Abstract
The liver contains a wide array of immune cells such as macrophages, dendritic cells, and lymphocytes. In addition, nonimmune liver cells participate in the immune system by producing soluble pattern recognition receptors (PRRs) and complement proteins. This liver-mediated immune system maintains the local defense against microbial infection as well as systemic homeostasis. In fact, patients with advanced liver diseases have a high risk for infection due to impaired biosynthesis of secreted PRRs. Thus, the liver is to be considered as an immune organ.
The innate immune system can promptly eliminate nonspecific pathogens using several types of PRRs, including Toll-like receptors, NOD-like receptors, RIG-I-like receptors, and complement proteins. Representative products that act through PRR signaling are cytokines, which have antibacterial and antiviral effects. These mediators are used to kill pathogens with the assistance of other immune cells. Indeed, mice deficient in PRRs are susceptible to infection. However, an excessive immune response may lead to a sustained elevation of harmful cytokines. As a result, mice deficient in PRRs also show rather mild liver injuries, indicating that the lack of PRR signaling can provide beneficial effects. Thus, the innate immune system has dual functions in the prevention of infection as well as the development of liver diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Components of the Innate Immune System in the Liver
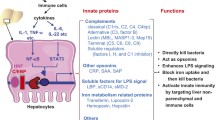
The innate immune system promptly eliminates nonspecific pathogens using membrane-bound pattern recognition receptors (PRRs), intracellular PRRs, phagocytic PRRs, and secreted PRRs. These PRRs include Toll-like receptors (TLRs), NOD-like receptors, RIG-I-like receptors, and complement proteins. TLRs are located on the cell surface or in endosomes. NOD-like receptors and RIG-I-like receptors are located in the cytosol and participate in intracellular immune surveillance. PRR signaling leads to the production of a wide variety of cytokines. In addition, complement proteins are secreted from liver cells such as hepatocytes. This hepatic innate immune system contributes to the prevention of microbial infection as well as the development of liver diseases (Fig. 1.1). We herein describe TLRs, NOD-like receptors, RIG-I, and their downstream molecules, and complement proteins in the liver, followed by a discussion of the association between the hepatic innate immune system and hepatic manifestations observed in liver diseases.
2 TLRs
2.1 TLRs, Adaptor Proteins, and TLR Ligands
TLRs are representative PRRs that recognize bacterial and viral components. In addition to pathogen-associated molecular patterns, TLRs recognize the extracellular matrix (ECM) and abnormal nucleic acids derived from the host cells. Currently, 10 and 13 TLRs have been identified in human and rodent, respectively (Table 1.1); the genes for TLR11, TLR12, and TLR13 do not exist in human [1]. TLRs are located on the cell surface (TLR1, TLR2, TLR4, TLR5, TLR6) or in intracellular vesicles (TLR3, TLR7, TLR8, TLR9). Although more than 10 TLRs have been identified, TLR signaling is divided into two pathways, namely, MyD88- and TRIF-dependent pathways. TLR3 uses only the TRIF-dependent pathway, while TLR4 uses both MyD88- and TRIF-dependent pathways. The other TLRs use the MyD88-dependent pathway only. The majority of proinflammatory cytokines and chemokines depend on the MyD88 pathway, while TRIF-dependent pathways are associated with the production of type I interferons (IFNs). However, cross talk between these pathways exists at downstream molecules. Thus, the MyD88-dependent pathway can also induce type I IFNs, and TRIF signaling can induce proinflammatory cytokines. MyD88-deficient mice and TRIF-deficient mice exhibit different phenotypes regarding liver injuries. Mice deficient in TLR4, which use both adaptor proteins, are protected from the development of nonalcoholic steatohepatitis (NASH) and alcoholic liver disease (ALD), which share several features. Interestingly, TRIF- but not MyD88-deficient mice exhibit decreased inflammation in an ALD model [2, 3], whereas MyD88-deficient mice show decreased inflammation in a NASH model [4]. TNF-α is a key cytokine in the progression of NASH and ALD according to the results of gene-modified mice. Although TNF-α production depends largely on the MyD88 pathway [5], the TRIF/IRF3 pathway may also transactivate the TNF-α gene in hepatic macrophages.
The liver has a unique blood supply system; 70–80 % is supplied from the gut via the portal vein with the remaining from the hepatic artery. Hence, the liver is constantly exposed to gut-derived factors including bacterial products, environmental toxins, and food antigens [6]. The hepatic immune system is strictly regulated as a barrier between the gastrointestinal tract and whole body. Although the gut microbiota is a source of TLR ligands, the hepatic cells can remove these gut-derived TLR ligands without harmful effects under normal physiological conditions, referred as liver tolerance. However, this tolerance is broken in liver diseases, leading to the susceptibility to TLR ligands. In addition, plasma levels of gut-derived TLR ligands are elevated during liver injury [7], which is caused by increased gut permeability and compositional changes in the gut microbiota. As a result, a larger amount of TLR ligands translocate into the portal circulation and stimulate liver cells to produce many cytokines. For instance, ethanol feeding disrupts the tight junctions of intestinal epithelial cells and increases gut permeability. TLR ligands migrate into the portal vein and then stimulate macrophages to produce TNF-α in the liver. TNF-α further promotes gut permeability by disrupting the tight junctions of the intestinal epithelium. In addition, ethanol feeding induces compositional changes in the gut microbiota. In a murine ALD model, the relative abundance of Bacteroidetes, a group of Gram-negative bacteria, is increased, whereas commensal probiotics, including Lactobacillus, are decreased [8]. Indeed, TLR4-deficient mice are protected from alcoholic liver injury [9]. Alcohol feeding further induces the overgrowth of intestinal bacteria, which can alter the metabolism with intestinal inflammation. Antibiotic treatment to sterilize the gut can reduce the severity of liver injury in ALD [10]. These data indicate that the gut microbiota contributes to the pathogenesis of liver diseases as a source of TLR ligands.
