Abstract
Osteosarcoma developing in elderly persons has several characteristic features. A predilection for the axial skeleton, particularly the pelvis, has been noted. Secondary osteosarcoma due to preexisting conditions such as irradiated bone, Paget’s disease, fibrous dysplasia, and bone infarct is occasionally observed in the elderly. Because osteosarcoma is relatively rare in the aged persons, diagnostic delay is common, with this exerting a possibly adverse effect on prognosis. Treatment-related factors also may affect the prognosis of elderly patients with osteosarcoma. Difficulty in achieving wide resection is reported because axial bones are affected in a high proportion of elderly osteosarcoma patients. Tolerability and completion rate of chemotherapy are lower in the elderly as compared with younger patients. In this chapter, tumor-related features and treatment-related characteristics are described in elderly patients with osteosarcoma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Osteosarcoma is known to have a double peak of incidence. Most osteosarcomas occur in adolescents during the growth spurt, with a second peak in the seventh and eighth decades [1]. Elderly patients with osteosarcoma have several particular problems including etiology, diagnostic difficulty, unusual site of predilection, advanced stage at diagnosis, and feasibility of multidisciplinary treatment in the same manner as that for younger patients with osteosarcoma.
In most developed world countries, the age of 65 years is accepted as the definition of “elderly” person. The United Nations has accepted that age over 60 years may be usually indicated as old age (http://www.who.int/healthinfo/survey/ageingdefnolder/en/ (Accessed July 26, 2014.)). In the context of osteosarcoma treatment, the peak age of onset is between 10 and 20 years, and most clinical therapeutic trials for patients with osteosarcoma have excluded those over the age of 40 years. For this reason physicians involved in the treatment of osteosarcoma have tended to variously define “elderly” as over 40, over 50, or over 60 years.
In this chapter, barriers to adequate diagnosis and therapeutic approach and the prognosis and prognostic factors of the present status are outlined for elderly patients with osteosarcoma, based on the findings of previous reports. Additionally, perspectives of novel therapeutic possibility are provided for this group.
2 Secondary Osteosarcoma
Osteosarcomas arising in adolescents are commonly de novo neoplasms, although in elderly patients they occasionally occur in the setting of a preexisting condition such as irradiated bone, Paget’s disease, and bone infarct [2–6] (Table 5.1).
In secondary osteosarcoma, previous studies indicated that the prognosis of elderly patients is dependent on the presence of preexisting conditions. Generally, osteosarcomas arising from a preexisting condition have a dismal prognosis, particularly from Paget’s disease of bone [2, 7]. However, little progress has been made in the treatment of Paget’s sarcoma in the last 60 years [8]. The proportion of Paget’s disease in secondary osteosarcoma as a preexisting condition has been differently reported even from Western countries (Table 5.1). Because osteosarcoma arising from Paget’s disease has been reported to be uncommon in Japan [9], two subsequent studies from Japan have reconfirmed the unlikelihood of this disease as an underlying pathological condition in this country. The significance of Paget’s disease of bone as a preexisting condition of osteosarcoma thus differs markedly among countries.
The carcinogenic effect of radiation is one of the crucial concerns in the treatment of patients with primary malignancy. Prior radiation of bone is a well-known risk factor for the development of secondary osteosarcoma [2, 6, 10]. The median latency period between radiotherapy for the primary cancer and the development of postradiation sarcoma ranges from 8 to 16 years. Grimer et al. reported that the time lag between radiotherapy and subsequent development of osteosarcoma ranged from 6 to 23 years [11]. Historically, radiotherapy was less used for patients with cancer in Japan than those in Western countries. However, comparing the past few decades, radiotherapy has recently assumed more prominent roles in the treatment of cancer. Thus it is likely that the number of secondary osteosarcomas, particularly radiation-induced osteosarcoma (Fig. 5.1), will increase in the near future. Grimer et al. reported that analyses of 41 patients with radiation-induced osteosarcoma confirmed that cure can be achieved even in the older (over 40 years) group of patients if treated aggressively with multidisciplinary treatment including surgery and chemotherapy [11], which is consistent with other previous studies [2, 6]. Contrary results have also been reported. Lewis et al. reported that despite multi-agent chemotherapy, postradiation osteosarcoma continues to have a poor prognosis. Possible contributing factors may include the advanced age and worsened performance status of this patient population [12].
