Abstract
Recent progresses on materials and device structures for solution-processed organic light-emitting devices (OLEDs) are discussed. Several solution-processable materials such as fluorescent oligomer, phosphorescent dendrimer, lithium complex, zinc oxide, and polyvinylpyridine are designed and synthesized for achieving multilayer structure. The successful fabrication of solution-processed white phosphorescent OLEDs and tandem OLEDs will pave the way toward printable, low-cost, and large-area solid-state lighting application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Solution processes such as spin coating, inkjet printing, slot-die coating, or spray coating for organic light-emitting devices (OLEDs) are fascinating due to their potential advantages for a production of large-area devices at low cost, although vacuum evaporation (dry) processes are much ahead of the solution processes from the mass production point of view. One of the key solutions to improve the performance of the devices is stacking of a number of successive layers of different functional materials. This multilayer structure allows for the separation of the charge-injecting, charge-transporting, and light-emitting functions to different layers, which leads to a dramatic increase in efficiency and lifetime. In the second section, we discuss our recent studies on fluorescent oligomers [1], phosphorescent dendrimers [2, 3], electron injection materials [4], and polymer binders [5] for solution-processed OLEDs. In the third section, we focus on solution-processed multilayer phosphorescent OLEDs using small molecules. On the basis of estimates from a solvent resistance test of small host molecules, we demonstrate that covalent dimerization or trimerization instead of polymer material can afford conventional small host molecules sufficient resistance to alcohols used for processing upper layers. This allows us to construct multilayer OLEDs through subsequent solution-processing steps, achieving record-high power efficiencies of 34 lm W−1 at 100 cd m−2 for solution-processed white phosphorescent OLEDs [6]. In the fourth section, we discuss the fabrication of a tandem OLED comprising two light-emitting units (LEUs) and a charge generation layer (CGL) between the indium tin oxide (ITO) anode and aluminum (Al) cathode using solution-based processes to simultaneously improve the luminance and device stability. A hybrid process of spin coating and thermal evaporation was utilized for the fabrication [7]. Each LEU with the configuration of first LEUs was fabricated using the spin coating method. Ultrathin (1 nm) Al is deposited as the electron injection layer (EIL) in the first unit, and molybdenum oxide is subsequently deposited as the CGL by thermal evaporation. Tandem OLEDs using hybrid processes showed almost twice the current efficiency of each light-emitting unit (LEU). Additionally, fully solution-processed tandem OLEDs consisting of two LEUs and a CGL between the anode and cathode is fabricated. A zinc oxide (ZnO) and polyethyleneimine-ethoxylated (PEIE) nanoparticle bilayer is used as the EIL in the first LEU and phosphomolybdic acid hydrate (PMA) as the electron acceptor of the CGL. Appropriate choice of solvents during spin coating of each layer ensures that a nine-layered structure is readily fabricated using only solution-based processes [8]. The determined driving voltage and efficiency of the fabricated tandem OLED are the sums of values of the individual LEUs.
8.2 Solution-Processable Materials
8.2.1 Fluorescent Oligomer
The π-conjugated polymers have been extensively studied as solution-processable emitting materials for the field of OLEDs since 1990 [9]. Precise control of molecular weight, end-group structure, and regioregular structure of the conjugated polymers for OLED has been established, but it is not possible to purify structural defects in a polymer chain itself thoroughly. However, monodisperse conjugated oligomers are able to have no structural defects and a better purity from conventional purification methods such as column chromatography, recrystallization, and sublimation. Four novel fluorescent dyes, bis(difluorenyl)amino-substituted carbazole 1, pyrene 2, perylene 3, and benzothiadiazole 4 as solution-processable light-emitting oligomer (Fig. 8.1a), are synthesized by palladium-catalyzed cross-coupling reaction. They are soluble in common organic solvents and can show a high glass transition temperature (T g) and a good film-forming ability. The energy levels are related to the electronic properties of the central core; the electron-donating carbazole compound showed the lowest ionization potential, and the electron-withdrawing benzothiadiazole compound showed the largest electron affinity. Emitting color can be easily controlled by a kind of central dyes, and outer fluorene oligomers can sterically prevent excimer formation between the emitting cores in a neat film. All compounds were purified from column chromatography and then thoroughly purified with a train sublimation for OLED application. These sublimable properties are one of the advantages compared with the π-conjugated polymers from the purity point of view, because it is difficult to separate low molecular impurities having similar polarity to the target compounds by column chromatography. In practice, such impurities are regarded as detrimental for device stability. OLEDs with the configuration as ITO/ poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT: PSS) (40 nm)/1-4 (50 nm)/ bis(2-methyl-8-quinolinolato) (biphenyl-4-olato)aluminum (BAlq) (50 nm)/LiF (0.5 nm)/Al (100 nm) were fabricated. PEDOT: PSS and the emitting layer were deposited by spin coating. BAlq and LiF/Al layers were deposited by evaporation under vacuum successively. Electroluminescence (EL) spectra of the compounds are well congruous with their photoluminescence (PL) spectrum of the film as shown in Fig. 8.1b, which showed the emission color derived from the central dye (1: sky blue, 2: blue green, 3: yellow, and 4: deep red) The outer oligofluorene groups did not affect the emission color because they have a wider energy gap than that of the central dye. The π-conjugations of the fluorene groups and the central dye do not seem to be fully delocalized. Photoluminescence quantum efficiency (PLQE) of the films is determined by using an integrating sphere system under nitrogen atmosphere. PLED of the compounds 1-3 exhibited higher than that of tris(8-quinolinolato)aluminum (Alq3) film (22 %), which was determined under the same conditions. Multicolor emissions from conjugated oligomer dyes having well-defined structures were achieved in their OLED fabricated from solution processes.
8.2.2 Phosphorescence Dendrimer
The combination of the solution process and the phosphorescent compounds can be an ideal choice to achieve low fabrication cost and high efficiency in OLEDs. For the solution process, substitution of functional dendrons on the complex is one of the approaches to solubilize it, and P. L. Burn group has done a lot of pioneering work on the dendrimer OLEDs [10, 11]. The dendron is bulky in volume, so that it can prevent intermolecular interaction between the emitting complexes, resulting in reduction of concentration quenching and high PLQE [12, 13]. From the OLED application point of view, those dendrons have to have enough high charge-transporting ability for low driving voltage[14] and have a larger triplet energy (T1) level than that of the core complex not to quench the triplet exciton of the complex [15, 16]. In phosphorescent OLEDs, m-carbazolylbenzene (mCP) is one of the well-known and widely used host materials, because its T1 level is high enough (3.0 eV) to confine the phosphorescent emission of the iridium complex, and has bipolar charge-transporting ability [17, 18]. 3,5-(N,N-di(4-(n-butyl)phenyl)amine) (DPA) is also used as hole transport substituent group. We designed and synthesized (mCP) 6 Ir and (DAP) 6 Ir (Fig. 8.2). The phosphorescent iridium complex, (mCP) 3 Ir, attached three mCP dendrons having alkyl groups and high efficiencies of the OLEDs using that complex. In (mCP) 3 Ir, mCP dendrons are attached on each phenyl ring of tris(2-phenylpyridinato)iridium(III) (Ir(ppy)3), and (mCP) 3 Ir is a facial isomer, so that the three mCP dendrons are attached spacially on the same side in the complex and surround only half a side of Ir(ppy)3 as shown in Fig. 8.3a. The fully surrounded Ir(ppy)3 by six host dendrons, (mCP) 6 Ir is shown in Fig. 8.3b. Both of the complexes showed higher PLQE in a neat film than that of half-surrounded (mCP) 3 Ir and (AP) 3 Ir, supporting well the results reported in the literature. PLQEs of the complexes in the neat film are important parameters to estimate the shielding effect of the surrounding dendrons to Ir(ppy)3. PLQEs of the toluene solution and the films were measured by using an integrating sphere system under 331 nm excitation. In a diluted solution, all complexes showed higher PLQE than 70 %, which are comparable to 85 % of unsubstituted Ir(ppy)3.