2.2 TLR2
TLR2 senses many components from pathogens, including Gram-positive bacteria, fungi, parasites, and viruses. TLR2 forms a heterodimer with either TLR1 or TLR6. The TLR2-TLR6 dimer recognizes diacylated lipopeptides, while the TLR2-TLR1 dimer recognizes triacylated lipopeptides in Gram-positive bacteria. Although TLR2 ligands induce proinflammatory cytokines and fibrogenic factors in in vitro experiments, the phenotype of TLR2-deficient mice varies depending on the model used. For instance, lesser degrees of hepatic steatosis and inflammation are observed in TLR2-deficient mice on a choline-deficient amino acid-defined (CDAA) diet [11], a NASH model. On the other hand, severe inflammation is induced by feeding methionine- and choline-deficient (MCD) diet [12]. Although both diets are well known as NASH models, the metabolic parameters largely differ between TLR2-deficient mice on a CDAA versus an MCD diet; the CDAA diet induces insulin resistance with obesity, while the MCD diet promotes insulin signaling with body weight loss. Enhanced sensitivity to lipopolysaccharide (LPS) likely accounts for the deterioration in TLR2-deficient mice on the MCD diet [12]. However, this phenomenon does not appear to occur in other obesity-inducible diets including the CDAA diet and a high-fat (HF) diet. Indeed, TLR2-deficient mice are protected from obesity and insulin resistance induced by the CDAA or HF diet. Currently, different gut microbiota compositions have been reported between obese and lean individuals; Firmicutes, Gram-positive bacteria, are increased in obese individuals, while Bacteroidetes, Gram-negative bacteria, are increased in lean individuals, as well as mice. These data suggest that compositional changes in the gut microbiota may provide different results.
Conflicting results have also been reported in experimental hepatocellular carcinoma (HCC) models. TLR2-deficient mice show an increased incidence of HCC induced by diethylnitrosamine (DEN) [13], suggesting that Gram-positive bacteria may protect from the development of HCC. The lack of TLR2 signaling decreases the infiltration of inflammatory cells and expression of proinflammatory cytokines, leading to the suppression of senescence and autophagy, which possess barrier function against HCC. However, inflammation is observed in most livers which develop HCC. On the other hand, Gram-positive bacteria contribute to the increased incidence of HCC in a model using a chemical carcinogen combined with HF diet [14]. In this model, Gram-positive bacteria Clostridium generate cytotoxic bile acids such as deoxycholic acid, which induce senescence of activated HSCs.
In contrast to the conflicting findings in NASH and HCC models, TLR2-deficient mice are not protected from ALD. TLR2 signaling is attenuated in monocytes isolated from patients with ALD [15]. In addition, Gram-negative bacteria are prominent in the gut of mice with ALD. These data suggest that TLR2 signaling has minor effects on the development of ALD. Furthermore, higher concentrations of TLR2 ligands are required for TLR2 activation compared with the TLR4 ligand LPS in in vitro experiments [16]. In this case, it is believed that LPS activates TLR4 signaling before TLR2 ligands are at sufficient concentrations to activate TLR2 in this case.
2.3 TLR3 and TLR7
TLR3 and TLR7 recognize double-strand and single-strand RNA, respectively. TLR3 and TLR7 ligands are derived from viruses, endogenous dying cells, and microRNAs. Although TLR3 and TLR7 signaling use different adaptor proteins, these TLRs are mostly responsible for IFN-mediated immune responses in liver diseases.
TLR3 recognizes hepatitis C virus (HCV) and subsequently induces type I IFNs. However, HCV can reduce the production of type I IFNs by inhibiting TLR3 signaling, which allows the replication of HCV. In in vitro experiments, HCV and its related proteins degrade TRIF, an adaptor protein for TLR3 [17]. TLR7 in DCs is believed to recognize HCV and its signaling is crucial for IFN production through IRF7. TLR7 agonists have been developed as agents for HCV treatment through IFN-mediated HCV elimination [18]. However, oral directly acting antiviral therapies for chronic HCV infection are currently widely used because these agents are highly effective and have a lower incidence of adverse effects. On the other hand, IFN-based therapies induce many adverse effects in patients. Thus, the use of IFN-based therapies is limited at present. HBV can also inhibit TLR signaling. The expression of TLR3 and IFN-β was significantly decreased in monocyte-derived DCs in patients with chronic HBV infection compared with normal subjects [19]. As expected, the expression of TLR3 was inversely correlated with the severity of hepatitis. Currently, nucleoside analogues (NAs) are widely used to treat patients with chronic HBV infection. The issues associated with NA administration include a long treatment period and the emergence of NA-resistant HBV. Because type I IFNs have anti-HBV effects and promote the seroconversion of HBe antigen [20], there are advantages utilizing IFN-mediated therapy in chronic HBV infection. Thus, TLR7 agonists have gained as an adjuvant therapy in combination with NAs.
Although TLRs have been originally identified as sensors for nonself-components, recent data demonstrate that TLRs recognize self-components, such as modified nucleic acids released from injured cells. In autoimmune diseases, including primary biliary cholangitis (PBC), TLR3-expressing immune cells recognize self-derived nucleic acids as nonself substances. The hepatic expression of TLR3 and IFN-α in PBC is higher than other liver diseases including autoimmune hepatitis and chronic HCV infection. Furthermore, there is a positive correlation between TLR3 and IFN-α/β expression in PBC [21]. TLR3 expression and its downstream molecule IFN-α are enhanced in the portal areas, where liver injury is most prominent in PBC. Indeed, macrophages and plasma cells express both TLR3 and IFN-α in the portal tract of PBC. These data suggest that the TLR3-TRIF pathway contributes to the development of PBC, and this signaling is a potential target for treatment of PBC. Although TLR3 recognizes double-strand RNA, the TLR3 ligands in PBC have not yet been identified. On the other hand, TLR7 expression was not increased in PBC.
In experimental models, TLR7-deficient mice show increased fibrosis in liver injuries induced by bile duct ligation or CCl4 [22]. Moreover, the antifibrotic effects of IFNs are suppressed in TLR7-deficient mice.
2.4 TLR4
TLR4 recognizes LPS, a cell wall component of Gram-negative bacteria. LPS binds to TLR4 after forming a complex between LPS and TLR4 coreceptors including MD2 and CD14, which do not have intracellular domains. TLR4 is a unique TLR that transduces its signal using two major adaptor proteins, MyD88 and TRIF. Upon TLR4 activation, the MyD88-dependent pathway is promptly initiated and subsequently activates its downstream molecules, IRAK1/IRAK4 and TRAF6, to induce NF-κB-related genes. Behind the initiation of MyD88 signaling, the TRIF-dependent pathway is activated through IKK/TAK1 and IRF3, resulting in the induction of IFN-inducible genes and NF-κB.
