Abstract
Skin sympathetic nerve activity (SSNA) is microneurographically recorded from the skin nerve fascicle in the peripheral nerves with properties of an irregular, pulse asynchronous burst with respiratory variation, followed by sweating and/or vasoconstriction, elicited by mental stress and arousal stimuli. It comprises sudomotor and vasoconstrictor nerve activities as well as vasodilator ones. SSNA function in thermoregulation in humans, however, is also elicited by mental stress or cognition. SSNA is lowest at thermoneutral temperature and enhanced in the presence of ambient warm and cool air. Burst amplitude is well correlated to sweat rate change or skin blood flow reduction rate. The clinical application of SSNA comprises the following: (1) clarification of sweating events, (2) clarification and diagnosis of anhidrosis or hyperhidrosis, (3) clarification of thermoregulatory function and diagnosis of thermoregulatory disorder, (4) clarification of pathophysiology and diagnosis of vascular disorders, (5) clarification of the relationship between cognitive function and SSNA, and (6) determination of pharmacological effects attributable to change in neuroeffector responses. Lastly, SSNA’s significance as a feedforward thermoregulatory tool is discussed since SSNA contributes more to rapid thermoregulatory response than thermoregulation using convectional thermotransmission. Especially feedforward thermoregulatory sweating response is estimated to be highly phylogenetic than vasoconstrictive response.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Sweating
- Vasoconstriction
- Core temperature
- Neural thermoregulatory pathway
- Convectional thermoregulatory pathway
- Feedforward thermoregulation
1 Introduction
Skin sympathetic nerve activity (SSNA) is microneurographically recorded from the skin nerve fascicle in the peripheral nerves and contributes to thermoregulatory function in humans. It controls the skin blood flow by regulating the arteriolar sphincter, erects the hair by constricting the arrector pili muscles, and excretes sweat from the sweat glands. Activation of the SSNA constricts the skin vessels, thereby suppressing the heat flux from the skin, namely, vasoconstrictor activity [1, 2]. Contrarily, it accelerates the sweating to cool the skin by evaporation through sudomotor nerve activity. This chapter focuses on the thermoregulatory function of the skin sympathetic nerve activity in humans and discusses how humans developed their civilization using this thermoregulatory capability.
In the present chapter, the recording method, the properties, the difference from the muscle sympathetic nerve activity, and the identification are described. Changes in SSNA under various environmental conditions, as well as the pathophysiology of SSNA, are described in an overall review by Mano et al. [3–7].
2 Recording Skin Sympathetic Nerve Activity
SSNA is recorded from the skin nerve fascicles by inserting a tungsten microelectrode into the peripheral nerves subcutaneously without anesthesia. The electrode is insulated with epoxy resin, and the tip is exposed to record the nerve impulses as the voltage from the reference electrode. The shaft diameter of the recording electrode is usually 100 μm, tip diameter is 1 μm, and impedance is 3–5 MΩ. The recorded signals are fed into a high-impedance bioamplifier and band-pass filters of 500–5 kHz and are displayed on an oscilloscope with sound monitoring. The signal is usually recorded in a magnetic data recorder or digitized with a sampling frequency of >5 kHz and stored on the hard disk of a personal computer.
In order to record SSNA, a three-step procedure should be undertaken: (1) detection of the nerve tract using electric stimulation, (2) penetration of the nerve fascicle with an electrode, and (3) moving the electrode tip to record the required nerve activity and to enhance the S/N ratio. When the electrode tip penetrates into the nerve fascicle, the subject feels paresthesia or a grasped sensation. Gentle touches to the skin or taps of the muscle belly determine whether the tip is situated in the skin or muscle fascicles, respectively.
SSNA is identified by the following criteria [3–7]: (1) spontaneous nonrhythmic efferent burst discharge from the skin fascicle; (2) followed by sweating or vasoconstriction after the burst discharge with a certain latency; (3) eliciting burst discharge by mental stimuli, sound, pain, or electric stimulation with a certain (~1 s) latency; (4) longer burst duration of ~300 ms compared with MSNA (~200 ms); and (5) eliciting burst discharge by sudden inspiration with a certain latency. The criterion for good recording is that the burst amplitude is double the noise level.
3 Quantification and Evaluation of Skin Sympathetic Nerve Activity
Since SSNA is recorded as the burst activities, the quantification of SSNA is derived from [burst number per minute (burst rate)], from [burst number per minute] × [mean burst amplitude] (total SSNA), or from [total burst area when the maximum burst amplitude is regarded as 1000]. SSNA contains sudomotor nerve activity and vasoconstrictor activity and, furthermore, vasodilator and pilomotor activities, and the analysis of SSNA requires the differentiation of these activities since these activities run in the same nerve fascicle interminglingly by using laser Doppler flowmetry and skin potential change.
4 Properties of Skin Sympathetic Nerve Activity
The role of SSNA is the thermal and mental control of sweating and skin blood flow, and its goal is to maintain the body temperature through sweating/vasoconstriction against environmental change of temperature, as well as mental stress [1, 2, 8–11].