Chemical structures of the dendronized iridium complex (Reprinted from Ref. [3]. Copyright 2012, with permission from Elsevier)
The optimized structures of: (a) half-surrounded (mCP)3Ir and (b) fully-surrounded (mCP)6Ir by PM6 calculation. The butyl groups were replaced to hydrogen in calculation (Reprinted from Ref. [3]. Copyright 2012, with permission from Elsevier)
This result demonstrated that these surrounding dendrons are optically inert and do not affect the emission efficiency of Ir(ppy)3 core. The fully surrounded complexes, (mCP) 6 Ir and (DAP) 6 Ir, showed high PLQE even in a neat film, which is comparable to PLQE in a dilute solution. On the other hand, the half-surrounded complexes, (mCP) 3 Ir and (DAP) 3 Ir, showed much lower PLQE in a neat film than that in a dilute solution. These complexes are facial isomers; therefore, in (mCP) 3 Ir and (DAP) 3 Ir, some spaces around pyridyl groups of Ir(ppy)3 core are opened, and their three-dimensional structure is like a hemisphere, resulting in only partial suppression of concentration quenching in a neat film of an iridium complex. However, in (mCP) 6 Ir and (DAP) 6 Ir, the bulky host dendrons fully surrounded Ir(ppy)3 and effectively prevented the intermolecular interaction between Ir(ppy)3s. There are still small amounts of reduction of PLQEs from a solution to a neat film, due to the concentration quenching even in the fully substituted complexes. The substituted host dendrons are not large enough to completely suppress the interaction between the core complexes. Adachi et al. reported that an average distance between iridium complexes in a doped film critically influenced PLQE [19]. Förster-type energy transfer between Ir(ppy)3 cores through an overlap of the emission and the absorption causes a decrease of neat film PLQE. If the average distance between iridium complexes is shorter than a Förster radius, a strong quenching occurs. The stronger quenching of (mCP) 3 Ir in the neat film than that of (mCP) 6 Ir is due to the shorter average distance between the cores derived from a smaller number of bulky host dendrons of (mCP) 3 Ir than that of (mCP) 6 Ir. Substitutions of more branched and larger dendrons to the core complexes are desirable to achieve the complete suppression of concentration quenching. Solution-processed OLEDs with (mCP) 6 Ir exhibited high efficiencies, 19 lm W−1, 32 cd A−1, and 12 % of external quantum efficiency (EQE) at 100 cd m−2, and 11 lm W−1, 25 cd A−1, 9.1 % at 1000 cd m−2. The energy levels of the surrounding dendrons intensely affected the charge injection into the emitting layer and the device performance.
8.2.3 Electron Injection Materials
Polymer light-emitting devices (PLEDs) employ low-work-function metals, such as cesium, barium, or calcium, as an electron injection layer (EIL) and a cathode to enhance the electron injection to the emitting layer. However, these metals and the cathode are highly reactive with atmospheric oxygen and moisture, which results in degradation of the device. To avoid these problems, stable alkali metal fluorides, such as LiF or CsF, are commonly used in the EIL of dry-processed OLEDs. Cs2CO3 has been reported to be an effective EIL material in solution-processed OLEDs because it is soluble in alcohol solvents and can be coated from solution. The solution-processed Cs2CO3 EIL exhibits a high electron injection ability that is comparable to that of alkali metals [20, 21]. However, Cs2CO3 still has some disadvantages: it is hygroscopic and unstable in air, and it requires an ultrathin thickness because it is an insulating material. A strong chemical reduction is known to occur between Cs2CO3 and the thermally evaporated Al cathode.
Lithium phenolate complexes could be used to form an excellent EIL, and the device performance was much less sensitive to the thickness of the coating of these complexes because of their high electron-transporting ability compared with insulating Cs2CO3 [22, 23]. The lithium phenolate complexes also have stability against oxidation and are less hygroscopic. We reported the efficient solution processing of an EIL based on the lithium quinolate complexes (Liq) that are dissolved into alcohol; in this EIL, a low driving voltage and improved stability of the PLEDs is achieved. Liq has high solubility in polar solvents, such as alcohols, and it has a smooth surface morphology. Therefore, Liq can be spin coated onto the emitting polymer; the device prepared with spin-coated Liq as an EIL exhibited a lower turn-on voltage and had a higher efficiency than the devices prepared with spin-coated Cs2CO3 or with thermally evaporated calcium.
On the other hand, ZnO nanoparticles have recently been reported to be air-stable electron injection materials in PLEDs [24, 25]. To improve the electron injection ability of the solution-processed EIL that had a thickness of more than 10 nm, we utilized ZnO nanoparticles as a host for Liq or Cs2CO3 (Fig. 8.4a). ZnO can enhance the electron injection characteristics through the addition of alkali metal salts [26, 27]. The ZnO nanoparticles, which were synthesized from a zinc acetate precursor [28], were well dispersed into 2-ethoxyethanol at a concentration of 10 mg ml−1.
(a) The chemical structure of Liq and the structure of the device. (b) Current density-voltage (solid symbol) and luminance-voltage (open symbol) characteristics (Reprinted with the permission from Ref. [4]. Copyright 2011 American Chemical Society)
The average diameter of the ZnO nanoparticles was estimated using transmission electron microscopy (TEM) and dynamic light scattering (DLS) and was approximately 10 nm, with the particles being monodispersed. The mixture of ZnO nanoparticles and Liq formed a thick film with a homogeneous and smooth surface, indicating that Liq is well dispersed around the ZnO nanoparticles. The combination of ZnO and Liq significantly reduced the driving voltage and improved the power efficiency compared to only ZnO or ZnO:Cs2CO3 (Fig. 8.4b). This inorganic–organic hybrid EIL is an effective approach for enhancing the efficiency and the stability of PLEDs, and the thickness can be sufficiently thick for reproducible large-scale devices.
8.2.4 Polymer Binder
The thickness of EILs comprised of compounds such as Liq and Cs2CO3 must be ultrathin (<2 nm) to achieve efficient electron injection characteristics due to their poor electron transport properties. However, precise thickness control in the range of a few nanometers is practically impossible for large-scale devices using solution processes such as spin coating and blade coating. In this context, only relatively thick EIL films (10–20 nm) can be mass produced for large PLEDs using solution processing. Herein, we report the use of a mixture of poly(vinylphenylpyridine) and Liq for solution-processable efficient, thick electron injection layers. Vinyl polymers with high solubilities in alcoholic solvents and good film-forming abilities, such as poly(4-vinylpyridine) (PV4Py) and poly[4-(4-vinylphenyl)pyridine] (PVPh4Py) (Fig. 8.5a), were used as binders for Liq, and the effects of the π-conjugation of the polymers on the electron transport and injection characteristics were investigated. The influence of the position of the nitrogen in the pyridine rings was also investigated using poly[2-(4-vinylphenyl)pyridine] (PVPh2Py) and poly[3-(4-vinylphenyl)pyridine] (PVPh3Py).
