Abstract
When applying luminescence dating to sediment deposited in aquatic environments, a key issue for accurate age determination is resetting of acquired luminescence in sediment by surface exposure (bleaching) before burial. The time needed for bleaching is known to vary among the signals used in three methods: optically stimulated luminescence (OSL), infrared stimulated luminescence (IRSL), and post-infrared IRSL (pIRIR). A comparison of luminescence ages from these different signals is therefore useful to assess whether a sample was fully bleached before burial. In a comparison of OSL, IRSL50/225 and pIRIR225 ages of eight samples of fine-grained sediment from a 294-cm-long sediment core from Lake Yogo, a small-catchment lake in central Japan, the IRSL50/225 and pIRIR225 ages were much older than the OSL ages. The IRSL50/225 residual signals were close to zero, and the difference between pIRIR225 and OSL signals was much larger than the pIRIR225 residual signals. Thus, IRSL50/225 and pIRIR225 signals were not completely bleached, which we attribute to the short sediment transport distance in this small catchment. Five corrected bulk radiocarbon (14C) ages agreed with the OSL ages, except for two intervals in which OSL ages were about 500 and 1,900 years older than the corrected 14C ages. These discrepancies are attributable to incomplete bleaching related to sediment transport, whereas the rest of the OSL ages show no evidence of incomplete bleaching. This study shows that even in samples in which the pIRIR225 and IRSL50/225 signals are not well-bleached, OSL dating yields accurate age estimates because of the faster bleaching rate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Lake sediments contribute to our understanding of past climate change in terrestrial areas (e.g., Colman et al. 1995; Williams et al. 1997). Depth profiles of lake sediment can provide proxy records that represent paleoenvironments (e.g., BDP-98 Members 2001; BDP-99 Members 2005). The determination of sedimentation age is critical for converting depth profiles of proxies to age profiles for reconstruction of paleoclimatic fluctuations. Radiocarbon (14C) dating is typically used for age determination of lake sediments for ages up to about 40 ka (e.g., Nara et al. 2005; Watanabe et al. 2007, 2009). Optically stimulated luminescence (OSL) and infrared stimulated luminescence (IRSL) dating methods also have the potential to date the depositional age of sediments when the acquired luminescence of sediment entering the lake is reset by sunlight (bleached) during its transport from the catchment area to the basin. The age range of OSL and IRSL dating is from the present to about 200 ka, and these methods have been successfully applied to lake sediments (e.g., Juschus et al. 2007; Zheng et al. 2010). Buylaert et al. (2013) successfully applied another luminescence method, post-IR infrared stimulated luminescence (pIRIR) dating, to K-feldspar in lake sediments older than about 70 ka. However, Zheng et al. (2010) showed that OSL ages of lake sediment younger than about 10 ka had been overestimated.
Murray et al. (2012) proposed an approach to assess the degree of bleaching based on the different bleaching rates for OSL and pIRIR signals: if feldspar pIRIR ages are close to quartz OSL ages, then the quartz must be completely bleached because the bleaching rate of the pIRIR signal in feldspar is much slower than that of the OSL signal in quartz. Their approach identifies well-bleached quartz independently of other age controls.
Before AD 1604, Lake Yogo was a small enclosed lake with a catchment area of 7.97 km2. At that time, it was artificially connected to the Yogo River and its catchment area expanded to 35.37 km2. The original Lake Yogo catchment is underlain by alluvial sediment and rocks of a Jurassic accretionary wedge, and it contains no volcanic rocks (Wakita et al. 1992). In applying OSL dating to this sediment, incomplete bleaching during sediment transport presents a significant potential problem because the initial catchment area was very small and the sediment may have traveled short distances.
This study tests the proposed approach of Murray et al. (2012) on lake sediment from a small catchment by using samples from Lake Yogo. As was done by Murray et al. (2012), we determined IRSL and pIRIR ages in addition to OSL ages. We also determined 14C ages from plant residue and the bulk sediment to provide independent age controls. We then compared luminescence and radiocarbon ages to assess the effectiveness of OSL dating of the Lake Yogo sediment core, and the validity of the approach of Murray et al. (2012) for dating lacustrine sediments from a small catchment.