Pressure hemihidrosis is the phenomenon that occurs asymmetrically on the unilateral trunk when pressured on the contralateral side. It was firstly reported by Kuno [12], and the mechanism that skin pressure applied to one side of the body produces an ipsilateral suppression and contralateral facilitation was reported (Takagi and Kobayashi 1955) [13]. After the introduction of SSNA, Sugiyama et al. [14] reported that skin pressure reduced ipsilaterally the amplitude of sudomotor bursts. Furthermore, Okagawa et al. [15] concluded that the ipsilateral suppression of cutaneous blood flow was not mediated by the vasoconstrictor nerve system although ipsilateral suppression of sweating elicited by skin pressure was mediated by the sudomotor nervous system, indicating that the occurrence of the spinal reflex due to skin pressure is not uniform between the sudomotor and the vasoconstrictor nerve systems, which represent different organizations at the level of the spinal cord.
In humans, SSNAs innervating the glabrous skin including the palm and sole and those innervating the hairy skin in the generalized body surface behave differently. The former mainly contain mental sudomotor/vasomotor activities, while the latter include thermoregulatory ones [8–10]. Furthermore, since sudomotor nerve activity includes vasodilatory activity, a comparison of these activities is useful in analysis of the regional differentiation of sympathetic nerve activity in humans [16].
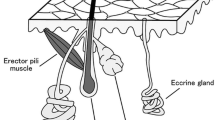
The sudomotor and vasoconstrictor activities are well correlated with the skin potential change and the rate of reduction of vasoconstriction, respectively; therefore, it is well documented that sympathetic skin response and sympathetic flow response measured by laser Doppler flowmetry are good reflections of sudomotor and vasoconstrictor nerve activity, respectively (Fig. 4.1).
Schematic illustration of skin sympathetic nerve activity. Skin sympathetic nerve activity mainly includes sudomotor nerve activity governing the sweat glands and vasoconstrictive nerve activity innervating the skin arteriolar presphincters of skin vessels. Sympathetic skin response is produced by the movement of sweat in the subcutaneous tissue, and output at the sweat gland orifice provides the sweat expulsion. In contrast, vasoconstriction by vasoconstrictor SSNA causes the reduction in skin blood flow measured by laser Doppler flowmetry
The following describes the basic properties of SSNA.
4.1 The Relationship Between SSNA Bursts and Sweating Rate and Skin Blood Flow (Fig. 4.2)
Sudomotor and vasoconstrictor components in skin sympathetic nerve activity. Traces are full-wave rectified and integrated skin sympathetic nerve activity (SSNA), skin blood flow measured by laser Doppler flowmetry, photoelectric plethysmogram, and sweat rate measured by the ventilated capsule method. The latency from the SSNA burst to the inflection of sweat rate identifies the property of the SSNA burst as sudomotor, and that to the reduction point of laser Doppler skin blood flow identifies as vasoconstrictor. The burst indicators are SM sudomotor, VC vasoconstrictor, and SM+VC mixed. The numbers after the burst indicators correspond to the sweat rate inflections and the skin blood flow changes [17]
The report by Bini et al. [1, 2] showed that laser Doppler flowmetry could enable the accurate measurement of skin blood flow, and sweat rate could be measured quantitatively by development of the ventilated capsule method. The full-wave rectified and integrated SSNA sudomotor and vasoconstrictor bursts were proved to be correlated with the slope of the sweat rate and the rate of reduction of skin blood flow by laser Doppler flowmetry, respectively [17].
4.2 Effect of Thermal Change in the Environment (Fig. 4.3)
Changes in sudomotor and vasoconstrictor components in skin sympathetic nerve activity depending on the ambient temperature from the tibial nerve and the peroneal nerve
The tibial SSNA innervating to the glabrous skin (sole) and the peroneal SSNA innervating to the hairy skin (dorsum pedis) change their components depending on the ambient temperature [10]
SSNA contributes to thermoregulation by enhancing or reducing its activity, which is lowest under thermoneutral conditions. This thermoneutral temperature depends on ethnic factors and is estimated to be 27 °C in the case of the Japanese [10].
SSNA changes depending on the recorded nerve when the environmental temperature changes. In the peroneal nerve innervating hairy skin, the lowest level was designated at the thermoneutral temperature, and vasoconstrictor SSNAs were predominant at 15 °C, while sudomotor SSNA is predominant at 35 °C. Therefore, the shape of the SSNA showed a hairpin curve, reaching its nadir at 27 °C. In contrast, changes in the tibial nerve SSNA showed the lowest value at 35 °C, with gradual enhancement as environmental temperature decreased. Since the tibial SSNA reflects the stress received from the environment, environmental cold stress enhances the vasoconstrictor SSNA [9, 10].
In this way, sudomotor and vasoconstrictor SSNAs contribute to human thermoregulatory function by altering its components depending on the environmental temperature.
4.3 Conduction Velocity of Skin Sympathetic Nerve Activity (Fig. 4.4)
Conduction velocity measurement in skin sympathetic nerve activity. Traces are integrated SSNA recorded at the medial malleolus, integrated SSNA recorded at the popliteal fossa, electrocardiogram, skin potential change, and skin blood flow change measured by laser Doppler flowmetry. The distance between the recording points at the medial malleolus and the popliteal fossa divided by the burst rise time differences provides the sudomotor and vasoconstrictor conduction velocities [18]
A double recording technique [4] enabled us to measure the conduction velocity of SSNA. Two microelectrodes were inserted into the proximal and distal sites of one nerve, the time differences between burst rises or burst peaks were measured, and the velocity was calculated by dividing the distance between the electrodes by this time difference. The conduction velocities of MSNA thus measured were reported to be 0.72 m/s in the median nerve and 1.09 m/s in the peroneal nerve, while those of SSNA were 0.93 m/s in sudomotor, 0.76 m/s in vasoconstrictor at rest, 1.12 m/s in sudomotor, and 0.81 m/s in vasoconstrictor in elicited bursts by electrical stimulation [18].
