Abstract
The transient and dynamic nature of changes that cancer cells undergo during metastasis cannot be explained by simple progressive accumulation of irreversible genetic alterations in the primary tumor. The ability of cancer cells to switch on and off different cellular processes such as epithelial-to-mesenchymal transition (EMT) and the ability to de-/re-differentiate allows them to respond to different cellular environments and to disseminate from the primary tumor to distant organs. This and many other features of metastatic tumor cells such as dormancy, active DNA repair, and resistance to apoptosis are shared by normal stem cells. It has been theorized that a subpopulation of cancer cells can acquire stem cell-like traits and aid in the process of metastasis. Moreover, the EMT and stem cellness support the ability of cancer cells to migrate and survive conventional therapies that predominantly target actively proliferating cells. Accordingly, characterization of genes and signal transduction pathways that regulate the EMT and stemness within the tumor mass are likely to shed new light on the complex molecular events that promote metastasis, invasion and resistance to therapy and could offer novel therapeutic targets for developing tailor-made intervention strategies. In this chapter, I discuss the evidence that aberrant expression of a stress-response protein, transglutaminase II (TG2), reprograms the inflammatory signaling networks in cancer cells that play fundamental roles in promoting drug resistance and metastatic competence by inducing EMT and the stem cell phenotype.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
With an estimated 7.4 million new cases and 4.6 million deaths every year, cancer continues to be a major health problem and cause of mortality worldwide. Available anticancer therapies predominantly target rapidly dividing cells in the primary tumor. Similarly, many recently launched targeted therapies are also based on identification of targets that are activated (due to genetic or epigenetic alterations) and drive proliferation of cells in the primary tumor. Although targeted therapies have been successful in reducing the toxicity, they have failed to yield appreciable survival benefits despite exuberant increases in treatment costs.
Due to advances in early diagnosis, surgery, and adjuvant treatments many early-stage or localized tumors are now considered curable. However, the same cannot be said of metastatic disease. In most cases, the prognosis of metastatic cancer remains grim. Metastatic tumors often exhibit resistance to most or all the available therapies. While it is often possible to moderately prolong the survival, eradication of metastatic disease is not possible, while side effects of treatment are significant. As a result of this, majority of these patients succumb to the disease. Although the clinical importance of metastasis has long been appreciated, understanding of molecular mechanisms that facilitate metastasis has lagged behind over the development of therapeutic modalities that target proliferating cells. This is partly due to the fact that metastasis is a highly complex and multistep process (Fig. 10.1) and each step requires coordinated action of many genes and pathways (Iiizumi et al. 2008).
Schematic representation of steps in the metastatic cascade. In order to metastasize, cancer cells must acquire the ability to break away from the primary tumor, break down the basement membrane, invade into the stroma (local invasion), enter into the blood circulation (intravasation) and manage to survive before they can arrest at a distant organ and grow into clinically detectable metastases
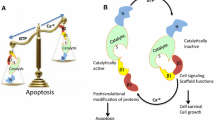
Cancer can spread to areas near the primary site (regional metastasis), or to parts of the body that are farther away (distant metastasis). When a tumor grows to more than 1 mm3 in size at the primary site, it induces growth of new blood vessels (neovascularization) in order to meet the increasing demand for oxygen and nutrients. Some tumor cells gain the growth advantage and acquire metastatic competence by accumulating additional genetic/epigenetic alterations. The first step in metastasis is the detachment of tumor cells from neighboring cells by deterring cell-cell and cell-extracellular matrix (ECM) adhesion, followed by degradation of the ECM (Slattum and Rosenblatt 2014). Once cancer cells break free from the main (primary) tumor they either enter the lymph system and end up in nearby lymph nodes (lymphatic spread) or enter the bloodstream (hematogenous spread) where they must survive the hostile environment including mechanical damage, lack of growth factors and the immune system. In the blood circulation, tumor cells often aggregate with platelets and fibrin and embolize in the capillaries or adhere to the endothelial cells like leukocytes at an inflammatory site. Surviving cells then extravasate and lodge at the secondary site to proliferate and colonize to make secondary/metastatic tumors (Fig. 10.1). Different processes during metastasis are controlled by a complex interplay between activated transcription factors and signaling pathways. In this chapter, I discuss evidence that aberrant expression of transglutaminase II (TG2) in epithelial cancer cells reprograms the inflammatory signaling networks that are implicated in conferring drug resistance and metastatic competence on cancer cells (Fig. 10.2).
