expresses cadherins-6b, -8, -13 and -20 (also called MN-cadherin) whereas the femorotibialis motor pool expresses cadherin-6b, -8, and -13 (Fig. 12.4c). Expression of cadherin-20 in the femorotibialis motor neurons results in their mixing with the adductor motor neurons. Additionally, removal of cadherin-20 function by expression of a dominant negative also causes mixing of the femorotibialis and adductor motor neurons. Expression of other cadherins not predicted to equalise expression between the two motor pools had no effect on pool segregation. These data argue that the specificity of motor pool segregation and motor neuron coalescence is driven by the specific nature of the combination of cadherins expressed in the motor neurons. A similar combinatorial code also operates in the segregation of cranial motor nuclei. Again, each cluster of cranial motor neurons expresses a different combination of cadherins and this drives specificity of coalescence and segregation of the motor neurons during development. Interestingly, with the notable exception of cadherin-13, all of the cadherins that drive motor pool segregation are members of the type II subfamily of cadherins. Furthermore, with the exception of cadherin-5, all type II cadherins are expressed differentially in motor neurons. This suggests that possibility that combinatorial type II cadherin expression is a major driver of specificity of cell-to-cell recognition within the developing nervous system. Combinatorial expression of cell adhesion molecules is an attractive and rather elegant mechanism for generating diversity to drive specificity of intercellular interactions. With relatively few different family members a large number of different combinations can be achieved. For example, with just 6 family members 462 different combinations are possible. However, the molecular nature of the display of different combinations of cadherins in an individual cell is currently not known. Additionally, considerable heterophilic interactions between different type II cadherin family members have been observed (Ahrens et al. 2002; Shimoyama et al. 2000; Katsamba et al. 2009). For example, cadherin-8 can bind to cadherin-9 and cadherin-11 can also bind to cadherin-8. A note of caution needs to be raised with analysis of these binding specificities. Classically, cadherin interaction specificity is assayed under conditions of a single cadherin being expressed in a single cell with that cell being challenged to interact with other cells expressing the same or different individual cadherins. How specificity of cadherin interaction manifests itself when multiple cadherins are expressed within a given cell has not been studied.
Motor neuron pool formation. Motor neurons pass through an early migratory phase (a) which coincides with a pan-motor neuron expression of cadherins. Following this, motor pool coalescence occurs (b). Differential cadherin expression is found in motor neuron pools and is instructive in motor pool coalescence (c). The refinement of cadherin expression occurs through neurotrophic factor expression in the limb which is read out by motor neurons, presumably via their axons (d)
Cadherin expression is also highly dynamic during motor neuron development. During spinal motor pool and cranial motor nucleus formation, the motor neurons pass through a phase that appears to have no differential cadherin expression. In other words, initially, motor neurons seem to express the same combination of cadherins with this expression being refined during the period of cell sorting. For spinal motor neurons, this cadherin refinement depends on a limb-derived source of the neurotrophic factor GDNF (Fig. 12.4d; Livet et al. 2002). In the GDNF knockout mutant mouse or in the absence of its cognate receptor, GFRα1, normally expressed within motor neurons, motor pool coalescence is perturbed (Haase et al. 2002). Cadherin expression is also perturbed in these mutant mice consistent with the role for cadherin expression in driving pool coalescence. The GDNF signals to motor neurons to express members of the ETS family of transcription factors in a pool-specific manner and it is this ETS expression that appears to drive the refinement of cadherin expression in the motor neurons. Interestingly, this GDNF signal is permissive for ETS and cadherin expression as the receptor is expressed in a pool-specific manner prior to the motor axons encountering the GDNF source in the limb.
The initial, pan-motor neuron, expression of cadherins plays a role in the migration of newly born motor neurons from the ventricular zone into the ventral horn of the developing spinal cord (Bello et al. 2012). This migration occurs on spinal radial glia, that act as guides for the motor neurons as they migrate. The cadherin expression in motor neurons during their migration might act to anchor the migration machinery within the cell providing traction for retrograde flow of actin to be used to force cell movement.
5 Cadherins in Synapses
One of the defining, and last, parts of neural development is the formation of functional synapses between postmitotic neurons. One could argue that this aspect of development continues throughout life as synaptic plasticity, the strengthening or weakening of synapses in response to circuit activity is a key feature of the functioning of the nervous system. Additionally, the genetic basis of many mental disorders can be traced to proteins whose function is predominantly in synapse function. Cadherins are implicated in many of the processes of synapse formation and plasticity (Arikkath and Reichardt 2008; Brigidi and Bamji 2011; Suzuki and Takeichi 2008; Tai et al. 2008; Takeichi 2007) and cadherin perturbations also underlie many psychiatric disorders.