11.2 Sample and Methods
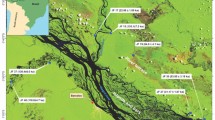
Samples for luminescence and 14C dating were taken from a 294-cm-long sediment core (YG11-3) from Lake Yogo. The core, which was obtained from the deepest part of the lake (12.5 m) by piston coring (Fig. 11.1), is composed of homogeneous brown silty clay without any distinctive features. Under subdued red light, samples were collected at 2-cm core intervals, and each sample was split in two. The first split was dried at 110 °C for 2 days and then used for measurement of water content and radioisotope concentration. The other split was not dried and was used for luminescence measurement. Plant residues were sampled from core depths of 182 and 278 cm.
11.2.1 Luminescence Dating
For luminescence measurement, wet samples were chemically treated at room temperature using 10 % hydrogen chloride to remove carbonate and then 10 % hydrogen peroxide to remove organic matter. Then, the 4–11 μm sediment size fractions were extracted by a method based on Stokes’ law by suspending the samples in a sodium oxalate solution. Portions of these polymineral fractions were used for IRSL and pIRIR dating, and the remaining fractions were further treated with 10 % hexafluorosilicic acid to remove feldspar and isolate the quartz fraction. The degree of contamination of the quartz fraction by feldspar, checked by observing IRSL emissions, was less than 1 % in all samples. The quartz fraction was then dated by OSL. The dried polymineral and quartz fractions (about 1.0 mg each) were suspended in acetone and placed on stainless steel disks 10 mm in diameter, after which the acetone was evaporated in an oven.
The luminescence signal was measured using a luminescence reader (DA-20, Risø) equipped with a 90Sr/90Y beta source at Geological Survey of Japan, AIST. Luminescence emissions in the ultraviolet region were measured through a Hoya U-340 filter for quartz OSL, and emissions in the blue-violet region were measured through Schott BG3, BG39, and GG400 filters, in ascending order, for polymineral IRSL and pIRIR. The single aliquot regenerative (SAR) protocol was applied to the determination of the equivalent dose D e (Table 11.1; Murray and Wintle 2000). The OSL signal was measured for 40 s at 125 °C. The IRSL signal was measured for 200 s at 50 °C (IRSL50/225), followed by pIRIR measurement for 200 s at 225 °C (pIRIR225). The luminescence signal was sampled every 0.1 s. The luminescence intensities were derived from the integral of the first 0.5 s (5 channels) and 2.0 s (20 channels) after subtracting the last 5 s for the OSL decay curve and the last 10 s for the IRSL and pIRIR decay curves. Radioisotope concentrations of each sample were measured by laser-ablation inductively coupled plasma mass spectrometry and x-ray fluorescence (Ito et al. 2009, 2011) and converted into annual dose rates by the method of Adamiec and Aitken (1998). Cosmic dose rate was estimated following Prescott and Hutton (1994).
11.2.2 14C Dating
14C dating of bulk sediment samples followed the protocol of Watanabe et al. (2009). Dried sediment was powdered, and then treated with 1.2 M HCl to remove carbonate. The sample was then combusted at 850 °C for 4 h with CuO wire, and the resulting CO2 was purified with ethanol, n-pentane, and liquid nitrogen in a vacuum line. The CO2 was reduced to graphite by heating with iron and hydrogen at 650 °C for 6 h.
The procedure for 14C dating of plant samples was after Nakamura et al. (2003). Small pieces of leaves were picked from the bulk sediment sample under the microscope and washed with distilled water to remove mineral grains. The leaves were treated with 1.2 M HCl and 1.2 M NaOH (acid-alkali-acid treatment), dried, and combusted. The resulting CO2 was purified and reduced to graphite by the same procedure used for the bulk sediment sample.
The 14C/12C ratio of graphite was measured with a Tandetron accelerator mass spectrometer (Model-4130, HVEE) at the Center for Chronological Research, Nagoya University. The measured ratio was corrected by δ13C, which was measured with an isotope ratio mass spectrometer (MAT-252, Thermo Finnigan). All 14C ages were converted to calendar years using the INTCAL09 data set (Reimer et al. 2009).
