Abstract
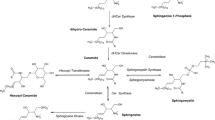
To investigate the biological functions and roles of sphingolipids, sensitive and compound-specific methods are required to measure their levels in biological samples. Liquid chromatography–mass spectrometry (LC-MS) using electrospray ionization (ESI) is suitable for the reliable simultaneous analysis of multiple compounds. In addition, the selected reaction monitoring (SRM) mode of tandem mass spectrometry (MS/MS) is effective to quantify with high sensitivity and selectivity. Therefore, LC-MS/MS came to be utilized for simultaneous analysis of the sphingolipids in vivo. Useful methods for the sphingolipids and related features are also summarized. The following protocol demonstrates information on determination of sphingolipids, especially sphingosine and sphingosine-1-phosphate, by LC-ESI-MS/MS in biological samples such as cell lysates, plasma, serum, or urine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sphingolipids
- Sphingosine
- Sphingosine-1-phosphate
- Dihydrosphingosine
- Phytosphingosine
- Dihydrosphingosine-1-phosphate
- Phytosphingosine-1-phosphate
- LC-MS/MS
1 Reagents
-
Sphingolipids (Avanti Polar Lipids)
-
C18 sphingosine (Sph)
-
C18 dihydrosphingosine (dhSph)
-
C18 phytosphingosine (pSph)
-
C18 sphingosine-1-phosphate (S1P)
-
C18 dihydrosphingosine-1-phosphate (dhS1P)
-
C18 phytosphingosine-1-phosphate (pS1P)
-
C17 sphingosine (C17-Sph)
-
C17 sphingosine-1-phosphate (C17-S1P)
-
Methanol for LC/MS
-
Chloroform for HPLC
-
Acetonitrile for LC/MS
-
Formic acid for LC/MS
-
Ammonium formate 1 mol/l solution for HPLC
-
Polytyrosine-1,3,6 calibration solution (Thermo Fisher Scientific)
-
Water (ultrapure grade)
2 Equipments
-
High performance liquid chromatography system
-
ESI-triple quadrupole mass spectrometer with analytical software
-
Polyether ether ketone (PEEK) tubing
-
HPLC column (ODS):
-
Example: CAPCELL PAK C18 ACR (3 μm, 1.0 mm i.d. × 150 mm, Shiseido) for analytical column and CAPCELL PAK ACR (3 μm, 1.0 mm i.d. × 35 mm, Shiseido) for trap column
-
Syringe (500 μl, Hamilton Company)
-
Centrifuge
-
Siliconized sample tube (2.0 ml, WATOSON)
-
YMC Duo Filter (0.2 μm, pore size, 4 mm inner diameter, YMC)
-
Micro tube mixer
-
Ultrasonic bath
-
Ultrasonic homogenizer
3 Prepare Stock Solutions and Working Solutions of Sphingolipids
Siliconized sample tubes and pipette tips are used. For quantitative analysis, stable isotope analogue internal standards are added to biological samples. The ratio between the internal standard and the compound of interest can be measured by LC/MS. However, many compounds in which acyl groups differ exist in sphingolipids. The separation and selective detection by MS will be difficult if the stable isotope-labeled compound overlaps the same m/z as the target compounds. Therefore, C17 (C17-Sph and C17-S1P) are used for sphingolipid analysis as the internal standard.
-
1.
Dissolve each powder of S1P, pS1P, C17-S1P, Sph, dhSph, and pSph in an appropriate volume of solution to prepare a stock solution; that is, Sph, C17-Sph, dhSph, and pSph are dissolved in methanol (1 mg/ml); S1P, C17-S1P, and pS1P are dissolved in methanol (0.04 mg/ml); dhS1P is dissolved in chloroform:methanol:H2O = 1.5:1.4:0.2 (0.04 mg/ml). Store the solutions at −80°C.
-
2.
Dilute the stock solutions with methanol to a final concentration of 10.0 μmol/l (solution AS1P, pS1P, dhS1P, Sph, dhSph, pSph), and then use it (them) for the optimization of a mass spectrometer.
-
3.