4.4 Change in Skin Sympathetic Nerve Activity During Sleep
In contrast to the MSNA change during sleep, which reduces its activity as sleep stage proceeds, SSNA changes were reported to be the component changes of sudomotor and vasoconstrictor. Generally, it is said that sympathetic nerve activity functions to cool the body during non-REM sleep, but to warm the body during REM sleep.
Takeuchi et al. [19] reported that SSNA reduces its activity until sleep stage 2 as the sleep stage proceeds, while in turn it increases during sleep stages 3 + 4. Kobayashi et al. [20] observed SSNA change during sleep by its effector responses and described that the sudomotor activity during NREM sleep is enhanced compared with that in REM sleep, while vasoconstrictor activity is enhanced during REM sleep compared with that in NREM sleep, the same tendency as reported by Noll et al. [21].
Thus, vasoconstrictor SSNA reduces its activity as the sleep stage proceeds, to cool the human body, while sudomotor SSNA is slightly enhanced to cool the human body during slow-wave NREM sleep (stages 3+4). Contrarily, vasoconstrictor is enhanced and sudomotor is reduced to warm the body during REM sleep.
4.5 Effect of Skin Sympathetic Nerve Activity on the Core Temperature Change
Upon exposure to a cold environment, vasoconstrictor SSNA is enhanced to reduce the heat dissipation from the skin surface, and thus, skin blood flow decreases. By this vasoconstriction, it has been observed that the core temperature, measured as the tympanic temperature (Tty), gradually increases with a certain latency, showing the usefulness of recording SSNA in studies of human thermoregulation [22] (Fig. 4.5).
Changes in body temperature and SSNA following acute local cooling. Traces are skin temperature of cooling site, skin temperature of non-cooling site, tympanic temperature, and SSNA burst rate with the sudomotor and vasoconstrictor components. Acute local cooling was provided by inserting both hands into a dry icebox [22]
When we analyzed the magnitude of increase in SSNA upon cold exposure and the increase in the core temperature, a significant correlation between increase in vasoconstrictor burst rate (%) and the rate of change of tympanic temperature was found (Fig. 4.6). This means that the greater the capability to change the SSNA upon cold exposure, the more excellent thermoregulatory function the individual possesses. The cross-correlation between the SSNA and the core temperature showed that the peak latency is approximately 10 min, meaning that it takes approximately 10 min to raise the core temperature upon cold exposure (Fig. 4.7) [22–25].
The relationship between the increase in vasoconstrictor component of SSNA taking the baseline reading as 100 % and the change rate of tympanic temperature per min
A significant correlation is observed between the two, indicating that the larger the individual who is capable of SSNA, the higher the tympanic temperature, i.e., the core temperature [22]
Cross-correlation between SSNA and tympanic temperature during ambient temperature drop. The cross-correlogram exhibited at its maximum at 10 min indicating that the SSNA discharge provides the maximum tympanic temperature rise at 10 min after the discharge [22]
In contrast, we also reported that rewarming from a cold environment of ~15 °C suppresses the vasoconstrictor SSNA (Fig. 4.8), and the greater suppression, rate of vasoconstrictive SSNA is associated with the larger suppression of the core temperature (Fig. 4.9) [25]. Cross-correlogram between SSNA discharge and the core temperature (tympanic temperature) change designated that the time delay exhibited ~7 min (Fig. 4.10), indicating that not only the cooling but also warming takes approximately 7–10 min to raise the core temperature through the convectional pathway.
SSNA suppression and tympanic temperature drop with ambient temperature decrease
The traces are room, skin, tympanic temperatures, SSNA, and skin blood flow. The room temperature was decreased to 10 °C and rewarmed to 28 °C. With the room temperature rise, SSNA was suppressed, and skin blood flow was increased, which enhanced the heat dissipation from the skin surface and resulted in a tympanic temperature drop
The relationships between the change rate of SSNA and the tympanic temperature change rate (a) and maximal change in tympanic temperature (b) The established significant correlation indicates the larger the capacity to enhance SSNA, the larger the tympanic temperature to change [23]
Cross-correlation between SSNA and tympanic temperature during ambient temperature rewarming The cross-correlogram exhibited at its maximum at 7 min indicating that the SSNA discharge suppression provides the maximum tympanic temperature drop at 7 min after the discharge suppression [25]
The height of integrated sudomotor SSNA is found to be proportional to the number of sweat glands observed with a video microscope [26]. This means that the height of integrated SSNA represents the driving force to expel sweating droplets, and it well correlates to the slope of the sweat rate.
4.6 Effect of Aging on Skin Sympathetic Nerve Activity in Humans
Although it is known that MSNA increases with advancing age, age-related changes in resting SSNA have not been clarified. Attenuation of peripheral effectors has been reported by Watanabe et al. [27]; however, another report described that SSNA in the elderly is decreased compared with that in the young and middle aged, and responsiveness to the thermal environment is also reduced [28].
In a recent study by Greaney et al. [29], it was observed that SSNA response to whole-body cooling is blunted in the elderly, which is related to a marked impairment in reflex cutaneous vasoconstriction, although cutaneous vascular responsiveness to adrenergic stimuli is not reduced and that to a non-thermoregulatory stimulus is preserved. Nevertheless, interindividual differences of resting SSNA are very large, which makes this issue difficult to investigate.