11.3 Results
11.3.1 OSL Dating
Quartz samples from Lake Yogo sediments exhibited OSL signals with a strong fast component (Fig. 11.2a), and the recycling ratio was close to unity. These samples thus differ from the volcanic quartz found elsewhere in Japan (e.g., Tsukamoto et al. 2003), which is characterized by anomalous fading and dominance of the slow component. Results of the preheat-plateau test, thermal transfer test, and dose recovery test for OSL analysis are presented in Fig. 11.3. The influence of hot bleaching was also investigated for these three tests, and the data with and without hot bleaching agreed within the standard error. The preheat-plateau test showed a plateau of D e around 1.6 Gy over the preheat temperature range from 160 to 280 °C (Fig. 11.3a). The thermal transfer was almost negligible at all preheat temperatures except for 300 °C (Fig. 11.3b).
For the dose recovery test, the natural sample was initially bleached using a blue LED to reduce the natural OSL to almost the background level at room temperature. After bleaching, the sample was given a beta dose of approximately 2.0 Gy, which is close to the natural D e , and then OSL was measured using preheat temperatures from 160 to 300 °C (Fig. 11.3c). The dose recovery ratio was close to unity at preheat temperatures from 160 to 280 °C. A preheat temperature of 200 °C for 10 s and a cut heat of 160 °C were chosen for all of our OSL measurements. Dose recovery tests were carried out on every sample at the preheat temperature of 200 °C, and the measured dose/given dose ratio was close to unity (1.01–1.03). The mean D e values of repeated OSL measurements were determined by the central age model of Galbraith et al. (1999) and are shown in Table 11.3. Aliquots were accepted for mean D e determination only if they yielded a recycling ratio within 1.0 ± 0.1.
11.3.2 IRSL50/225 and pIRIR225 Dating
Examples of polymineral IRSL50/225 and pIRIR225 decay curves are shown in Fig. 11.2b, c. Unbleachable residual signals of IRSL50/225 and pIRIR225 were estimated in parallel with the D e measurement and dose recovery test at a preheat temperature of 250 °C, following the protocol of Lowick et al. (2012). After the samples were exposed to natural sunlight for 2 days, the apparent residual doses were measured and plotted against the measured natural dose (Fig. 11.4). Although the residual dose of IRSL50/225 was negligible, the pIRIR225 signal contained a residual that was positively correlated with D e . The residual dose was thus calculated with an intercept value at D e = 0 after the method proposed by Sohbati et al. (2011). The residual dose of 5.08 ± 0.55 Gy was subtracted from all D e values of pIRIR225. Dose recovery tests were carried out on three samples (YG11-3-124, −245, and −349) at the preheat temperature of 250 °C, and the dose recovery ratio was close to unity (1.03–1.10 for IRSL50/225, 0.95–1.08 for pIRIR225). D e values of five aliquots were determined for both IRSL50/225 and pIRIR225, and their mean was used for final age determinations (Table 11.2).
The stability of the pIRIR225 signal was checked by estimating the fading rate (g-value) on every aliquot, which was measured and calculated following the conventional procedure (Huntley and Lamothe 2001; Auclair et al. 2003) and expressed as the percentage of luminescence decay in a decade. Most g-values for IRSL50/225 and pIRIR225 were 2.5–3.4 %/decade and smaller than 1.5 %/decade, respectively (Table 11.2). These g-values are consistent with the understanding that pIRIR225 signals are more stable than conventional IRSL signals (Thomsen et al. 2008; Buylaert et al. 2009; Thiel et al. 2010). A fading correction was not done for pIRIR signals, as Buylaert et al. (2012) suggested that samples with relatively low pIRIR290 g2days (1.44 %/decade) do not require the correction. To confirm that fading was negligible, it would be preferable to measure saturated samples (Buylaert et al. 2011). However, samples of that age were not available in the Lake Yogo sediment core. Therefore, we show pIRIR ages both uncorrected and corrected for fading. For IRSL50/225 dating, we used a fading correction because of the higher g-values.
Radioisotope concentrations are shown in Table 11.3. The alpha dose rates for fine-grained quartz and fine-grained polymineral samples were calculated using a-values of 0.04 and 0.08, respectively (Rees-Jones 1995). The water content of the samples decreased with increasing depth in the sediment core. We thus assumed an average water content for each sample as the mean between the water content of lake-floor sample YG11-3-001 and that of the sample. This modified water content (Table 11.3) was used for age determination. The calculated ages are shown in Table 11.4 and Fig. 11.5.