It is well known that LPS contributes to inflammation and liver fibrosis by stimulating liver cells to produce various cytokines. As expected, many reports have demonstrated that TLR4 signaling is associated with the progression of liver injuries. TLR4-deficient mice are protected from NAFLD/NASH, even mice on MCD diet, CDAA diet, and HF diet [23]. Apart from TLR2-deficient mice, other TLR signaling is not likely enhanced in TLR4-deficient mice.
2.5 TLR5
TLR5 is a receptor for flagellin, a component of bacterial flagella. TLR5-deficient mice spontaneously develop colitis and metabolic syndrome even in specific pathogen-free conditions [24], indicating that TLR5 signaling maintains gut homeostasis. Anti-flagellin antibody levels are low in TLR5-deficient mice and MyD88-deficient mice [25], suggesting that TLR5 signaling controls the production of anti-flagellin antibodies [26]. Many types of cells express TLR5 in the gut. Chassaing et al. have reported that mice lacking TLR5 specifically on intestinal epithelial cells exhibit colitis and metabolic syndrome similar to systemic TLR5-deficient mice [27], indicating that TLR5 on intestinal epithelial cells plays a key role in the development of manifestations observed in TLR5-deficient mice. Although initial studies have reported that compositional changes in the gut microbiota are a potential cause of manifestations observed in TLR5-deficient mice, recent findings demonstrate that the gut microbiota of TLR5-deficient mice is vastly diverse, that is, TLR5-deficient mice do not always exhibit colitis and steatohepatitis under different environmental conditions in other facilities [28]. Prolonged separated bleeding results in a different composition of gut microbiota in TLR-deficient mice [29]. Interestingly, there are minimal changes between TLR5-deficient mice and WT littermates regarding the composition of gut microbiota when they are housed at the same place. A time-series analysis of the gut microbiota provides further information particular to TLR5-deficient mice. TLR5-deficient mice show a greater diversity of gut microbiota depending on the conditions in which they are housed. The ability to stimulate TLR5 is not always equivalent in potency in flagellin-producing bacteria. For instance, flagellin from Salmonella typhimurium has a 1,000-fold greater ability to stimulate TLR5 compared with Helicobacter pylori [30]. Thus, minor changes in the gut microbiota may amplify the differences in gene expression in TLR5-deficient mice [31]. As a result, TLR5-deficient mice show a wide variety of manifestations due to the volatility of the gut microbiota and gene expression over time. TLR5 loss allows the expansion of specific gut microbiota that can cause injury to the intestinal epithelium. Carvalho et al. have reported that TLR5-deficient mice show sporadic increases of Proteobacteria including E. coli [32], which is associated with intestinal inflammation [33] and human Crohn’s disease [34]. Thus, TLR5 may be associated with the development of inflammatory bowel diseases [35].
2.6 TLR9
TLR9 recognizes bacteria- and virus-derived DNA and modified self-DNA from injured cells. TLR9 signaling induces proinflammatory cytokines and type I IFNs through the MyD88-dependent manner. These mediators contribute to the hepatic inflammation, liver fibrosis, and replication of hepatitis virus. TLR-mediated IFN production can prevent the replication of hepatitis virus including HBV. However, TLR9 expression and the subsequent production of IFN-α are reduced in mononuclear cells and DCs in patients with chronic HBV infection [36], suggesting that HBV inhibits TLR9 signaling.
The TLR9 ligand bacterial DNA is elevated in alcoholic liver injury [37] and NASH [4], in which gut permeability is increased. A TLR9 ligand stimulates macrophages to induce proinflammatory cytokines including IL-1β, which can also induce profibrogenic factors such as tissue inhibitor of metalloproteinase-1(TIMP-1). Indeed, TLR9-deficient mice are protected from inflammation and fibrosis in a CDAA diet-induced NASH model [4]. Thus, the stimulation of TLR9-mediated macrophages leads to inflammation and fibrosis.
In contrast to the NASH model, the role of TLR9 is complex in CCl4-induced inflammation and liver fibrosis [38]. Although the administration of a TLR9 ligand attenuates CCl4-induced liver fibrosis, TLR9-deficient mice show decreased fibrosis in the same model. A TLR9 ligand increases the number of NK cells in WT mice, in which TLR9 ligand-stimulated natural killer (NK) cells inhibit the proliferation of hepatic stellate cells (HSCs). However, HSCs undergo senescence in TLR9-deficient mice, resulting in decreased fibrosis. In the CCl4 model, different types of liver cells participate in TLR9-mediated liver injury. For instance, DCs are one of the cell types that abundantly express TLR9. In response to TLR9 ligands, DCs produce TNF-α that promotes inflammation-mediated fibrosis, whereas they also produce IFNs that suppress liver fibrosis. On the other hand, DCs can produce IL-10 that suppresses inflammation, while they can also produce proinflammatory IFNs. These data indicate that the role of TLR9 signaling depends on the disease models used and the function of the liver cells and cytokines.
2.7 TLR Polymorphisms
Experimental data have demonstrated that TLRs are associated with many liver disease models. Thus, single nucleotide polymorphisms (SNPs) in TLRs have been gained much attention in the development of human liver diseases. In chronic HCV infection and HCV-based HCC, patients with the TLR2 -196 to -174 del/ins polymorphism are at increased risk for HCC in HCV genotype 1 infection. Interestingly, these patients are characterized by higher viral load with similar clinical presentations, including serum alanine transaminase (ALT) levels and the stages of liver fibrosis. Monocytes isolated from patients with the TLR2 -196 to -174 del/ins polymorphism have low expression levels of TLR2 and IL-8 in response to TLR2 ligands [39], suggesting that a low immune response is a feature of patients with the TLR2 -196 to -174 del/ins polymorphism. On the other hand, patients with TLR2 rs3804099 C/T and rs3804100 C/T genotypes have a low risk of HCC [40]. Several studies have shown the association of TLR4 SNPs in HCV-related liver diseases. TLR4 D229G and T399I SNPs are reported to protect against hepatic inflammation and liver fibrosis [41]. These TLR4 SNPs are linked to liver cirrhosis in patients with chronic HCV infection [42] and the outcomes of liver transplantation [43]. TLR3 SNPs rs5743305 (T/A) and rs3775291 (C/T) were reported in patients with chronic HCV infection. However, no association was found between these SNPs and the clinical parameters [44]. The TLR3 L412F SNP may protect against acute graft rejection in the recipients of liver transplantation with HCV-related cirrhosis [45]. Most of the currently reported TLR SNPs have been associated with HCV-related liver diseases, although TLR SNPs may be found in association with nonviral hepatitis in the near future.