5.1 Cadherins in Synapse Formation and Function
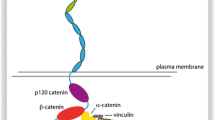
Synapses are small structures formed by axonal contact with the dendrites or soma of another neuron. They have a so-called presynaptic part, termed the active zone, which contains synaptic vesicles loaded with a neurotransmitter and a postsynaptic part that contains the receptors for the neurotransmitter. The receptors are anchored to the so-called postsynaptic density. For excitatory neurons, synapses are localised on a specialised dendritic structure known as a spine. Classical cadherins span the pre- and postsynaptic structures and are located at the outer edges of the active zone in mature synapses (Fannon and Colman 1996; Uchida et al. 1996). For example, N-cadherin and its associated catenins are found at synaptic junctions (Uchida et al. 1996). Their expression along with that of neuroligin has been shown to cooperate to regulate synapse formation (Aiga et al. 2011). Additionally, cadherins-11 and -13 can regulate the formation of both inhibitory and excitatory synapses (Paradis et al. 2007). General blockade of cadherin function using a blocking antibody results in smaller synapses with impaired function (Bozdagi et al. 2004, 2010). One of the major roles for cadherins, however, appears to be in dendritic spine morphogenesis. Cadherins are required for the formation of spines. Inhibition of cadherin function results in abnormal shapes of spines, such as their length and spine loss (Abe et al 2004; Mysore et al. 2007; Togashi et al 2002). These functions of cadherins require the cytoplasmic binding partners of cadherins such as αN-catenin and p120 catenin. p120 catenin is also important in the maturation of dendritic spines and this requires the Rho family of GTPases (Elia et al. 2006). Cadherins are also involved in the more general structure of synapses. For example, cadherins recruit PSD95 and synapsin to spines (Togashi et al. 2002) and bind to an AMPA receptor thus regulating its localisation in the synapse (Dunah et al. 2005; Nuriya and Huganir 2006; Saglietti et al. 2007). Spines are highly dynamic structures, this presumably being important for strengthening or weakening of synapses in response to neuronal activity. A major mechanism of synapse strengthening is a process called long term potentiation (LTP) whereby, following prolonged stimulation, a given input elicits a larger synaptic output (Bliss and Lomo 1973). LTP is associated with enlargement of spines which requires N-cadherin protein. The dynamic interplay between structural and functional plasticity at spines is also illustrated in the changes to the strength of cadherin adhesion related to the activity-dependent concentration of calcium ions at the synaptic cleft (Tai et al. 2008). Cadherin activation can also influence intracellular levels of calcium ions (Bixby et al. 1994; Chadborn et al. 2002; Marrs et al. 2009; Sheng et al. 2013). These phenomena could indicate that cadherins could act as activity sensors at the synapse and thus be intimate players in regulating the scaling of synaptic responses to prolonged activity (Thalhammer and Cingolani 2014). In addition to the biophysical changes to cadherin function at synapses, the recruitment and retention of cadherins at the synapse is also regulated by activity. Cadherins can be cleaved by proteases, for example, N-cadherin is processed by both ADAM10 and PS1/γ-secretase in a manner that depends on NMDA receptor activity (Monea et al. 2006; Reiss et al. 2005; Uemura et al. 2006; Malinverno et al. 2010). Interestingly, one of the cytoplasmic fragments of N-cadherin generated by proteolysis (N-cad/CTF2) induces the destruction of CREB-binding proteins. CREB-dependent gene expression is critical to synapse plasticity offering a transcriptional link between activity-dependent cadherin function and longer term changes to synapses (Marambaud et al. 2003; Uemura et al. 2006; Alberini 2009; Lonze and Ginty 2002). NMDA Receptor activation also reduces the rate of endocytosis of N-cadherin at the synapse (Tai et al. 2007). Thus, multiple mechanisms of activity-dependent changes to cadherin function feed into changes in synapse function.
