Abstract
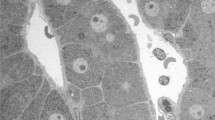
Soluble proteins and glycogen particles, which are easily lost upon conventional chemical fixation, have been reported to be better preserved in paraffin-embedded sections by “cryobiopsy” combined with freeze-substitution fixation (FS). In this part, we mentioned the distribution of glycogen in living mouse livers under (1) food restriction and (2) local circulation loss conditions with periodic acid-Schiff (PAS) staining by cryobiopsy. (1) Livers of fully fed mice showed high PAS-staining intensity in the cytoplasm of all hepatocytes, showing much glycogen in all hepatocytes. However, treatment with α-amylase clarifies that hepatocytes around the central vein preserve more glycogen than the hepatocytes around the portal vein. At 6 or 12 h after fasting, PAS-staining intensity markedly decreased in restricted areas of zone I near the portal tracts. This result was the same as that of specimens gotten from individual mouse. On the other hand, glycogen distributions were different in local circulation loss condition which caused by temporarily clipping of liver tissues followed by recovery of blood circulation. (2) In the liver tissues in which blood was recirculated for 1 h after the 30-min anoxia, PAS staining was still observed in zone II and also in restricted areas of zone I far from the portal tracts. We propose that the level of consumption or production of glycogen particles could vary in zone I, depending on the distance from the portal tracts. Thus, cryobiopsy combined with FS enabled us to examine time-dependent changes in glycogen distribution in the liver tissues of living mice. This combination might be applicable to the clinical evaluation of human liver tissues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cryobiopsy enables us to repeatedly obtain multiple tissue samples from a single mouse under different experimental conditions [1, 2]. In this chapter, we focused on the distribution of glycogen in the mouse liver and introduced their consumption pattern clarified by cryobiopsy . And then how to distinguish consumption from leakage by damage was mentioned. A large amount of glycogen in the livers is an important energy source to be metabolized into blood glucose to sustain animal life [3]. Glycogen metabolism was related to not only feeding conditions but also ischemia -reperfusion and stress of animal livers [4–8]. Ischemia and reperfusion typically occur in liver transplants and result in various types of liver damage and reactions [9–14]. Therefore, it is necessary to precisely determine time-dependent changes in the glycogen storage of hepatocytes in paraffin -embedded tissues, which reflect their living state s . In conventional preparation with chemical fixative s in a typical buffer solution, the artificial loss of glycogen particle s is much higher than in various cryotechniques [15].
2 Distinguishable Differences of a Tiny Amount of Glycogen in Hepatocytes
Cryobiopsy of liver tissues was performed on anesthetized mice [2, 16]. Briefly, after exposure of target liver lobes, a piece of tissue was quickly pinched off using handmade cryoforceps attached to sponge reservoirs of liquid nitrogen on Keisei Osteotrite Tweezers® (F-2051 or F-2052, Keisei Medical Industrial Co., Ltd., Niigata, Japan), which had already been precooled in liquid nitrogen (LN2, −196 °C). The cryobiopsied liver tissues were immediately immersed in liquid isopentane -propane cryogen (−193 °C) precooled in LN2 and then transferred into acetone containing 2 % paraformaldehyde at about −80 °C for starting freeze-substitution fixation (FS) [2, 16]. Cryobiopsy-FS specimen is able to choose usual paraffin embedding or sucrose embedding for frozen section. In this chapter, we’re showing paraffin-embedded specimens [1]. The paraffin-embedded specimens were treated in the same way as conventional specimens. To detect glycogen in hepatocytes , deparaffinized sections were incubated in periodic acid followed by Schiff’s solution and sodium metabisulfite. In mouse liver tissues of fully fed mice prepared by cryobiopsy , high-intensity PAS staining was observed as a strong red-purple color in the cytoplasm of all hepatocytes (Fig. 51.1a). However, treatment with α-amylase for serial sections digested the glycogen in hepatocytes and clarified that the hepatocytes around central vein s kept much more glycogen in their cytoplasm than those around the portal vein s (Fig. 51.1b). Compared with glycogen of fully fed mice, the intensity of PAS staining after fasting for 6 h became slightly weaker in hepatocytes (Fig. 51.1c). After 12-h fasting, the PAS-staining intensity had clearly decreased around almost all portal tracts (Fig. 51.1d). Most hepatocytes in parts of zone I far from the portal veins were still more PAS positive (arrowheads in Fig. 51.1d).