3 NOD-Like Receptors
NOD-like receptors (NLRs) and its related proteins form a large molecular platform referred to as the inflammasome, in which procaspase-1 is processed to active caspase-1, and the subsequent maturation of IL-1 and IL-18. There are 22 members of NLRs in human and 34 in mouse [46]. Although NLRs were initially identified as sensors for pathogens, emerging data demonstrate that NLRs also recognize danger-associated molecular patterns (DAMPs), such as ATP and uric acids. Of the NLRs, NACHT, LRR and PYD domains-containing protein 3 (NLRP3) has been well investigated in the development of liver injuries including acetaminophen-induced liver injury, ischemic reperfusion liver injury, alcoholic liver injury, and NASH. The NLRP3-mediated inflammasome requires two steps for activation: an increase in NLRP3 expression and the aggregation of inflammasome components. First, TLR ligands such as LPS increase the NLRP3 expression through NF-κB. Then, various DAMPs induce the aggregation of inflammasome components and process caspase-1 activation.
Of the human liver diseases, NASH is characterized by increased inflammasome activation compared with chronic HCV infection [47]. Many substances that can stimulate inflammasome components are increased in NASH. For instance, saturated fatty acid and cholesterol crystal are substances that can form a complex of inflammasome components in NASH [48, 49]. These lipids activate the inflammasome when its components are sufficiently expressed. In addition, host-derived modified DNA has been shown to function as a DAMP. TLR9-deficient mice on a CDAA diet show an interesting cytokine profile. These mice exhibit lesser degrees of steatosis and inflammation compared with WT mice. Although WT macrophages produce several proinflammatory cytokines in response to a TLR9 ligand, only IL-1β expression is suppressed in TLR9-deficient mice on a CDAA diet [4]. The CDAA diet increases several components of the inflammasome including NLRP3, NLRP1, ASC, AIM-2, and caspase-1 [11]. This diet also increases the number of apoptotic cells and bacterial DNA levels, which can aggregate inflammasome components. Thus, the inflammasome is activated in an experimental NASH model.
One of the final products of inflammasome activation is IL-1β, which is increased at the mRNA and protein levels in experimental NASH models. Mice deficient in either IL-1R or IL-1β are protected from steatosis, cell death, and liver fibrosis, indicating that IL-1β promotes the progression of NASH. IL-1β has multiple functions in the development of steatohepatitis. IL-1β increases steatosis by activating PPAR-α and DGAT2, a converter of diglyceride to triglyceride. In addition, IL-1β may induce cell death in lipid-laden hepatocytes or support TNF-α-mediated cell injury [50].
4 RIG-I
RIG-I-like receptors (RLRs) include RIG-I, melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2). These PRRs sense RNA viruses such as HCV and subsequently produce type I IFNs. RIG-I is expressed by most cells at a low level with resting state in the cytosol. Once RIG-I senses RNA pathogens, RIG-I moves to the mitochondrial-associated membrane and engages MAVS (Cardif), which initiates the signaling cascade. Finally, IFNs and proinflammatory cytokines are produced through IRF3 and NF-κB activation. Type I IFNs are used for the treatment of chronic HBV/HCV infections because they have potent antiviral effects. Because IFNs can increase the expression of RIG-I, the activation of RIG-I signaling is a target for the treatment of viral hepatitis. However, HCV protein NS3/4A inhibits IFN production by degrading MAVS [51] as well as the TLR3 adaptor protein TRIF. HCV NS5A also suppresses RIG-I signaling [52] as well as TLR4-mediated antiviral effects. Although hepatocytes contribute to the production of type I IFNs by recognizing HCV through RIG-I, HCV can evade this signaling. Hence, IFN treatment alone is not sufficient for eradicating HCV. At present, directly acting antivirus agents are available as anti-HCV agents, including asunaprevir and daclatasvir, which are inhibitors of HCV proteins N3/4A and NS5A, respectively. These agents are highly effective against HCV without mutations at Y93 and L31 in the NS5 region. Although HBV is a DNA virus, RIG-I senses HBV [53, 54], which inhibits the RIG-I pathway by downregulating MAVS. As a result, the production of type I and III IFNs is suppressed in hepatocytes, which allows for the replication of HBV.
5 Complements
The liver is responsible for the production of 80–90 % of complement proteins [6]. The complement system comprises more than 35 proteins. There are three pathways for activating the complement system: the classical, lectin, and alternative pathways. All three pathways use C3 to activate the complement cascade. After C3 is cleaved into C3a and C3b, C3b cleaves C5 into C5a and C5b, and the C5b-9 complex is formed to attack infected cells (Fig. 1.2).
Although the primary role of the complement system is to defend against microbial infection, its activation is closely associated with the development of liver diseases. For instance, patients with nonalcoholic fatty liver disease (NAFLD) had increased plasma concentrations of complement proteins and complement activation [55] because IL-6 and TNF-α levels, which enhance the hepatic production of complement proteins, are increased in these patients. In addition, gut-derived products and apoptotic cells that activate the complement cascade are increased in NASH [56]. As a result, the hepatic deposition of complement proteins is correlated with the severity of NASH, an advanced stage of NAFLD. NAFLD is a predictor for type 2 diabetes, and the major cause of death in NAFLD patients is cardiovascular diseases. Thus, complements produced by the liver may be associated with systemic diseases, including diabetes and cardiovascular diseases.
In experimental ALD models, the deposition of C3, C8, and C9 is increased in the liver [57, 58]. C5 is also associated with the development of ALD. C5-deficient mice show low expression levels of proinflammatory cytokines and attenuated histological inflammation in ALD [59]. In a liver fibrosis model by bile duct ligation, mice deficient in C5 (Hc0/Hc0 mice) showed an attenuation of liver fibrosis at an early time point [60]. Decreased leukocyte infiltration and TNF-α expression accounted for the decreased fibrosis. Because hepatic macrophages and HSCs produce proinflammatory cytokines and profibrogenic factors through C5 activation, the blockade of C5R1 attenuates hepatic inflammation and fibrosis in mice.