5.2 Cadherins in Neural Disorders
Synaptic disfunction is believed to play a role in some disorders of the nervous system. A growing body of evidence suggests that at least some of the phenotypes found in these disorders may have a genetic basis linked to cadherin loci (Bhalla et al. 2008; Rose et al. 1995; Singh et al. 2010; Wang et al. 2009). Mutations in cadherin genes have been found in a wide spectrum of different disorders including autism spectrum disorders (Crepel et al. 2014), schizophrenia and bipolar disorder, and addiction-related disorders. For example, cadherin-13 has been linked to autism spectrum disorder, attention-deficit and hyperactivity disorder, schizophrenia, and addiction disorders (Børglum et al. 2014; Chapman et al. 2011; Johnson et al. 2006; Lasky-Su et al. 2008; Lesch et al. 2008; Treutlein et al. 2009). Members of the catenin family of cadherin-interacting proteins are also linked to neural disorders with αN-catenin and δ-catenin mutations found in schizophrenia and severe intellectual disability (Chu and Liu 2010; Medina et al. 2000). Exactly how cadherin/catenin mutations are involved in these disorders is not currently clear. It seems likely that synaptic functions of cadherins underpin their role in neural disorders but other functions of cadherins in circuit formation in general may also play a role (Gleeson 2001).
6 Summary
Cadherins play key roles throughout the development of the nervous system. The expression of cadherins is highly dynamic and regulated at both the transcriptional and posttranslational level. Far from being relatively simple homophilic molecular adhesives, the multiple binding partners of cadherins indicate that they play an important part in orchestrating many aspects of neural development both in the embryo as well as throughout life. There is still much to learn about the roles of cadherins in disorders of the nervous system. Additionally, it seems clear that we have only begun to scratch the surface of the diversity of functions that cadherins play in neural development.
References
Abe K, Chisaka O, Van Roy F, Takeichi M (2004) Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat Neurosci 7:357–363
Ahrens T, Pertz O, Haussinger D et al (2002) Analysis of heterophilic and homophilic interactions of cadherins using the c-Jun/c-Fos dimerization domains. J Biol Chem 277:19455–19460
Aiga M, Levinson JN, Bamji SX (2011) N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures. J Biol Chem 286:851–858
Alberini CM (2009) Transcription factors in long-term memory and synaptic plasticity. Physiol Rev 89:121–145
Andrews GL, Mastick GS (2003) R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci 23:9873–9880
Arikkath J, Reichardt LF (2008) Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci 31:487–494
Astick M, Tubby K, Mubarak WM et al (2014) Central topography of cranial motor nuclei controlled by differential cadherin expression. Curr Biol 24:2541–2547
Babb SG, Marrs J (2004) E-cadherin regulates cell movements and tissue formation in early zebrafish embryos. Dev Dyn 230:263–277
Barnes SH, Price SR, Wentzel C, Guthrie SC (2010) Cadherin-7 and cadherin-6B differentially regulate the growth, branching and guidance of cranial motor axons. Development 137:805–814
Bekirov IH, Nagy V, Svoronos A et al (2008) Cadherin-8 and N cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus 18:349–363
Bellion A, Baudoin J-P, Alvarez C (2005) Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J Neurosci 25:5691–5699
Bello SM, Millo H, Rajebhosale M, Price SR (2012) Catenin-dependent cadherin function drives divisional segregation of spinal motor neurons. J Neurosci 32:490–505
Bhalla K, LuoY BT et al (2008) Alterations in CDH15 and KIRREL3 in patients with mild to severe intellectual disability. Am J Hum Genet 83:703–713
Bixby JL, Lilien J, Reichardt LF (1988) Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol 107:353–361
Bixby JL, Grunwald GB, Bookman RJ (1994) Ca2+ influx and neurite growth in response to purified N-cadherin and laminin. J Cell Biol 127:1461–1475
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Borchers A, David R, Wedlich D (2001) Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 128:3049–3060
Børglum AD, Demontis D, Grove J et al (2014) Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry 19:325–333