Prepare seven working solutions (1000, 200, 20.0, 10.0, 2.0, 1.0, and 0.2 nmol/l) using each stock solution (AS1P, pS1P, dhS1P, Sph, dhSph, pSph) as described in Table 26.1. Store at −80 °C.
Table 26.1 Preparation of seven working solutions -
4.
Prepare IS mixture 1 (each 1.0 μmol/l) and IS mixture 2 (each 200 nmol/l) of the internal standard containing C17-S1P and C17-Sph. Store at −80 °C.
-
5.
For each calibration curve, mix equal amounts of working solutions and IS mixture 2 (final concentration of sphingolipids are 500, 100, 10, 5.0, 1.0, 0.5, 0.1 nmol/l, and C17-S1P and C17-Sph are 100 nmol/l) to determine the concentration–response relationship.
-
6.
Prepare quality control (QC) samples by spiking blank plasma or biological samples with stock solution. QC samples should be prepared at four levels: high-level QC (HQC), middle-level QC (MQC), low-level QC (LQC), and lower low-level QC (LLQC).
-
7.
Prepare a deproteinization solution, 0.1 % formic acid in methanol.
4 Protocol for Sample Preparation from Plasma
Before analysis, all frozen samples (−80 °C) should be thawed and allow to equilibrate at 4 °C (or on ice). Do not leave the blood samples on ice before preparing plasma because red blood cells are a source of the plasma S1P. Thus, it is necessary to be prepared for plasma fraction from whole blood as soon as possible. Sample preparation for MS is recommended to utilize deproteinization of an organic solvent such as methanol (Fig. 26.1) as follows:
-
1.
Transfer 10 μl plasma into a siliconized sample tube.
-
2.
Add 80 μl deproteinization solution (0.1 % formic acid in methanol).
-
3.
Add 10 μl IS mixture 1 (final concentration of IS is 100 nmol/l).
Tthe additional volume of IS mixture should be changed to match the standard calibration curve if you need concentration steps for sample preparation.
-
4.
Close the tube and vortex for 30 s.
-
5.
Then, homogenize for 5 min in an ultrasonic bath on a tube floater.
-
6.
Centrifuge at 16,400 g for 10 min at 4 °C and separate supernatant.
-
7.
The supernatant (approximately 100 μl) should be passed through a filter (0.2 μm pore size, 4 mm inner diameter).
-
8.
Inject 10 μl filtered solution into the chromatographic system using an automatic injector. That is, each internal standard is 1 pmol/on column.
5 Protocol for Sample Preparation from Tissue
The sample preparation for tissue is also shown in Fig. 26.1 as follows:
-
1.
Tissue (approximately 50 mg) is placed into a siliconized sample tube (2.0 ml).
-
2.
Add 400 μl deproteinization solution.
-
3.
Add 50 μl IS mixture 1 (final concentration of IS is 100 nmol/l).
The additional volume of IS mixture should be changed to match the standard calibration curve if you need concentration steps for sample preparation.
-
4.
Close the tube and vortex for 30 s.
-
5.
Then, homogenize for 30 s by an ultrasonic cell disruptor.
-
6.
Centrifuge at 16,400 g for 10 min at 4 °C.
-
7.
Transfer 100 μl supernatant to another siliconized tube (2.0 ml).
-
8.
Pass the supernatant through a filter (0.2 μm pore size, 4 mm inner diameter, YMC).
-
9.
Inject 10 μl filtered solution into the chromatographic system using an automatic injector.
6 Optimization of H-ESI-MS/MS by Infusion of Sphingolipids
A triple quadrupole mass spectrometer equipped with an ESI source is used for sphingolipids analysis such as TSQ Quantum Ultra with heated electrospray ionization (H-ESI) probe (Thermo Fisher Scientific). Because carryover of samples based on interactions with metal components and the phosphate group of lipids is a concern, PEEK tubing and connector are useful and are recommended. To optimize the sensitivity of sphingolipids, several parameters such as spray voltage, sheath gas pressure, auxiliary gas flow rate, capillary temperature, tube lens offset voltage, and vaporizer temperature need to be optimized.
-
1.