TG2-regulated signaling promotes drug resistance and a metastatic phenotype. Smoldering inflammation due to recruitment of immune cells at the tumor site induces aberrant expression of TG2 in epithelial cancer cells. Owing to its scaffold function, TG2 binds to and degrades the inhibitory IκBα protein, resulting in the release and constitutive activation of NF-κB. Activated NF-κB, in complex with TG2, translocates to the nucleus where it binds to HIF-1α promoter and results in its transcriptional regulation and protein expression. Increased expression of HIF-1α, in turn, induces the expression of transcription repressors such as Snail, Zeb, and Twist. Collectively, TG2/NF-κB/HIF-1-induced alterations result in acquisition of an EMT phenotype and stem cell traits. Membrane-bound TG2, on the other hand, can interact with integrin and growth factor receptors (such as platelet-derived growth factor receptor and epithelial growth factor receptor) and induce mitogenic/cell survival signaling. Extracellular TG2 stabilizes the matrix by crosslinking its component proteins, adding to the shear force and promoting metastatic signaling. Ammonia, which is produced as a byproduct of TG2 catalytic activity, may have an important role in protecting tumor cells from acid toxicity caused by increased lactic production due to the Warburg effect (Katt et al. 2015). In a nutshell, aberrant expression of TG2 reprograms the inflammatory signaling networks which initiate a series of inside-out and outside-in signaling pathways to confer an aggressive phenotype in cancer cells
10.2 Inflammation and Cancer
Although genetic alterations are essential for cancer development, they are not sufficient to cause disease progression. In addition to genetic alterations, early-stage tumors require some ancillary changes to become invasive (Radisky and Radisky 2007). It is now becoming clear that the inflammatory microenvironment favors the expansion of genomic aberrations and progression of cancer (Mantovani 2009). While acute or physiological inflammation is predominantly a self-limiting process with therapeutic significance, chronic or pathological inflammation frequently leads to the progression of various chronic diseases, including cancer (Grivennikov and Karin 2010). Many epidemiological, animal, and clinical studies have supported the concept that chronic inflammation promotes and exacerbates malignancy (Demaria et al. 2010). Several types of cancer arise in the setting of chronic inflammation, suggesting a strong link between inflammation and cancer and, for that reason, tumors are often referred to as wounds that never heal (Mantovani et al. 2008; Grivennikov et al. 2010 ). It is estimated that nearly 25 % of all cancers are etiologically linked to chronic inflammation and infection (Hussain and Harris 2007). Chronic inflammation can impact tumorigenesis during various phases such as cellular proliferation, transformation, apoptosis evasion, survival, invasion, angiogenesis and metastasis (Mantovani et al. 2008; Aggarwal et al. 2006). Immune cells that are continuously recruited to the tumor site produce a number of pro-inflammatory molecules within the tumor microenvironment that can provoke a complex signaling network and enable tumor cells to extravasate through the stroma, resulting in tumor progression (Colotta et al. 2009). My intent here is not to elaborate on details about cancer progression or inflammation, but rather to discuss the relationship between TG2 expression and tumor-promoting inflammation as part of an effort to develop therapeutic approaches aimed at targeting TG2 to treat refractory cancers and to prevent metastatic progression of early-stage tumors.
10.2.1 TG2 and Cancer
TG2 is structurally and functionally a complex protein that comprises four structural domains (Fig. 10.3a). Each domain has a specific function, which permits TG2 to catalyze enzymatic as well as scaffold functions (Eckert et al. 2014). Its traditional role is that of a calcium-dependent enzymatic activity that crosslinks protein to form covalently linked protein complexes (Fig. 10.3b). However, it has also been shown to function as a G-protein intimately involved in cell signaling (Eckert et al. 2014), to modulate transcriptional complexes to drive gene expression (Tatsukawa et al. 2009; Kumar and Mehta 2012; Verma and Mehta 2007; Belkin 2011), to form complexes with other proteins to alter target protein function and to be secreted where it functions in the extracellular environment to regulate cell adhesion and motility (Eckert et al. 2014; Belkin 2011).