Bozdagi O, Valcin M, Poskanzer K et al (2004) Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol Cell Neurosci 27:509–521
Bozdagi O, Wang XB, Nikitczuk JS et al (2010) Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci 30:9984–9989
Bradke F, Dotti CG (2000) Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 10:574–581
Brigidi GS, Bamji SX (2011) Cadherin-catenin adhesion complexes at the synapse. Curr Opin Neurobiol 21:208–214
Cappello S, Attardo A, Wu X et al (2006) The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci 9:1099–1107. doi:10.1038/nn1744
Carver EA, Jiang R, Lan Y et al (2001) The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition the mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol 21:8184–8188. doi:10.1128/MCB.21.23.8184
Chadborn N, Eickholt B, Doherty P, Bolsover S (2002) Direct measurement of local raised subplasmalemmal calcium concentrations in growth cones advancing on an N-cadherin substrate. Eur J Neurosci 15:1891–1898
Chang L, Blain D, Bertuzzi S, Brooks BP (2006) Uveal coloboma: clinical and basic science update. Curr Opin Ophthalmol 17:447–470. doi:10.1097/01.icu.0000243020.82380.f6
Chapman NH, Estes A, Munson J et al (2011) Genome-scan for IQ discrepancy in au- tism: evidence for loci on chromosomes 10 and 16. Hum Genet 129:59–70
Chen S, Lewis B, Moran A, Xie T (2012) Cadherin-mediated cell adhesion is critical for the closing of the mouse optic fissure. PloS One 7:1–8. doi:10.1371/journal.pone.0051705
Chenn A, Walsh C (2003) Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex 13:599–606. doi:10.1093/cercor/13.6.599
Chenn A, Zhang YA, Chang BT, McConnell SK (1998) Intrinsic polarity of mammalian neuroepithelial cells. Mol Cell Neurosci 11:183–193. doi:10.1006/mcne.1998.0680
Chow RL, Lang RA (2001) Early eye development in vertebrates. Annu Rev Cell Dev Biol 17:255–296
Chu TT, Liu Y (2010) An integrated genomic analysis of gene function correlation on schizophrenia susceptibility genes. J Hum Genet 55:285–292
Ciruna B, Rossant J (2001) FGF signalling regulates mesoderm cell Fate specification and morphogenetic movement at the primitive streak. Dev Cell 1:37–49. doi:10.1016/S1534-5807(01)00017-X
Clay MR, Halloran MC (2014) Cadherin 6 promotes neural crest cell detachment via F-actin regulation and influences active Rho distribution during epithelial-to-mesenchymal transition. Development 141:2506–2515. doi:10.1242/dev.105551
Coles EG, Taneyhill LA, Bronner-Fraser M (2007) A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol 312:533–544. doi:10.1016/j.ydbio.2007.09.056
Costa MR, Wen G, Lepier A et al (2008) Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development 135:11–22. doi:10.1242/dev.009951
Crepel A, De Wolf V, Brison N et al (2014) Association of CDH11 with non-syndromic ASD. Am J Med Genet B Neuropsychiatr Genet 165:391–398
Dady A, Blavet C, Duband JL (2012) Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn 241:1333–1349. doi:10.1002/dvdy.23813
Das RM, Storey KG (2014) Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 343:200–204. doi:10.1126/science.1247521
de Anda FC, Pollarolo G, Da Silva JS et al (2005) Centrosome localization determines neuronal polarity. Nature 436:704–708
de Anda F, Gartner A, Tsai LH, Dotti CG (2008) Pyramidal neuron polarity axis is defined at the bipolar stage. J Cell Sci 121:178–185
de Anda FC, Meletis K, Ge X et al (2010) Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci 30:10391–10406
Demireva EY, Shapiro LS, Jessell TM, Zampieri N (2011) Motor neuron position and topographic order imposed by β - and γ-catenin activities. Cell 147:641–52
Doe CQ (2008) Neural stem cells: balancing self-renewal with differentiation. Development 135:1575–1587. doi:10.1242/dev.014977
Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8:1454–1468
Duan X, Krishnaswamy A, De la Huerta I, Sanes JR (2014) Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158:793–807
Dunah AW, Hueske E, Wyszynski M (2005) LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci 8:458–467
Dupin E, Creuzet S, Le Douarin NM (2006) The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol 589:96–119
Elia LP, Yamamoto M, Zang K, Reichardt LF (2006) p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron 51:43–56