5 Clinical Application of Skin Sympathetic Nerve Activity
5.1 Investigation of Sweating Physiology
Thermoregulatory sweating is only observed in some primates, including humans. The phenomenon of “sweat expulsion,” which involves the emission of sweat from the sweat gland orifices, has been clarified to correspond to SSNA burst discharges [30]. The more impulses are included in sudomotor bursts, the more sweat glands are driven by SSNA bursts, and the more sweating is facilitated, which contributes to the thermoregulation of the human body.
5.2 Investigation of Anhidrosis/Hypohidrosis
Anhidrosis/hypohidrosis is defined as an absence of sweating. It may have multiple etiologies, but the recording of SSNA can identify the site of the lesion (Fig. 4.11) [31–36]. Attenuated SSNA represents neurogenic anhidrosis/hypohidrosis, and no change or activation, especially activation under heat exposure, indicates that a lesion is at a sweat gland. In neurogenic anhidrosis/hypohidrosis, a peripheral origin increases the burst rate, while a central origin does not exhibit an SSNA change (Fig. 4.12).
Secretion of individual sweat glands and various effector responses to spontaneous sudomotor bursts during mild sweating. Skin blood flow, sweat rate, skin potential activity, and skin sympathetic nerve activity recorded from the tibial nerve are indicated. The lower panel indicates the timing of sweat secretion from individual sweat glands observed on a small area of the sole by a videomicroscope. The numerals 1–3 along the vertical bar represent individual glands. Two sudomotor bursts (S with no asterisk) elicited sweat secretion from three or five glands (Nos. 6, 12, 16, 18, 20). Another sudomotor burst (S with asterisk) did not elicit any sweat secretion, although the burst caused small responses in skin potential and local sweat rate. Skin vasoconstrictor bursts (shown as V) induced a transient reduction in skin blood flow. One of the sudomotor bursts contains the vasoconstrictor component. Three vertical lines show the onsets of sudomotor burst, skin potential response, and sweat response
SSNA from acquired idiopathic generalized anhidrosis (AIGA). Traces are ① raw trace of SSNA, ② integrated SSNA, ③ laser Doppler skin blood flow, ④ photoelectric plethysmogram, and ⑤ sweat rate (hidrogram) in the control subject, and in AIGA patient in baseline recording (a), and after electric stimulation (b). No sweat rate increase was observed after electric stimulation at the wrist joint [37]
Idiopathic and secondary anhidrosis and hypohidrosis exist, among which acquired idiopathic generalized anhidrosis (AIGA, 36, 32, 33, 34, 35) is characterized by a generalized absence of sweating without other autonomic and neurologic dysfunctions. AIGA is classified into three subgroups: (1) sudomotor neuropathy, (2) idiopathic pure sudomotor failure (IPSF), and (3) sweat gland failure (SGF), with each subgroup presenting a different pathogenesis. Sudomotor neuropathy is neurogenic and may sometimes be accompanied by vasoconstrictor neuropathy (Fig. 4.13). IPSF is the most common presentation and is characterized by loss of sweating in the absence of any neurological features or destruction of sweat glands by skin biopsy. IPSF is associated with cholinergic urticaria and estimated to be of autoimmune origin. In SGF, skin biopsy demonstrates the destruction and devastation of the sweat glands, which suggests the possibility of the resultant devastation of IPSF. SSNA is normal or even enhanced in IPSF and SGF without neurological deficits but with a generalized absence of sweating, sometimes associated with heat intolerance, especially upon heat exposure [37–39].
Changes in skin blood flow, sweat rate, skin potential change, and integrated skin sympathetic nerve activity followed by sound/electric stimulations in acquired idiopathic generalized anhidrosis (a) and autonomic neuropathy (b) (a) In acquired idiopathic generalized anhidrosis, resting SSNA is enhanced, reflex response to sudden sound is favorable, and skin potential change exhibits fair response, although no sweating response is observed (b) In autonomic neuropathy, since both sudomotor and vasoconstrictor SSNA are disordered, reduced resting SSNA is observed, and electric stimulation elicits lowered reflex bursts, reduced skin potential change, as well as no sweat response, and no skin blood flow change
Since IPSF has been proved to be improved by steroid pulse therapy, early diagnosis of the disease by microneurography and skin biopsy is recommend [40].
5.3 Investigation of Hyperhidrosis
Hyperhidrosis is defined as an excess of sweating beyond the amount required to return an elevated body temperature to normal, with a distinction between primary and secondary forms. Among primary forms, idiopathic palmoplantar hyperhidrosis is frequently found in Mongoloids, with profuse sweating in their palms and soles. The condition may become socially and professionally debilitating. Idiopathic palmoplantar hyperhidrosis begins in childhood and frequently runs in families. Epidemiologically, it has been estimated to occur 0.6–1 % of the population, while its rate has been reported to be 2.8 % in the United States [41].
The definition of palmoplantar hyperhidrosis is not quantitatively determined in terms of sweating more than a certain amount, but rather that the patient feels that the hyperhidrotic state is troublesome. It affects men and women equally and most commonly occurs among people aged 25–64 years, some of whom may have been affected since early childhood; ~30–50 % have another family member afflicted, implying a genetic predisposition [40]. In 2006, a primary palmar hyperhidrosis locus was reported to be mapped to 14q11.2–q13 [42].