In the UV–vis absorption spectra, the PVPhPys exhibited smaller energy gaps than that of PV4Py, because the additional phenyl group participates in extended π-conjugation compared with only the pyridine group (Fig. 8.5b). Among the PVPhPys, PVPh2Py exhibited a bathochromically shifted absorption peak compared to those of PVPh3Py and PVPh4Py. The greater π-conjugation of PVPh2Py is probably due to the greater planarity of the structure of 2-phenylpyridine, which results because of the absence of a hydrogen at the ortho position and the consequent reduced steric hindrance. The influence of the concentration of Liq in a PVPh4Py:Liq mixture in the performance of the ultrathin layers of approximately 1.6 nm was investigated. In addition to devices prepared with EIL layers comprised of PVPh4Py with 10, 30, 50, or 70 wt%, two control devices were fabricated using ultrathin layers of only Liq and PVPh4Py.
The observed EL spectra of various devices are identical to the emission from F8BT, and no emission was observed from TFB or Liq. This result indicated that the holes and the electrons were confined within the F8BT and that the recombination of the charges occurred only in the F8BT. The device with 100 wt% PVPh4Py exhibited a high turn-on driving voltage of 3.0 V and driving voltages of 5.6 and 8.4 V at 100 and 1000 cd m−2, respectively. The EQE of 0.6 % observed at 1000 cd m−2 for the devices with the 100 wt% PVPh4Py layer were lower than those of the device with PVPh4Py doped with Liq. This result suggested that PVPh4Py itself has a poor electron injection property due to its shallow LUMO level of 1.9 eV. However, the device performance dramatically improved when Liq was added to the PVPh4Py. The driving voltage of the devices with PVPh4Py:Liq decreased with increasing Liq concentration from 10 to 70 wt% due to increased electron injection into the F8BT from the Al cathode. The external quantum efficiencies were 4.9–6.9 %. These driving voltages and efficiencies were nearly equivalent to those of the device with the ultrathin EIL layer comprised of 100 wt% Liq. In the device with 10 wt% Liq, balanced charge ratio resulted in the highest power efficiency of 23 lm W−1 and an EQE of 6.9 % at 1000 cd m−2. Notably, this power efficiency is the highest value reported in the literatures to date for devices with F8BT as the emissive layer (EML) [29, 30]. These results indicate that while PVPh4Py itself is not effective as an EIL, mixing it with Liq does not deteriorate the electron injection properties of Liq and improves the driving voltages and efficiencies of the devices.
The performance of devices with EILs of different thicknesses comprised of the mixtures of PVPh4Py and Liq was investigated. The three types of EILs with thicknesses of 1.6, 8.6, and 16 nm were deposited from solutions with different Liq concentrations using different spin-coating speeds. As the EILs’ thickness was increased from 1.6 nm to 16 nm, the driving voltages increased and the EQEs decreased. However, the increase in the voltage and the decrease in the EQE were suppressed in the EILs comprised of PVPh4Py and Liq compared to those for the EIL comprised of 100 wt% Liq. The device with a 50 wt% mixed EIL exhibited the least dependence on the layer thickness, and the lowest driving voltage and the highest EQE for all of the devices was observed for an EIL with thickness of 16 nm. The high driving voltage and low efficiency of the device with the thick Liq layer is attributed to the poor electron transport properties of Liq itself. Conversely, mixing PVPh4Py with Liq could improve the electron transport properties of the EIL, and the driving voltage remained low, even for a thick EIL. Figure 8.6a shows that the UV absorption edge of the film prepared from the mixture of Liq and PVPh4Py with 50 wt% Liq was red shifted by 15 nm, corresponding to 0.11 eV, compared to that of the pure Liq film. Conversely, both the HOMO levels of Liq and the mixture of 50 wt% Liq and PVPh4Py were the same at 5.5 eV, as determined via photoelectron yield spectroscopy. Consequently, the mixture of Liq and PVPh4Py had a smaller energy gap than that of Liq due to the lower LUMO level of the mixture than that of Liq. Therefore, to understand the distribution of the HOMO and LUMO level in Liq, DFT calculations were conducted. The HOMO is not located on the Li atom (Fig. 8.6b), but the LUMO is associated with the Li atom (Fig. 8.6c). These results suggest that the interactions between the Li atom of Liq and the pyridine ring of PVPh4Py affected the LUMO level. The reduced dependence of EIL performance on the layer thickness will be advantageous for the large-area coating processes, because it is difficult using solution processing to form uniform thin films with an accuracy of a few nanometers.
(a) UV-vis absorption spectra films of Liq alone and Liq with PVPh4Py film. (b) HOMO and (c) LUMO of Liq with structures optimized in the grand states by DFT calculation (Reproduced from Ref. [5] by permission of Jon Wiley & Sons Ltd)
The position of the nitrogen in the pyridine rings had slight influence on the electron injection properties in the ultrathin layers (Fig. 8.7a). Conversely, in the devices with thin EILs (approximately 8.6 nm), the position of the nitrogen in the pyridine rings of the polymers had a greater influence on the driving voltage and efficiency. The driving voltage increased in the order PVPh4Py < PVPh3Py < PVPh2Py, Alternatively, the EQE increased in the order PVPh2Py < PVPh3Py < PVPh4Py (Fig. 8.7b). These results suggest that the position of the nitrogen in the pyridine rings significantly affects the electron transport properties of the polymers, rather than the electron injection properties. Sasabe et al. previously reported that the electron mobility of a series of oligo phenylpyridine derivatives was strongly affected by the position of the nitrogen in the pyridine rings due to C-H ⋯ N hydrogen bonding interactions [31]. The glass transition temperatures (T g) were determined via differential scanning calorimetry (DSC). The T g of PVPh4Py was observed at 185 °C, which is higher than that of PV4Py (146 °C) due to the more rigid structure of the phenylpyridine. The T g of PVPh4Py was also higher than those of PVPh2Py (162 °C) and PVPh3Ph (140 °C), suggesting that the location of the nitrogen at the 4-position of the PVPh4Py enables stronger intermolecular hydrogen bonding interactions than those in PVPh3Py and PVPh2Py. The denser packing of PVPh4Py that results from the stronger hydrogen bonding interactions probably leads to the enhanced electron transport properties observed for the thick films.