Fannon AM, Colman DR (1996) A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron 17:423–434
Farkas LM, Huttner WB (2008) The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol 20:707–715. doi:10.1016/j.ceb.2008.09.008
Gartner A, Fornasiero EF, Dotti CG (2012a) N-cadherin: a new player in neuronal polarity. Cell Cycle 11:2223–2224
Gartner A, Fornasiero EF, Munck S et al (2012b) N-cadherin specifies first asymmetry in developing neurons. EMBO J 31:1893–1903
Gärtner A, Fornasiero EF, Dotti CG (2014a) Cadherins as regulators of neuronal polarity. Cell Adh Migr 14:1–8
Gärtner A, Fornasiero EF, Valtorta F, Dotti CG (2014b) Distinct temporal hierarchies in membrane and cytoskeleton dynamics precede the morphological polarization of developing neurons. J Cell Sci 127:4409–4419
Gilmore EC, Walsh CA (2013) Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscp Rev Dev Biol 2:461–478. doi:10.1002/wdev.89
Gleeson JG (2001) Neuronal migration disorders. Ment Retard Dev Disabil Res Rev 7:167–171
Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6:777–788. doi:10.1038/nrm1739
Gregory W, Edmondson J (1988) Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J Neurosci 8:1728–1738
Haase G, Dessaud E, Garcès A (2002) GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron 35:893–905
Halbleib JM, Nelson WJ (2006) Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20:3199–3214. doi:10.1101/gad.1486806
Hall BK (2008) The neural crest and neural crest cells in vertebrate development and evolution. TripleC. doi:10.1007/128
Hall R, Erickson C (2003) ADAM 10: an active metalloprotease expressed during avian epithelial morphogenesis. Dev Biol 256:146–159
Hatakeyama J, Bessho Y, Katoh K et al (2004) Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development 131:5539–5550. doi:10.1242/dev.01436
Hatakeyama J, Wakamatsu Y, Nagafuchi A et al (2014) Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development 141:1671–1682. doi:10.1242/dev.102988
Hatta K, Takeichi M (1986) Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320:447–449. doi:10.1038/320447a0
Hazan RB, Qiao R, Keren R et al (2004) Cadherin switch in tumor progression. Ann N Y Acad Sci 1014:155–163. doi:10.1196/annals.1294.016
Higginbotham HR, Gleeson JG (2007) The centrosome in neuronal development. Trends Neurosci 30:276–283
Hirano S, Takeichi M (2012) Cadherins in brain morphogenesis and wiring. Physiol Rev 92:597–634
Hirano M, Hashimoto S, Yonemura S et al (2008) EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. J Cell Biol 182:1217–1230. doi:10.1083/jcb.200712086
Hong E, Brewster R (2006) N-cadherin is required for the polarized cell behaviours that drive neurulation in the zebrafish. Development 133:3895–3905. doi:10.1242/dev.02560
Inuzuka H, Redies C, Takeichi M (1991) Differential expression of R- and N-cadherin in neural and mesodermal tissues during early chicken development. Development 113:959–967
Johnson C, Drgon T, Liu QR et al (2006) Pooled association genome scanning for alcohol dependence using 104,268 SNPs: validation and use to identify alcoholism vulnerability loci in unrelated individuals from the collaborative study on the genetics of alcoholism. Am J Med Genet B Neuropsychiatr Genet 141:844–853
Junghans D, Hack I, Frotscher M et al (2005) Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn 233:528–539. doi:10.1002/dvdy.20365
Kadowaki M, Nakamura S, Machon O et al (2007) N-cadherin mediates cortical organization in the mouse brain. Dev Biol 304:22–33
Katsamba P, Carroll K, Ahlsen G et al (2009) Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc Natl Acad Sci U S A 106:11594–11599
Kay JN, De la Huerta I, Kim IJ (2011) Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci 31:7753–7762
Kostetskii I, Moore R, Kemler R, Radice GL (2001) Differential adhesion leads to segregation and exclusion of N-cadherin-deficient cells in chimeric embryos. Dev Biol 234:72–79. doi:10.1006/dbio.2001.0250
Lasky-Su J, Neale BM, Franke B et al (2008) Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet 147:1345–1354
Lee CH, Gumbiner BM (1995) Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev Biol 171:363–373. doi:10.1006/dbio.1995.1288
Lein W-H, Klezovitch O, Fernandez TE, et al (2006) αE-catenin controls cerebral cortical size by regulating hedgehog signalling pathway. 311:1609–1612. doi:10.1126/science.1121449.