70-year-old male. He received 60 Gy of radiotherapy for the treatment of his prostate cancer 7 years before. He developed radiation-induced osteosarcoma in his right pubic bone (white arrows) (a). Axial sections of CT image (b) and MR images of T1 weighted (c) and T2 weighted (d) show extraosseously invading tumor (green arrows)
3 Delay in Diagnosis
Malignancies of bone are commonly metastases from other primary malignancies in elderly patients, possibly resulting in the misdiagnosis and/or delay in diagnosis of osteosarcoma. Okada et al. reported that the duration between development of symptoms and referral to a specialist ranged from 1 to 276 months (mean 18 months). Clinical and radiological misdiagnoses at the initial presentation were observed in 15 (23 %) of the 64 cases. The length of time in the delay of the diagnosis ranged from 3 to 12 months (average, 6 months) [13]. Nishida et al. reported that although the median duration from the initial presentation at any clinic to the definitive diagnosis of osteosarcoma at specialist centers was 2 months, the mean duration from presentation was 5.2 months. There were 23 cases in which it took more than 5 months between presentation and the making of the correct diagnosis. In many cases, the lesion was considered to be a bone metastasis from carcinoma at another site [14]. A possible reason for such a misdiagnosis is that rare periosteal reactions in osteosarcoma in elderly patients (Fig. 5.2) were previously reported [15], occasionally leading to the radiological diagnosis of metastatic disease of bone.
78-year-old female. Primary osteosarcoma developed in her left shaft of femur. X-ray indicated permeative osteolytic lesion without obvious periosteal reaction (white arrows) (a and b). T2-weighted axial image (c) and STIR coronal image (d) demonstrated the extraosseously invading tumorous lesion (green arrows)
The results of these Japanese studies agree with those of another report in which the median time interval from onset of symptoms to diagnosis was 4 months [16]. In the younger population, the median time interval has been reported to be 10 weeks [17].
A major concern is the association between delay in diagnosis and patient prognosis. A previous report indicated that misdiagnosis is associated with a poor prognosis in elderly patients with osteosarcoma. The 10-year survival rate of patients with inadequate diagnoses was much lower than that of patients with adequate diagnoses, although the difference was not statistically significant [13]. In contrast, another study demonstrated that a prolonged latency period before the correct diagnosis was made was not an individual prognostic factor [14].
4 Site of Predilection
The knee region is affected in approximately 62 % of adolescents with osteosarcoma [18]. Several reports have shown a predilection for axial localization in elderly persons with osteosarcoma (Table 5.2), including Longhi’s report in which 4 (9.3 %) of 43 patients were noted to have had a primary tumor in the knee area and 10 (23 %) a tumor in the axial skeleton [16]. Grimer’s study based on 481 cases over 40 years revealed that 21 % of cases had tumor in the axial bones [11]. Other series including primary and secondary osteosarcoma reported higher rates (27–38 %) of axial skeleton as the site of involvement in elderly patients [5, 13, 14, 19]. Secondary osteosarcoma due to conditions such as postradiation sarcoma and Paget’s sarcoma often occurs in the pelvis [2, 7, 8], contributing to a higher tumor incidence in axial bone. However, Manoso et al. and Iwata et al.’s studies focusing on primary osteosarcoma in the elderly [20, 21] also demonstrated the predilection of primary osteosarcoma for axial bones (19 % and 49 %, respectively). Taking these results together, it may be that primary osteosarcoma in elderly patients is also more likely to involve axial bone (Fig. 5.3).
5 Stage at Diagnosis
The Cooperative Osteosarcoma Study Group (COSS) had a uniform treatment protocol for two decades. The registration was not limited to the young patients with localized limb tumors, but included all patients with osteosarcoma. The results of this study [22] reflecting the long-term outcome of a wide spectrum of patients with high-grade osteosarcoma after the introduction of multi-agent chemotherapy may be representative of the whole osteosarcoma cohort, thereby facilitating comparison with those of elderly patients with osteosarcoma. In this study, of 1702 patients, 1491 (87.6 %) presented with localized disease, and 211 (12.4 %) had distant metastases.