Although the plasma concentration of complement is elevated in early stages of liver diseases, the activity is suppressed at advanced stages of liver diseases due to impaired biosynthesis and increased consumption [61]. This condition results in infectious diseases in patients with liver diseases, such as spontaneous bacterial peritonitis, a common complication of liver cirrhosis. C3 and C5 activation is reported to recruit neutrophils to the site of infection, which prevent the expansion of microbial infection by promoting phagocytic activity and opsonization. However, these defense mechanisms weaken due to low levels of complement. In liver transplantation, the recipients have a higher risk of infection when the donor liver has a genetically decreased capacity of complement production [62]. In an experimental murine model, mice deficient in decay-accelerating factor (CD55/DAF), an inhibitor of complement, demonstrated severe steatosis and elevated ALT levels following ethanol feeding. Collectively, these studies show that appropriate concentrations of complement proteins provide a healthy condition.
6 Innate Immune System and Hepatic Manifestations
6.1 Inflammation
Many liver cells are engaged in the development of inflammation, in which macrophages contribute to both the progression and suppression of inflammation (Fig. 1.3). Several classifications have been proposed to divide inflammatory and anti-inflammatory macrophages. One of the representatives is the M1/M2 classification [63], which depends on the catabolism of L-arginine. M1 macrophages express inducible nitric oxide synthase (iNOS), which converts L-arginine to L-citrulline and nitric oxide. M1 macrophages are classical macrophages that produce proinflammatory cytokines. On the other hand, M2 macrophages express arginase I, which converts L-arginine to L-ornithine. M2 macrophages are referred to as alternatively activated macrophages and demonstrate anti-inflammatory responses. The abundance of M1 macrophages and M2 macrophages is altered in systemic diseases including liver diseases. For instance, the switch from M2 anti-inflammatory macrophages to M1 inflammatory macrophages occurs in the liver of mice with NASH [64]. The number of macrophages expressing the M2 markers is increased during mild liver injury caused by ALD and obesity compared with those during severe injury [65], suggesting that M2 macrophages have protective roles. These are debating on the M1/M2 polarization in Kupffer cells, hepatic tissue macrophages. Wan et al. have reported that Kupffer cells include both M1 and M2 phenotypes [66]. Of Kupffer cells, the M2 type is 20 % and 40 % in Th1-biased C57/BL6 mice and Th2-biased Balb/c mice, respectively. T helper cell-derived Th1 and Th2 cytokines can activate M1 and M2 macrophages, respectively. In Th2-biased Balb/c mice, ethanol feeding increases expression of the M2 markers but not M1 markers in macrophages. In this model, M2-type Kupffer cells induce the apoptosis of M1 Kupffer cells by an IL-10-mediated arginase-dependent mechanism [66]. As a result, Th2-biased Balb/c mice are resistant against alcohol-induced liver injury and high-fat-induced steatosis.
Ly6C, a glycoprotein on the cell surface, has emerged as a marker for bone marrow-derived inflammatory macrophages. We have previously reported that Ly6C-positive macrophages are recruited in experimental NASH along with the progression of inflammation [67]. Interestingly, CCR2-deficient mice show a decreased number of Ly6C macrophages in the liver, suggesting that CCR2 contributes to the recruitment of Ly6C macrophages. In other injury models, the recruitment of Ly6C macrophages depends on CCR2 expression [68]. In acetaminophen-induced liver injury, Ly6Chigh macrophages are predominantly recruited in the inflammatory phase, while Ly6Clow macrophages are recruited in the recovery phase [69]. In CCl4-induced liver injury, Ly6Chigh macrophages are recruited in both the inflammatory and recovery phases. On the other hand, Ly6Clow macrophages are predominantly recruited in the recovery phase and contribute to tissue repair by producing angiogenetic factors and inhibiting of neutrophils [68]. Thus, the expression of Ly6C on macrophages is a potential marker to know hepatic inflammation. Although the phenotype of M1 macrophages is similar to that of Ly6Chigh macrophages, these macrophages are not always identical.
Several investigators have described the phenotype of inflammatory macrophages using classical markers including CD11b and CD68, which are receptors for C3b and oxidized low-density lipoprotein (LDL), respectively. Kinoshita et al. have reported that F4/80 and CD11b double-positive macrophages are recruited from the circulation, spleen, and BM. These recruited macrophages possess an increased ability to produce proinflammatory cytokines [70]. On the other hand, F4/80 and CD68 double-positive macrophages are classical hepatic macrophages Kupffer cells and have potent phagocytic ability and ROS production.
DCs, a small population in the liver, are comprised from heterogeneous populations and exhibit vastly different functions depending on the expressing surface markers. Monocyte-derived DCs (moDCs) contribute to inflammation by producing TNF-α in the bile duct ligation model [71]. moDCs expressing CD11b differentiate into inflammatory DCs, which are recruited to inflammatory sites in a CCR2-dependent manner. The phenotype of these moDCs is similar to M1 macrophages that produce proinflammatory cytokines in response to TLR ligands. On the other hand, CD1c+ DCs secrete anti-inflammatory cytokine IL-10 in response to LPS [72]. CD1c+ DCs represent a major population in the liver among the three major classes of DCs [73]. DCs are mainly located in the portal area of the liver, where the cells have the advantage to detect foreign bodies from the gut. However, hepatic DCs represent an immature phenotype and have decreased antigen-presenting ability compared with DCs in the skin and spleen. Thus, hepatic DCs are associated with hepatic tolerance as well as the suppression of inflammation in the liver.