Infuse a polytyrosine-1,3,6 calibration solution directly into the ESI source with a syringe pump to tune and calibrate.
-
2.
Save the tune method and calibration files.
-
3.
Fill a clean syringe with 10 μg/ml sphingolipid solution and place in the syringe holder of the syringe pump of MS.
-
4.
Set up the MS detector to tune in H-ESI/MS mode and start infusion. The MS/MS transitions will be detected in the full scan mode (m/z 50–500). Optimal conditions of tube lens offset and collision energy for SRM analysis employ the transition of the [M + H]+ precursor ions to their product ions of S1P, dhS1P, pS1P, Sph, dhSph, and pSph. The parameters of ionization, especially vaporizer temperature, sheath gas pressure, and auxiliary gas pressure, should be retuned after LC condition is fixed because they depend on the flow rate of LC. Example of defined parameters of ionization and MS/MS transitions are described in Table 26.2.
Table 26.2 Parameters of ionization and MS/MS transition for SRM
7 Separation Condition of Liquid Chromatography (LC)-MS/MS
The basic LC instrumentation for MS consists of degassing equipment, pumps, an automatic injector, columns, and a column oven with PEEK tubing and connector. To check carryover, make a blank injection during the course of multiple runs as needed. For low carryover, washing and cleaning of the needle attached to an automatic injector may be effective with more than one washing/cleaning solvent. An online solid-phase extraction may be valid for ultramicroanalysis. Our LC system for sphingolipids analysis is described in Fig. 26.2. The sample is injected by an autosampler and located to a trap column by eluent C for 7 min at position A (1–6). After changing from position A to B of a six-port valve, trapped compounds are eluted from the trap column and loaded to the analytical column by eluent A and B for 10 min at position B (1–2).
Isocratic elution or gradient elution may be chosen. The column selection (phase material, inner diameter, length, etc.) and optimization of the mobile phase are very important for stable analysis. On sphingolipid analysis using reverse-phase chromatography, pH 4.0 of the mobile phase is especially important (Table 26.3).
Generally, an ODS column is selected for sphingolipids analysis. However, a peak-tailing problem may be detected because of an interaction between a phosphate group of sphingolipids and the residual silanol group (imperfect hydration state) or a metal ion inside a ODS column. Therefore, column that has as reduced a residual silanol group as possible to improve the peak-tailing problem should be selected for sphingolipids analysis. To conduct good analysis, the improvement of peak shape should be aimed at evaluating a number of theoretical stages and a symmetry factor carefully, and this leads to the rise of sensitivity.
8 Preparation of the Mobile Phase
-
1.
Mobile phase A [5 mM HCOONH4 in H2O, pH 4.0 (HCOOH)] is prepared by mixing 99.5 ml water and 0.5 ml 1 mol/l ammonium formate solution, and then adjusting to pH 4.0 with formic acid (~9.6 μl).
-
2.
Mobile phase B [5 mM HCOONH4 in H2O/CH3CN = 5/95, pH 4.0 (HCOOH)] is prepared by mixing 95 ml acetonitrile, 4.5 ml water, and 0.5 ml 1 mol/l ammonium formate solution, and then adjusting to apparent pH 4.0 with formic acid (~1160 μl).
9 Condition of Ionization by LC-MS/MS
The parameters of ionization using LC change depending on the flow rate, especially vaporizer temperature, sheath gas pressure, and auxiliary gas pressure. Our optimal parameters for Nanospace SI 2-Quantum Ultra system are described in Table 26.4. SRM chromatograms are shown in Fig. 26.3.
10 Preparation of Calibration Curve and Determination of Sphingolipids by LC-MS/MS
-
1.
All peaks were integrated automatically by Xcalibur software (Thermo Fisher Scientific).
-
2.
The unknown sphingolipid amount is estimated from the calibration curves by the ratios of their peak areas to that of IS, which regression coefficients are given with appropriate software. For example, a calibration curve fitting a straight line with nonlinear regression for S1P and Sph is shown in Fig. 26.4a, b, which was drawn using GraphPad Prism (GraphPad Software, Inc.) from the experimental data with 1/x weighting method, i.e., y = mx + c, where y is area ratio, x is analyte level, m is the gradient of the line, and c is its intercept with the y-axis.