Lepage SE, Bruce AEE (2010) Zebrafish epiboly: mechanics and mechanisms. Int J Dev Biol 54:1213–1228. doi:10.1387/ijdb.093028sl
Lesch KP, Timmesfeld N, Renner TJ et al (2008) Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. J Neural Transm 115:1573–1585
Liu Q, Marrs JA, Azodi E et al (2004) Differential expression of cadherins in the developing and adult zebrafish olfactory system. J Comp Neurol 478:269–281
Livet J, Sigrist M, Stroebel S et al (2002) ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 35:877–892
Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35:605–623
Malinverno M, Carta M, Epis R et al (2010) Synaptic localization and activity of ADAM10 regulate excit- atory synapses through N-cadherin cleavage. J Neurosci 30:16343–16355
Marambaud P, Wen PH, Dutt A et al (2003) A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114:635–645
Marín O, Valiente M, Ge X, Tsai L-H (2010) Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol 2:1–21. doi:10.1101/cshperspect.a001834
Marrs GS, Theisen CS, Bruses JL (2009) N-cadherin modulates voltage activated calcium influx via RhoA, p120-catenin, and myosin-actin interaction. Mol Cell Neurosci 40:390–400
Martin-Belmonte F, Perez-Moreno M (2011) Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 12:23–38. doi:10.1038/nrc3169
Masai I, Lele Z, Yamaguchi M (2003) N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development 130:2479–2494
Matsunaga M, Hatta K, Nagafuchi A, Takeichi M (1988a) Guidance of optic nerve fibres by N-cadherin adhesion molecules. Nature 334:62–64
Matsunaga M, Hatta K, Takeichi M (1988b) Role of N-cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron 1:289–295. doi:10.1016/0896-6273(88)90077-3
Matsunaga E, Nambu S, Oka M, Iriki A (2013) Differential cadherin expression in the developing postnatal telencephalon of a New World monkey. J Comp Neurol 521:4027–4060
Mayor R, Theveneau E (2013) The neural crest. Development 140:2247–2251. doi:10.1242/dev.091751
Medina M, Marinescu RC, Overhauser J, Kosik KS (2000) Hemizygosity of delta-catenin (CTNND2) is associated with severe mental retardation in cri-du-chat syndrome. Genomics 63:157–164
Monea S, Jordan BA, Srivastava S et al (2006) Membrane localization of membrane type 5 matrix metalloproteinase by AMPA receptor binding protein and cleavage of cadherins. J Neurosci 26:2300–2312
Mysore SP, Tai CY, Schuman EM (2007) Effects of N-cadherin disruption on spine morphological dynamics. Front Cell Neurosci 1:1. doi:10.3389/neuro.03.001.2007
Nakagawa S, Takeichi M (1995) Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development 121:1321–1332. doi:10.1016/0168-9525(95)90550-2
Nakaya Y, Sheng G (2008) Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ 50:755–766. doi:10.1111/j.1440-169X.2008.01070.x
Nelson J, Nusse R (2004) Convergence of Wnt, β-Catenin, and Cadherin pathways. Science (80–) 303:1483–1487. doi:10.1126/science.1094291
Nishimura T, Takeichi M (2009) Remodeling of the Adherens Junctions During Morphogenesis. In: Lecuit T (ed) Tissue remodeling and epithelial morphogenesis, 1st edn. Elsevier, San Diego, pp 33–49
Noles SR, Chenn A (2007) Cadherin inhibition of β -catenin signaling regulates the proliferation and differentiation of neural precursor cells. Mol Cell Neurosci 35:549–558. doi:10.1016/j.mcn.2007.04.012
Nuriya M, Huganir RL (2006) Regulation of AMPA receptor trafficking by N-cadherin. J Neurochem 97:652–661
Oblander SA, Brady-Kalnay SM (2010) Distinct PTPmu-associated signaling molecules dif- ferentially regulate neurite outgrowth on E-, N-, and R-cadherin. Mol Cell Neurosci 44:78–93
Oblander SA, Ensslen-Craig SE, Longo FM, Brady-Kalnay SM (2007) E-cadherin promotes retinal ganglion cell neurite outgrowth in a protein tyrosine phosphatase mu dependent manner. Mol Cell Neurosci 34:481–492
Ozair MZ, Kintner C, Brivanlou AH (2013) Neural induction and early patterning in vertebrates. Wiley Interdiscip Rev Dev Biol 2:479–498. doi:10.1002/wdev.90
Paradis S, Harrar DB, Lin Y et al (2007) An RNAi-based approach identifies molecules required for gluta- matergic and GABAergic synapse development. Neuron 53:217–232
Park K-S, Gumbiner BM (2010) Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development 137:2691–2701. doi:10.1242/dev.050096