Schematic representation of TG2 structure and functions. (a). TG2 comprises four major structural domains (depicted by colored rectangles). Each domain has a specific function (shown in bold text under the respective domain). (b). In the presence of calcium, TG2 catalyzes an irreversible crosslinking reaction between peptide-bound glutamine and peptide-bound lysine residues. In the GTP-bound form TG2 participates in multiple signaling pathways. (c). The TG2-catalyzed crosslinking reaction is regulated by Ca2+, GTP, and the redox potential. Binding of Ca2+ (dissociation constant ~60 μM) is essential for TG2 to acquire a catalytically active or ‘open’ conformation. In contrast, binding of GTP/GDP (dissociation constant ~1.6 μM) renders TG2 in a catalytically inactive or ‘closed’ conformation. Under physiological conditions, high levels of GTP, a low redox potential, and low free Ca2+ (<0.5 μM) are likely to keep intracellular TG2 in a catalytically inactive state. Due to high calcium and low GTP levels in an extracellular environment, TG2 can be expected to be in a catalytically active state. However, even in extracellular environments a large fraction of TG2 remains in an inactive form due to disulfide bonding. Thioredoxin 1 has been suggested to be a physiological activator of oxidized TG2. In catalytically inactive state, TG2 acts as a scaffold protein and results in the activation of various transcription factors and signaling pathways. In a catalytically active state, it catalyzes highly stable protein crosslinking, resulting in apoptotic death if inside the cell or stabilizes the matrix if outside the cell
Three important physiological regulators of TG2 catalytic function include Ca2+, GTP/GDP, and redox potential (Eckert et al. 2014; Klöck and Khosla 2012). Binding of Ca2+ to TG2 induces the catalytically active ‘open’ conformation, while binding to GTP or GDP promotes the catalytically inactive ‘closed’ conformation (Fig. 10.3c). Because most intracellular TG2 is GTP bound and intracellular calcium concentrations are low, TG2 stays in the catalytically inactive form under physiological conditions. In the ‘closed’ form, TG2 serves as a scaffold protein and acts as a signaling protein by binding and altering the function and stability of some key effector proteins. Increase in intracellular calcium due to cell damage or other stressors causes a major change in TG2 conformation to become catalytically active (extended), leading to TG2-catalyzed crosslinking of cellular proteins and apoptotic death. In contrast, in the extracellular environment, which has a considerably lower concentration of GTP/GDP and an abundance of free calcium, TG2 can be expected in a catalytically active (open) form. However, most TG2 even in extracellular environments is enzymatically inactive due to intra-molecular disulfide bonding. Under oxidizing conditions, the disulfide bond between Cys230 and Cys370 facilitates the formation of the more stable Cys370-Cys371 disulfide bond that inactivates TG2 (see Chap. 14). The reactivation of extracellular TG2 can be achieved under favorable redox potential or alternatively by protein cofactor thioredoxin (Klöck and Khosla 2012). Once activated, TG2 can crosslink extracellular matrix (ECM) component proteins and stabilize the matrix for increased cell attachment, cell motility and outside-in signaling.
Recent studies have identified the important contribution of TG2 in cancer progression. TG2 expression is markedly increased in multiple tumors and tumor cell lines, especially in those selected for resistance to chemotherapy or isolated from metastatic sites (Mehta et al. 2010; Verma et al. 2006; Yuan et al. 2008; Satpathy et al. 2007; Park et al. 2002; Mehta et al. 2004; Budillon et al. 2011; Mangala et al. 2007; Oh et al. 2011). At a functional level, TG2 expression is associated with reduced expression of tumor suppressor genes, increased synthesis and deposition of fibronectin and collagen, stabilized ECM, reactivation of an embryonically regulated process, called epithelial-to-mesenchymal transition (EMT), enhanced drug resistance, and enhanced metastatic ability. Conversely, inhibition of TG2 by small-molecule inhibitors, antisense RNA or small inhibitory RNA (siRNA) results in reduced invasion and metastasis and increased sensitivity of cancer cells to chemotherapeutic drugs. These events are predominantly regulated via the scaffold function of TG2 whereby TG2 binds and modify the function and/or stability of key signaling proteins.