8.3 Solution-Processed Multilayer Small-Molecule OLEDs
8.3.1 Solubility of Small-Molecule Materials
Small-molecule-based OLEDs typically consist of four or more multiple layers of different materials in precise optoelectrical design. Such multilayer structures allow for the separation of the charge-injecting, charge-transporting, and light-emitting functions to the different layers, thus leading to a marked increase in efficiency and lifetime [32–34]. Although stepwise vacuum evaporation easily achieves the required multilayer structures of small molecules at the expense of high manufacturing cost, it is more challenging in the case of solution processing, because depositing one layer would dissolve the layer beneath it. To achieve the multilayer structures by solution processing, research efforts have focused on π-conjugated polymers that afford a robust hydrophobic layer, on which a hydrophilic layer can be deposited from orthogonal solvents, such as water or water/alcohol mixture [35]. In situ cross-linking reactions have also been explored to afford covalently bound structures that are highly resistant to processing solvents [36–38]. Despite their high mechanical robustness and compatibility with subsequent solution processing, polymers are plagued by limited reproducibility in the device performance because of batch-to-batch variations with respect to molecular weight, polydispersity, regioregularity, and purity. Moreover, their efficiencies are still far below the fluorescent tubes [39]. The highest reported power efficiency of white polymer LEDs is 25 lm W−1 thus far [40, 41]. On the other hand, small molecules are very attractive because they have a well-defined molecular structure that offers more reproducibility of synthesis procedures and better understanding of molecular structure–device performance relationships. However, their thin-film assemblies, most of which are amorphous in nature, are easily broken up, even by the orthogonal solvents, because small molecules typically attach to each other only by weak intermolecular forces such as van der Waals, H-bonding, and π–π stacking interactions. Consequently, the highest reported efficiency of solution-processed small-molecule OLEDs still relies on a vacuum-evaporated electron-transporting layer (ETL), which is not practical for low-cost mass production of scalable devices [42]. Herein, we demonstrate highly efficient small-molecule OLEDs in which quadruple organic layers, including a molecular-emitting layer (EML) and ETL, are fully solution processed. The key feature of the devices is the use of newly developed small host molecules in the EML, which are sufficiently resistant to the orthogonal solvents such as alcohols, used for processing upper ETLs, thus allowing us to construct the multilayer structure through subsequent solution-processing steps. While a robust host polymer is typically required to realize the multilayer structure, we simply modified conventional host molecules by covalent dimerization or trimerization to afford sufficient resistance to alcohols. With this approach, record-high efficiencies have been achieved for solution-processed blue, green, and white OLEDs.
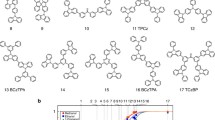
Through experiments with 17 host molecules over a wide range of molecular weight from 243 to 1146 (Fig. 8.8), we found that their resistance to alcohols remarkably increasing molecular weight. Figure 8.8b shows the normalized remaining thickness of molecular thin films after rinsing with a variety of alcohols as a function of molecular weight, as measured by ultraviolet–visible absorption spectroscopy. From the best-fit cumulative distribution function, we determined that the threshold molecular weights for achieving 95 % remaining thickness were 775, 811, 849, and 767 for methanol, ethanol, 1-propanol, and 2-propanol, respectively. This result demonstrates that even conventional host molecules can be compatible with the subsequent solution process simply by covalent dimerization or trimerization to exceed the threshold molecular weights, eliminating the need for polymeric counterparts. In addition, this figure covers a wide variety of building blocks, including arene, carbazole, triphenylamine, fluorene, benzothiophene, and even polar moieties such as benzophenone, pyridine, and triazine, making this approach broadly applicable. We also note that our approach enables subsequent solution processing onto the EML without having to use water, which is detrimental to device efficiency and stability [43–45].
Solvent resistance of molecular thin films. (a) Molecular structures of the host molecules arranged in order of increasing molecular weight. (b) Plots of normalized remaining thickness of the molecular thin films after rinsing with a variety of alcohols as a function of molecular weight. The solid lines represent the best fit to the cumulative distribution function (Reprinted by permission from Macmillan Publishers: Ref. [6], copyright 1993)
8.3.2 Green and Blue Phosphorescent OLEDs
On the basis of the above estimates, we elected to use two host molecules for green phosphorescent OLEDs: 3,3′:6′,3″-ter(9-phenyl-9H-carbazole) (TPCz) and 3,3′,6,6′-tetrakis(9-phenyl-9H-carbazol-3-yl) benzophenone (TCzBP). The schematic energy diagram of the host molecules is shown in Fig. 8.8b. When mixing the two host molecules in an EML, holes should preferentially reside in the shallow highest occupied molecular orbital of TPCz and electrons in the deep lowest unoccupied molecular orbital of TCzBP. Consequently, we can accurately optimize charge balance in the device by varying the ratio of the two host molecules to achieve high efficiency. By using the two molecules as hosts for Ir(ppy)3, we fabricated green phosphorescent OLEDs, in which quadruple organic layers were fully solution processed. The device configuration was ITO (130 nm)/ PEDOT:PSS (30 nm)/TFB (20 nm)/host:12 wt% Ir(ppy)3 (30 nm)/ 2,2′,2″-(1,3,5-benzinetriyl)tris(1-phenyl-1-H-benzimidazole) (TPBi) (50 nm)/ Liq (1 nm)/Al (100 nm) (Fig. 8.9a). In these devices, TPBi was elected as an ETL because of its sufficient solubility in methanol, enabling the subsequent solution processing on the molecular EML. The most common host polymer poly(N-vinylcarbazole) (PVK) was also used for comparison. By mixing a 1:1 ratio of TPCz and TCzBP, we achieved a power efficiency of 52 lm W−1 at 100 cd m−2. Indeed, the peak power efficiency reached an extremely high value of 96 lm W−1. The corresponding external quantum efficiency (EQE) was 23 %, which remained as high as 22 % and 20 % at 100 cd m−2 and 1,000 cd m−2, respectively. We also note that there is no perceivable change in luminance as a function of viewing angle (Lambertian factor: 1.03), eliminating the possibility of overestimating the efficiencies.
Device structure of the solution-processed OLEDs. (a) Schematic of the optimized device and molecular structures of the materials used. (b) Schematic energy-level diagram of the materials Reprinted by permission from Macmillan Publishers: Ref. [6], copyright 1993)
Despite the impressive efficiencies of the green phosphorescent OLEDs, our initial attempt at using standard blue phosphorescent emitter bis(2-(4,6- difluorophenyl)pyridine) (picolinate)iridium(III) (FIrpic) resulted in poor efficiencies. In the blue OLEDs, 4,4′-(3,3′-bi(9H-carbazole)-9,9′-diyl)bis(2,6-diphenyl)benzene (BCzTPh) and 4,4′-(3,3′-bi(9H-carbazole)-9,9′-diyl)bis(N,N-diphenyl)aniline (BCzTPA) were employed as host molecules, and 2-propanol- soluble 1,3-bis(3-(diphenylphosphoryl)phenyl)benzene (BPOPB) was used as an ETL. The device configuration was ITO (130 nm)/PEDOT:PSS (30 nm)/TFB (20 nm)/host:12 wt% FIrpic (30 nm)/BPOPB (45 nm)/Liq (1 nm)/Al (100 nm) (Fig. 8.9a). While these host molecules have a sufficiently high T1 level for efficient exothermic energy transfer to the phosphorescent blue emitter, the resulting device exhibited considerable emission from the host molecules at around 420 nm and with a low power efficiency of 6.5 lm W−1 at 100 cd m−2. Alternatively, with three-coordinated tris(2-(4,6-difluorophenyl)pyridine)iridium(III) (Ir(Fppy)3), the device efficiencies significantly increased to 36 lm W−1 for power efficiency and 20 % for EQE at 100 cd m−2. It is intriguing to note that Ir(Fppy)3 performs five times as well as FIrpic in power efficiency, although these two blue emitters possess almost identical optoelectrical properties. In these devices, the majority of excitons would be generated near the EML/ETL interface because of the relatively large injection barrier between them (Fig. 8.9b) and would subsequently be harvested by the doped blue phosphorescent emitter. In addition, we have previously reported that the direct electron-trapping process of a blue phosphorescent emitter played a major role in efficient electron injection at the EML/ETL interface in evaporated OLEDs [46]. We thus hypothesized that polar picolinate ligand-containing FIrpic would dissolve away from the EML surface upon the subsequent solution processing of the ETL from the 2-propanol solution, resulting in the unwanted host emission and poor device efficiencies.