6.2 Steatosis
Hepatic steatosis is observed in NAFLD/NASH and ALD as a result of imbalance between the lipid input and output. Interestingly, the innate immune system is associated with the development of hepatic steatosis (Fig. 1.3). For example, the polarization of hepatic macrophages is linked to the development of steatosis, in which the polarization of M1 macrophages contributes to steatosis. TNF-α is a potential mediator of inducing steatosis by several mechanisms [23]. TNF-α induces insulin resistance by inhibiting insulin receptors, insulin receptors substrate-1, and GLUT4 expression. Insulin resistance promotes subsequent release of free fatty acids (FFAs) from adipocytes. Increased serum FFA levels further promote insulin resistance. On the other hand, TNF-α increases the uptake of FFAs and cholesterol accumulation in hepatocytes by inducing LDL receptor and by inhibiting the efflux of cholesterol [74], resulting in aberrant steatosis. A representative chemokine CCL2 contributes to the development of steatosis by recruiting TNF-α-producing macrophages, resulting in an increased lipid accumulation in the liver. CCL2 also promotes steatosis by increasing lipid synthesis through PEPCK activity. In addition, CCL2 inhibits lipid efflux through Apo B suppression in hepatocytes. Hepatocytes have been reported not to express CCR2, a receptor for CCL2, suggesting that hepatocytes use other pathways. Indeed, mice deficient in these proinflammatory cytokines or chemokine exhibit decreased hepatic steatosis.
In contrast to M1 macrophages, steatosis is mild when macrophages are polarized to the M2 phenotype in human ALD and obese individuals [75]. In addition, M2 Kupffer cells inhibit steatosis in ALD and NASH through the production of IL-6 [65]. These data indicate that M2 macrophages reduce the accumulation of lipids in the liver. On the other hand, TGF-β promotes lipid accumulation in hepatocytes through SMAD signaling [76], although certain M2 macrophages produce TGF-β. Because M2 macrophages can be divided into at least three subpopulations, further investigation is required to elucidate the mechanism by which M2 macrophages contribute to the development of steatosis.
6.3 Liver Fibrosis
Liver fibrosis is a common consequence of any chronic liver diseases. The innate immune system also contributes to the development of liver fibrosis (Fig. 1.3). HSCs are well known as the primary cells that promote liver fibrosis by producing ECM proteins, such as type I collagen. Recently, the immunologic functions of HSCs have gained much attention in liver fibrosis because HSCs express PRRs, including TLR2, TLR4, TLR9, and complement receptor C5R1. TLR ligands further increase the expression of chemokines and PRRs in HSCs. Although TLR ligands do not always directly drive HSCs to produce collagen, some TLR ligands render HSCs to sense TGF-β, a potent fibrogenic factor. Bambi, a pseudoreceptor for TGF-β, is a molecular target of TLR ligands, which can downregulate the expression of Bambi in HSCs [77]. As a result, HSCs stimulated with the TLR4 ligand LPS show enhanced TGF-β signaling, which accelerates the transformation from HSCs to collagen-producing myofibroblast-like cells. Thus, TLR4 signaling in HSCs promotes liver fibrosis through the downregulation of Bambi. Although TLR2 ligands can decrease the expression of Bambi in HSCs, the role of TLR2 on liver fibrosis is dependent on the models. For example, TLR2-deficient mice are not predispose to liver fibrosis induced by bile duct ligation [77], whereas TLR2-deficient mice showed decreased fibrosis in a NASH model induced by a CDAA diet [11]. A higher concentration of ligands is required to stimulate TLR2 in HSCs [16], suggesting that limited conditions allow the predominant activation of TLR2 signaling in the liver. In mice, a CDAA diet induces obesity, in which Gram-positive bacteria are abundant in the gut. Thus, an increase in Gram-positive bacteria is a potential cause of TLR2 activation in mice on a CDAA diet.
Macrophages play a role in the progression, as well as regression, of liver fibrosis. Ly6Chigh macrophages have been reported to promote liver fibrosis by producing higher levels of TGF-β and proinflammatory cytokines. These Ly6Chigh macrophages are recruited from circulating monocytes. On the other hand, resident Kupffer cells contribute to liver fibrosis regardless of Ly6C expression [78]. Macrophages are one of the sources of TGF-β, a potent activator of HSCs. In addition, hepatic macrophages produce IL-1 and TNF in response to TLR ligands. IL-1 and TNF subsequently prolong the survival period of HSCs [78]. These proinflammatory cytokines induce TIMP-1 expression, which can inhibit apoptosis of HSCs. In contrast to Ly6Chigh macrophages and Kupffer cells, Ly6Clow macrophages contribute to resolution of liver fibrosis [79]. Ly6Clow macrophages are derived from Ly6Chigh macrophages and are distinct from typical M2 macrophages [79], which contribute to tissue remodeling by producing TGF-β.
The contribution of DCs to liver fibrosis is exceptionally complex. DCs regulate NK cells to kill activated HSCs, resulting in the resolution of liver fibrosis. In addition, DCs secrete large concentration of matrix metalloproteinases (MMPs), including MMP-9 [80]. MMPs play a role in the resolution of ECM, resulting in the improvement of liver fibrosis. These data show that DCs are able to attenuate liver fibrosis. On the other hand, a subset of DCs significantly can increase in number during bile duct ligation-induced liver fibrosis [81], suggesting that DCs promote liver fibrosis. A recently published paper has demonstrated that hepatic DCs play a lesser role in liver fibrosis [78]. Further investigation is required to address the role of DCs during liver fibrosis.
NK cells are considered to suppress liver fibrosis by killing activated HSCs. The ability of NK cells to kill activated HSCs is reduced in chronic exposure to ethanol via several molecules including NKG2D, TRAIL, FAS ligands, and IFN-γ [82]. In addition, increased TGF-β, a potential inhibitor of NK cells, suppresses the function of NK cells.
6.4 Hepatic Malignancies
HCC is the primary cancer caused by the chronic HBV/HCV infection, NASH, and exposure to alcohol/aflatoxin. The innate immune system has dual functions in the development of HCC. Although TLR4-deficient mice are protected from precancerous liver injuries, conflicting data exist regarding the role of TLR4 in experimental HCC models using chemical carcinogens. TLR4-deficient mice exhibit a decreased incidence of HCC induced by DEN [83] or DEN combined with CCl4 [84]. TLR4 deficiency suppresses the production of proinflammatory cytokines and ECM proteins, suggesting that TLR4-mediated inflammation and fibrosis contribute to the development of HCC. In these settings, TLR4 expression on non-parenchymal cells plays an important role in tumor growth by producing growth factors, ECM, and angiogenetic factors. HCC generally develops during chronic inflammation and liver fibrosis, in which tumor-associated macrophages and cancer-associated fibroblasts are components of the tissue microenvironment of the tumors. Indeed, the depletion of macrophages or fibroblasts inhibits tumor growth in mice [85, 86]. In addition to macrophages and fibroblasts, emerging data demonstrate that human HCC cells express TLRs, and the expression is correlated with the prognosis and tumor size [87]. TLR ligands can activate TLR signaling in human HCC cell lines. The TLR4 ligand LPS induces the expression of IL-6, a tumor-promoting factor, and the proliferation of HCC cell lines [88]. In addition, LPS induces the epithelial-mesenchymal transition of HCC cells. Thus, TLR4 signaling in tumor cells is also activated during the development of tumors.