Regarding the cohort of elderly patients with primary and secondary osteosarcoma, Longhi et al. reported the results of 43 osteosarcoma patients over 65 years of whom 13 had metastatic disease (30.2 %) and 30 (69.8 %) localized disease [16]. Nishida et al. reported that 12 (13 %) of 91 patients had distant metastases at diagnosis [14]. According to the results of Okada et al. who analyzed patients over 50 years, 11 % had lung metastasis at the initial presentation [13]. In the cohort of elderly patients with only primary osteosarcoma, Grimer et al. described that 48 (18 %) of 270 patients with high-grade primary osteosarcoma over 40 years had distant metastasis at diagnosis [11]. Manoso et al. noted a lower proportion of the cases with distant metastasis at diagnosis (5 %) in their cases over 40 years. Iwata et al. reported that 28 % of patients with primary osteosarcoma over 40 years had distant metastasis at diagnosis [20]. In this way the reported proportion of distant metastasis at diagnosis in the cohort of elderly patients has been reported to range from 5 to 30 %. Stage at diagnosis, particularly with distant metastasis, is slightly higher compared with the all-age cohort (12.4 %) [22]. It is likely that the feasibility of chemotherapy and/or surgical treatment and response to chemotherapy may be more important for elderly patients with osteosarcoma.
6 Treatment Modality
6.1 Surgery
As discussed later, “operability” has been reported as a significant good prognostic factor for elderly patients with osteosarcoma in several studies [14, 16, 20]. However, as was described above, the axial skeleton, in particular the pelvis, is significantly more often affected in elderly patients with osteosarcoma, which makes definitive surgery more difficult to achieve. A previous report, reflecting the whole osteosarcoma cohort based on 1702 cases, indicated that 113 (6.6 %) of 1702 cases retained macroscopic residual tumor at the primary tumor site [22]. Among the 113 patients with macroscopic residual tumor, 84 had not been surgically treated, while 29 had residual tumor after definitive surgery.
In their cohort of elderly patients over 40 years, Carsi et al. reported that 42 (89 %) of 47 cases were operable [19]. According to Grimer’s study of a cohort over 40 years, of 238 cases with high-grade nonmetastatic extremity primary osteosarcoma, 212 (92 %) underwent amputation or limb salvage surgery, while of elderly patients with pelvic osteosarcoma, 37 (51 %) of 72 cases underwent surgery [11]. Iwata et al. investigated elderly patients with primary osteosarcoma over 40 years, of whom 63 (73 %) of 86 received surgical treatment [20]. Nishida et al. noted that 81 (85 %) of 95 cases received surgical treatment in an analysis of patients over 60 years [14]. Of the cohort of aged over 65 years, 74.4 % underwent surgery [16]. Together, the predilection of osteosarcoma in the elderly for axial bone may be the main factor precluding the achievement of complete resection of primary tumors.
Another concern arises in terms of limb salvage surgery when osteosarcoma arises in an extremity. In contrast to an incidence of limb salvage in the younger population of approximately 90 % [23], in Longhi’s study of patients over 65 years it was limited to only 18 patients (56 %) [16]. In Nishida et al.’s investigation of patients over 60 years, 22 (27 %) of the 81 patients underwent amputation and 59 (73 %) limb salvage surgery [14]. As described in Grimer’s study, the proportion having limb salvage was higher in the under 60-year age group (64 %) compared with those over 60 years (49 %) [11], indicating that the rate of limb salvage as a definitive surgery may decrease with increasing age. However, as described later in this chapter, considering that the feasibility of definitive surgery has been a significant prognostic factor in several reports [14, 16, 20] with multivariate analysis, operable cases should be treated aggressively to improve the prognosis.
6.2 Chemotherapy
Chemotherapy has made a major contribution to improving patient prognosis for children, adolescents, and young adults with osteosarcoma [24, 25]. However, such efficacy was not confirmed in a study of relatively elderly patients [5]. In addition, because elderly patients may not tolerate aggressive multi-agent chemotherapy, patients over 40 years who develop osteosarcoma are commonly excluded from clinical trials of treatment for it.