To verify the hypothesis, we performed depth-profiling measurements of the devices by time-of-flight secondary ion mass spectrometry (TOF-SIMS). These measurements involved Ar2500 + gas cluster ion beam etching starting [47] from the ETL surfaces to the underlying substrates. This direction of etching allows for collecting the composition of the EMLs without alternating their original position at the EML/ETL interfaces (peak fronts), while etching and primary ion beams cause peak tailing. Figure 8.10 displays the TOF-SIMS depth profiles of the solution-processed devices with the different blue phosphorescent emitters, FIrpic and Ir(Fppy)3, in comparison with reference devices with a vacuum-evaporated ETL, for which a well-defined interface is expected to exist between the EML and ETL. We focused on [C22H12F4IrN2]+ ions with m/z = 573 as signatures both of FIrpic and Ir(Fppy)3 to obtain sufficient intensity in the dilute emitters embedded in the host matrix. We also monitored the corresponding molecular ions for the other molecules. The depth resolution was 11.5 nm under our experimental conditions. One observed from the TOF-SIMS depth profiles that the composition at the solution-processed EML/ETL interface was significantly varied between the FIrpic and Ir(Fppy)3 system. Ir(Fppy)3 showed a slight reduction in intensity in the interface region of roughly 10–20 nm for the solution-processed device, whereas a noticeable reduction in FIrpic intensity occurred almost over the entire EML, particularly at the EML/ETL interface. The corresponding reduction in the concentration of the blue emitters was also quantitatively observed by high-performance liquid chromatography analysis; the reduction in concentration of emitters was 48 % and 8 % for FIrpic and Ir(Fppy)3, respectively, upon rinsing with pure 2-propanol. These results indicate that almost half of the FIrpic molecules dissolved from the EML while depositing the ETL and the host molecules were compositionally rich in the resulting interface. On the other hand, the Ir(Fppy)3 molecules existed over the entire EML, including the interface even after the deposition of the ETL. The improved device efficiencies upon introduction of Ir(Fppy)3 therefore arise from the efficient electron injection at the EML/ETL interface through the direct electron-trapping process of Ir(Fppy)3. We also note that a very small amount of the EML composition migrated and uniformly distributed into the solution-processed ETL.
TOF-SIMS depth profiles of the blue OLEDs with different emitters (top: FIrpic and bottom: Ir(Fppy)3). The dashed lines represent depth profiles of the devices using solution-processed BPOPB. The solid lines represent data for reference devices using evaporated BPOPB (Reprinted by permission from Macmillan Publishers: Ref. [6], copyright 1993)
8.3.3 White Phosphorescent OLEDs
We fabricated solution-processed multilayer white OLEDs by incorporating green-emitting Ir(ppy)3 (0.2 wt%) and red-emitting tris(2-phenyl-1-quinoline)iridium(III) (Ir(phq)3) (0.7 wt%) into the blue EML. Although the EL spectra of white OLEDs typically depend on current density (in other words, luminance) [20, 48, 49], the resulting solution-processed white OLED surprisingly showed no perceived change in the EL spectra under varying current density. The corresponding color shift in the Commission internationale de l’éclairage (CIE) coordinates was as small as Δx,y = 0.002, 0.002 between 100 and 1,000 cd m−2. For comparison, the evaporated ETL exhibited relatively strong green and red emissions and a gradual blue shift in the CIE coordinates of Δx,y = 0.054, 0.002 between 100 and 1,000 cd m−2.
The stable EL spectra can be explained if the green and red emitters were partially washed away from the EML surface upon the solution processing of the ETL, causing electron trapping and recombination preferentially on the blue emitter at the EML/ETL interface. This would provide uniform exciton distribution among the three emitters, and thus the stable EL spectra, because the only exciton generation path left for the green and red emitters is energy transfer from the blue emitter staying at the EML/ETL interface. This hypothesis was confirmed with PL spectroscopy, showing that the red and green emissions of the EML decreased upon rinsing with pure 2-propanol. Similar stable EL spectra have also been observed in evaporated OLEDs, in which the blue emitter is placed at the recombination interface and spatially separated from other emitters as in this case.
Remarkably, we achieved a high power efficiency of 34 lm W−1 and an EQE of 21 % at 100 cd m−2 for a white emission with a color rendering index (CRI) of 70 and CIE coordinates of 0.43, 0.43 without the use of any outcoupling enhancement. The peak power efficiency and EQE reached 45 lm W−1 and 22 %, respectively. To the best of our knowledge, these efficiencies are considerably higher than the highest efficiencies ever reported for white polymer LEDs. In addition, when outcoupling all the photons trapped in the glass substrate, these efficiencies increase by a factor of 1.96 as confirmed by using an index-matched hemisphere lens. As a result, the maximum achievable power efficiency and EQE are expected to be 88 lm W−1 and 41 %, respectively. We also note that the solution-processed device showed lower driving voltages and higher efficiencies compared with the corresponding device with an evaporated ETL. The superior performance with a solution-processed ETL was also observed in the green phosphorescent OLEDs using TPBi as an ETL.
8.4 Solution-Processed Tandem OLEDs
8.4.1 Solution-Evaporation Hybrid Tandem OLEDs
Whereas the luminance of OLEDs increases with the current density, high currents promote the degradation of the organic materials [50]. To simultaneously improve the luminance and device stability, Kido et al. developed tandem OLEDs, comprising several stacked LEUs interconnected by CGLs [51]. In general, tandem OLED fabricated by evaporation can have more than ten layers between the anode and the cathode [52–58]. Whereas stepwise vacuum evaporation-based processes can generate multilayered structures, such structures are a challenge to solution-processing techniques because solution-based coating of one layer can dissolve the layer beneath it. Electron injection from CGL into the first LEU is a key factor impacting the characteristics of tandem OLEDs. In vacuum-processed devices, an alkali metal [52, 54, 55] or a bilayer of alkali metal halide and Al [58] effectively enhances electron injection and is used in the EIL of the first LEU. The CGL is composed of electron-accepting materials such as MoO3, [55] V2O5, [54] and WO3, [59] and electron-donating materials such as arylamine derivatives. It is important to match the Fermi level of electron-accepting materials and the HOMO level of electron-donating materials. However, such metals, metal halides, and metal oxides are not readily solution processable because of their poor solubility in organic solvents.
A hybrid process of spin coating and thermal evaporation was utilized for the tandem OLEDs’ fabrication. Each LEU with the configuration of PEDOT:PSS/LE-polymer/EIL was fabricated by spin coating. Ultrathin Al was deposited as the EIL in the first unit, and MoO3 was subsequently deposited as the CGL by thermal evaporation. Low-work-function metals cannot be used as the EIL for solution-based processing of tandem devices because of their high reactivity with organic solvents, which results in severe degradation of the device. Cs2CO3-doped ZnO nanoparticles were used as an EIL on the LE polymer to improve the electron injection from the cathode.