Conversely, Wang et al. have demonstrated that the lack of TLR4 signaling promotes the development of DEN-induced HCC. A deficiency in TLR4 signaling inhibits the function of DNA repair protein Ku70 and senescence [89], which are potential factors for cancer promotion. Although the discrepancy of these studies remains unknown, TLR4 signaling in different cell types may lead to conflicting data.
The liver is rich in lymphocytes including NK cells and NKT cells. For instance, approximately 30–50 % of human hepatic lymphocytes are NK cells. NK cells have antitumor effects by killing tumor cells. However, the function and the number of NK cells are decreased in hepatic malignancies, resulting in a poor prognosis. NK cells express a wide variety of immune recognition receptors that recognize tumor cells. The expression of several receptors is decreased in HCC, allowing tumor cells to evade the anticancer immune system [90]. Thus, new immune therapies to restore the function of NK cells are under development.
7 Concluding Remarks
The innate immune system in the liver contributes to the prevention against microbial infection. Recent work has demonstrated that the innate immune system is also closely associated with the development of liver diseases. The involvement of the innate immune system in liver diseases is a complex process, in which many liver cells and mediators participate in the modulation of disease activity. Thus, innate immunity contributes to the amelioration, as well as deterioration, of liver diseases. Therefore, further investigations are required to determine how to control the innate immune system in order to prevent microbial infection as well as liver diseases.
References
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84.
Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31.
Zhao X-J, Dong Q, Bindas J, Piganelli JD, Magill A, Reiser J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–56.
Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–34.e7.
Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, et al. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem Biophys Res Commun. 2005;328:383–92.
Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–36.
Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252–8.
Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105.
Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8.
Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–24.
Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577–89.
Rivera CA, Gaskin L, Allman M, Pang J, Brady K, Adegboyega P, et al. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol. 2010;10:52.
Lin H, Yan J, Wang Z, Hua F, Yu J, Sun W, et al. Loss of immunity-supported senescence enhances susceptibility to hepatocellular carcinogenesis and progression in Toll-like receptor 2-deficient mice. Hepatology. 2013;57:171–82.
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101.
Pimentel-Nunes P, Roncon-Albuquerque R, Gonçalves N, Fernandes-Cerqueira C, Cardoso H, Bastos RP, et al. Attenuation of toll-like receptor 2-mediated innate immune response in patients with alcoholic chronic liver disease. Liver Int. 2010;30:1003–11.
Paik Y-H, Lee KS, Lee HJ, Yang KM, Lee SJ, Lee DK, et al. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest. 2006;86:676–86.
Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009;83:9824–34.
Funk E, Kottilil S, Gilliam B, Talwani R. Tickling the TLR7 to cure viral hepatitis. J Transl Med. 2014;12:129.
Li N, Li Q, Qian Z, Zhang Y, Chen M, Shi G. Biochemical and biophysical research communications impaired TLR3 / IFN- b signaling in monocyte-derived dendritic cells from patients with acute-on-chronic hepatitis B liver failure: relevance to the severity of liver damage. Biochem Biophys Res Commun. 2009;390:630–5.
Yeh M-L, Peng C-Y, Dai C-Y, Lai H-C, Huang C-F, Hsieh M-Y, et al. Pegylated-interferon alpha therapy for treatment-experienced chronic hepatitis B patients. PLoS One. 2015;10:e0122259.
Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-yoshida Y, et al. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Investig. 2005;85:908–20.
Roh YS, Park S, Kim JW, Lim CW, Seki E, Kim B. Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis. Hepatology. 2014;60:237–49.
Miura K, Seki E, Ohnishi H, Brenner DA. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic Fatty liver disease. Gastroenterol Res Pract. 2010;2010:362847.
Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31.
Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571–81.
Letran SE, Lee SJ, Atif SM, Uematsu S, Akira S, McSorley SJ. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol. 2011;41:29–38.
Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363–77.e17.
Flores-Langarica A, Marshall JL, Hitchcock J, Cook C, Jobanputra J, Bobat S, et al. Systemic flagellin immunization stimulates mucosal CD103+ dendritic cells and drives Foxp3+ regulatory T CELL and IgA responses in the mesenteric lymph node. J Immunol. 2012;189:5745–54.
Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–56.
Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, Peek RM. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis. 2004;189:1914–20.
Leifer CA, Mcconkey C, Li S, Chassaing B, Gewirtz AT, Ley RE. Linking genetic variation in human Toll-like receptor 5 genes to the gut microbiome’ s potential to cause inflammation. Immunol Lett. 2014;162:3–9.
Carvalho FA, Koren O, Goodrich JK, Johansson ME V, Nalbantoglu I, Aitken JD, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–52.
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5.
Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology. 2004;127:412–21.
Sheridan J, Mack DR, Amre DK, Israel DM, Cherkasov A, Li H, et al. A Non-synonymous coding variant (L616F) in the TLR5 gene is potentially associated with Crohn’s disease and influences responses to bacterial flagellin. PLoS One. 2013;8:1–6.
Xu N, Yao HP, Lv GC, Chen Z. Downregulation of TLR7/9 leads to deficient production of IFN-?? from plasmacytoid dendritic cells in chronic hepatitis B. Inflamm Res. 2012;61:997–1004.
Romics L, Dolganiuc A, Kodys K, Drechsler Y, Oak S, Velayudham A, et al. Selective priming to Toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555–64.
Abu-Tair L, Axelrod JH, Doron S, Ovadya Y, Krizhanovsky V, Galun E, et al. Natural killer cell-dependent anti-fibrotic pathway in liver injury via toll-like receptor-9. PLoS One. 2013;8:1–11.