Chemotherapeutic agents used for elderly patients with osteosarcoma have been basically the same as those used for young patients, namely, cisplatin, doxorubicin, ifosfamide, and methotrexate (Table 5.3). Several reports have described the tolerability of chemotherapy for elderly patients with osteosarcoma. Grimer et al. noted that chemotherapy was used in 154 patients, with only 29 % of patients over 60 years receiving it in contrast to 80 % of those under 60 years. No patient over 60 years was recorded to have completed the full planned course of chemotherapy, whereas 73 % of those under 60 years did so. There were four treatment-related deaths associated with the use of chemotherapy [11]. Nishida et al. reported that the mean age of patients administered chemotherapy was 67 ± 4.6 years, while that of those not administered it was 71 ± 6.6 years (P = 0.0007). No deaths in their series were caused by chemotherapy. Twelve of 51 cases with chemotherapy could tolerate similar doses and regimens to those used for young patients [14]. Manoso et al. reported that among 40 patients who received chemotherapy, six were unable to complete the full course of chemotherapy because of toxicity. An additional seven patients required a reduction in dosage during treatment. No deaths were caused by chemotherapy [21]. Longhi et al. reported that in patients over 65 years, none received methotrexate due to its high nephrotoxicity. All patients received ifosfamide and an anthracycline. All drugs were delivered at doses reduced by 20 % as compared with standard protocols and for a reduced number of cycles. Twenty-nine (67 %) of 43 patients did not receive chemotherapy, of whom 10 were excluded because of impaired cardiac or renal function. The physician decided not to conduct chemotherapy due to the patient’s age in 15 cases [16].
A most important point of interest to physicians is whether chemotherapy can improve the prognosis of elderly patients with osteosarcoma. Grimer et al., in a review of 481 patients older than age 40 years in the EMSOS study, found that chemotherapy significantly improved survival. Patients treated with chemotherapy had a 5-year OS of 51 % compared with 39 % in those patients undergoing surgery alone [11]. Manoso et al. reported the results of the Memorial Sloan Kettering experience in which multimodality treatment with surgery and chemotherapy statistically improved survival (P = 0.02) compared with surgery alone in patients with high-grade osteosarcoma. The chemotherapy group had a median survival of 113 months versus the surgery-only group with a median survival of 5 months. The subset analysis of patients older than 60 years with high-grade disease showed that the chemotherapy significantly increased the overall survival (P < 0.0008). The median survival with chemotherapy was 68 months as compared to 5 months without chemotherapy. However, the two groups had only seven patients each [21]. Bacci et al. reported the results of the Rizzoli experience in which the 5-year overall survival rate was 70 % in patients aged 41–60 years with chemotherapy, which is similar to that of 296 patients under 40 years treated with a more aggressive protocol of chemotherapy (5-year overall survival, 71 %) [26].
In contrast to these beneficial effects of chemotherapy, Okada et al. reported that in a group of 22 patients receiving preoperative chemotherapy, 82 % of cases showed a poor response rate. They were unable to show any beneficial impact of chemotherapy on prognosis [13]. Carsi et al. showed that 35.7 % of their patients received chemotherapy, but found no difference in survival [19]. Lewis et al. reported that current chemotherapy regimens fail to impact survival in postradiation osteosarcoma [12]. Nishida et al. reported that chemotherapy had no impact on patient survival in those aged over 60 years [14]. Iwata et al. reported that in patients without metastasis at diagnosis who subsequently underwent definitive surgery, chemotherapy had no significant impact on survival (with chemotherapy, 63.4 %; without chemotherapy, 65.2 %), despite the absence of any significant differences in the tumor site, stage, or surgical margin between the groups [20].
No definitive conclusion was drawn from previous reports due to the heterogeneity of the cohort studies, chemotherapeutic regimens, and their indications. Prospective, multi-institutional, well-directed clinical trials will be needed to help clarify the role of chemotherapy for elderly patients with osteosarcoma.