The surface morphology of a spin-coated metal oxide nanoparticle layer appears to be rough, with many gaps due to agglutination of nanoparticles. Consequently, the thin layer of metal oxide nanoparticles cannot protect the first LEU organic layer from the spin-coating solvent of the second LEU organic layer. Thus, we chose PV4Py as a binder to improve the film morphology of the ZnO:Cs2CO3 mixture and facilitate the formation of a uniform and dense film to prevent the solvent from soaking into the first LEU. The thermally evaporated MoO3 layer is insoluble in organic solvents, such as toluene, p-xylene, and dichlorobenzene. Thus, an electron-donating layer can be spin coated on top of the MoO3 layer. Poly(4-butylphenyl-diphenyl-amine) (poly-TPD) was used as an electron-donating and hole-transporting layer and was spin coated onto the MoO3 layer using a dichlorobenzene solution. This combination of MoO3 and poly-TPD as a prospective CGL may be successful because bilayers of MoO3 and arylamine derivatives, such as NPD or TPD, can work as an efficient CGL in tandem OLEDs [55]. Poly-TPD is insoluble in toluene and p-xylene; therefore, an LE polymer such as F8BT can be spin coated onto the poly-TPD layer using a p-xylene solution without dissolving the bottom layer. The efficient solution-based processing of EILs in the CGL containing MoO3/poly-TPD bilayers was employed for the construction of a tandem device as shown in Fig. 8.11a.
(a) Device structure of the 1st-LEU, 2nd-LEU, and tandem OLED. (b) Current efficiency–current density characteristics of the 1st-LEU, 2nd-LEU, and tandem OLED (Reproduced from Ref. [7] by permission of The Royal Society of Chemistry)
At high luminance values of 1000 cd m−2, first LEU and second LEU exhibited efficiencies of 6 cd A−1 and 4 cd A−1, respectively (Fig. 8.11b). The efficiency of second LEU was lower than that of the first LEU due to the decrease in the charge balance and the increase in the driving voltage for MoO3 as an HIL. Nevertheless, the current efficiency of the tandem device increased to 10 cd A−1, which is the sum of the efficiency of the two single devices. The LUMO level of poly-TPD was 2.3 eV, which was just shallow enough to block electrons from the second LEU to the first LEU [60]. The conduction band (CB) of ZnO was 7.4 eV, which was deep enough to block holes from the first LEU to the second LEU. Thus, the emissions are attributed to the recombination of charges that were generated in the CGL without current leakage. These results demonstrate that MoO3/poly-TPD can function as an effective CGL.
8.4.2 Fully Solution-Processed Tandem OLEDs
Recently, solution-processed tandem OLEDs were reported by Colsmann and coworkers [61] with an inverted structure of polymer OLEDs. To the best of our knowledge, there is no report on the tandem OLEDs having the regular configuration of ITO anode and Al cathode. Figure 8.12 shows tandem OLED structure comprising two LEUs (first LEU and second LEU) and a CGL between the anode and the cathode using only solution-based processes. The driving voltage and efficiency of the fabricated tandem OLED are the sums of corresponding values of the component LEUs. These results demonstrate that the solution-processed CGL successfully generated electrons and holes and that the generated electrons and holes were injected into first LEU and second LEU, respectively, when a voltage was applied, resulting in charge recombinations in each LEU. Recently, PEIE and ZnO nanoparticles have been reported as efficient electron-collecting layers between a semiconducting organic layer and a cathode in organic photovoltaics (OPV) [62, 63] and electron injection layers between a cathode and the semiconducting organic layer in OLEDs [64–66]. These properties emerge from their ability to reduce the work function of the cathode. PEIE along with ZnO nanoparticles in the EIL of first LEU and F8BT as the emitting polymer were used. The PEIE and ZnO nanoparticles can be coated as EILs onto the F8BT layer from 2-ethoxyethanol solution and dispersion, respectively. Furthermore, it is required that these EILs not be soluble in the solvent used for coating subsequent layers, which include layers of p-type electron-accepting and hole-transporting materials in second LEU, consisting of the CGL. The solvent used for coating the electron acceptor cannot be water or alcohol, both of which dissolve PEIE.
Layered sequence of individual LEUs and the tandem OLED (Reproduced from Ref. [8] by permission of John Wiley & Sons Ltd)
Therefore, we chose phosphomolybdic acid hydrate (MoO3)12 · H3PO4 · (H2O) x (PMA) [67] as the electron acceptor of the CGL and acetonitrile as the solvent; PMA is soluble in acetonitrile, whereas PEIE, ZnO, and F8BT are not. Furthermore, TFB is chosen as the HTL of second LEU. For coating, a solution of TFB is prepared in p-xylene because p-xylene does not dissolve PMA, PEIE, or ZnO. However, F8BT is soluble in p-xylene; therefore, we carefully monitored the tendency of ZnO and/or PEIE layers to resist the dissolution of underlying F8BT in p-xylene using AFM surface images and the intensity of UV–Vis absorption after rinsing with the solvent (Fig. 8.13). Rinsing PEIE-coated F8BT with p-xylene reduces the thickness of the underlying F8BT layer, as indicated by the reduction in the intensity of UV–Vis absorption (Fig. 8.13a). The loss of F8BT is attributed to the nonuniform deposition of the PEIE layer (from its solution in 2-ethoxyethanol) on the F8BT layer due to the large difference in the surface energies of the compounds. Consequently, the roughness of the PEIE (0.67 nm) allows p-xylene to readily permeate into the F8BT layer. Similar dissolution of the F8BT underlayer on rinsing ZnO-coated F8BT is observed (Fig. 8.13b). It is likely that the roughness of the ZnO layer (2.19 nm) permits p-xylene to permeate the layer and dissolve F8BT. It must be noted here that both ZnO and PEIE are not soluble in p-xylene. To ensure the formation of a uniform layer of PEIE, we first deposited a layer of ZnO nanoparticles onto F8BT using a dispersion of the same in 2-ethoxyethanol. Once the ZnO layer is dried, it is not able to be redispersed into 2-ethoxyethanol. Subsequently, PEIE (in 2-ethoxyethanol) is uniformly coated onto the ZnO layer. The surface roughness of the F8BT/ZnO/PEIE layer (30 nm) is much smaller than that observed for F8BT/ZnO or F8BT/PEIE. Absence of any change in the intensity of UV–Vis absorption on rinsing F8BT/ZnO/PEIE with p-xylene (Fig. 8.13c) clearly demonstrates that a uniformly coated PEIE layer can prevent p-xylene. When compared with the device containing EIL composed only of ZnO, the driving voltage of first LEU device is lower and the efficiency is higher, demonstrating the superior electron injection property of the ZnO/PEIE bilayer. The second LEU device (Fig. 8.12b), fabricated with the electron acceptor PMA as HIL, showed a similar low driving voltage, indicating that holes and electrons were generated at the interface of electron acceptor PMA and electron donor TFB, and these two layers worked properly as solution-processed CGL. These devices corresponding to the first LEU and the second LEU were combined to fabricate a solution-processed tandem OLED (Fig. 8.12c), which consists of nine layers except electrodes.