10.2.2 TG2-Regulated Inflammatory Signaling
Both intracellular and extracellular TG2 can regulate cell-signaling pathways that facilitate cell survival, cell motility, cell attachment, and invasive behavior (Fig. 10.2). Multiple reports have documented that aberrant expression of TG2 in epithelial cancer cells results in constitutive activation of focal adhesion kinase, Akt, NF-κB, and HIF-1α signaling pathways (Eckert et al. 2014; Kumar and Mehta 2012; Verma and Mehta 2007; Belkin 2011), which play fundamental roles in cancer progression by inducing EMT and promoting drug resistance and metastasis (Lu et al. 2006; Monti and Gariboldi 2011; Kumar et al. 2011). For example, constitutively activated NF-κB is an important hallmark of advanced-stage cancer. It can regulate multiple downstream genes that are known to protect cells from cell death, promote invasiveness, or induce EMT and stem cell properties (Gupta et al. 2010). Moreover, activation of NF-κB is considered central to inflammation-induced tumor progression (Aggarwal et al. 2006). TG2 expression promotes constitutive activation of NF-κB via a canonical yet non-conventional pathway (see Chap. 5). TG2 binds to the inhibitory protein IκBα and results in its rapid degradation in a proteasomal-independent pathway (Kumar and Mehta 2012). This results in reduced levels of IκBα, permitting release and activation of NF-κB, its translocation to the nucleus and transactivation of multiple downstream target genes (Gupta et al. 2010). TG2-induced activation of NF-κB is independent of its catalytic functions as a catalytically inactive mutant (C277S) of TG2 was fully competent for activating NF-κB and downstream events (Grivennikov and Karin 2010; Gupta et al. 2010; Mantovani 2009). TG2 also binds to the p65 subunit of activated NF-κB and is recruited to the NF-κB binding site in the promoter sequence of Snail (Kim et al. 2010) and HIF-1a (Kumar and Mehta 2012), resulting in their transcriptional regulation. Downregulation of TG2 by a gene-specific siRNA attenuated NF-κB activation and inhibited HIF-1α expression (Kumar and Mehta 2012). Like NF-κB, HIF-1α expression is considered a negative prognostic factor because of its ability to promote chemoresistance, angiogenesis, invasiveness, metastasis, resistance to cell death, altered metabolism, and genomic instability (Wilson and Hay 2011). Moreover, TG2-regulated NF-κB and HIF-1α activation play an important role in initiation and induction of EMT due to their ability to regulate Snail, Twist, and Zeb1 transcription repressors (Kumar and Mehta 2012; Figure 2).
In addition to regulating intracellular signaling, TG2 in an extracellular environment can support outside-in signaling for increased cell growth, cell survival, and invasive functions. For example, it is well known that cell-surface TG2 can regulate integrin-mediated signaling through direct and stable interaction with β1, β3, and β5 integrin (Eckert et al. 2014; Belkin 2011; Nurminskaya and Belkin 2012). TG2 has a strong binding affinity for the gelatin-binding region of fibronectin and can interact strongly with fibronectin on one hand and integrin on the other to enhance integrin-fibronectin interaction. This interaction facilitates cell attachment to the matrix and activates integrin-mediated signaling. For example, integrin-TG2 interaction modulates the integrin-dependent activation of FAK, Src, p190RhoGAP, and increased levels of GTP-bound RhoA and its downstream target ROCK, leading to increased focal adhesion and actin stress fiber formation and enhanced actomyosin contractility (Nurminskaya and Belkin 2012). Based on this information, Dr. Matie’s group ventured on screening a library of small-molecule inhibitors that could block fibronectin/integrin interaction (Yakubov et al. 2014). A compound identified in this screen (TG53) was able to inhibit adhesion of ovarian cancer cells to fibronectin and mitigated their migration and invasion. Contrary to the previous report, the authors observed that extracellular TG2 was able to activate NF-κB via a non-canonical pathway in ovarian tumors and the enzyme activity was required for this activation (Yakubov et al. 2013). TG2-induced activation of NF-κB was mediated through the hyaluronan receptor, CD44. The NF-κB activation by extracellular TG2 upregulated CD44 expression and induced EMT, contributing to increased invasiveness and peritoneal dissemination of ovarian cancer. These observations support the concept that extracellular TG2 can also play an important role in cancer progression and metastasis.