The activated sudomotor as well as vasoconstrictor SSNA governing the palms (median nerve) and soles (tibial nerve) was first reported in 1997, while that governing hairy skin (peroneal nerve) is not enhanced as such (Figs. 4.13 and 4.14). The intermingling of sudomotor and vasoconstrictor in the same nerve fascicle causes not only palmar hyperhidrosis but also vasoconstriction-caused hypothermia in the affected skin areas [32]. Hyperhidrosis observed in amyotrophic lateral sclerosis [43] or Guillain-Barré syndrome [30] is also caused by this enhanced SSNA.
Tibial SSNA and peroneal SSNA in palmoplantar hyperhidrosis and their responses to mental arithmetic (psychological load). Traces are raw tibial SSNA and its full-wave rectified and integrated trace, innervating the sole, and peroneal SSNA and its full-wave rectified and integrated trace, innervating the dorsum pedis. The resting tibial SSNA is enhanced compared with peroneal SSNA, and the response to the mental arithmetic was markedly enhanced. The resting peroneal SSNA is not so activated, and the response is not so prominent. These traces indicate that the SSNA response to mental stimuli only is markedly enhanced [32]
5.4 Investigation of Thermoregulatory Function and Thermoregulatory Disorders
There are large interindividual differences in human thermoregulatory function, and we have reported that the activation capacity of vasoconstrictive SSNA determines individual thermoregulatory function [22, 23, 25]. Individuals who can suppress SSNA can minimize the change in core temperature. Thus, human cold tolerance and heat tolerance are acquirable by acclimatization by altering the sympathetic nerve activity toward environmental thermal change [24].
In hypothalamic thermodysregulation, microneurographic SSNA recording revealed the hypofunction of the hypothalamic warm neurons, whereas the cold neurons worked effectively, which suppressed sweating and resulted in thermodysregulation [27].
5.5 Investigation of Pathophysiology and Diagnosis of Vasomotor Disease
Diseases due to aberrant vasoconstriction, for example, Raynaud’s disease and Buerger’s disease, are sometimes treated and improved by sympathectomy. SSNA from these disorders with a certain perturbation presents different responses from that in normal subjects [24, 44–47]. These recordings are beneficial to assess the effectiveness of nerve block or sympathectomy.
5.6 Investigation of Association Between Cognitive Function and Sympathetic Nerve Activity
Studies on the association between cognitive function and sympathetic nerve activity were initiated by the analysis of reflex latency in Parkinson’s disease [48], in which delay of reflex latency was reported.
One of the event-related potentials, P300, has a close relationship with cognitive function. Simultaneous recordings of event-related potential and SSNA with an oddball paradigm have revealed that cognition of the target elucidates a significantly strong sympathetic response compared with that upon no cognition, which indicates that the cognitive process strongly influences central sympathetic outflow in humans. Furthermore, analysis of the presence of reflex bursts and summation of event-related potentials clarified that the early component of P300 is closely related to the origin of SSNA [49].
5.7 Evaluation of Drug Effects on Nerve-Effector Responses
The relationships between the height of integrated burst discharge and sweat acceleration, and between that and reduction rate of skin blood flow, have been established (Fig. 4.15). Analysis of the slope of the regression lines can enable evaluation of drug effects in cases when such effects cannot be clarified by analysis of the changes in effector organs.
Tibial SSNA and peroneal SSNA in palmoplantar hyperhidrosis and their responses to ambient temperature rise (psychological and physical load). Traces are, from the top to the bottom, tibial SSNA, its full-wave rectified and integrated trace, peroneal SSNA, and its integration at the ambient temperature of 25 °C in resting (a), during heating to ambient temperature of 30 °C (b), and 30 °C in resting (c). Resting SSNA was enhanced in the tibial and gradually augmented with an ambient temperature rise in both, but the augmentation was more prominent in the tibial SSNA. The degree of enhancement was greater in the mental load than in the physical [17]
For example, sweat acceleration and vasoconstrictive effects of prostaglandin E1 can be clarified by analyzing the relationship between sudomotor SSNA and sweat expulsion and vasoconstrictor SSNA and the rate of reduction of laser Doppler skin blood flow, respectively, even if the baseline measurement of both sweat rate and skin blood flow indicated no changes. The attenuating effect of prostaglandin E1 on sudomotor and vasoconstrictive effects could be confirmed by the changes in the slope of the regression line from a steep gradient to a gradual gradient by the intravenous administration of prostaglandin E1 (Figs. 4.16 and 4.17) [50].
The relationship between SSNA and laser Doppler skin blood flow. There established a significant correlation between the amplitude of integrated SSNA burst (h) and the slope of sweat rate rise (tan θ, sweat acceleration); the logarithm of reduction rate of skin blood flow log (b/a ×100) [17]
Effect of prostaglandin E1 administration on the relationships between logarithm of integrated SSNA and reduction rate of skin blood flow (a) Administration of prostaglandin E1 reduces the slope of the regression line between the logarithm of integrated SSNA and the rate of reduction of skin blood flow. Prostaglandin E1 administration reduces the regression line between log (integrated SSNA amplitude) and reduction rate of laser Doppler skin blood flow (b) Administration of prostaglandin E1 reduces the slope of the regression line between the logarithm of integrated SSNA and the rate of reduction of laser Doppler skin blood flow dose dependently [50]
6 Significance of SSNA as a Feedforward Tool
6.1 Environmental Temperature and Thermal Conduction
There are two ways to conduct information on the environmental temperature to the hypothalamus. One is a neural pathway that conducts the thermal information through a neural network, while the other is a convectional pathway that provides information through circulation by warmed or cooled blood to the hypothalamus.