11.3.3 14C Dating
The bulk 14C ages of samples YG11-3-246 and YG11-3-342 were generally about 300 years older than the plant residue 14C ages of the adjoining samples YG11-3-245 and YG11-3-343 (Table 11.4). This overestimation is ascribed to the well-known old-carbon effect (e.g., Watanabe et al. 2009). We assumed that 300 years is the average overestimation of the bulk 14C ages and subtracted it to yield the corrected bulk 14C ages, which were compared with the OSL ages in this study.
11.4 Discussion
The IRSL50/225 and pIRIR225 ages were much older than the OSL ages (Table 11.4), and the residual could not account for the excess. The slower bleaching rates for IRSL and pIRIR signals thus appear to have contributed to the excess in equivalent dose. Lake Yogo has a small catchment characterized by a short sediment transport distance, which would provide less opportunity for bleaching by sunlight and result in incomplete bleaching of IRSL and pIRIR signals. Nevertheless, except for samples YG11-3-001, YG11-3-245, and YG11-3-343, five OSL ages were concordant with corrected bulk 14C ages, suggesting that there was generally enough sunlight exposure to reset the OSL signal. The IRSL signal also showed evidence of more complete bleaching than the pIRIR signal.
With regard to the three exceptional samples, there are two possible explanations for why the OSL ages were not concordant with the corrected bulk 14C ages. First, for YG11-3-245 and YG11-3-343, the OSL ages were probably older than the corrected bulk 14C ages because of incomplete bleaching. Mass movements such as river floods and landslides may lead to less exposure to sunlight and account for the poor bleaching of OSL. These two samples showed a larger difference between OSL and pIRIR ages than other samples, which is consistent with a short exposure to sunlight because the difference between the OSL and pIRIR residual signals is large for bleaching times of only a few seconds (Murray et al. 2012). In other samples, quartz was well bleached for OSL dating before deposition and gave reliable ages concordant with 14C ages. Second, for sample YG11-3-001, from near the top of the core (8 cm depth), both OSL and corrected bulk 14C ages may be overestimated. After 1960, the water balance of Lake Yogo came under artificial control for irrigation and flood-control purposes (Shimada et al. 2002); thus, changes in the organic carbon source and transport processes may have resulted in overestimated corrected bulk 14C and OSL ages. The presence of radioactivity from 137Cs (42.5 ± 5.7 Bq/kg) is evidence for the deposition of YG11-3-001 after 1960.
For coarse-grained sediments, the effects of bleaching on age determinations are best evaluated by the single-grain method and corresponding age models (such as MAM, Galbraith et al. 1999). However, for fine-grained sediments, the methods used in this study are most suitable. On the other hand, comparisons of quartz OSL and pIRIR ages (Murray et al. 2012) may be in effective in establishing the accuracy of OSL ages when the OSL signal is completely reset but IRSL and pIRIR signals are not fully reset, because of the difference in bleaching rates for the three signals. In that situation, one solution is to compare luminescence ages with an independent age control, and this study using sediment from Lake Yogo is an example of that approach’s success. Laboratory studies suggest that sun exposure of no more than a few tens of seconds is enough to perturb OSL age estimates (Murray et al. 2012). If bleaching rates of OSL, IRSL, and pIRIR signals are accurately known, these may be used to constrain the approximate time of sediment’s sunlight exposure before burial. Although OSL is the most reliable technique for age determination of young sediment, the degree of sunlight exposure in a given sediment system may be important for estimating effects of incomplete bleaching when applying the pIRIR method to much older sediments.
11.5 Summary
-
1.
The IRSL50/225 and pIRIR225 ages were much older than the OSL ages in samples from Lake Yogo, whereas the OSL ages were generally concordant with the corrected bulk 14C ages. The difference in bleaching rates of these three luminescence signals appears to have contributed to these age differences. In the case of Lake Yogo, there was enough sunlight exposure to mainly reset the OSL signal but not enough to reset the IRSL and pIRIR signals. Therefore, the bleaching period and conditions are key issues for accurate age determination of samples from small catchment areas.
-
2.