While intracellular TG2 can accelerate the synthesis and deposition of new ECM, extracellular TG2 can stabilize it by catalyzing the crosslinking of ECM component proteins (Fig. 10.2). It is likely that TG2-induced changes in the ECM contribute to a desmoplastic response in growing tumors. Indeed, the gene expression profile of TG2-overexpressing cells revealed increased expression of collagen, fibronectin, laminin, matrix metalloproteinases (MMP), growth factors such as TGFβ and platelet-derived growth factor and integrins (Kumar and Mehta, unpublished results) – the genes that represent key constituents of the desmoplastic response. TG2 can directly or indirectly regulate the expression and function of most of these effectors. ECM remodeling and stiffening are known to affect tumor behavior. Breast cancer progression, for example, requires collagen crosslinking, ECM stiffening, and increased focal adhesion formation (Levental et al. 2009). Lysyl oxidase-induced crosslinking of collagen, for example, results in ECM stiffening which is associated with increased focal adhesion formation, enhanced PI3 kinase (PI3K) activity, and increased tumor invasion. On the basis of these observations, it is tempting to speculate that TG2-catalyzed crosslinking and deposition of the ECM could play a role in promoting a malignant phenotype. Indeed, in a recent study, Lee et al. (2015) reported that extracellular TG2 catalyzes crosslinking of collagen in a pancreatic cancer milieu and promotes fibroblast proliferation and tumor growth. These results imply that inhibition of TG2 could be a promising target to block pancreatic cancer growth.
10.3 TG2-Regulates EMT, Stemness, and Glucose Metabolism
EMT is an evolutionarily conserved cellular process that plays crucial roles in the differentiation of tissue and organs during normal development of multicellular organisms (Thiery et al. 2009). It is characterized by loss of apical-basal cellular polarity, which results in the transition of polarized epithelial cells into a mesenchymal phenotype (Nieto and Cano 2012). In addition, EMT plays an important role during tissue repair but adversely can also promote organ fibrosis and cancer progression by conferring invasive and migratory properties on cancer cells (Thiery et al. 2009; Kalluri 2009). Recent evidence suggests that EMT can induce stem cell properties and prevent apoptosis (Dave et al. 2012).
During cancer progression, EMT is considered to be the first important step in metastatic dissemination of tumor cells. It allows tumor cells to detach from neighboring cells, invade tissues and survive in hostile environments. During EMT, the polarized epithelial cells undergo multiple morphological and biochemical changes that enable them to acquire a mesenchymal phenotype. Tumor cells with a mesenchymal phenotype exhibit enhanced migratory functions, invasiveness, and resistance to apoptosis, all of which are important hallmarks of metastatic tumors (Dave et al. 2012; Kalluri 2009).
Stable expression of TG2 in epithelial cancer cells is associated with morphological and molecular alterations that are characteristic of EMT (Kumar et al. 2010, 2012; Cao et al. 2012; Satpathy et al. 2007; Lin et al. 2011). Thus, overexpression of TG2 in mammary and ovarian cancer cells resulted in loss of epithelial markers and gain of mesenchymal markers. Moreover, TG2-induced EMT in cancer cells was accompanied by increased cell motility, invasiveness, and anchorage-independent growth (Fig. 10.4). TG2 overexpression in mammary epithelial cells resulted in loss of apico-basal polarity and disrupted their ability to form organized acinar structures when grown in 3D Matrigel cultures (Fig. 10.5). Unlike TG2-deficient cells, TG2-overexpressing cells grew into irregular spheroids with fragmented basement membrane and no defined lumen (Fig. 10.5). Conversely, suppression of TG2 by siRNA resulted in reversal of EMT (MET) as revealed by a gain in E-cadherin expression and loss of Snail1 and Zeb1 expression (Kumar et al. 2010).