The thermal sensation is transmitted to the thermoregulatory center in the hypothalamus, situated at the preoptic area and anterior hypothalamus. Warming this area facilitates heat dissipation, through vasodilatation and sweating. Inversely, cooling this area suppresses heat dissipation, through vasoconstriction or shivering. Thus, activation of these neurons facilitates sympathoactivation. After thermoreception at the preoptic area and anterior hypothalamus, the generation of comfortableness and uncomfortableness is involved with amygdala, orbitofrontal cortex, or cingulate cortex. This central command descends through the periaqueductal gray and raphe nucleus, connects to the rostral ventrolateral medulla as the premotor neuron, and exchanges the neurons at the intermediolateral nucleus and sympathetic ganglion. The descending sympathetic outflow to the skin thus governs the sweat glands and skin vasculature to control the body temperature.
6.2 Chronological Delay in Thermoregulation Using Convectional and Neural Pathway
The time delay from vasoconstrictive SSNA discharge to the core temperature rise should be ~7–14 min, from the correlative analysis of SSNA and core temperature. In the same fashion, the time delay from the stimulation to the warm neurons to the core temperature drop through vasodilatation or sweating should be also ~7–14 min. Thus, thermoregulation using a convectional pathway, that is, negative feedback, requires approximately 10 min depending on the individual; contrarily, the time delay from the environmental temperature change through the neural pathway to thermoregulatory SSNA discharge is within 1 min. Therefore, employing this rapid neural pathway, namely, the feedforward network, is efficient to control the human body temperature.
In this way, feedforward control by this neural pathway has the merit of rapid initiation of thermoregulatory control compared with the convectional pathway which takes approximately 10 min, through the activation or suppression of SSNA. This capacity to activate/suppress the SSNA determines the thermoregulatory capacity through the feedforward neural pathway.
The reason why two thermo-informative pathways have developed in humans is that the convectional pathway, possessed at phylogenetically older levels, takes too long to transmit the thermoregulatory information to the central nervous system. This accelerated the development of the feedforward and neural pathway, which takes only one tenth of the time of the convectional pathway. In particular, thermoregulation by sweating is recognized in the primates, which is assumed to be phylogenetically high level of thermoregulation.
6.3 Phylogenetic Considerations
Differentiation of sudomotor and vasoconstrictor components of SSNA and correlative study with evoked potentials have revealed that the cognitive potential, P300, has close associations with sudomotor SSNA (Fig. 4.18) and somatosensory potential, N140, has close associations with vasoconstrictor SSNA (Fig. 4.19) [51]. These findings suggest that the sweating responding to neural transmission is a higher level of thermoregulatory function associated with cognition and behavior, which are phylogenetically higher-level cortical functions than skin vasoconstriction induced by stimulation of the skin or cold exposure.
Sudomotor SSNA is closely associated with cognition. Integrated SSNA with skin potential change (SSR sympathetic skin response) is assumed as sudomotor SSNA (SSR+), and SSNA without skin potential change is assumed as SSR− SSNA (a). The amplitude of integrated SSNA burst with SSR+ response accompanies with a significantly large P300 amplitude (b) in the evoked potential (in Cz and Pz,), whereas there is no significant difference in N140 (c)
Vasoconstrictor SSNA is closely associated with somatosensory sensation. Integrated SSNA with skin blood flow change (SFR, sympathetic flow response) is assumed as vasoconstrictor SSNA (SFR+) and without skin blood flow change is assumed as SSR− SSNA (a). The amplitude of integrated SSNA burst with SSR+ response accompanies with significantly large N140 amplitude (b) in the evoked potential (in Cz), whereas there is no significant difference in P300 (c)
This hypothesis that “mental sweating necessitates recognition whereas somatosensory stimulation is important for vasoconstriction” is supported by the fact that “mental sweating disappears but vasoconstriction continues under general anesthesia” and that “vasoconstriction is induced by the cold sensation to sensory loss skin.”
In this way, the development of humans is considered to have occurred by them widening their activity under various thermal environments, preventing extreme body temperature rises or drops through more rapid thermoregulation using feedforward control of the body temperature via neural thermoregulatory control.
7 Conclusion
The recording techniques, properties, clinical applications, and significance of sympathetic nerve activity to the skin have been described. Time series analysis with other variables would clarify the various aspects of SSNA in humans. The significance of SSNA as a phylogenetic consideration should be clarified in future studies.
References
Bini G, Hagbarth K-E, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol. 1980;306:537–52.
Bini G, Hagbarth K-E, Hynninen P, Wallin BG. Regional similarities and differences in thermoregulatory vaso- and sudomotor tone. J Physiol. 1980;306:553–65.
Mano T. Sympathetic nerve mechanisms of human adaptation to environments – findings obtained by recent microneurographic studies. Environ Med. 1990;34:1–35.
Mano T, Iwase S, Sugiyama Y. Double recording technique of sympathetic nerve traffic in microneurography. In: Shibasaki H, Kimura J, editors. Recent advances in clinical neurophysiology. Amsterdam: Elsevier Science; 1996. p. 416–9.
Mano T. Microneurographic research on sympathetic nerve response to environmental stimuli in humans. Jpn J Physiol. 1998;48:99–114.