Park K-S, Gumbiner BM (2012) Cadherin-6B stimulates an epithelial mesenchymal transition and the delamination of cells from the neural ectoderm via LIMK/ cofilin mediated non-canonical BMP receptor signaling. Dev Biol 366:232–243. doi:10.1016/j.biotechadv.2011.08.021.Secreted
Perego C, Vanoni C, Massari S et al (2002) Invasive behaviour of glioblastoma cell lines is associated with altered organisation of the cadherin-catenin adhesion system. J Cell Sci 115:3331–3340
Pollarolo G, Schulz JG, Munck S, Dotti CG (2011) Cytokinesis remnants define first neuronal asymmetry in vivo. Nat Neurosci 14:1525–1533
Powell SK, Rivas RJ, Rodriguez-Boulan E, Hatten ME (1997) Development of polarity in cerebellar granule neurons. J Neurobiol 32:223–236
Price SR, De Marco GNV, Ranscht B, Jessell TM (2002) Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell 109:205–16
Radice GL, Rayburn H, Matsunami H et al (1997) Developmental defects in mouse embryos lacking N-cadherin. Dev Biol 181:64–78. doi:10.1006/dbio.1996.8443
Rakic P (2003) Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex 13:541–549. doi:10.1093/cercor/13.6.541
Rasin M-R, Gazula V-R, Breunig JJ et al (2007) Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 10:819–827. doi:10.1038/nn1924
Redies C, Takeichi M (1993) N-and R-cadherin expression in the optic nerve of the chicken embryo. Glia 8:161–171
Redies C, Engelhart K, Takeichi M (1993) Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J Comp Neurol 333:398–416
Reiss K, Maretzky T, Ludwig A et al (2005) ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J 24:742–752
Riehl R, Johnson K, Bradley R et al (1996) Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron 17:837–848
Rose O, Grund C, Reinhardt S et al (1995) Contactus adherens, a special type of plaque-bearing adhering junction containing M-cadherin, in the granule cell layer of the cerebellar glomerulus. Proc Natl Acad Sci U S A 92:6022–6026
Rousso DL, Pearson CA, Gaber ZB et al (2013) Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron 74:314–330. doi:10.1038/nature11130.Reduced
Rungger-Brändle E, Ripperger JA, Steiner K et al (2010) Retinal patterning by Pax6-dependent cell adhesion molecules. Dev Neurobiol 70:764–780. doi:10.1002/dneu.20816
Saglietti L, Dequidt C, Kamieniarz K et al (2007) Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 54:461–477
Schiffmacher AT, Padmanabhan R, Jhingory S, Taneyhill LA (2014) Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol Biol Cell 25:41–54. doi:10.1091/mbc.E13-08-0459
Schmid RS, McGrath B, Berechid BE et al (2003) Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci U S A 100:4251–4256. doi:10.1073/pnas.0630496100
Sheng L, Leshchyns’ka I, Sytnyk V (2013) Cell adhesion and intracellular calcium signaling in neurons. Cell Commun Signal 11:94. doi:10.1186/1478-811X-11-94
Shikanai M, Nakajima K, Kawauchi T (2011) N-Cadherin regulates radial glial fiber-dependent migration of cortical locomoting neurons. Commun Integr Biol 4:326–330. doi:10.4161/cib.4.3.14886
Shimizu T, Yabe T, Muraoka O et al (2005) E-cadherin is required for gastrulation cell movements in zebrafish. Mech Dev 122:747–763. doi:10.1016/j.mod.2005.03.008
Shimoyama Y, Tsujimoto G, Kitajima M, Natori M (2000) Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J 349:159–167
Shoval I, Ludwig A, Kalcheim C (2007) Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development 134:491–501. doi:10.1242/dev.02742
Singh SM, Castellani C, O’Reilly R (2010) Autism meets schizophrenia via cadherin pathway. Schizophr Res 116:293–294
Solecki DJ, Model L, Gaetz J (2004) Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci 7:1195–1203
Solnica-Krezel L, Sepich DS (2012) Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol 28:687–717
Suzuki SC, Takeichi M (2008) Cadherins in neuronal morphogenesis and function. Dev Growth Differ 1:S119–S130
Swartling FJ, Savov V, Persson AI et al (2012) Distinct neural stem cell populations give rise to disparate brain tumors in response to N-MYC. Cancer Cell 21:601–613. doi:10.1016/j.ccr.2012.04.012
Tai CY, Mysore SP, Chiu C, Schuman EM (2007) Activity-regulated N-cadherin endocytosis. Neuron 54:771–785
Tai CY, Kim SA, Schuman EM (2008) Cadherins and synaptic plasticity. Curr Opin Cell Biol 20:567–575
Takahashi T, Nowakowski RS, Caviness VS (1993) Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci 13:820–833