Glycogen distribution of cryobiopsied liver tissue specimens stained with PAS . (a) Fully PAS-stained hepatocytes are clearly observed in the specimen without α-amylase treatment. (b) Hepatocytes around the central vein (CV) exhibit higher-intensity PAS staining than those around the portal tract (P) after treatment with α-amylase. (c) Slightly weaker PAS staining is detected around some portal tracts after 6-h fasting. (d) In the liver tissues of mice fasted for 12 h, PAS-staining intensity around the portal tracts is markedly decreased, but still observed in parts of zone I (arrowheads). CV central vein, P portal vein . Bars: 100 μm
3 Repeatable Cryobiopsy for Individual Mouse
Cryobiopsy was developed for clinical and repeated collection as substitute for our “in vivo cryotechnique .” To examine that the cryobiopsy was time-dependently repeatable, one piece from an area of liver tissues was obtained by cryobiopsy (Fig. 51.2a-i), and another area was cryobiopsied for 6 h (Fig. 51.2c-ii). Temporary bleeding from the tissue area pinched with the cryoforceps was quickly stopped by covering it with instant glue (Fig. 51.2b). During the continuous experiments, all the mice were warmed on an electrically heated plate to maintain their body temperature. In the liver tissues of an almost fully fed mouse, PAS staining was observed in almost all hepatocytes (Fig. 51.2a), with a slightly lower intensity in some hepatocytes around portal vein s . After 6-h fasting, the PAS-staining intensity of hepatocytes had clearly decreased around the portal tracts (Fig. 51.2b), but other hepatocytes far from the portal veins in zone I were still PAS positive (arrowheads in Fig. 51.2b). Thus, the time-dependent changes in glycogen distribution can be clearly evaluated in a single mouse liver by cryobiopsy.
Schematic representation of a series of cryobiopsy processes to obtain liver tissue specimens from anesthetized mice. A liver specimen is initially pinched off at one area with a pair of cryoforceps (cryobiopsy (i) in (a)), and bleeding is immediately stopped with an instant glue (b). After 6-h fasting, another liver tissue specimen is similarly obtained at a second area with the cryoforceps (cryobiopsy (ii) in (c)). (d) In the specimen of cryobiopsy (i), intense PAS staining is observed in almost all hepatocytes , the exception being those around portal vein s (P). (e) The PAS-staining intensity of the area of cryobiopsy (ii) is found to be decreased around portal tracts. Note the strongly PAS-positive hepatocytes remaining in parts of the middle of zone I (arrowheads). CV central vein , P portal vein. Bars: 100 μm
4 Detection of Different Consumption Under Local Circulation Loss Condition
To examine the effects of blood circulation on glycogen consumption, temporary stopping and reflowing of blood circulation were produced by binding with a circular clip and removing it (Fig. 51.3a). To prevent drying of the liver surface tissues, abdominal skin was quickly sutured, and a cotton sheet-absorbing physiological saline was placed on the mouse abdomen throughout the experiment. Temporary hypoxia of some parts of the clipped liver tissues had already been confirmed by measuring the oxygen saturation of similar liver tissues with confocal Raman cryomicroscopy , as previously reported [17]. After stopping blood circulation for 30 min and reflowing for 1 h, cryobiopsy of the restricted areas was performed on the inside (Fig. 51.3b-I) or outside (Fig. 51.3b-II) of the clipped liver tissues. In the liver tissues inside the clipped areas, the PAS -staining intensity was markedly decreased in certain areas around the portal vein s of zone I and also the central vein s (zone III) (Fig. 51.3c). Even in liver tissues outside the clipped areas (Fig. 51.3c, d), the PAS-staining intensity heterogeneously decreased as a mosaic pattern in all zonal areas. Thus, the PAS staining of liver tissues by cryobiopsy clearly revealed zonal difference s in glycogen storage after reflowing of blood circulation in living mice and probably reflects zonal metabolic functions in vivo.
Schematic representation of the experiment about anoxic blood circulation ; an area of the mouse liver tissue is clipped to stop the blood circulation for 30 min (a). One hour after removing the clip, some liver tissue specimens are obtained inside or outside the clipped area with cryoforceps (cryobiopsy (I) and (II) in (b)). Liver tissues were pinched off from inside ((c) cryobiopsy (I)) or outside ((d) cryobiopsy (II)) clipped areas, as shown in (b). (c) Marked reductions in PAS -staining intensity are detected in zone III and local areas of zone I near the portal tracts inside the clipped areas. Staining is still observed in areas far from the portal tracts in zone I (arrowheads). (d) The PAS-staining intensity is heterogeneously detected in all areas of the liver tissue outside the clipped area. CV central vein , P portal vein . Bars: 100 μm
5 Conclusion
The cryobiopsy presented here is a simple manual technique to capture all biological materials of living mouse liver s in rapidly frozen ice crystal s , the characteristics of which probably reflect their living state s [1, 2, 16]. This does not require resection of a target organ with continuous blood circulation in vivo, results in neither ischemia nor anoxia , and enables us to obtain multiple tissue samples from a single animal and any areas of the target organ at different times. The subsequent freeze substitution in acetone with chemical fixative s is useful to preserve many glycogen particle s in hepatocytes and avoids their removal during the preparation steps. Thus, the cryobiopsy technique combined with freeze-substitution fixation for PAS staining is a reliable tool to visualize changes in glycogen storage in hepatocytes of living animal livers. This text is mainly quoted from Reference [18].