AFM images of the films, and UV–Vis absorption spectra of the pristine and p-xylene-rinsed films: (a) Glass/F8BT (80 nm)/PEIE (20 nm), (b) Glass/F8BT (80 nm)/ZnO (10 nm), and (c) Glass/F8BT (80 nm)/ZnO (10 nm)/PEIE (20 nm). The Rq is square surface roughness and the Ra is average surface roughness (Reproduced from Ref. [8] by permission of John Wiley & Sons Ltd)
The insolubility of each layer in the solvent used for coating the subsequent layer is carefully monitored, allowing for the successful stacking of the nine layers on the ITO substrate by a series of solution processes. Finally, Al is deposited in vacuum, and solution processing of the electrodes remains a formidable challenge. The driving voltage of the tandem OLED is nearly equal to the sum of the driving voltages of individual LEU devices at low current density. The driving voltage of the tandem OLED increases gradually. In tandem OLEDs, the two LEUs are connected in series; therefore, the current in each LEU must be the same, whereas the voltage must be the sum of the voltages applied across each LEU. This additive driving voltage in the tandem OLED demonstrates that the interfaces in the device do not offer large resistance to increase voltage, and in particular, the electrons accepted by PMA from TFB in the CGL are smoothly injected into the F8BT of first LEU through the electron-injecting ZnO/PEIE bilayers. Investigation into the stability of the device at the same current density (Fig. 8.14) shows that the absolute value of the device lifetime is strongly dependent on the nature of the materials used. However, a distinct difference between the single LEU device and the tandem OLED is observed. The tandem OLED and first LEU show similar degradation tendencies in terms of the drop in luminance with increasing voltage, although the luminance of the tandem OLED is twofold higher than that of first LEU. This observation shows that the solution-based process used for stacking the two LEUs does contribute to device instability; the advantage of the tandem OLED manifests in the form of high luminance for the lifetime of the device. The stability of second LEU extends to periods much longer than that observed in case of the tandem OLED or first LEU. The luminance of second LEU, however, is much lower than that of the tandem OLED due to lower efficiency. One clear reason for the longer lifetime of second LEU is the absence of the PEDOT:PSS layer, recognized widely as being unstable. Another possible reason can be the ZnO/PEIE bilayer, which is found in the tandem OLED and the other individual LEUs, in common, that show relatively short lifetimes.
Lifetime of the devices at the same current density (7.5 mA cm−2). Initial luminances were 800 cdm−2 for the 1st-LEU, 300 cdm−2 for the 2nd-LEU and 1200 cdm−2 for the tandem OLED (Reproduced from Ref. [8] by permission of John Wiley & Sons Ltd)
8.5 Summary
In summary, solution-processed OLEDs have promising results of large area processing and low fabrication cost for lighting application. Several approaches have been studied to achieve efficiencies of solution-processed OLEDs. In this chapter, we discussed our recent works: 1) solution-processable materials and 2) solution-processed multilayer structure. Fluorescent oligomers and phosphorescent dendrimers are synthesized as solution-processable light-emitting dyes having well-defined structures. Liq, Cs2CO3, and ZnO nanoparticles are also studied as a solution-processed electron injection material. In addition, series of (vinylphenyl)pyridine-based polymer binders, PVPh2Py, PVPh3Py, and PVPh4Py, are synthesized for thick layers that can be mass produced for large-area coating using solution processing. In the solution-processed multilayer white phosphorescent OLEDs using small molecules, we achieved high power efficiencies of 34 lm W−1 at 100 cd m−2 with stable electroluminescence spectra under varying current density. In addition, a tandem OLED consisting of two LEUs and a CGL between the anode and the cathode is fabricated using only solution-based processes. Appropriate choice of solvents during spin coating of each layer ensures that a nine-layered structure is readily fabricated using only solution-based processes. The determined driving voltage and efficiency of the fabricated tandem OLED are the sums of values of the individual LEUs. These results indicate that the CGL formed by the solution-based process successfully generates electrons and holes under applied voltage. The formed electrons are efficiently injected into the first LEU through the ZnO/PEIE bilayer, and the holes are injected into the second LEU. The successful fabrication of solution-processed white phosphorescent OLEDs and tandem OLEDs will pave the way toward printable, low-cost, and large-area white light sources.
References
Y.-J. Pu, M. Higashidate, K. Nakayama, J. Kido, J. Mater. Chem. 18, 4183 (2008)
N. Iguchi, Y.-J. Pu, K. Nakayama, M. Yokoyama, J. Kido, Org. Electron. 10, 465 (2009)
Y.-J. Pu, N. Iguchi, N. Aizawa, H. Sasabe, K. Nakayama, J. Kido, Org. Electron. 12, 2103 (2011)
T. Chiba, Y.-J. Pu, M. Hirasawa, A. Masuhara, H. Sasabe, J. Kido, ACS Appl. Mater. Interfaces 4, 6104 (2012)
T. Chiba, Y.-J. Pu, S. Takahashi, H. Sasabe, J. Kido, Adv. Funct. Mater. 24, 6038 (2014)
N. Aizawa, Y.-J. Pu, M. Watanabe, T. Chiba, K. Ideta, N. Toyota, M. Igarashi, Y. Suzuri, H. Sasabe, J. Kido, Nat. Commun. 5, 5756 (2014)
T. Chiba, Y.-J. Pu, H. Sasabe, J. Kido, Y. Yang, J. Mater. Chem. 22, 22769 (2012)
Y.-J. Pu, T. Chiba, K. Ideta, S. Takahashi, N. Aizawa, T. Hikichi, J. Kido, Adv. Mater. 27, 1327 (2015)
J.H. Burroughes, D.D.C. Bradley, A.R. Brown, R.N. Marks, K. Mackay, R.H. Friend, P.L. Burn, A.B. Holmes, Nature 347, 539 (1990)
P.L. Burn, S.C. Lo, I.D.W. Samuel, Adv. Mater. 19, 1675 (2007)
S.C. Lo, P.L. Burn, Chem. Rev. 107, 1097 (2007)