For incompletely bleached samples, comparisons between OSL and independent age controls may be more reliable than comparisons between different luminescence techniques. However, overestimated ages may be useful to constrain the approximate time of sunlight exposure before burial, based on the results of laboratory bleaching studies. For old samples, incomplete bleaching of the pIRIR signal is negligibly small compared to D e and the pIRIR method may be used to determine the sedimentation age.
References
Adamiec G, Aitken M (1998) Dose-rate conversion factors: update. Ancient TL 16:37–50
Auclair M, Lamothe M, Huot S (2003) Measurement of anomalous fading for feldspar IRSL using SAR. Radiat Meas 37:487–492
BDP-98 Members (2001) The new BDP-98 600-m drill core from Lake Baikal: a key late Cenozoic sedimentary section in continental Asia. Quatern Int 80–81:19–36
BDP-99 Members (2005) A new Quaternary record of regional tectonic, sedimentation and paleoclimate changes from drill core BDP-99 at Posolskaya Bank, Lake Baikal. Quatern Int 136:105–121
Buylaert JP, Murray AS, Thomsen KJ, Jain M (2009) Testing the potential of an elevated temperature IRSL signal from K-feldspar. Radiat Meas 44:560–565
Buylaert JP, Thiel C, Murray AS, Vandenberghe DAG, Yi S, Lu H (2011) IRSL and post-IR residual doses recorded in modern dust samples from the Chinese Loess Plateau. Geochronometria 38:432–440
Buylaert JP, Jain M, Murray AS, Thomsen KJ, Thiel C, Sohbati R (2012) A robust feldspar luminescence dating method for Middle and Late Pleistocene sediments. Boreas 41:435–451
Buylaert JP, Murray AS, Gebhardt AC, Sohbati R, Ohlendorf C, Thiel C, Wastegard S, Zolitschka B, The PASADO, Team S (2013) Luminescence dating of the PASADO core 5022-1D from Laguna Potrok Aike (Argentina) using IRSL signals from feldspar. Quatern Sci Rev 71:70–80
Colman SM, Peck JA, Karabanov EB, Carter SJ, Bradbury JP, King JW, Williams DF (1995) Continental climate response to orbital forcing form biogenic silica records in Lake Baikal. Nature 378:21–28
Galbraith RF, Roberts RG, Laslett GM, Yoshida H, Olley JM (1999) Optical dating of single grains of quartz from Jinmium rock shelter, northern Australia: part 1, experimental design and statistical models. Archaeometry 41:339–364
Huntley DJ, Lamothe M (2001) Ubiquity of anomalous fading in K-feldspars and the measurement and correction for it in optical dating. Can J Earth Sci 38:1093–1106
Ito K, Hasebe N, Sumita R, Arai S, Yamamoto M, Kashiwaya K, Ganzawa Y (2009) LA-ICP-MS analysis of pressed powder pellets to luminescence geochronology. Chem Geol 262:131–137
Ito K, Hasebe N, Hasebe A, Arai S (2011) The matrix effect on 238U and 232Th measurements using pressed powder pellets by LA-ICP-MS. Geochem J 45:375–385
Juschus O, Preusser F, Melles M, Radtke U (2007) Applying SAR-IRSL methodology for dating fine-grained sediments from Lake El’gygytgyn, north-eastern Siberia. Quatern Geochronol 2:187–194
Lowick SE, Trauerstein M, Preusser F (2012) Testing the application of post IR-IRSL dating to fine grain waterlain sediments. Quatern Geochronol 8:33–40
Murray AS, Wintle AG (2000) Luminescence dating of quartz using an improved single-aliquot regenerative- dose protocol. Radiat Meas 32:57–73
Murray AS, Thomsen KJ, Masuda N, Buylaert JP, Jain M (2012) Identifying well-bleached quartz using the different bleaching rates of quartz and feldspar luminescence signals. Radiat Meas 47:688–695
Nakamura T, Oda T, Tanaka A, Horiuchi K (2003) High precision 14C age estimation of bottom sediments of Lake Baikal and Lake Hovsgol by AMS. Gekkan Chikyu 42, Kaiyoushuppan, Tokyo, pp 20–31. (in Japanese)
Nara F, Tani Y, Soma Y, Soma M, Naraoka H, Watanabe T, Horiuchi K, Kawai T, Oda T, Nakamura T (2005) Response of phytoplankton productivity to climate change recorded by sedimentary photosynthetic pigments in Lake Hovsgol (Mongolia) for the last 23,000 years. Quatern Int 136:71–81