References
Bai Y, Ohno N, Terada N, Saitoh S, Nakazawa T, Nakamura N, Katoh R, Ohno S (2009) Immunolocalization of serum proteins in xenografted mouse model of human tumor cells by various cryotechniques. Histol Histopathol 24:717–728
Ohno N, Terada N, Bai Y, Saitoh S, Nakazawa T, Nakamura N, Naito I, Fujii Y, Katoh R, Ohno S (2008) Application of cryobiopsy to morphological and immunohistochemical analyses of xenografted human lung cancer tissues and functional blood vessels. Cancer 113:1068–1079
Bollen M, Keppens S, Stalmans W (1998) Specific features of glycogen metabolism in the liver. Biochem J 336:19–31
Hammad ES, Striffler JS, Cardell RR Jr (1982) Morphological and biochemical observations on hepatic glycogen metabolism in mice on a controlled feeding schedule. II. Streptozotocin-diabetic mice. Dig Dis Sci 27:692–700
He XS, Ma Y, Wu LW (2005) Dynamical changing patterns of glycogen and enzyme histochemical activities in rat liver graft undergoing warm ischemia injury. World J Gastroenterol 11:2662–2665
Dodero F, Benkoel L, Hardwigsen J, Campan P, Lambert R, Botta-Fridlund D, Le Treut YP, Chamlian A (1999) Effect of ischemia-reperfusion on the heterogeneous lobular distribution pattern of glycogen content and glucose-6-phosphatase activity in human liver allograft. Cell Mol Biol 45:1209–1215
Frederiks WM, Marx F (1990) The effect of ischemia on glycogen phosphorylase activity in rat liver: a quantitative histochemical study. Anal Cell Pathol 2:347–355
Domenicali M, Vendemiale G, Serviddio G, Grattagliano I, Pertosa AM, Nardo B, Principe A, Viola A, Trevisani F, Altomare E, Bernardi M, Caraceni P (2005) Oxidative injury in rat fatty liver exposed to ischemia-reperfusion is modulated by nutritional status. Dig Liver Dis 37:689–697
Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, Roselló-Catafau J (2000) Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol 279:163–171
Sumimoto R, Southard JH, Belzer FO (1993) Livers from fasted rats acquire resistance to warm and cold ischemia injury. Transplantation 55:728–732
Miller K, Halow J, Koretsky AP (1993) Phosphocreatine protects transgenic mouse liver expressing creatine kinase from hypoxia and ischemia. Am J Physiol 265:1544–1551
Bradham CA, Srachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters JJ, Thurman RG, Brenner DA (1997) Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology 25:1128–1135
Bendinelli P, Piccoleti R, Maroni P, Bernelli-Zazzera A (1996) The MAP kinase cascades are activated during post-ischemic liver reperfusion. FEBS Lett 398:193–197
Selzner M, Selzner N, Jochum W, Graf R, Clavien PA (2007) Increased ischemic injury in old mouse liver: an ATP-dependent mechanism. Liver Transpl 13:382–390
Ohno N, Terada N, Ohno S (2006) Histochemical analyses of living mouse liver under different hemodynamic conditions by “in vivo cryotechnique”. Histochem Cell Biol 126:389–398
Fujii Y, Ohno N, Li Z, Terada N, Baba T, Ohno S (2006) Morphological and histochemical analyses of living mouse livers by new ‘cryobiopsy’ technique. J Electron Microsc 55:113–122
Terada N, Ohno N, Saitoh S, Ohno S (2008) Application of “in vivo cryotechnique” to detect erythrocyte oxygen saturation in frozen mouse tissues with confocal Raman cryomicroscopy. J Struct Biol 163:147–154
Saitoh Y, Terada N, Saitoh S, Ohno N, Fujii Y, Ohno S (2010) Histochemical approach of cryobiopsy for glycogen distribution in living mouse livers under fasting and local circulation loss conditions. Histochem Cell Biol 133:229–239
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Saitoh, Y., Terada, N., Ohno, N., Ohno, S. (2016). Histochemical Approach of Cryobiopsy for Glycogen Distribution in Living Mouse Livers Under Fasting and Local Circulation Loss Conditions. In: Ohno, S., Ohno, N., Terada, N. (eds) In Vivo Cryotechnique in Biomedical Research and Application for Bioimaging of Living Animal Organs. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55723-4_51
Download citation
DOI: https://doi.org/10.1007/978-4-431-55723-4_51
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55722-7
Online ISBN: 978-4-431-55723-4
eBook Packages: MedicineMedicine (R0)