Mano T. Muscular and cutaneous sympathetic nerve activity. In: Appenzeller O, editor. The autonomic nervous system (1), handbook of clinical neurology, vol. 174. Amsterdam: Elsevier Science; 1999. p. 649–65.
Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol. 2006;117:2357–84.
Iwase S, Sugenoya J, Sugiyama Y, Mano T, Yamamoto K. Skin sympathetic nerve activity in the peroneal and the tibial nerves. Ann Rep Res Inst Environ Med Nagoya Univ. 1990;40:308–13.
Iwase S, Sugenoya J, Mano T, Sugiyama Y, Hakusui S, Yamamoto K. Reflex burst of skin sympathetic nerve activity and sudomotor/vasoconstrictor activity in humans. Ann Rep Res Inst Environ Med Nagoya Univ. 1991;41:1990.
Okamoto T, Iwase S, Sugenoya J, Mano T, Sugiyama Y, Yamamoto K. Different thermal dependency of cutaneous sympathetic outflow to glabrous and hairy skin in humans. Eur J Appl Physiol Occup Physiol. 1994;68:460–4.
Sugenoya J, Iwase S, Mano T, Ogawa T. Identification of sudomotor activity in cutaneous sympathetic nerves using sweat expulsion as the effector response. Eur J Appl Physiol Occup Physiol. 1990;61:302–8.
Kuno Y. The physiology of human perspiration. London: Churchill; 1934.
Takagi K, Kobayashi S. Skin pressure-vegetative reflex. Acta Med Biol. 1955;4:31–57.
Sugiyama Y, Watanabe T, Takeuchi S, Iwase S, Mano T. Asymmetric discharges of skin sympathetic nerve in humans—with special reference to pressure hemihidrosis. Environ Med. 1992;36:207–10.
Okagawa T, Sugenoya J, Iwase S, Mano T, Suzumura A, Matsumoto T, et al. Occurrence of the spinal reflex due to skin pressure in sudomotor and cutaneous vasoconstrictor nerve system of humans. Auton Neurosci. 2003;105:62–70.
Sugenoya J, Iwase S, Mano T, Sugiyama Y, Ogawa T, Nishiyama T, et al. Vasodilator component in sympathetic nerve activity destined for the skin of the dorsal foot of mildly heated humans. J Physiol. 1998;507(Pt 2):603–10.
Iwase S, Mano T, Sugenoya J, Sugiyama Y, Matsukawa T, Yamamoto K. Microneurographic study on sympathetic control of sweating and skin blood flow. In: Mabuchi K, editor. Advanced techniques and clinical applications in biomedical thermology. Chur: Harwood; 1994. p. 147–62.
Kondo M, Iwase S, Mano T, Kuzuhara S. Direct measurement of human sympathetic nerve conduction velocity. Muscle Nerve. 2004;29:128–33.
Takeuchi S, Iwase S, Mano T, Okada H, Sugiyama Y, Watanabe T. Sleep-related changes in human muscle and skin sympathetic nerve activities. J Auton Nerv Syst. 1994;47:121–9.
Kobayashi R, Koike Y, Hirayama M, Ito H, Sobue G. Skin sympathetic nerve function during sleep—a study with effector responses. Auton Neurosci. 2003;103:121–6.
Noll G, Elam M, Kunimoto M, Karlsson T, Wallin BG. Skin sympathetic nerve activity and effector function during sleep in humans. Acta Physiol Scand. 1994;151:319–29.
Sawasaki N, Iwase S, Mano T. Effect of skin sympathetic response to local or systemic cold exposure on thermoregulatory functions in humans. Auton Neurosci. 2001;87:274–81.
Michikami D, Iwase S, Kamiya A, Fu Q, Mano T, Suzumura A. Interrelations of vasoconstrictor sympathetic outflow to skin core temperature during unilateral sole heating in humans. Auton Neurosci. 2001;91:55–61.
Iwase S, Sawasaki N, Michikami D, Cui J, Kamiya A, Niimi Y, et al. Effect of activation and suppression of vasoconstrictive skin sympathetic nerve activity on the core temperature in humans. Auton Nerv Syst. 2001;38:7–10.
Iwase S, Cui J, Wallin BG, Kamiya A, Mano T. Effects of increased ambient temperature on skin sympathetic nerve activity and core temperature in humans. Neurosci Lett. 2002;327:37–40.
Nishiyama T, Sugenoya J, Matsumoto T, Iwase S, Mano T. Irregular activation of individual sweat glands in human sole observed by a videomicroscopy. Auton Neurosci. 2001;88:117–26.
Watanabe H, Shindo K, Ida H, Tanaka H, Nagasaka T, Shiozawa Z. Aging effects of sympathetic reflex activities on skin nerves. Gerontology. 2003;49:366–73.
Grassi G, Seravalle G, Turri C, Bertinieri G, Dell’Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation. 2003;108:729–35.
Greaney JL, Stanhewics AE, Kenney WL, Alexander LM. Impaired increases in skin sympathetic nerve activity contribute to age-related decrements in reflex cutaneous vasoconstriction. J Physiol. 2015. doi:10.1113/JP270062 [Epub ahead of print].
Yamamoto K, Sobue G, Iwase S, Nagamatsu M, Mano T, Mitsuma T. Skin sympathetic nerve activity in Guillain-Barré syndrome: a microneurographic study. J Neurol Neurosurg Psychiatry. 1997;63:537–41.