Takeichi M (2007) The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci 8:11–20
Takeichi M, Inuzuka H, Shimamura K et al (1990) Cadherin subclasses: differential expression and their roles in neural morphogenesis. Cold Spring Harb Symp Quant Biol 55:319–325
Tanabe K, Takahashi Y, Sato Y et al (2006) Cadherin is required for dendritic morphogenesis and synaptic terminal organization of retinal horizontal cells. Development 133:4085–4096
Tanaka T, Serneo FF, Higgins C et al (2004) Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol 165:709–721
Taneyhill LA, Schiffmacher AT (2013) Chapter 3: The Cell Biology of Neural Crest Cell Delamination and EMT. In: Trainor P (ed) Neural Crest Cells: Evolution, Development and Disease. Elsevier Inc, Taramani, pp 1–22
Taneyhill LA, Coles EG, Bronner-Fraser M (2007) Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134:1481–1490. doi:10.1242/dev.02834
Temple S (2001) The development of neural stem cells. Nature 414:112–117. doi:10.1038/35102174
Teng J, Rai T, Tanaka Y et al (2005) The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol 7:474–482. doi:10.1038/ncb1249
Tepass U, Truong K, Godt D et al (2000) Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol 1:91–100. doi:10.1038/35040042
Thalhammer A, Cingolani LA (2014) Cell adhesion and homeostatic synaptic plasticity. Neuropharmacology 78:23–30
Theveneau E, Mayor R (2012) Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol 366:34–54. doi:10.1016/j.ydbio.2011.12.041
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-Mesenchymal Transitions in Development and Disease. Cell 139:871–890. doi:10.1016/j.cell.2009.11.007
Togashi H, Abe K, Mizoguchi A et al (2002) Cadherin regulates dendritic spine morphogenesis. Neuron 35:77–89
Tomaselli KJ, Neugebauer KM, Bixby JL et al (1988) N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron 1:33–43
Treutlein J, Cichon S, Ridinger M et al (2009) Genome-wide association study of alcohol dependence. Arch Gen Psychiatry 66:773–784
Tsuchiya B, Sato Y, Kameya T et al (2006) Differential expression of N-cadherin and E-cadherin in normal human tissues. Arch Histol Cytol 69:135–145
Uchida N, Honjo Y, Johnson KR et al (1996) The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol 135:767–779
Uemura K, Kihara T, Kuzuya A et al (2006) Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. Neurosci Lett 402:278–283
Vallin J, Girault JM, Thiery JP, Broders F (1998) Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev 75:171–174. doi:10.1016/S0925-4773(98)00099-9
Wang K, Zhang H, Ma D et al (2009) Common genetic variants on 5p14.1 associate with autism spectrum dis- orders. Nature 459:528–533
Zhadanov AB, Provance DW, Speer CA et al (1999) Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr Biol 9:880–888. doi:10.1016/S0960-9822(99)80392-3
Zhang J, Woodhead GJ, Swaminathan SK et al (2010) Cortical neural precursors inhibit their own differentiation via N- cadherin maintenance of beta-catenin signaling. Dev Cell 18:472–479. doi:10.1016/j.devcel.2009.12.025.Cortical
Zhang J, Shemezis JR, Mcquinn ER et al (2013) AKT activation by N-cadherin regulates beta-catenin signaling and neuronal differentiation during cortical development. Neural Dev 8:1. doi:10.1186/1749-8104-8-7
Zhong Y, Brieher WM, Gumbiner BM (1999) Analysis of C-cadherin regulation during tissue morphogenesis with an activating antibody. J Cell Biol 144:351–359. doi:10.1083/jcb.144.2.351
Zhu X, Zhang J, Tollkuhn J et al (2006) Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753. doi:10.1101/gad.1444706
Zmuda JF, Rivas RJ (1998) The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil Cytoskel 41:18–38
Zohn IE, Li Y, Skolnik EY et al (2006) p38 and a p38-Interacting Protein Are Critical for Downregulation of E-Cadherin during Mouse Gastrulation. Cell 125:957–969. doi:10.1016/j.cell.2006.03.048
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Brayshaw, L.L., Price, S.R. (2016). Cadherins in Neural Development. In: Suzuki, S., Hirano, S. (eds) The Cadherin Superfamily. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56033-3_12
Download citation
DOI: https://doi.org/10.1007/978-4-431-56033-3_12
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56031-9
Online ISBN: 978-4-431-56033-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)