TG2-induced changes in epithelial cells. Stable expression of TG2 in epithelial cancer cells is associated with their trans-differentiation into mesenchymal looking cells (EMT) (a). Such differentiation endows cancer cells with the ability to invade (b) and acquire the ability to grow in an anchorage-independent manner (c), increases cell motility as determined by a wound-healing scratch assay (d), and acquire a stem cell phenotype as established by enrichment of mammosphere-forming cells (e)
TG2 induces tumorigenic phenotype. Morphology of acinar structures (a, upper panel) formed when TG2-deficient mammary epithelial cells (MCF-10A) were grown in Matrigel for 12 days. TG2 overexpression, however, resulted in loss of their ability to form such defined structures; instead cells grew as tumoroid structures with many cells in the periphery invading through the Matrigel (a, lower panel). Moreover, significant loss in basement membrane integrity (b) as well as in E-cadherin expression (c) was observed in TG2-overexpressing cells. Blue staining- DAPI
It is now generally believed that small subset of cells within a tumor, termed cancer stem cells (CSCs) or tumor-initiating cells (TICc), are responsible for tumor sustenance and regrowth after chemotherapy (Lacerda et al. 2010). Because CSCs/TICs exhibit intrinsic resistance to therapy, their number would be expected to increase after chemotherapy. Indeed, the gene expression profile of residual tumors that survived after chemotherapy closely resembled the EMT gene signature (Creighton et al. 2009; Calcagno et al. 2010). Interestingly, TG2-induced EMT in breast cancer and ovarian cancer cells is accompanied by acquisition of stem cell characteristics (Kumar et al. 2011; Cao et al. 2012). TG2-expressing cells showed enhanced ability to form mammospheres, self-renewal ability, and cellular plasticity (Fig. 10.4) – important traits of mammary stem cells. Mammospheres derived from TG2-expressing cells differentiated into complex secondary structures when grown in Matrigel and treated with prolactin. Importantly, cells in these secondary structures differentiated into Muc1-positive (luminal marker) and/or integrin α6-positive (basal marker) cells (Kumar et al. 2012).
Increased glucose uptake and its metabolism via glycolysis (even in the presence of oxygen) is another important hallmark of metastatic cancers. This metabolic reprogramming is essential for successful growth and survival of tumor cells in distant tissues and as a source of substrates for biomass generation. TG2 expression reprograms glucose metabolism as a result of constitutive activation of NF-κB and HIF1α. Thus, TG2 overexpression resulted in increased glucose uptake, increased lactate production and decreased oxygen consumption rates by mitochondria (Kumar et al. 2014). Experimental suppression of TG2 attenuated HIF-1α and reversed these downstream events in mammary epithelial cells. Moreover, downregulation of either TG2, p65/RelA or HIF-1α by gene-specific siRNAs enabled epithelial cells to restore normal glucose uptake, lactate production, mitochondrial respiration and glycolytic protein expression (Kumar et al. 2014). These results imply that aberrant expression of TG2 in epithelial cancer cells is an important modulator of glucose metabolism and it facilitates metabolic alterations owing to its ability to activate NF-κB and its downstream target HIF-1α. A TG2-induced shift in glucose metabolism helps cancer cells to survive under stressful conditions and makes them competent to survive and colonize in distant organs.
10.4 Conclusion and Clinical Perspective
Taken together, these observations suggest that aberrant expression of TG2 in cancer cells is a key event during cancer progression. It promotes drug resistance and metastatic competence in epithelial cancer cells owing to its ability to reprogram the inflammatory signaling networks as outlined in Fig. 10.2. These signaling pathways, in turn, play fundamental roles in inducing EMT, stemness, and altering glucose metabolism. Clinical evidence also supports this contention; elevated expression of TG2 in tumor samples is associated with poor survival rates in patients, resistance to therapy, and increased incidence of metastasis (Verma et al. 2006; Mehta et al. 2004; Hwang et al. 2008). Also, these patients show shorter relapse-free and metastasis-free survival after adjustment for known prognostic factors such as tumor size, lymph node metastasis, age, and hormone-receptor status (Ai et al. 2008). Therefore, inhibition of TG2 represents an attractive therapeutic option to reverse chemoresistance and intervention of metastatic progression. As a proof-of-concept, TG2-siRNA has been successfully used to reverse chemoresistance and to inhibit metastatic progression in preclinical models both in vitro and in vivo (Verma et al. 2008; Hwang et al. 2008). However, due to limited clinical evidence to support the effectiveness of siRNA as a therapeutic approach, its use in patients with cancer warrants further studies for optimization of siRNA delivery and safety in preclinical models.