Donadio V, Montagna P, Nolano M, Cortelli P, Misciali C, Pierangeli G, et al. Generalised anhidrosis: different lesion sites demonstrated by microneurography and skin biopsy. J Neurol Neurosurg Psychiatry. 2005;76:588–91.
Iwase S, Ikeda T, Kitazawa H, Hakusui S, Sugenoya J, Mano T. Altered response in cutaneous sympathetic outflow to mental and thermal stimuli in primary palmoplantar hyperhidrosis. J Auton Nerv Syst. 1997;64:65–73.
Iwase S. Acquired idiopathic generalized anhidrosis. Nihon Iji Shimpo. 2003;4136:1–10.
Iwase S. Acquired idiopathic generalized anhidrosis. Auton Nerv Syst. 2003;40:335–42.
Miyazoe S, Matsuo H, Ohnishi A, Tajima F, Fujishita S, Ichinose K, et al. Acquired idiopathic generalized anhidrosis with isolated sudomotor neuropathy. Ann Neurol. 1998;44:378–81.
Nakazato Y, Tamura N, Ohkuma A, Yoshimaru K, Shimazu K. Idiopathic pure sudomotor failure: anhidrosis due to deficits in cholinergic transmission. Neurology. 2004;63:1476–80.
Murakami K, Sobue G, Iwase S, Mitsuma T, Mano T. Skin sympathetic nerve activity in acquired idiopathic generalized anhidrosis. Neurology. 1993;43:1137–40.
Hakusui S, Yasuda T, Yanagi T, Yamamoto K, Iwase S, Ikeda T, et al. Pathophysiology of 2 cases of acquired anhidrosis. Auton Nerv Syst. 1996;33:366–70.
Yamamoto K, Sobue G, Iwase S, Kumazawa K, Mitsuma T, Mano T. Possible mechanism of anhidrosis in a symptomatic female carrier of Fabry’s disease: an assessment by skin sympathetic nerve activity and sympathetic skin response. Clin Auton Res. 1996;6:107–10.
Chen YC, Wu CS, Chen GS, Khor GT, Chen CH, Huang P. Identification of subgroups of acquired idiopathic generalized anhidrosis. Neurologist. 2008;14:318–20. doi:10.1097/NRL.0b013e318173e818.
Haider A, Solish N. Focal hyperhidrosis: diagnosis and management. Can Med Assoc J. 2005;172(1):69–75. doi:10.1503/cmaj.1040708.PMC543948.
Higashimoto I, Yoshiura K, Hirakawa N, Higashimoto K, Soejima H, Otoki T, et al. Primary palmar hyperhidrosis locus maps to 14q11.2-q13. Am J Med Genet A. 2006;140A(6):567–72. doi:10.1002/ajmg.a.31127.PMID16470694.
Shindo K, Watanabe H, Ohta E, Nagasaka T, Shiozawa Z, Takiyama Y. Sympathetic sudomotor neural function in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:39–44. doi:10.3109/17482968.2010.508529. Epub 2010 Aug 25.
Iwase S, Sawasaki N, Michikami D, Cui J, Kamiya A, Niimi Y, et al. Effects of activation and suppression of vasoconstrictor nerve activity in skin sympathetic nerve activity on core temperature change in humans. Auton Nerv Syst. 2001;38:641–4.
Iwase S, Okamoto T, Mano T, Kamiya A, Niimi Y, Qi F, et al. Skin sympathetic outflow in Buerger’s disease. Auton Neurosci. 2001;87:286–92.
Sakakibara H, Iwase S, Mano T, Watanabe T, Kobayashi F, Furuta M, et al. Skin sympathetic activity in the tibial nerve triggered by vibration applied to the hand. Int Arch Occup Environ Health. 1990;62:455–8.
Hakusui S, Iwase S, Sugenoya J, Mano T. Microneurographic analysis of skin sympathetic nerve activity under cold environment in humans —with special reference to Raynaud’s phenomenon—. Environ Med. 1989;33:103–6.
Ishida G, Nakashima K, Takahashi K. Skin nerve sympathetic activity reflex latency in Parkinson’s disease. Acta Physiol Scand. 1990;81:121–4.
Ito H, Sugiyama Y, Mano T, Okada H, Matsukawa T, Iwase S. Skin sympathetic nerve activity and event-related potentials during auditory oddball paradigms. J Auton Nerv Syst. 1996;60:129–35.
Iwase S, Yamamoto K, Miwa C, Kamiya A, Niimi Y, Fu Q, et al. Skin sympathetic neuroeffector response is attenuated dose-dependently by systemic prostaglandin E1injection in humans. Neurosci Lett. 2000;292:191–4.
Kuwahara Y, Tsukahara R, Iwase S, Shimizu Y, Nishimura N, Sugenoya J, et al. Arousal electrical stimuli evoke sudomotor activity related to P300, and skin vasoconstrictor activity related to N140 in humans. Clin Neurophysiol. 2015;126:933–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan
About this chapter
Cite this chapter
Iwase, S., Nishimura, N., Kuwahara, Y., Sugenoya, J. (2017). Skin Sympathetic Nerve Activity and Thermoregulatory Control in Humans. In: Iwase, S., Hayano, J., Orimo, S. (eds) Clinical Assessment of the Autonomic Nervous System. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56012-8_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-56012-8_4
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56010-4
Online ISBN: 978-4-431-56012-8
eBook Packages: MedicineMedicine (R0)