-
3.
Estimate the concentration of unknown sample using the line equation.
For the analysis of sphingolipids by MS in various biological samples, ionization of matrix effects are taken into account. A matrix effect is the phenomenon that increases or decreases in the sensitivity of the target compound in a tested sample. To perform an exact determination quantity, a matrix effect has to be reduced as much as possible. Therefore, the validation test needs to be carried out using a practical biological sample, that is, plasma, liver, or brain, for developing the determination method, and evaluated accuracy and precision of reproducibility from intraday and interday assay. The analytical method validation is referenced from generally published guidance, i.e., Bioanalytical Method Validation; Food and Drug Administration.
This section describes a protocol for analysis of sphingolipids by LC-MS/MS in a biological sample. However, the present method was specific for 1-acyl sphingolipids, and there are other sphingo base compounds and many species of diacyl sphingolipids of glycosphingolipids, sphingomyerine, ceramide, and their phosphates. We recommend replacing the analytical column with a C8-based column, or using ultrahigh performance liquid chromatography, because the simultaneous analysis for these compounds takes 1–2 h using an ODS (C18-based) column [1]. The useful methods for sphingolipids and related features are summarized in Table 26.5. Although the performance of sphingolipids profiling depends on the improvement of equipment, this basic protocol is utilized for analysis in future studies.
References
Kasumov T, Huang H, Chung YM, Zhang R, McCullough AJ, Kirwan JP (2010) Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem 401:154
Wewer V, Brands M, Dormann P (2014) Fatty acid synthesis and lipid metabolism in the obligate biotrophic fungus Rhizophagus irregularis during mycorrhization of Lotus japonicus. Plant J 79:398
Liebisch G, Drobnik W, Reil M, Trumbach B, Arnecke R, Olgemoller B, Roscher A, Schmitz G (1999) Quantitative measurement of different ceramide species from crude cellular extracts by electrospray ionization tandem mass spectrometry (ESI-MS/MS). J Lipid Res 40:1539
Han X (2002) Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem 302:199
Gaskell SJ, Edmonds CG, Brooks CJ (1976) Applications of boronate derivatives in the study of ceramides by gas-liquid chromatography-mass spectrometry. J Chromatogr 126:591
Camera E, Picardo M, Presutti C, Catarcini P, Fanali S (2004) Separation and characterisation of sphingoceramides by high-performance liquid chromatography-electrospray ionisation mass spectrometry. J Sep Sci 27:971
Couch LH, Churchwell MI, Doerge DR, Tolleson WH, Howard PC (1997) Identification of ceramides in human cells using liquid chromatography with detection by atmospheric pressure chemical ionization-mass spectrometry. Rapid Commun Mass Spectrom 11:504
Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A (2010) Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J Lipid Res 51:3074
Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A (2009) Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol 579:443
Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH Jr (2009) Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res 50:1692
Scherer M, Leuthäuser-Jaschinski K, Ecker J, Schmitz G, Liebisch G (2010) A rapid and quantitative LC-MS/MS method to profile sphingolipids. J Lipid Res 51:2001
Scherer M, Bottcher A, Schmitz G, Liebisch G (2011) Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Biochim Biophys Acta 1811:68
Merritt MV, Sheeley DM, Reinhold VN (1991) Characterization of glycosphingolipids by supercritical fluid chromatography-mass spectrometry. Anal Biochem 193:24
Norberg P, Månsson JE, Liljenberg C (1991) Characterization of glucosylceramide from plasma membranes of plant root cells. Biochim Biophys Acta 1066:257
Legnini E, Orsini JJ, Mühl A, Johnson B, Dajnoki A, Bodamer OA (2012) Analysis of acid sphingomyelinase activity in dried blood spots using tandem mass spectrometry. Ann Lab Med 32:319