Prescott JR, Hutton JT (1994) Cosmic ray contributions to dose rates for luminescence and ESR dating: large depths and long-term time variations. Radiat Meas 23:497–500
Rees-Jones J (1995) Optical dating of young sediments using fine-grain quartz. Ancient TL 13:9–14
Reimer PJ, Baillie MGL, Bard E, Bayliss A, Beck JW, Blackwell PG, Bronk Ramsey C, Buck CE, Burr GS, Edwards RL, Friendrich M, Grootes PM, Guilderson TP, Hajdas I, Heaton TJ, Hogg AG, Hughen KA, Kaiser KF, Kromer B, McCormac FG, Manning SW, Reimer RW, Richards DA, Southon JR, Talamo S, Turney CSM, van der Plicht J, Weyhenmeyer CE (2009) IntCal09 and Marine09 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon 51:1111–1150
Shimada T, Kashiwaya K, Hyodo M, Masuzawa T (2002) Hydro-environmental fluctuation in a lake-catchment system during the Late Holocene inferred from Lake Yogo sediments. Trans Jpn Geomorphol Union 23:415–431. (in Japanese with English abstract)
Sohbati R, Murray AS, Buylaert JP, Ortuño M, Cunha PP, Masana E (2011) Luminescence dating of Pleistocene alluvial sediments affected by the Alhama de Murcia fault (eastern Betics, Spain) – a comparison between OSL, IRSL and post-IR IRSL ages. Boreas 41:250–262
Thiel C, Coltorti M, Tsukamoto S, Frechen M (2010) Geochronology for some key sites along the coast of Sardinia (Italy). Quatern Int 222:36–47
Thomsen KJ, Murray AS, Jain M, Bøtter-Jensen L (2008) Laboratory fading rates of various luminescence signals from feldspar-rich sediment extracts. Radiat Meas 43:1474–1486
Tsukamoto S, Rink WJ, Watanuki T (2003) OSL of tephric loess and volcanic quartz in Japan and an alternative procedure for estimation D e from a fast OSL component. Radiat Meas 37:459–465
Wakita K, Harayama S, Kano K, Mimura K, Sakamoto T, Hiroshima T, Komazawa M (1992) Geological map, 1: 200,000, Gifu. Geological Survey of Japan, Tsukuba
Watanabe T, Nakamura T, Kawai T (2007) Radiocarbon dating of sediments from large continental lakes (Lakes Baikal, Hovsgol and Erhel). Nucl Instrum Method Phys Res B 259:565–570
Watanabe T, Nakamura T, Watanabe Nara F, Kakegawa T, Horiuchi K, Senda R, Oda T, Nishimura M, Inoue Matsumoto G, Kawai T (2009) High-time resolution AMS 14C data sets for Lake Baikal and Lake Hovsgol sediment cores: changes in radiocarbon age and sedimentation rates during the transition from the last glacial to the Holocene. Quatern Int 205:12–20
Williams DF, Peck J, Karabanov EB, Prokopenko AA, Kravchinsky V, King J, Kuzmin MI (1997) Lake Baikal record of continental climate response to orbital insolation during the past 5 million years. Science 278:1114–1117
Zheng YE, Zhou LP, Zhang JF (2010) Optical dating of the upper 22 m of cored sediments from Daihai Lake, northern China. Quatern Geochronol 5:228–232
Acknowledgments
We thank Akihiro Hasebe and Haruka Hayashi for their help with the LA-ICP-MS and XRF analyses, respectively. This work was supported by a JSPS Research Fellowship (24–810) for Young Scientists from the Japan Society for the Promotion of Science (Ito) and Grants-in-Aid for Scientific Research (A-23240120).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Ito, K. et al. (2015). Comparison of Luminescence Dating Methods on Lake Sediments from a Small Catchment: Example from Lake Yogo, Japan. In: Kashiwaya, K., Shen, J., Kim, J. (eds) Earth Surface Processes and Environmental Changes in East Asia. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55540-7_11
Download citation
DOI: https://doi.org/10.1007/978-4-431-55540-7_11
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55539-1
Online ISBN: 978-4-431-55540-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)