Alternatively, the use of small-molecule inhibitors that can bind and inhibit TG2 may offer an alternative strategy to block TG2 signaling. Increased awareness about TG2’s role in multiple pathological conditions (inflammation, tissue fibrosis, cancer, organ degenerative disorders, neurodegenerative disorders, celiac disease) has tickled interest by many groups to develop inhibitors against TG2 in a hope that some potent and selective compounds with therapeutic potential may soon be discovered (Keillor et al. 2015). A systematic study with larger cohort of patients to establish whether TG2 is a promising target for reversing chemoresistance and inhibiting metastatic progression is warranted. If TG2, as discussed in this chapter, turns out to be an important mediator of the metastatic cascade, it could not only offer a novel therapeutic target for treatment drug-resistant (refractory) and metastatic (recurrent) tumors (which together account for more than 90 % of cancer-related deaths) but also offer a promising diagnostic marker for early stratification of aggressive tumors.
References
Aggarwal BB, Shishodia S, Sandur SK et al (2006) Inflammation and cancer: how hot is the link? Biochem Pharmacol 72:1605–1621
Ai L, Kim WJ, Demircan B et al (2008) The transglutaminase 2 gene (TGM2), a potential molecular marker for chemotherapeutic drug sensitivity, is epigenetically silenced in breast cancer. Carcinogenesis 29:510–518
Belkin AM (2011) Extracellular TG2- emerging functions and regulation. FEBS J 278:4704–4716
Budillon A, Carbone C, Di GE (2011) Tissue transglutaminase: a new target to reverse cancer drug resistance. Amino Acids 44:63–72
Calcagno AM, Salcido CD, Gillet JP et al (2010) Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst 102:1637–1652
Cao L, Shao M, Schilder J et al (2012) Tissue transglutaminase links TGF-β, epithelial to mesenchymal transition and a stem cell phenotype in ovarian cancer. Oncogene 31:2521–2534
Colotta F, Allavena P, Sica A et al (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081
Creighton CJ, Li X, Landis M et al (2009) Residual breast cancers after conventinal therapy display mesenchymal as well as tumro initiating features. Proc Natl Acad Sci U S A 106:13820–13825
Dave B, Mittal V, Tan NM, Chang JC (2012) Epithelial-mesenchymal transition, cancer stem cells and treatment reisistance. Breast Cancer Res 14:202
Demaria S, Pikarsky E, Karin M et al (2010) Cancer and inflammation: promise for biologic therapy. J Immunother 33:335–351
Eckert RL, Kaartinen MT, Nurminskaya M et al (2014) Transglutaminase regulation of cell function. Physiol Rev 94:383–417
Grivennikov SI, Karin M (2010) Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 20:65–71
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29:405–434
Hussain SP, Harris CC (2007) Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 121:2373–2380
Hwang JY, Mangala LS, Fok JY et al (2008) Clinical and biological significance of tissue transglutaminase (TG2) in ovarian carcinoma. Cancer Res 68:5849–5858
Iiizumi M, Liu W, Pai SK et al (2008) Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim Biophys Acta 1786:87–104
Kalluri R (2009) EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Invest 119:1417–1419
Katt WP, Antonyak MA, Cerione RA (2015) Simultaneously targeting tissue transglutaminase and kidney type glutaminase sensitizes cancer cells to acid toxicity and offers new opportunities for therapeutic intervention. Mol Pharm 12:46–55
Keillor JW, Apperley KYP, Akbar A (2015) Inhibitors of tissue transglutaminase. Trends Pharmacol Sci 36:32–40
Kim Y, Eom S, Kim K et al (2010) Transglutaminase II interacts with rac1, regulates production of reactive oxygen species, expression of snail, secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation. Mol Immunol 47:1010–1022
Klöck C, Khosla C (2012) Regulation of the activities of the mammalian transglutaminase family of enzymes. Protein Sci 21:1781–1791
Kumar S, Mehta K (2012) Tissue transglutaminase constitutively activates HIF-1alpha promoter and nuclear factor-kappaB via a non-canonical pathway. PLoS One 7:e49321
Kumar A, Xu J, Brady S et al (2010) Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PLoS One 5:e13390
Kumar A, Gao H, Xu J et al (2011) Evidence that aberrant expression of tissue transglutaminase promotes stem cell characteristics in mammary epithelial cells. PLoS One 6:e20701