7 Prognosis and Prognostic Factors
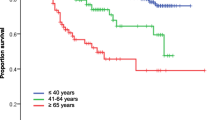
The prognosis of elderly patients with osteosarcoma has been shown to be worse than that of younger patients. Of the cohort of elderly patients with both primary and secondary osteosarcoma, Huvos et al. indicated a 5-year overall survival of only 8 % in the whole cohort, being however, 37 % in primary osteosarcoma and 7.5 % in secondary osteosarcoma [5]. Longhi et al. showed that in patients over 65 years, overall survival was 22 % at 5 years and 45 % when patients with distant metastasis at diagnosis were excluded [16]. Okada et al. revealed the overall survival at 5 years to be 55.5 % in patients over 50 years. Nishida et al. reported an overall survival rate of 46.7 % at 5 years in patients over 60 years including those with low-grade malignancy. Patients with high-grade malignancy showed 42.8 % survival at 5 years and patients with extremity location 51.9 % [14].
Regarding a cohort limited to primary osteosarcoma, Grimer et al. reported an overall survival rate of 46 % at 5 years in high-grade and conventional osteosarcoma without metastasis at diagnosis [11]. Carsi et al. showed the overall survival at 5 years to be 42 % in their cohort over 40 years (distant metastasis at diagnosis: 17 %) [19]. Iwata et al. described the 5-year overall survival to be 38.8 % in their cohort aged over 40 years. Prognosis was poor compared with results from other studies, but their cohort included 24 patients (28 %) with AJCC stage IV [20]. Manoso analyzed 58 patients with primary osteosarcoma over 40 years including 3 with initial distant metastasis and revealed that overall survival was 58 % at 5 years and 44 % at 10 years. Focusing on high-grade tumors, overall survival was 53 % at 5 years and 41 % at 10 years [21]. Bacci et al. reported that patients with nonmetastatic extremity osteosarcoma aged 41–60 years showed a 5-year overall survival of 70 % [26].
The results of whole-aged cohort including distant metastasis at diagnosis showed overall survival rates at 5 years and 10 years to be 65.3 % and 59.8 %, respectively [22]. Compared to this survival rate, outcome in the elderly patients including initial distant metastasis was lower (8–58 %) at 5 years as shown above.
Prognostic factors in the elderly patients with osteosarcoma have been variously reported (Table 5.4). In general, inoperable cases, distant metastasis at diagnosis, large size, and older age were reported as poor prognostic factors. Of interest, site of upper limb (or proximal humerus) was shown to be a poor prognostic factor in two studies [11, 27].
8 Perspective
Osteosarcoma in the elderly had some characteristic features including unusual site of predilection, high proportion of secondary osteosarcomas as compared to adolescents, delay in diagnosis, higher proportion of amputation in extremity location, and poor tolerability of chemotherapy. Of the tumor-related variables, none can be modified, whereas diagnosis and treatment-related factors may be improved and/or newly innovated in the near future.
Raising awareness regarding the occurrence of osteosarcoma in the elderly population is important for its early diagnosis, particularly in developed countries in which society is experiencing unprecedented aging. Prospective, well-organized, multi-institutional studies should be planned for elderly osteosarcoma patients and should help to clarify the indications of chemotherapy in greater detail. Targeted therapy will be introduced into the area of osteosarcoma in the future. Although osteosarcoma is a group of heterogeneous tumors, pazopanib is now being used for patients with soft tissue sarcomas, which are also composed of heterogeneous tumors.
A novel therapeutic tool is particle radiotherapy. The efficacy of heavy ion radiotherapy has already been reported in patients with primary malignant bone tumors including osteosarcoma [28, 29]. Given that osteosarcoma in the elderly often arises in the axial skeleton where wide resection is difficult to achieve and surgical treatment-related morbidity is a concern, charged particle radiation therapy including carbon ion radiotherapy and proton therapy are promising tools for elderly patients with osteosarcoma (Fig. 5.4). Accumulated data will be needed to more precisely elucidate their fascinating details and clarify issues such as efficacy and indications.
83-year-old female. Primary osteosarcoma occurred in her right ilium adjacent to sacrum (white arrows) (a). She received carbon ion radiotherapy of 70.4 GyE. Six years after carbon ion radiotherapy, axial section of CT image indicates postradiation fractures (green arrows) (b). Coronal section of CT shows the osteogenic response in the irradiated field (orange arrows) (c). She could walk with T-cane at the latest follow-up
References
Dorfman HD, Czerniak B. Osteosarcoma. In: Dorfman HD, Czemiak B, editors. Bone tumors. St. Louis: Mosby; 1998. p. 128–252.