Seefelder W, Schwerdt G, Freudinger R, Gekle M, Humpf HU (2002) Liquid chromatography/electrospray ionisation-mass spectrometry method for the quantification of sphingosine and sphinganine in cell cultures exposed to fumonisins. J Chromatogr B Anal Technol Biomed Life Sci 780:137
Blaas N, Schüürmann C, Bartke N, Stahl B, Humpf HU (2011) Structural profiling and quantification of sphingomyelin in human breast milk by HPLC-MS/MS. J Agric Food Chem 59:6018
Fischbeck A, Krüger M, Blaas N, Humpf HU (2009) Analysis of sphingomyelin in meat based on hydrophilic interaction liquid chromatography coupled to electrospray ionization-tandem mass spectrometry (HILIC-HPLC-ESI-MS/MS). J Agric Food Chem 57:9469
Van Veldhoven PP, De Ceuster P, Rozenberg R, Mannaerts GP, de Hoffmann E (1994) On the presence of phosphorylated sphingoid bases in rat tissues. A mass-spectrometric approach. FEBS Lett 350:91
Mano N, Oda Y, Yamada K, Asakawa N, Katayama K (1997) Simultaneous quantitative determination method for sphingolipid metabolites by liquid chromatography/ionspray ionization tandem mass spectrometry. Anal Biochem 244:291
Lieser B, Liebisch G, Drobnik W, Schmitz G (2003) Quantification of sphingosine and sphinganine from crude lipid extracts by HPLC electrospray ionization tandem mass spectrometry. J Lipid Res 44:2209
Lan T, Bi H, Liu W, Xie X, Xu S, Huang H (2011) Simultaneous determination of sphingosine and sphingosine 1-phosphate in biological samples by liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 879:520
Murph M, Tanaka T, Pang J, Felix E, Liu S, Trost R, Godwin AK, Newman R, Mills G (2007) Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: potential biomarkers for cancer diagnosis. Methods Enzymol 433:1
Schmidt H, Schmidt R, Geisslinger G (2006) LC-MS/MS-analysis of sphingosine-1-phosphate and related compounds in plasma samples. Prostaglandins Other Lipid Mediat 81:162
Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC (2005) Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem 339:129
Berdyshev EV, Gorshkova IA, Usatyuk P, Zhao Y, Saatian B, Hubbard W, Natarajan V (2006) De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal 18:1779
Jiang X, Han X (2006) Characterization and direct quantitation of sphingoid base-1-phosphates from lipid extracts: a shotgun lipidomics approach. J Lipid Res 47:1865
Cutignano A, Chiuminatto U, Petruzziello F, Vella FM, Fontana A (2010) UPLC–MS/MS method for analysis of sphingosine 1-phosphate in biological samples. Prostaglandins Other Lipid Mediat 93:25
Sun D, Froman BE, Orth RG, MacIsaac SA, Larosa T, Dong F, Valentin HE (2009) Identification of plant sphingolipid desaturases using chromatography and mass spectrometry. J Chromatogr Sci 47:895
Jin YX, Cui XH, Paek KY, Yim YH (2012) A strategy for enrichment of the bioactive sphingoid base-1-phosphates produced by Hypericum perforatum L. in a balloon type airlift reactor. Bioresour Technol 123:284
Saigusa D, Shiba K, Inoue A, Hama K, Okutani M, Iida N, Saito M, Suzuki K, Kaneko T, Suzuki N, Yamaguchi H, Mano N, Goto J, Hishinuma T, Aoki J, Tomioka Y (2012) Simultaneous quantitation of sphingoid bases and their phosphates in biological samples by liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Bioanal Chem 403:1897
Zhao YY, Liu J, Cheng XL, Bai X, Lin RC (2012) Urinary metabonomics study on biochemical changes in an experimental model of chronic renal failure by adenine based on UPLC Q-TOF/MS. Clin Chim Acta 413:642
Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51:3299
Lydic TA, Busik JV, Reid GE (2014) A monophasic extraction strategy for the simultaneous lipidome analysis of polar and nonpolar retina lipids. J Lipid Res 55:1797
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Takahashi, T. et al. (2015). Determination of Sphingolipids by LC-MS/MS. In: Yokomizo, T., Murakami, M. (eds) Bioactive Lipid Mediators. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55669-5_26
Download citation
DOI: https://doi.org/10.1007/978-4-431-55669-5_26
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55668-8
Online ISBN: 978-4-431-55669-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)