Kumar A, Xu J, Sung B et al (2012) Evidence that GTP-binding domain but not catalytic domain of transglutaminase 2 is essential for epithelial-to-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 14:R4
Kumar S, Donti TR, Agnihotri N, Mehta K (2014) Transglutaminase 2 reprogramming of glucose metabolism in mammary epithelial cells via activation of inflammatory signaling pathways. Int J Cancer 134:2798–2807
Lacerda L, Pusztai L, Woodward WA (2010) The role of tumor initiating cells in drug resistance of breast cancer: implications for future therapeutic approaches. Drug Resist Updat 13:99–108
Lee J, Condello S, Yakubov B et al (2015) Tissue transglutaminase mediated tumor-stroma interaction promotes pancreatic cancer progression. Clin Cancer Res 21:4482–4493
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Lin CY, Tsai PH, Kandaswami CC et al (2011) Role of tissue transglutaminase 2 in the acquisition of a mesenchymal-like phenotype in highly invasive A431 tumor cells. Mol Cancer 10:87
Lu H, Ouyang W, Huang C (2006) Inflammation, a key event in cancer development. Mol Cancer Res 4:221–233
Mangala LS, Fok JY, Zorrilla-Calancha IR et al (2007) Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 26:2459–2470
Mantovani A (2009) Cancer: inflaming metastasis. Nature 457:36–37
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Mehta K, Fok J, Miller FR et al (2004) Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res 10:8068–8076
Mehta K, Kumar A, Kim HI (2010) Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol 80:1921–1929
Monti E, Gariboldi MB (2011) HIF-1 as a target for cancer chemotherapy, chemosensitization and chemoprevention. Curr Mol Pharmacol 4:62–77
Nieto MA, Cano A (2012) The epithelial-mesenchymal transition under control: global programs to regulate epithelial pasticity. Semin Cancer Biol 22:361–368
Nurminskaya MV, Belkin AM (2012) Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol 294:1–97
Oh K, Ko E, Kim HS et al (2011) Transglutaminase 2 facilitates the distant hematogenous metastasis of breast cancer by modulating interleukin-6 in cancer cells. Breast Cancer Res 13:R96
Park KS, Kim HK, Lee JH et al (2002) Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. J Cancer Res Clin Oncol 136:493–502
Radisky E, Radisky DC (2007) Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord 8:279–287
Satpathy M, Cao L, Pincheira R et al (2007) Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res 67:7194–7202
Slattum GM, Rosenblatt J (2014) Tumor cell invasion: an emerging role for basal epithelial cell extrusion. Nat Rev Cancer 14:495–501
Tatsukawa H, Fukaya Y, Frampton G et al (2009) Role of transglutaminase 2 in liver injury via cross-linking and silencing of transcription factor Sp1. Gastroenterology 136:1783–1795
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Verma A, Mehta K (2007) Transglutaminase-mediated activation of nuclear transcription factor-kappaB in cancer cells: a new therapeutic opportunity. Curr Cancer Drug Targets 7:559–565
Verma A, Wang H, Manavathi B et al (2006) Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res 66:10525–10533
Verma A, Guha S, Diagaradjane P et al (2008) Therapeutic significance of elevated tissue transglutaminase expression in pancreatic cancer. Clin Cancer Res 14:2476–2483
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Yakubov B, Chelladurai B, Schmitt J et al (2013) Extracellular tissue transglutaminase activates non-canonical NF-κB signaling and promotes metastasis in ovarian cancer. Neoplasia 15:609–619
Yakubov B, Chen L, Belkin AM et al (2014) Small molecule inhibitors target the tissue transglutaminase and fibronectin interaction. PLoS One 9:e89285
Yuan L, Behdad A, Siegel M et al (2008) Tissue transgluaminase 2 expression in meningiomas. J Neurooncol 90:125–132
Acknowledgments
Supported in part by Bayer Healthcare Pharmaceutical Grants4Targets.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Mehta, K. (2015). Transglutaminase II and Metastasis: How Hot Is the Link?. In: Hitomi, K., Kojima, S., Fesus, L. (eds) Transglutaminases. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55825-5_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-55825-5_10
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55823-1
Online ISBN: 978-4-431-55825-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)