Bielack SS, et al. Combined modality treatment for osteosarcoma occurring as a second malignant disease. Cooperative German-Austrian-Swiss Osteosarcoma Study Group. J Clin Oncol. 1999;17(4):1164.
Dahlin DC, Coventry MB. Osteogenic sarcoma. A study of six hundred cases. J Bone Joint Surg Am. 1967;49(1):101–10.
Duffaud F, et al. Osteosarcomas of flat bones in adolescents and adults. Cancer. 2000;88(2):324–32.
Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer. 1986;57(7):1442–9.
Huvos AG, et al. Postradiation osteogenic sarcoma of bone and soft tissues. A clinicopathologic study of 66 patients. Cancer. 1985;55(6):1244–55.
Shaylor PJ, et al. Paget’s osteosarcoma – no cure in sight. Sarcoma. 1999;3(3–4):191–2.
Mankin HJ, Hornicek FJ. Paget’s sarcoma: a historical and outcome review. Clin Orthop Relat Res. 2005;438:97–102.
Naka T, et al. Osteosarcoma versus malignant fibrous histiocytoma of bone in patients older than 40 years. A clinicopathologic and immunohistochemical analysis with special reference to malignant fibrous histiocytoma-like osteosarcoma. Cancer. 1995;76(6):972–84.
Mindell ER, Shah NK, Webster JH. Postradiation sarcoma of bone and soft tissues. Orthop Clin North Am. 1977;8(4):821–34.
Grimer RJ, et al. Osteosarcoma over the age of forty. Eur J Cancer. 2003;39(2):157–63.
Lewis VO, et al. Outcome of postradiation osteosarcoma does not correlate with chemotherapy response. Clin Orthop Relat Res. 2006;450:60–6.
Okada K, et al. Second primary osteosarcoma with rosette-like structure in a patient with retinoblastoma. Virchows Arch. 2004;445(4):421–4.
Nishida Y, et al. Osteosarcoma in the elderly over 60 years: a multicenter study by the Japanese Musculoskeletal Oncology Group. J Surg Oncol. 2009;100(1):48–54.
de Santos LA, et al. Osteogenic sarcoma after the age of 50: a radiographic evaluation. AJR Am J Roentgenol. 1978;131(3):481–4.
Longhi A, et al. Osteosarcoma in patients older than 65 years. J Clin Oncol. 2008;26(33):5368–73.
Bacci G, et al. Delay in diagnosis of high-grade osteosarcoma of the extremities. Has it any effect on the stage of disease? Tumori. 2000;86(3):204–6.
Campanacci M. Bone tumors. Philadelphia: Lippincott-Verlag; 1999. p. 1418–68.
Carsi B, Rock MG. Primary osteosarcoma in adults older than 40 years. Clin Orthop Relat Res. 2002;397:53–61.
Iwata S, et al. Prognostic factors in elderly osteosarcoma patients: a multi-institutional retrospective study of 86 cases. Ann Surg Oncol. 2014;21(1):263–8.
Manoso MW, et al. De novo osteogenic sarcoma in patients older than forty: benefit of multimodality therapy. Clin Orthop Relat Res. 2005;438:110–5.
Bielack SS, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–90.
Ferrari S, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23(34):8845–52.
Meyers PA, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5–15.
Rosen G, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49(6):1221–30.
Bacci G, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities in patients aged 41–60 years: outcome in 34 cases treated with adriamycin, cisplatinum and ifosfamide between 1984 and 1999. Acta Orthop. 2007;78(3):377–84.
Song WS, et al. Prognosis of extremity osteosarcoma in patients aged 40–60 years: a cohort/case controlled study at a single institute. Eur J Surg Oncol. 2010;36(5):483–8.
Imai R, et al. Cervical spine osteosarcoma treated with carbon-ion radiotherapy. Lancet Oncol. 2006;7(12):1034–5.
Kamada T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20(22):4466–71.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Nishida, Y. (2016). Osteosarcoma in the Elderly: Clinical Features and Outcome. In: Ueda, T., Kawai, A. (eds) Osteosarcoma. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55696-1_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55696-1_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55695-4
Online ISBN: 978-4-431-55696-1
eBook Packages: MedicineMedicine (R0)