Nischalke HD, Coenen M, Berger C, Aldenhoff K, Müller T, Berg T, et al. The toll-like receptor 2 (TLR2) -196 to -174 del/ins polymorphism affects viral loads and susceptibility to hepatocellular carcinoma in chronic hepatitis C. Int J Cancer. 2012;130:1470–5.
Junjie X, Songyao J, Minmin S, Yanyan S, Baiyong S, Xiaxing D, et al. The association between Toll-like receptor 2 single-nucleotide polymorphisms and hepatocellular carcinoma susceptibility. BMC Cancer. 2012;12:57.
Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–8.
Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306.
Dhillon N, Walsh L, Krüger B, Ward SC, Godbold JH, Radwan M, et al. A single nucleotide polymorphism of Toll-like receptor 4 identifies the risk of developing graft failure after liver transplantation. J Hepatol. 2010;53:67–72.
Yin S, Gao B. Toll-like receptor 3 in liver diseases. Gastroenterol Res Pract. 2010;1–7.
Citores MJ, Baños I, Noblejas A, Rosado S, Castejon R, Cuervas-Mons V. Toll-like receptor 3 L412F polymorphism may protect against acute graft rejection in adult patients undergoing liver transplantation for hepatitis C-related cirrhosis. Transplant Proc. 2011;43:2224–6.
Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev. 2015;95:149–78.
Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–44.
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT-H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15.
Rajam̈aki K, Lappalainen J, Öörni K, Välimäki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:1–9.
Petrasek J, Dolganiuc A, Csak T, Kurtjones EA, Szabo G. Type i interferons protect from toll-like receptor 9associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140:697–708.
Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72.
Hiet M-S, Bauhofer O, Zayas M, Roth H, Tanaka Y, Schirmacher P, et al. Control of temporal activation of hepatitis C virus-induced interferon response by domain 2 of nonstructural protein 5A. J Hepatol. 2015;63(4):829–37.
Wei C, Ni C, Song T, Liu Y, Yang X, Zheng Z, et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 2010;185:1158–68.
Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, et al. Article the RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123–32.
Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, et al. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009;50:1809–17.
Ogden CA, DeCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95.
Järveläinen HA, Väkevä A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105:57–63.
Bykov IL, Väkevä A, Järveläinen HA, Meri S, Lindros KO. Protective function of complement against alcohol-induced rat liver damage. Int Immunopharmacol. 2004;4:1445–54.
Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Medof ME, et al. {A figure is presented}differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–26.
Schmitt J, Roderfeld M, Sabrane K, Zhang P, Tian Y, Mertens JC, et al. Complement factor C5 deficiency significantly delays the progression of biliary fibrosis in bile duct-ligated mice. Biochem Biophys Res Commun. 2012;418:445–50.
Bird G, Senaldi G, Panos M, Rolando N, Alexander G, Vergani D, et al. Activation of the classical complement pathway in spontaneous bacterial peritonitis. Gut. 1992;33:307–11.
Verspaget HW, Berger SP, Daha MR, Frölich M. Mannose binding lectin gene polymorphisms confer a major. Gastroenterology. 2005;129:408–14.
Sica A, Mantovani A. Science in medicine macrophage plasticity and polarization : in vivo veritas. J Clin Invest. 2012;122:787–95.
Seth RK, Das S, Pourhoseini S, Dattaroy D, Igwe S, Ray JB, et al. M1 polarization bias and subsequent nonalcoholic steatohepatitis progression is attenuated by nitric oxide donor DETA NONOate via inhibition of CYP2E1-induced oxidative stress in obese mice s. J Pharmacol Exp Ther. 2015;352:77–89.
Wan J, Benkdane M, Alons E, Lotersztajn S, Pavoine C. M2 kupffer cells promote hepatocyte senescence: an IL-6-dependent protective mechanism against alcoholic liver disease. Am J Pathol. 2014;184:1763–72.
Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2013;130–42.
Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1310–21.
Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74.
Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–53.
Kinoshita M, Uchida T, Sato A, Nakashima M, Nakashima H, Shono S, et al. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53:903–10.
Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234:90–104.
Kassianos AJ, Hardy MY, Ju X, Vijayan D, Ding Y, Vulink AJE, et al. Human CD1c (BDCA-1) + myeloid dendritic cells secrete IL-10 and display an immuno-regulatory phenotype and function in response to Escherichia coli. Eur J Immunol. 2012;42:1512–22.
Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901–11.
Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48:770–81.
Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217–26.
Yang L, Roh YS, Song J, Zhang B, Liu C, Loomba R, et al. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology. 2014;59:483–95.
Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32.
Pradere J-P, Kluwe J, De Minicis S, Jiao J-J, Gwak G-Y, Dapito DH, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–73.
Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–95.
Yen JH, Kocieda VP, Jing H, Ganea D. Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem. 2011;286:38913–23.
Bleier JI, Katz SC, Chaudhry UI, Pillarisetty VG, Kingham TP, Shah AB, et al. Biliary obstruction selectively expands and activates liver myeloid dendritic cells. J Immunol. 2006;176:7189–95.
Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–58.
Yu L-X, Yan H-X, Liu Q, Yang W, Wu H-P, Dong W, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–33.
Dapito DH, Mencin A, Gwak G-Y, Pradere J-P, Jang M-K, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–16.
Fan Q, Jing Y, Yu G, Kou X, Ye F, Gao L, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial – mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–8.
Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907.
Eiró N, Altadill A, Juárez LM, Rodríguez M, González LO, Atienza S, et al. Toll-like receptors 3, 4 and 9 in hepatocellular carcinoma: relationship with clinicopathological characteristics and prognosis. Hepatol Res. 2014;44:769–78.
Wang L, Zhu R, Huang Z, Li H, Zhu H. Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig Dis Sci. 2013;58:2223–36.
Wang Z, Yan J, Lin H, Hua F, Wang X, Liu H, et al. Toll-like receptor 4 activity protects against hepatocellular tumorigenesis and progression by regulating expression of DNA repair protein Ku70 in mice. Hepatology. 2013;57:1869–81.
Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol. 2015;12:292–302.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Miura, K., Ohnishi, H. (2016). Innate Immunity and the Liver. In: Ohira, H. (eds) The Liver in Systemic Diseases. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55790-6_1
Download citation
DOI: https://doi.org/10.1007/978-4-431-55790-6_1
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55789-0
Online ISBN: 978-4-431-55790-6
eBook Packages: MedicineMedicine (R0)