T. Sato, D.-L. Jiang, T. Aida, J. Am. Chem. Soc. 121, 10658 (1999)
A.W. Freeman, S.C. Koene, P.R. Malenfant, M.E. Thompson, J.M. Fréchet, J. Am. Chem. Soc. 122, 12385 (2000)
S. Gambino, S.G. Stevenson, K.A. Knights, P.L. Burn, I.D. Samuel, Adv. Funct. Mater. 19, 317 (2009)
S.-C. Lo, R.E. Harding, C.P. Shipley, S.G. Stevenson, P.L. Burn, I.D. Samuel, J. Am. Chem. Soc. 131, 16681 (2009)
J.J. Kim, Y. You, Y.-S. Park, J.-J. Kim, S.Y. Park, J. Mater. Chem. 19, 8347 (2009)
V. Adamovich, J. Brooks, A. Tamayo, A.M. Alexander, P.I. Djurovich, B.W. D’Andrade, C. Adachi, S.R. Forrest, M.E. Thompson, New J. Chem. 26, 1171 (2002)
B.W. DAndrade, S.R. Forrest, J. Appl. Phys. 94, 3101 (2003)
Y. Kawamura, J. Brooks, J.J. Brown, H. Sasabe, C. Adachi, Phys. Rev. Lett. 96, 017404 (2006)
J. Huang, G. Li, E. Wu, Q. Xu, Y. Yang, Adv. Mater. 18, 114 (2006)
J. Huang, Z. Xu, Y. Yang, Adv. Funct. Mater. 17, 1966 (2007)
J. Endo, T. Matsumoto, J. Kido, Jpn. J. Appl. Phys. 41, L800 (2002)
Y.-J. Pu, M. Miyamoto, K. Nakayama, T. Oyama, Y. Masaaki, J. Kido, Org. Electron. 10, 228 (2009)
D. Kabra, M.H. Song, B. Wenger, R.H. Friend, H.J. Snaith, Adv. Mater. 20, 3447 (2008)
H.J. Bolink, E. Coronado, J. Orozco, M. Sessolo, Adv. Mater. 21, 79 (2009)
H.J. Bolink, H. Brine, E. Coronado, M. Sessolo, Adv. Mater. 22, 2198 (2010)
D. Kabra, L.P. Lu, M.H. Song, H.J. Snaith, R.H. Friend, Adv. Mater. 22, 3194 (2010)
B. Sun, H. Sirringhaus, Nano Lett. 5, 2408 (2005)
L.P. Lu, D. Kabra, R.H. Friend, Adv. Funct. Mater. 22, 4165 (2012)
B.R. Lee, E.D. Jung, J.S. Park, Y.S. Nam, S.H. Min, B.S. Kim, K.M. Lee, J.R. Jeong, R.H. Friend, J.S. Kim, S.O. Kim, M.H. Song, Nat. Commun. 5, 4840 (2014)
H. Sasabe, D. Tanaka, D. Yokoyama, T. Chiba, Y.-J. Pu, K.-i. Nakayama, M. Yokoyama, J. Kido, Adv. Funct. Mater. 21, 336 (2011)
C.W. Tang, S.A. Vanslyke, Appl. Phys. Lett. 51, 913 (1987)
J. Kido, M. Kimura, K. Nagai, Science 267, 1332 (1995)
S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lussem, K. Leo, Nature 459, 234 (2009)
P.K.H. Ho, J.S. Kim, J.H. Burroughes, H. Becker, S.F.Y. Li, T.M. Brown, F. Cacialli, R.H. Friend, Nature 404, 481 (2000)
X.H. Yang, D.C. Muller, D. Neher, K. Meerholz, Adv. Mater. 18, 948 (2006)
R.Q. Png, P.J. Chia, J.C. Tang, B. Liu, S. Sivaramakrishnan, M. Zhou, S.H. Khong, H.S.O. Chan, J.H. Burroughes, L.L. Chua, R.H. Friend, P.K.H. Ho, Nat. Mater. 9, 152 (2010)
N. Aizawa, Y.-J. Pu, T. Chiba, S. Kawata, H. Sasabe, J. Kido, Adv. Mater. 26, 7543 (2014)
S. Reineke, M. Thomschke, B. Lussem, K. Leo, Rev. Mod. Phys. 85, 1245 (2013)
B.C. Krummacher, V.E. Choong, M.K. Mathai, S.A. Choulis, F. So, F. Jermann, T. Fiedler, M. Zachau, Appl. Phys. Lett. 88, 113506 (2006)
F. Huang, P.I. Shih, C.F. Shu, Y. Chi, A.K.Y. Jen, Adv. Mater. 21, 361 (2009)
B.H. Zhang, G.P. Tan, C.S. Lam, B. Yao, C.L. Ho, L.H. Liu, Z.Y. Xie, W.Y. Wong, J.Q. Ding, L.X. Wang, Adv. Mater. 24, 1873 (2012)
T. Ikeda, H. Murata, Y. Kinoshita, J. Shike, Y. Ikeda, M. Kitano, Chem. Phys. Lett. 426, 111 (2006)
H. Yamamoto, J. Brooks, M.S. Weaver, J.J. Brown, T. Murakami, H. Murata, Appl. Phys. Lett. 99, 33301 (2011)
H.T. Nicolai, M. Kuik, G.A.H. Wetzelaer, B. de Boer, C. Campbell, C. Risko, J.L. Bredas, P.W.M. Blom, Nat. Mater. 11, 882 (2012)
H. Sasabe, N. Toyota, H. Nakanishi, T. Ishizaka, Y.J. Pu, J. Kido, Adv. Mater. 24, 3212 (2012)
I. Yamada, J. Matsuo, N. Toyoda, Nucl. Inst. Methods Phys. Res. B 206, 820 (2003)
J.H. Zou, H. Wu, C.S. Lam, C.D. Wang, J. Zhu, C.M. Zhong, S.J. Hu, C.L. Ho, G.J. Zhou, H.B. Wu, W.C.H. Choy, J.B. Peng, Y. Cao, W.Y. Wong, Adv. Mater. 23, 2976 (2011)
S.Y. Shao, J.Q. Ding, L.X. Wang, X.B. Jing, F.S. Wang, J. Am. Chem. Soc. 134, 20290 (2012)
R. Meerheim, K. Walzer, M. Pfeiffer, K. Leo, Appl. Phys. Lett. 89, 61111 (2006)
T.N.T. Matsumoto, J. Endo, K. Mori, N. Kawamura, A. Yokoi, J. Kido, SID 03 Digest, 979, 2003
L.S. Liao, K.P. Klubek, C.W. Tang, Appl. Phys. Lett. 84, 167 (2004)
T. Tsutsui, M. Terai, Appl. Phys. Lett. 84, 440 (2004)
F.W. Guo, D.G. Ma, Appl. Phys. Lett. 87, 173510 (2005)
H. Kanno, R.J. Holmes, Y. Sun, S. Kena-Cohen, S.R. Forrest, Adv. Mater. 18, 339 (2006)
M.Y. Chan, S.L. Lai, K.M. Lau, M.K. Fung, C.S. Lee, S.T. Lee, Adv. Funct. Mater. 17, 2509 (2007)
T.W. Lee, T. Noh, B.K. Choi, M.S. Kim, D.W. Shin, J. Kido, Appl. Phys. Lett. 92, 43301 (2008)
T. Chiba, Y.-J. Pu, R. Miyazaki, K. Nakayama, H. Sasabe, J. Kido, Org. Electron. 12, 710 (2011)
M.H. Ho, T.M. Chen, P.C. Yeh, S.W. Hwang, C.H. Chen, Appl. Phys. Lett. 91, 233507 (2007)
G. Sarasqueta, K.R. Choudhury, J. Subbiah, F. So, Adv. Funct. Mater. 21, 167 (2011)
S. Hofle, A. Schienle, C. Bernhard, M. Bruns, U. Lemmer, A. Colsmann, Adv. Mater. 26, 5155 (2014)
Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A.J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun, J. Kim, M. Fenoll, A. Dindar, W. Haske, E. Najafabadi, T.M. Khan, H. Sojoudi, S. Barlow, S. Graham, J.L. Bredas, S.R. Marder, A. Kahn, B. Kippelen, Science 336, 327 (2012)
A.K. Kyaw, D.H. Wang, V. Gupta, J. Zhang, S. Chand, G.C. Bazan, A.J. Heeger, Adv. Mater. 25, 2397 (2013)
T. Xiong, F. Wang, X. Qiao, D. Ma, Appl. Phys. Lett. 93, 123310 (2008)
Y.H. Kim, T.H. Han, H. Cho, S.Y. Min, C.L. Lee, T.W. Lee, Adv. Funct. Mater. 24, 3808 (2014)
S. Hofle, A. Schienle, M. Bruns, U. Lemmer, A. Colsmann, Adv. Mater. 26, 2750 (2014)
Y. Zhu, Z. Yuan, W. Cui, Z. Wu, Q. Sun, S. Wang, Z. Kang, B. Sun, J. Mater. Chem. A 2, 1436 (2014)
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Chiba, T., Pu, YJ., Kido, J. (2015). Solution-Processed Organic Light-Emitting Devices. In: Ogawa, S. (eds) Organic Electronics Materials and Devices. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55654-1_8
Download citation
DOI: https://doi.org/10.1007/978-4-431-55654-1_8
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55653-4
Online ISBN: 978-4-431-55654-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)